Abstract
Aging effects on sleep are important to consider for the practicing pulmonologist due to the increase in prevalence of major respiratory disorders as well as the normal changes that occur in sleep patterns with aging. Typically, aging is associated with decreases in the amount of slow wave sleep and increases in stage 1 and 2 non–rapid eye movement sleep, often attributed to an increased number of spontaneous arousals that occur in the elderly. Elderly individuals tend to go to sleep earlier in the evening and wake earlier due to a phase advance in their normal circadian sleep cycle. Furthermore the development of sleep-related respiratory disorders such as obstructive sleep apnea (OSA) and central sleep apnea or Cheyne-Stokes respiration (CSA-CSR) associated with congestive heart failure (CHF) occur with increasing prevalence in the elderly. The development of such disorders is often of major concern because they are associated with systemic hypertension and cardiovascular disease, metabolic disorders such as diabetes, and impaired neurocognition. The present review reflects the current understanding of the normal changes in sleep patterns and sleep needs with advancing age, in addition to the effect that aging has on the predisposition to and consequences of OSA and CSA-CSR associated with CHF.
Keywords: Aging, sleep patterns, sleep apnea, congestive heart failure
Sleep is a vital physiological process with important restorative functions that are essential for optimal day-time functioning. Insufficient or poor quality sleep has been associated with neurocognitive impairments,1,2 end-organ dysfunction and chronic health conditions,3–5 and increased mortality.6–8 Importantly, aging is associated with both qualitative and quantitative changes in our sleep pattern and distribution. For instance, in infancy, sleep duration is at a lifetime maximum, with newborn infants sleeping for ~16 hours each day. This sleep requirement decreases through childhood, reaching 7 to 8 hours in adulthood. Although less well studied, there is evidence that sleep duration further decreases from young adulthood into our older years. However, such an idea remains controversial, with other studies suggesting that the amount of sleep does not change; instead sleep in the elderly becomes more fragmented and is usually consolidated through daytime naps. To date, several factors, such as cumulative health problems and changes in circadian influences have been hypothesized as mechanisms underlying reduced sleep quality in the elderly. In addition, a major factor contributing to poor-quality sleep is the presence of sleep-related disorders, which occur with increasing frequency among elderly people.9–11
The study of aging in the sleep field has been challenging for several reasons, many of which are true for aging research in general. First, the prevalence of comorbidities is high in the elderly, leading to discussion about what is normal aging. On the one hand, some would argue to exclude comorbidities and study the healthy elderly. On the other hand, some argue that these “super-healthy” individuals are not a representative or generalizable sample. Thus an ongoing debate exists regarding the definition of the term healthy aging. Second, some sleep research protocols can be somewhat onerous, and the ability to make careful assessments in vulnerable populations can be compromised. Thus the number of sleep papers focused on elderly cohorts is somewhat modest. Third, clinical samples reflect referral biases that are complicated in the field of sleep and aging. For example, the elderly may be less prone to sleepiness from sleep deprivation or sleep apnea; thus one could argue that they may be less likely to be referred with a clinical complaint. Similarly, the elderly may be more isolated than younger cohorts, and fewer bed partners and family members may be in a position to recognize a potential abnormality during sleep. In this article we review the existing literature in the context of our clinical and research experience. We review the current understanding aging’s impact on sleep as well as the pathogenesis and consequences of sleep-related disorders, namely obstructive sleep apnea (OSA) and central sleep apnea or Cheyne-Stokes respiration (CSA-CSR), including its association with congestive heart failure (CHF).
PHYSIOLOGICAL CHANGES IN SLEEP PATTERNS WITH AGE
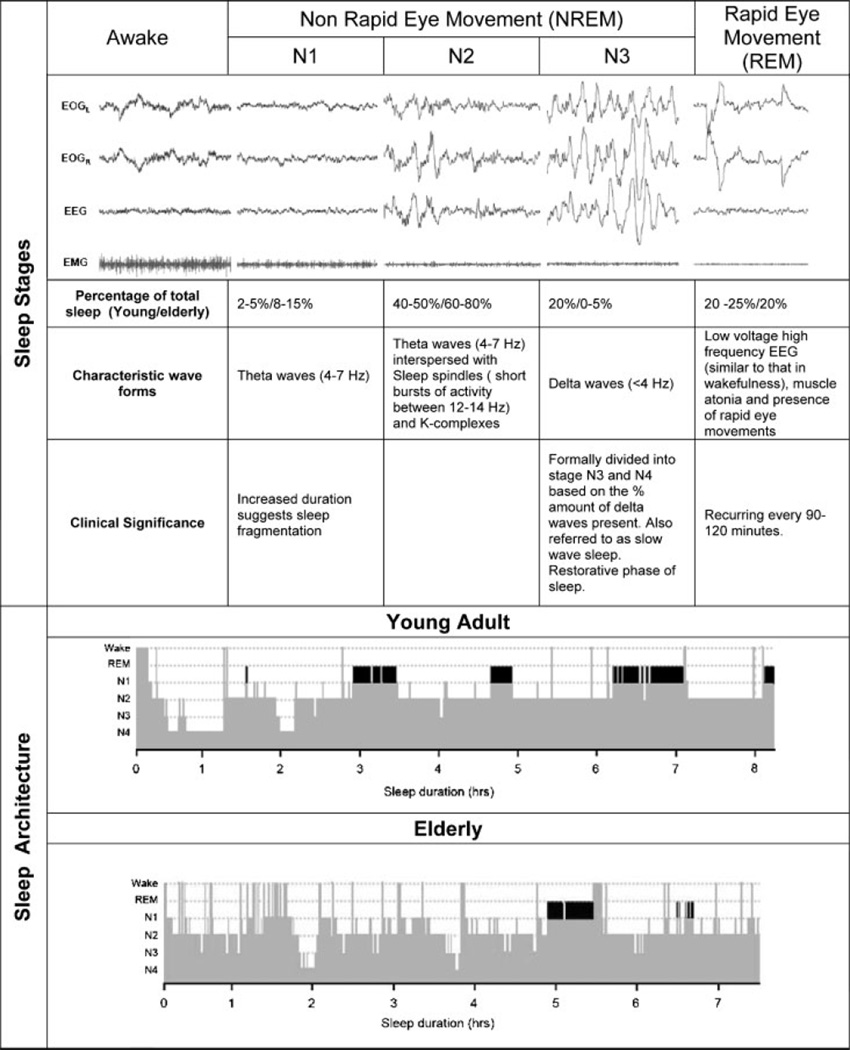
Accurate monitoring of sleep via polysomnography involves recording of cortical activity from the electroencephalogram (EEG), as well as recording of eye movements, muscle tone, and cardiorespiratory activity. Sleep is characterized by both non–rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. NREM sleep is further subdivided into three stages; N1 (formerly stage 1), N2 (stage 2), and N3 (stages 3 and 4) corresponding to increasing depth of sleep and differentiated by a specific electroencephalographic (EEG) frequency (Fig. 1, top panel). The transition from wakefulness to NREM sleep is associated with a reduction in ventilation and heart rate as well as a small reduction in metabolic rate, possibly due to relative autonomic stability. In contrast, during REM sleep ventilation becomes highly variable, and cardiovascular activity becomes irregular due to bursts of sympathetic nerve activity unique to REM sleep.
Figure 1.
Effect of aging on the architecture of sleep. The top panel depicts the different stages of sleep and their associated characteristics. The bottom panel is an example of a typical hypnogram from a healthy young adult aged 24 years and a healthy elderly individual aged 72 years. It demonstrates the cycling between non–rapid eye movement (NREM) and rapid eye movement (REM) (black bars) throughout the night, with more stage 3 and 4 NREM sleep or slow wave sleep (SWS) during the early part of the night and the increased proportion of REM in the early hours. Note that elderly individuals have a reduction in SWS and REM as well as the frequent arousals/awakenings. N1–N4, non–rapid eye movement sleep stages 1 through 4.
The distribution of sleep stages across the night, commonly referred to as sleep architecture, is displayed via a hypnogram (Fig. 1, bottom panel). In healthy adults, sleep progresses through NREM stages N1 through N3 followed by a period of REM occurring ~90 minutes into sleep. This sleep state cycling is a fundamental feature of sleep, although there is a reduction in N3 and increase in REM sleep as the night progresses. Sleep is punctuated by brief arousals (defined as an increase in EEG frequency to >8 Hz lasting longer than 3 seconds) and awakenings (periods of wakefulness >30 seconds). As we age, however, important changes in the patterns or structure of sleep occur, which have been the topic of several excellent reviews12–14 that are summarized in Table 1 and illustrated in Fig. 1B. A meta-analysis of 65 studies representing 3577 healthy subjects has shown that the total amount of sleep decreases linearly with age with a loss of ~10 minutes per decade.15 Sleep architecture also changes with age. Up to the age of 60 years, the percentage of N3 sleep decreases linearly at 2% per decade. The percentage of REM sleep also diminishes, although the decline is more subtle. As with N3, the percentage of REM sleep appears to plateau after age 60. The net result of these changes is an increase in N1 and N2. In addition, sleep efficiency continues to decline due to increased sleep latency, arousals from sleep and time awake after sleep onset; however, the mechanisms responsible for these changes in sleep architecture are unclear. Although it has been postulated that these changes reflect age-related reduction in EEG amplitude produced by the increase in electrical impedance of the skull and scalp, they are more likely a reflection of age-related neural degeneration and changes in hormonal systems.16
Table 1.
Typical Changes that Occur in Sleep Patterns with Age
| Total sleep time decreases |
| Sleep onset or latency becomes delayed |
| Increased daytime napping |
| Increase in awakenings and arousals |
| Decreased sleep efficiency |
| Increased stage 1 and 2 sleep |
| Decreased stage 3 and 4 sleep or slow wave sleep (SWS) |
| Circadian phase advanced (i.e., early to bed and early to rise) |
| Decreased rapid-eye movement (REM) sleep |
| Fewer sleep cycles through per night |
Numerous studies have also identified an interesting gender disparity in these age-related changes in sleep architecture. Although many of these individual studies give conflicting results, a meta-analysis of 65 studies of healthy adults indicates that men are more affected by aging than women. In this analysis, men were shown to have decreased total sleep time, decreased percentage of N3 and REM sleep, and increased percentage of N2 and wake time after sleep onset compared with women.15 On the other hand women had increased sleep latencies when compared with men. These findings are of particular interest given that women often self-report shorter and poorer sleep compared with men.17 This greater perception of sleep difficulty in women is cited as the reason why women use hypnotics at a greater frequency than men.18 The results of this meta-analysis on gender differences in age-related changes in sleep architecture were confirmed the same year with data from the Sleep Heart Health Study (n = 2685).11 Overall, whether this gender difference in sleep architecture is a result of hormonal changes or environmental factors remains to be elucidated.
In addition to the global changes that occur in the sleep architecture, several studies have examined the changes that occur in the microstructure of sleep. Such studies have demonstrated an age-related decrease in both spontaneous K-complexes and sleep spindle densities,19,20 as well as the number and amplitude of evoked K-complexes.21 The findings of these studies have been interpreted to reflect an age-related alteration of thalamocortical regulatory mechanisms, which could be used to identify the changes in neurobiology of the brain with advancing age.
Whether sleep need changes with aging is a more complex question; the relevant literature having been reviewed by Bliwise.14 A large number of investigations that have used either surveys or laboratory sleep assessments to address this question have reported that sleep time is reduced in the elderly; however, other studies have reported no change or even an increase in sleep time. Consolidation of such findings is difficult because the results are complicated by whether total sleep time was assessed objectively or was self-reported and whether daytime napping was included, given that ~15% of people aged over 55 years are reported to nap four to seven times per week.22 One current view is that total sleep time may not necessarily decrease with age, but the way in which sleep is consolidated becomes altered (i.e., daytime napping). Such a hypothesis is strengthened by findings that elderly individuals are sleepier during the day, measured by tests like the multiple sleep latency test, indicating a reorganization of sleep disruption. On the one hand, there is evidence that napping in healthy individuals improves daytime functioning.23 On the other hand, there is evidence that napping is associated with a higher risk of all-cause mortality in some studies.24–26 Although excessive daytime somnolence is common in the elderly and may be indicative of cognitive impairment,27 it is often secondary to a comorbidity and its treatment or an underlying sleep disorder.28 Thus, when sleep need is assessed by both total sleep time and daytime functioning, healthy elderly people are no sleepier than their younger counterparts. Interestingly, a recent study by Duffy et al29 showed that healthy elderly individuals were less sleepy and performed better in tests of alertness and attention than young subjects after sleep deprivation supporting the concept that sleep need may reduce with age. Of note though, a recent Spanish study investigating sleep duration in the elderly has shown that health-related quality of life was reduced at sleep durations less than or greater than 7 hours, particularly at the extreme sleep durations (<5 h and >10 h).30
CHANGES IN SLEEP QUALITY AND CIRCADIAN RHYTHMS
Along with the reported changes in sleep architecture come reports of decreasing sleep quality. More specifically, difficulties in initiating sleep have been reported in 13 to 45%, disrupted sleep in 20 to 65%, early morning awakening in 15 to 54%, and nonrestorative sleep in 11%31 of older adults. The question then remains: Are these changes in sleep quality simply a part of aging, or are they secondary to other comorbidities that arise with advancing age? Much of the evidence points to the latter. For example, after controlling for comorbidities (e.g., chronic pain, depression, heart disease), the high rates of insomnia reported in the elderly population become considerably lower. The age-related increase in insomnia is abolished when social satisfaction and activity status are considered using adequate statistical control.32 This finding is further supported by a study of 11,924 Canadians, which showed no association between aging and insomnia.33 Importantly, reductions in reported difficulties of sleep initiation and maintenance are associated with improved health.34 Foley et al35 reported that from a cohort of over 9000 people over 65 years of age, only 12% reported an absence of sleep complaints, but that aging generally was not associated with more frequent complaints after adjusting for health status. In addition, Vitiello et al36 found in two large research cohorts that only 1.35% and 3.14% of adults >60 years of age had major sleep complaints or disorders after screening for medical and psychiatric illnesses. Lastly, data from the 2003 National Sleep Foundation Survey from people aged 55 to 84 years revealed that ~40% of those with major comorbidity perceived their sleep to be of only fair to poor quality.37 This is striking when compared with those without medical conditions in whom only 10% perceive their sleep quality as fair to poor. Combined, these epidemiological studies suggest that many of the age-related sleep problems are secondary to medical illness rather than to aging per se. Although much of the increased prevalence of insomnia in the elderly is secondary in nature, primary insomnia can still occur. Treatment of primary insomnia was recently reviewed38 and can involve both nonpharmacological and pharmacological therapy. Nonpharmacological therapy, such as cognitive behavioral therapy, is effective but can be time consuming and difficult to employ in the primary care setting. Pharmacological options include hypnotics, which have been shown to be beneficial in the treatment of insomnia in the elderly, although safety profiles require monitoring.
In addition to changes in sleep quality, elderly individuals often tend to go to bed earlier and wake up earlier when compared with younger individuals. Although this finding was originally attributed to a phase advance of the circadian oscillator,39,40 recent evidence suggests that the age-related advance in sleep–wake timing may result from an inability to sustain sleep at particular circadian phases or be attributed to the presence of evening napping and advanced illumination.41,42 Advanced age is characterized by a deterioration of the circadian variation of many physiological processes that play an important role in homeostasis (e.g., sleep–wake cycle, core body temperature, performance, alertness, and secretion of many hormones). In humans the circadian rhythm is generated by the circadian pacemaker that is situated in the suprachiasmatic nucleus (SCN) of the hypothalamus and synchronized to 24 hours, primarily by the light–dark cycle acting via the SCN. Aging is associated with malfunction or decrease in sensitivity of the SCN to environmental cues to adjust circadian rhythm to a natural 24-hour day/night cycle, which may explain why older people are generally less tolerant of shift work and jet lag. One key sleep-promoting hormone, melatonin, is released in the evening during darkness. Melatonin concentrations decrease from puberty, eventually reaching levels similar to daytime concentrations in old age,43 which may contribute to the increased frequency of sleep-related disorders with aging.44
Other Factors Influencing Sleep Problems in the Elderly
Behavioral and environmental factors may also contribute to the frequent awakenings and the inability to sleep well in the elderly. Poor sleep hygiene, dietary habits, excessive daytime napping,22 and increased nocturia45 may be detrimental to nocturnal sleep. Environmental factors such as noise can affect the quality of sleep,46 particularly because elderly individuals have more N1 and N2 sleep, and may be responsible for causing further arousals and disruption in the elderly. In addition, elderly individuals experience a higher prevalence of medical conditions that make sleep consolidation difficult, including chronic cardiac or pulmonary disease, and any condition associated with chronic discomfort, such as arthritis. Unfortunately, medications taken for the symptoms of these conditions may also promote insomnia. The prevalence of several sleep disorders also increases with aging, including restless legs syndrome and REM behavior disorder. Furthermore, a common sleep disorder that impairs an elderly individual’s ability to achieve consolidated sleep is sleep-disordered breathing.
SLEEP-DISORDERED BREATHING
Sleep-disordered breathing (SDB) is a broad term that encompasses a range of breathing disorders, from primary snoring through to upper airways resistance syndrome and obstructive sleep apnea. Less common, but seen primarily in the aging person, is central sleep apnea, which is often associated with CHF.
OBSTRUCTIVE SLEEP APNEA
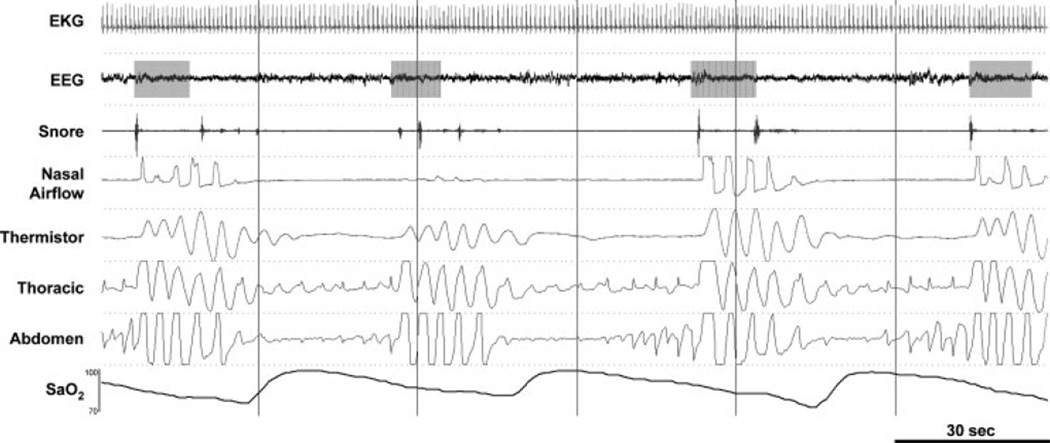
Obstructive sleep apnea (OSA) is a common syndrome in the middle-aged U.S. population, being present in at least 4% of adult men and 2% of adult women.47 The disorder is characterized by repetitive collapse (apnea) or partial collapse (hypopnea) of the pharyngeal airway during sleep.48–51 These airway obstructions lead to increasingly powerful respiratory efforts until the airway reopens and breathing is restored, often in association with an arousal from sleep (Fig. 2). These transient events also expose the sufferer to intermittent hypoxia and hypercapnia, large swings in intrathoracic pressure, as well as surges in sympathetic activation, all of which have important deleterious consequences.
Figure 2.
An example of obstructive sleep apnea (OSA). Polysomnographic example from a clinical study in a patient with severe OSA (apnea/hypopnea index = 55.5 events per hour). Note that despite the repeated respiratory efforts to breathe (thoracic and abdomen), there is no nasal airflow, indicating the airway has become obstructed. Such obstructions are often associated with repeated oxygen desaturations and arousals (gray boxes) from sleep. EKG, electrocardiogram; EEG, electroencephalogram (C3-A2); SaO2, arterial blood oxygen saturation.
Effects of Aging on the Pathophysiology of OSA
Aging is known to be a major factor contributing to the risk of OSA, with increases in age associated with apnea prevalence. Ancoli-Israel et al found that 62% of community-dwelling individuals over 60 years of age had a respiratory disturbance index ≥10/h (number of apneas and hypopneas per hour of sleep).52 Similarly the Sleep Heart Health Study, one of the largest epidemiological studies to date, found stepwise increases in the prevalence of SDB with advancing age; it is noteworthy that ~20% of subjects aged over 60 years had an RDI ≥15/h.53 In addition, Mehra et al recently showed that 26% of older men in a large cohort of almost 3000 had an RDI ≥15/h, with aging being an independent risk factor in multivariate logistic regression analyses. Even individuals who are healthy, nonobese, and asymptomatic for OSA demonstrate a marked increase in the respiratory disturbance index with age, especially for men.54,55
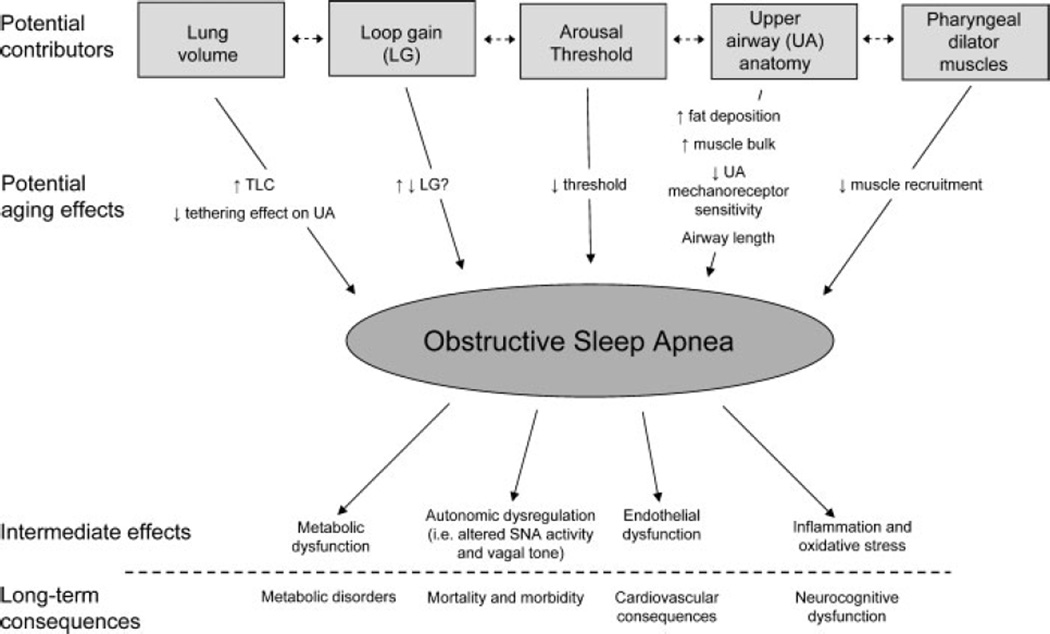
The exact mechanisms whereby aging increases the risk of OSA are not completely understood. Although several physiological factors or phenotypical traits have been identified, the amount each trait contributes to an individual’s OSA is likely to vary with different combinations of traits that are present in each patient. The majority of data suggest that an anatomically small pharyngeal airway is a key factor in the development of upper airway (UA) obstruction with pharyngeal dilator muscles compensating for the anatomical deficiency during wakefulness, but not during sleep. The ability to maintain a patent airway is a balance between (1) the amount of soft tissue located in the bony compartment created by the mandible and spinal column and (2) the ability or strength of the pharyngeal dilator muscles to contract. It is therefore not surprising that many OSA patients have a reduced airway lumen due to increased soft tissue and a compromised pharyngeal anatomy. In addition to UA anatomy, several phenotypic traits are likely to be important: the ability of pharyngeal dilator muscles to respond to chemical and mechanical stimuli during sleep, the ease with which arousal from sleep is triggered by obstructed breathing efforts (arousal threshold), changes in lung volume and recoil (since increased end-expiratory lung volume can tether open the upper airway) and the effect of ventilatory control stability (loop gain). It is likely that aging may be associated with important changes in one or a combination of the factors that may contribute to OSA in such groups (summarized in Fig. 3).
Figure 3.
Summary of the potential aging effects on the pathogenesis of obstructive sleep apnea (OSA). Schematic of the various phenotypical traits or factors that contribute to OSA and how aging may affect them to increase the incidence of OSA in the elderly. Furthermore both the intermediate effects of OSA and the long-term consequences are also depicted. Refer to the text for further detail.
UPPER AIRWAY ANATOMY AND COLLAPSIBILITY
The UA in the human is composed of numerous muscles and soft tissue but lacks bony or rigid support. As such there are several anatomical and physiological influences that bias toward a collapsible airway. Perhaps the most obvious factor predisposing to OSA is the size or caliber of the airway; a large airway will be less prone to collapse than a small airway. Most studies that have examined age-related changes in the UA have used imaging techniques such as magnetic resonance imaging (MRI) or computed topography (CT) and acoustic reflection. Malhotra et al used MRI studies of the upper airway and observed increased deposition of parapharyngeal fat in healthy older individuals as compared with younger controls56 that was independent of body mass index (BMI), suggesting a preferential deposition of parapharyngeal fat with aging. Furthermore, Martin et al measured UA caliber using acoustic reflection in 114 subjects over the age range of 16 to 74 years and found that all UA dimensions, except at the oropharyngeal junction, decreased modestly with age.57
There has also been controversy in the published data regarding the effects of aging on pharyngeal resistance and collapsibility, with some groups showing similar findings in older versus younger individuals, whereas others have reported pharyngeal compromise in the elderly.57–60 Eikermann et al61 recently observed increased pharyngeal collapsibility during sleep in older as compared with younger individuals. However, an increase in the predisposition to pharyngeal collapse due to anatomy has not been shown in all investigations. Mayer et al measured airway size using CT and reported that older patients (>63 years) had larger UAs at all pharyngeal levels than the youngest group of patients (<52 years).62 Similarly, Burger et al reported an increase in pharyngeal airway lumen area with aging at functional residual capacity (FRC); however, only normal subjects were included, potentially leading to influences of survivor effects and selection bias.63 Thus it is difficult to draw firm conclusions from the available imaging data about the changes in upper airway and the predisposition toward collapse with age.
In addition to the size of the airway, the length of the airway has been suggested to also be an important predisposing factor to the pathogenesis of OSA, especially in males.64 Specifically, it has been proposed that as the length of the airway increases, it becomes more prone to collapse. Such an effect is not simply limited to males because some data have shown that there are changes in pharyngeal airway length at menopause that likely adversely affect pharyngeal mechanics in females.56,64–66 As such, the current data suggest that changes in the length of the airway may be important in the predisposition to OSA with aging.
UPPER AIRWAY DILATOR MUSCLE ACTIVITY
UA patency depends not only on the anatomy of the UA but also on the ability of the UA dilator muscles to be recruited. Unfortunately, there have been only a few studies addressing the impact of aging on pharyngeal dilator muscle function and responsiveness. The ability of the genioglossus muscle (the major pharyngeal dilator muscle) to respond to increases in pharyngeal negative pressure is impaired with aging, yielding a more vulnerable airway.56,67 Although such studies recently reported marked impairment in UA protective reflexes in association with aging, these studies were conducted in healthy subjects during wakefulness. Whether aging influences on pharyngeal airway dilator muscle recruitability are important in mediating pharyngeal compromise in the elderly is also unclear. One report demonstrated a diminished genioglossus muscle response to hypoxia in association with aging, although the mechanistic importance to OSA of this observation is unclear.68 Nonetheless, taken in aggregate, the current evidence strongly suggests that the anatomical susceptibility (i.e., UA anatomy and physiology) to OSA appears to worsen with age.
LUNG VOLUME CHANGES
The indirect effect that changes in lung volume have on airway patency is also an important factor. An increase in lung volume can apply a caudal traction force on the trachea and larynx. Such traction can induce longitudinal tension on the UA, reduce the intraluminal pressure required to close and reopen the UA, as well as decrease the pressure exerted on the UA walls by surrounding tissues,69,70 all of which may assist in patency. There have been several important human studies that have suggested that increases in end-expiratory lung volume can provide a protective influence on the UA.71,72 Furthermore, studies have demonstrated that manipulations in end-expiratory lung volume during sleep have a marked effect on pharyngeal collapsibility in controls and those with OSA.73–75 There are also data demonstrating that the FRC is reduced in supine obese patients76 and that FRC tends to decrease following sleep onset.77
Lung compliance is known to increase with aging as a result of airspace dilatation and remodeling of lung parenchyma (“senile emphysema”).78,79 Furthermore, obesity can cause reductions in the FRC, which may also increase the propensity for pharyngeal collapse. The existing data might therefore suggest that aging-related decrements in lung elastic recoil could compromise pharyngeal mechanics.80,81 In theory, older individuals may have less lung volume tethering on the UA than younger individuals.78,79 However, there have been no systematic studies addressing the hypothesis that the aging predisposition to pharyngeal collapse is mediated (at least in part) via reduced end-expiratory lung volume tethering during sleep reported to date.
AROUSAL THRESHOLD
Arousals from sleep due to UA obstruction occur when ventilatory drive reaches a certain threshold: often termed the arousal threshold.82 The arousal from sleep is an important protective mechanism used to reopen the obstructed airway and ameliorate the hypoxemia associated with the hypopnea or apnea. However, the aging effects on arousal threshold have been minimally studied. Indeed, elderly people are known to have an increased number of spontaneous arousals from sleep,11,83,84 suggesting that older adults may have a lower arousal threshold. In contrast, recent studies examining how the arousal threshold changes with age have found no difference,61,85 suggesting that aging does not have a major impact on the threshold. Thus controversy remains regarding aging effects on arousal threshold.
VENTILATORY CONTROL STABILITY OR LOOP GAIN
The stability of the respiratory control system is also an important factor in the pathogenesis of OSA.86 The stability of the ventilatory system is often described using the engineering concept of loop gain (LG). LG is formally defined as a unitless number that characterizes the sensitivity of control systems with negative feedback. In respiratory control, LG is the product of the combined sensitivity of the peripheral and central chemoreceptors (i.e., controller gain) and the efficiency of the lungs and muscles that produce ventilation to determine the level of O2 and CO2 in the blood for any given ventilatory drive (i.e., plant gain). Mathematically, LG can be defined as the size of a ventilatory correction or response divided by the size of the disturbance prior. For example, if ventilation is disturbed in some way, such as by a hypopnea, there will be a fall in arterial O2 and a rise in CO2, and ventilation will increase as a consequence when the event is terminated. If the magnitude of the response is larger than the disturbance then LG will exceed 1, and breathing disturbance will grow in amplitude and result in unstable breathing. Conversely, if LG is less than 1, breathing will remain stable.
There are several features of the ventilatory pattern in elderly individuals which suggest that the chemical control of breathing is unstable. For example, an increased proportion of central apneas in elderly patients with sleep apnea have been reported.52,87–89 Furthermore, when compared with healthy young adults, the prevalence of periodic breathing in the elderly is increased, especially in those with CHF90 (see later discussion). Importantly, if the stability of the respiratory controller decreases with age, it could contribute to the increased prevalence of OSA in the elderly. In contrast, studies have shown that chemosensitivity is actually unchanged91 or even reduced with age.92–94 Although chemosensitivity is only one factor that contributes to the chemical control of breathing, studies of chemosensitivity suggest that the system actually becomes more stable with age. In accordance with such studies, a recent investigation demonstrated that LG values for healthy, elderly patients with or without OSA (when measured with the proportional assist ventilation technique) were quite low.95 Although this study was limited by the small number of elderly patients with OSA that were studied, the data indicate that ventilatory control is quite stable in the elderly. This finding, combined with the uncertainty surrounding the role of changes in the arousal threshold with advancing age, suggests that OSA in the elderly can primarily be attributed to changes in the anatomy and physiology of the UA.
Consequences of OSA
The consequences of OSA fall broadly into two domains: (1) neurocognitive dysfunction, such as excessive daytime sleepiness and decreased quality of life resulting from sleep fragmentation96–98 and (2) metabolic dysfunction99–101 and cardiovascular disease (including atherosclerosis, stroke, myocardial infarction, hypertension, and CHF), which are likely the result of the intermittent hypoxia and sympathetic stimulation during OSA.48,102,103 Although the prevalence of OSA tends to increase with age, a recent report demonstrates that the clinical significance and severity of OSA may actually decrease.104 Interestingly, although OSA probably results in increased morbidity and mortality during middle age, it has been suggested that OSA may have a protective role in patients aged ≥70 years,105 perhaps due to underlying factors that enhance survival or as a result of the development of protective mechanisms. However, such a proposal still remains controversial because the effects that aging has on such consequences of OSA are complex and not necessarily independent of each other.
NEUROCOGNITIVE FUNCTION
Cognitive impairment is known to increase with age, from a prevalence of up to 19% in individuals <75 years to 29% in those >85 years.106 Furthermore, 47.2% of individuals over the age of 85 years are known to have dementia, including Alzheimer disease.107,108 Because SDB is more prevalent in the elderly, it is plausible that the presence of a sleep disorder could worsen dementia. SDB occurs more frequently in patients with Alzheimer disease than in nondemented older subjects, and its severity is correlated with the degree of cognitive impairment in this group. Interestingly there is limited evidence that treatment of sleep apnea may reverse some symptoms of dementia in some studies.109 and it has been suggested that practitioners should consider SDB in the differential diagnosis of reversible dementia in older patients.110
In middle-aged individuals, good evidence shows that the intermittent hypoxemia and arousals associated with frequent airway obstructions result in fragmented sleep and contribute to impaired cognitive functions such as memory, attention, and learning.96–98 However, the association between OSA and neurocognitive impairment in elderly individuals is complex, and may be confounded by the increased prevalence of other comorbidities.109,111,112 Two studies in a group of elderly Japanese American men have reported no association between SDB and cognition,34,113 whereas several other studies have reported that the presence of SDB in the elderly increases the risk of cognitive impairment,27,112,114 especially in older women.115 Additionally, some studies have suggested common genetic features that link OSA and dementia based on ApoE genes.115,116 Another possibility is that some dementia patients actually have pseudodementia that is caused by depression, a condition whose incidence is known to increase in incidence with untreated sleep apnea.53 As such, the mechanism underlying the link between these two disorders still requires further investigation.
OBESITY AND METABOLIC DYSFUNCTION
Obesity is one of the most common public health issues that the developed world faces and is well known to be linked to the prevalence of OSA.117 Aging may increase the risk for OSA because the prevalence of obesity increases in the elderly.117 Indeed, a recent study followed 427 community-dwelling elderly individuals for 18 years to examine the natural history of SDB. The authors reported that the observed changes in RDI were associated with changes in BMI, independent of age.118 Undoubtedly the presence of obesity imposes a mechanical disadvantage to the upper airway (i.e., increased fat around both the UA and the trunk promotes UA collapse and reduced FRC). Obesity also strongly increases the risk for the development of metabolic syndromes such as insulin resistance and glucose intolerance, both of which are more prevalent in the elderly.119,120 A recent investigation showed a similar age-related increase in the number of individuals with metabolic syndrome as compared with those with OSA.121 Furthermore, the presence of OSA, independent of obesity, is also a very strong risk factor for the development of adult-onset diabetes as well as a variety of adverse cardiovascular outcomes such as hypertension.122,123 Because the potential for serious OSA-related cardiovascular events may increase with advanced age, these findings have important implications for the elderly.124
SYSTEMIC HYPERTENSION AND OTHER CARDIOVASCULAR CONSEQUENCES
Although there are many studies highlighting the association between cardiovascular morbidity in the middle-aged population,125–128 very few studies have specifically investigated the cardiovascular sequelae in the aged. While there is strong evidence for a link between OSA and hypertension in the middle-aged, interestingly this may not hold true for the elderly. Haas and colleagues129 conducted a large cross-sectional analysis of 6120 participants of the Sleep Heart Health Study and found no significant relationship between hypertension and SDB in those who were over 60 years of age. Because this study was not longitudinal, it is possible that this finding may have been confounded by a survivor effect; that is, the elderly subjects sampled might have been inherently less susceptible to the adverse effects of OSA versus young to middle-aged people. However, a recent study suggested that the lack of association between hypertension and OSA in the elderly may be due to the reduced heart rate and blood pressure responses associated with arousal.130 These relatively new findings demonstrate the need for new studies that investigate the effect of treatment of OSA on cardiovascular outcomes in the elderly.
There is also good evidence in the literature which demonstrates that OSA may worsen left ventricular function and contribute to the development of CHF.131,132 Cross-sectional data from the Sleep Heart Health Study demonstrated that individuals are 2.38 times more likely to have heart failure in the presence of OSA defined as an AHI >11/h, which was the largest cardiovascular risk reported.131 A recent, prospective longitudinal study in a population-based cohort of subjects ranging from 70 to 100 years of age demonstrated that the presence of severe OSA increases the risk of ischemic stroke in the elderly.133 In elderly individuals, the presence of heart failure is linked to myocardial infarction and atrial fibrillation,134 which are also linked to the presence of OSA.
CONGESTIVE HEART FAILURE AND CENTRAL SLEEP APNEA
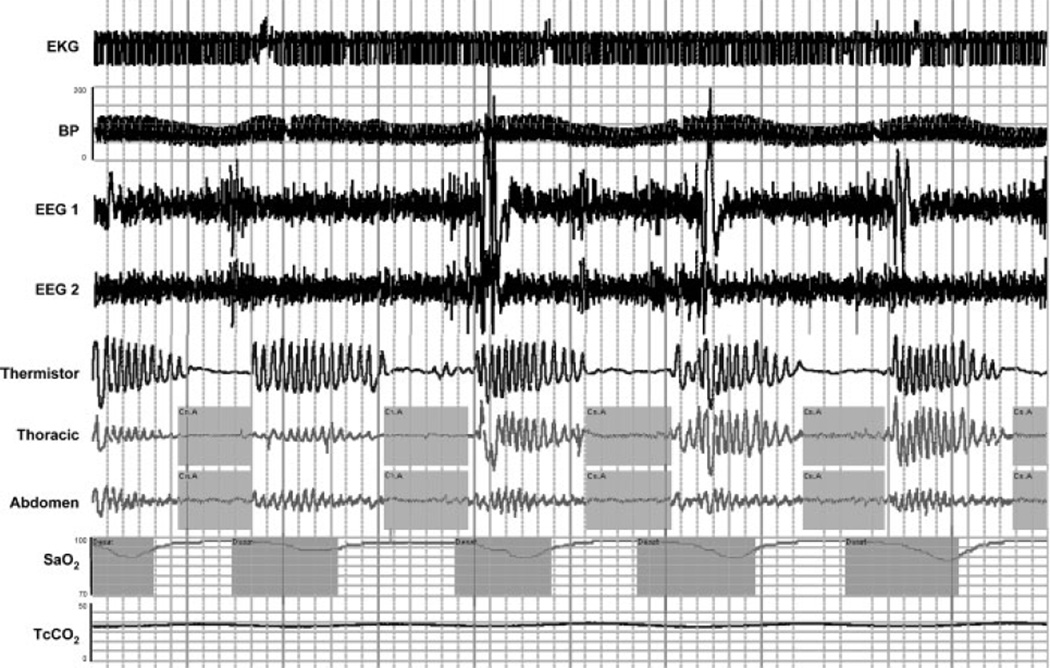
CHF is a condition that affects more than 5 million individuals in the United States. Despite available treatments, CHF is still associated with major morbidity and mortality. Significant reduction in cardiac output causes an accumulation of blood in the veins and can lead to lung congestion, which may progress to pulmonary edema. Edema may be localized to the extremities in right heart failure, but it may also cause pulmonary congestion and interfere with the regulation of breathing in addition to causing orthopnea. Patients with CHF may have a variety of sleep-related problems, such as disrupted sleep, reduced sleep efficiency, paroxysmal nocturnal dyspnea, and a general feeling of fatigue.135 Furthermore, the appearance of OSA and central sleep apnea (CSA) is quite common in patients with CHF,135–138 although the relative prevalence of CSA versus OSA varies across studies.90,139–141 Because considerable overlap exists in the pathogenesis of OSA and CSA, CHF may be associated with both conditions, although for this section we will focus primarily on CSA. CSA in patients with CHF often manifests as a type of periodic breathing called Cheyne–Stokes respiration (commonly referred to as CSA-CSR), which is characterized by a cyclic pattern of waxing and waning breaths with periods of apnea (Fig. 4). In contrast to OSA, CSA-CSR is specifically defined by a lack of respiratory effort during the phase of zero airflow, resulting in repetitive periods of insufficient ventilation and compromised gas exchange. Furthermore the cycle time of CSA-CSR is quite long (60 to 90 seconds) as compared with other forms of CSA.
Figure 4.
An example of Cheyne-Stokes respiration associated with congestive heart failure. Polysomnographic example from a clinical study in a patient with congestive heart failure and central sleep apnea (CHF-CSA). Note the waxing and waning that occur in respiration and presence of repeated central apneas. Like OSA, the repeated apneas are often associated with repeated oxygen desaturations, arousals, and fluctuations in blood pressure and heart rate. EKG, electrocardiogram; BP, blood pressure; EEG, electroencephalogram (C4-A1, O2-A1); SaO2, arterial blood oxygen saturation; TcCO2, transcutaneous carbon dioxide.
The Effects of Aging on the Pathophysiology and Consequences of CHF and CSA-CSR
CSA-CSR has long been established to be due to instability in feedback control involved in the chemical regulation of breathing.142–144 It is well documented that all CHF patients have increased circulation delays, and those with CSA-CSR often have increased controller gains135,145 (i.e., increased peripheral chemosensitivity), and both would be predicted to increase LG, thus contributing to the development of CSA-CSR. However, because the prolonged circulation delays are similar in CHF patients with and without CSA-CSR, circulation delay is thought to be a predisposing factor rather than causal. Additionally, decreases in lung volume associated with the supine position (a factor that increases the plant gain because it reduces the capacity to dampen oscillations in PaO2 and PaCO2) may help explain the increased propensity for CSA in this position.146 However, the contribution of plant gain to the genesis of CSA-CSR in CHF has been suggested to be less important when compared with increases in delay and chemosensitivity.144 There are several other factors associated with CHF that may predispose such patients to CSA-CSR, such as hypocapnia and decreased cerebrovascular reactivity to CO2. Patients with CSA-CSR have significantly lower PaCO2 both awake and asleep due to the chronic hyperventilation when compared with CHF patients without CSA-CSR.135,147 Such hyperventilation may be caused by increased controller gain, increased adrenergic stimulation, or stimulation of vagal and irritant afferents by pulmonary congestion and edema. Nonetheless, such hypocapnia places patients close to their apneic threshold and increases the propensity for apnea.
Aging is known to be a major factor affecting the risk of CSA-CSR associated with CHF. This was best characterized by a comprehensive study that examined 450 patients with CHF, and showed that age >60 years was a major risk factor for CSA-CSR in CHF patients.90 Other factors that have been independently associated with the presence of CSA-CSR in patients with systolic heart failure are male sex, awake hypocapnia (PaCO2 <38 mm Hg), and the presence of atrial fibrillation and ventricular arrhythmias.136,140,148 A current topic that is still under debate is whether CSA-CSR is simply a consequence of poor cardiac function, or whether CSA-CSR exerts an independent effect on the failing heart. In accordance with the former, the normal aging process is characterized by cellular, functional, and structural changes that bias toward cardiomyopathy. For instance normal aging has been shown to result in decreases in diameter and wall thickness of the arteries, which causes stiffening of the arterial walls, decreased volume elasticity, and increased blood pressure. When normal aging is compounded by the addition of one or more types of organic heart disease, such as CHF, it can lead to further deterioration of function. An investigation of 128 CHF patients admitted to hospital reported that elderly patients with CHF had relatively greater vasoconstriction (or decreased compliance) and blunted heart rate responsiveness associated with increased circulating norepinephrine when compared with younger patients with CHF.149 Another contributing factor to the progression of myocardial failure and premature mortality may be the presence of any undiagnosed OSA. The presence of untreated OSA and the increased prevalence of OSA in the elderly may contribute to the development of heart failure. Further deterioration may cause the appearance of CSA-CSR. Once the abnormal pattern of breathing becomes established, it may play an important role in CHF progression, and most evidence suggests that the presence of CSA-CSR is associated with a significant increase in the risk for cardiac transplantation or death150–152
Treatment of SDB
To date, there is a paucity of data examining the treatment efficacy of SDB in older individuals. Continuous positive airway pressure (CPAP) remains the treatment of choice for individuals with OSA, but it is not always tolerated well, especially in the elderly. A recent, retrospective review of 44 older individuals with OSA showed that their CPAP adherence significantly declined with increasing age.153 In contrast, another recent study reported that elderly individuals with both OSA and Alzheimer disease were able to tolerate therapeutic CPAP treatment,154 suggesting that CPAP treatment may have benefits in certain elderly subpopulations. Other common treatments of SDB include the use of oral appliances or surgery. However, the practicality and the efficacy of such interventions in the elderly have not been thoroughly assessed. Conservative management of OSA through such things as weight loss, limiting alcohol consumption, and avoidance of the supine position during sleep should be stressed and recommended. Although less well studied in the elderly, certain myorelaxant hypnotics (e.g., benzodiazepines) may worsen apnea and should probably be avoided.155
The use of CPAP as a treatment has been widely studied for individuals with CSA-CSR. While it has been shown to be effective at reducing CSA-CSR in patients with CHF, the largest, randomized, multicenter trial that has been conducted to date did not show improved survival with CPAP use.156 Because CPAP does not seem to reduce CSA-CSR for a large number of patients with CHF, other forms of treatment have been investigated. These include forms of noninvasive ventilation (i.e., adaptive pressure support ventilation), oxygen therapy and drugs such as theophylline,157 β-blockers,158 and acetazolamide159 (all which may have efficacy in reducing CSA-CSR). Importantly, whether these treatments are as efficacious in elderly individuals remains an unresolved and important area of research. The current treatment of choice for CSA-CSR remains optimization of medical therapy for CHF.
SUMMARY AND FUTURE DIRECTIONS
Aging is associated with a variety of changes that affect sleep and the emergence of sleep-disordered breathing. However, it is important to distinguish between treatable complaints and the normal age-associated changes in sleep patterns. Frequent arousals are common and should not necessarily prompt treatment interventions other than providing explanation and reassurance. Elderly patients should be advised that it is normal to awaken several times during the night, and that such awakenings will not seriously interfere with their next-day functioning as long as they can get back to sleep in a reasonable period of time. Quality and amount of sleep in the elderly is affected by many factors that include current medical conditions such as arthritis and depression as well as the presence of sleep-disordered breathing. The prevalence of SDB increases with advanced age regardless of the mechanism, although at a certain age it may become less harmful for the elderly. Future research is needed to improve the characterization of apnea phenotypes in the elderly as well as to determine whether the CSA-CSR is a consequence of CHF or contributes independently to the morbidity of CHF. Given the severe consequences associated with OSA and CHF, such research should focus on how to effectively treat such conditions. Lastly, because benefits of treatment may be modest in the elderly, treatment decisions need to be individualized for each patient.
ACKNOWLEDGMENTS
The authors are grateful to Professor Matthew Naughton of the Alfred Hospital, General Respiratory and Sleep Medicine Service, for providing the polysomnography example of CSR associated with CHF. The authors also thank Scott Sands for his assistance with the description of the loop gain of the respiratory control system. Dr. Edwards and Dr. O’Driscoll are recipients of the Thoracic Society of Australia and New Zealand/Allen and Hanburys respiratory research fellowship. Dr. Malhotra is principal investigator on grants R01 HL085188; R01 HL73146; R01 HL090897; K24 HL 093218; and AHA 0840159N.
REFERENCES
- 1.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7:161–166. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara M, De Gennaro L, Casagrande M, Bertini M. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology. 2000;37:440–446. [PubMed] [Google Scholar]
- 3.Weissman MM, Greenwald S, Niño-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–250. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 4.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- 5.Scheen AJ, Byrne MM, Plat L, Leproult R, Van Cauter E. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol. 1996;271(2 Pt 1):E261–E270. doi: 10.1152/ajpendo.1996.271.2.E261. [DOI] [PubMed] [Google Scholar]
- 6.Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6:102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- 7.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1995;18:149–157. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleepdisordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinberg I. Changes in sleep cycle patterns with age. J Psychiatr Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 10.Gillin JC, Duncan WC, Murphy DL, et al. Age-related changes in sleep in depressed and normal subjects. Psychiatry Res. 1981;4:73–78. doi: 10.1016/0165-1781(81)90010-x. [DOI] [PubMed] [Google Scholar]
- 11.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 12.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 13.Feinsilver SH, Hertz G. Sleep in the elderly patient. Clin Chest Med. 1993;14:405–411. [PubMed] [Google Scholar]
- 14.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 26–42. [Google Scholar]
- 15.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 16.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg JF, Miedema HME, Tulen JHM, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32:1367–1375. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blazer D, Hybels C, Simonsick E, Hanlon JT. Sedative, hypnotic, and antianxiety medication use in an aging cohort over ten years: a racial comparison. J Am Geriatr Soc. 2000;48:1073–1079. doi: 10.1111/j.1532-5415.2000.tb04782.x. [DOI] [PubMed] [Google Scholar]
- 19.Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002;113:1615–1622. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- 20.Wauquier A. Aging and changes in phasic events during sleep. Physiol Behav. 1993;54:803–806. doi: 10.1016/0031-9384(93)90095-w. [DOI] [PubMed] [Google Scholar]
- 21.Crowley K, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002;11:129–140. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- 22.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 23.Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005;53:48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- 24.Burazeri G, Gofin J, Kark JD. Siesta and mortality in a Mediterranean population: a community study in Jerusalem. Sleep. 2003;26:578–584. doi: 10.1093/sleep/26.5.578. [DOI] [PubMed] [Google Scholar]
- 25.Bursztyn M, Stessman J. The siesta and mortality: twelve years of prospective observations in 70-year-olds. Sleep. 2005;28:345–347. [PubMed] [Google Scholar]
- 26.Tanabe N, Iso H, Seki N, et al. JACC Study Group. Daytime napping and mortality, with a special reference to cardiovascular disease: the JACC study. Int J Epidemiol. 2010;39:233–243. doi: 10.1093/ije/dyp327. [DOI] [PubMed] [Google Scholar]
- 27.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–208. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 28.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 29.Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57:1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faubel R, Lopez-Garcia E, Guallar-Castillón P, et al. Sleep duration and health-related quality of life among older adults: a population-based cohort in Spain. Sleep. 2009;32:1059–1068. [PMC free article] [PubMed] [Google Scholar]
- 31.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 32.Ohayon MM, Zulley J, Guilleminault C, Smirne S, Priest RG. How age and daytime activities are related to insomnia in the general population: consequences for older people. J Am Geriatr Soc. 2001;49:360–366. doi: 10.1046/j.1532-5415.2001.49077.x. [DOI] [PubMed] [Google Scholar]
- 33.Sutton DA, Moldofsky H, Badley EM. Insomnia and health problems in Canadians. Sleep. 2001;24:665–670. doi: 10.1093/sleep/24.6.665. [DOI] [PubMed] [Google Scholar]
- 34.Foley DJ, Monjan AA, Masaki KH, Enright PL, Quan SF, White LR. Associations of symptoms of sleep apnea with cardiovascular disease, cognitive impairment, and mortality among older Japanese-American men. J Am Geriatr Soc. 1999;47:524–528. doi: 10.1111/j.1532-5415.1999.tb02564.x. [DOI] [PubMed] [Google Scholar]
- 35.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 36.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–559. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 37.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SR, Weiss JS. Review of insomnia pharmacotherapy options for the elderly: implications for managed care. Popul Health Manag. 2009;12:317–323. doi: 10.1089/pop.2008.0047. [DOI] [PubMed] [Google Scholar]
- 39.Richardson GS, Carskadon MA, Orav EJ, Dement WC. Circadian variation of sleep tendency in elderly and young adult subjects. Sleep. 1982;5(Suppl 2):S82–S94. doi: 10.1093/sleep/5.s2.s82. [DOI] [PubMed] [Google Scholar]
- 40.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275(5 Pt 2):R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 41.Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 42.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 43.Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:1–220. [PubMed] [Google Scholar]
- 45.Endeshaw Y. Clinical characteristics of obstructive sleep apnea in community-dwelling older adults. J Am Geriatr Soc. 2006;54:1740–1744. doi: 10.1111/j.1532-5415.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 46.Zepelin H, McDonald CS, Zammit GK. Effects of age on auditory awakening thresholds. J Gerontol. 1984;39:294–300. doi: 10.1093/geronj/39.3.294. [DOI] [PubMed] [Google Scholar]
- 47.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton GS, Solin P, Naughton MT. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34:420–426. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 49.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan CE, Issa FG. Obstructive sleep apnea. Clin Chest Med. 1985;6:633–650. [PubMed] [Google Scholar]
- 51.Guilleminault C, Motta J, Mihm F, Melvin K. Obstructive sleep apnea and cardiac index. Chest. 1986;89:331–334. doi: 10.1378/chest.89.3.331. [DOI] [PubMed] [Google Scholar]
- 52.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in communitydwelling elderly. Sleep. 1991;14:486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young T, Shahar E, Nieto FJ, et al. Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 54.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–248. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoch CC, Reynolds CF, III, Monk TH, et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990;13:502–511. doi: 10.1093/sleep/13.6.502. [DOI] [PubMed] [Google Scholar]
- 56.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119:72.e9–72.e14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 58.Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–1839. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 59.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol. 2001;90:981–988. doi: 10.1152/jappl.2001.90.3.981. [DOI] [PubMed] [Google Scholar]
- 60.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med. 2000;162:1627–1632. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- 61.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayer P, Pépin JL, Bettega G, et al. Relationship between body mass index, age and upper airway measurements in snorers and sleep apnoea patients. Eur Respir J. 1996;9:1801–1809. doi: 10.1183/09031936.96.09091801. [DOI] [PubMed] [Google Scholar]
- 63.Burger CD, Stanson AW, Sheedy PF, II, Daniels BK, Shepard JW., Jr Fast-computed tomography evaluation of age-related changes in upper airway structure and function in normal men. Am Rev Respir Dis. 1992;145(4 Pt 1):846–852. doi: 10.1164/ajrccm/145.4_Pt_1.846. [DOI] [PubMed] [Google Scholar]
- 64.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 65.Huang Y, White DP, Malhotra A. Use of computational modeling to predict responses to upper airway surgery in obstructive sleep apnea. Laryngoscope. 2007;117:648–653. doi: 10.1097/MLG.0b013e318030ca55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 68.Klawe JJ, Tafil-Klawe M. Age-related response of the genioglossus muscle EMG-activity to hypoxia in humans. J Physiol Pharmacol. 2003;54(Suppl 1):14–19. [PubMed] [Google Scholar]
- 69.Kairaitis K, Byth K, Parikh R, Stavrinou R,Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30:179–186. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 70.Wheatley JR, Amis TC. Mechanical properties of the upper airway. Curr Opin Pulm Med. 1998;4:363–369. doi: 10.1097/00063198-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–1331. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 72.Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis. 1990;141(4 Pt 1):854–860. doi: 10.1164/ajrccm/141.4_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- 73.Heinzer R, White DP, Malhotra A, et al. Effect of expiratory positive airway pressure on sleep disordered breathing. Sleep. 2008;31:429–432. doi: 10.1093/sleep/31.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 76.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 77.Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol. 1990;68:2034–2041. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]
- 78.Teramoto S. Age-related changes in lung structure and function in the senescence-accelerated mouse (SAM): SAM-P/1 as a new model of senile hyperinflation of lung. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1361. [PubMed] [Google Scholar]
- 79.Teramoto S, Ishii M. Aging, the aging lung, and senile emphysema are different. Am J Respir Crit Care Med. 2007;175:197–198, author reply 198. doi: 10.1164/ajrccm.175.2.197. [DOI] [PubMed] [Google Scholar]
- 80.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 81.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol. 1991;70:1328–1336. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 82.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 83.Mathur R, Douglas NJ. Frequency of EEG arousals from nocturnal sleep in normal subjects. Sleep. 1995;18:330–333. doi: 10.1093/sleep/18.5.330. [DOI] [PubMed] [Google Scholar]
- 84.Boselli M, Parrino L, Smerieri A, Terzano MG. Effect of age on EEG arousals in normal sleep. Sleep. 1998;21:351–357. [PubMed] [Google Scholar]
- 85.Qureshi TA, Chowdhuri S, Pidgeon J, Pranathiageswaran S, Badr MS. Effect of aging on the apneic threshold during NREM sleep [abstract] Am J Respir Crit Care Med. 2009;179:A6350. [Google Scholar]
- 86.Younes M. The physiological basis of central apnea and periodic breathing. In: Simmons DH, editor. Current Pulmonology. Chicago: Year Book Medical Publishers; 1989. pp. 265–326. [Google Scholar]
- 87.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men, I: Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 88.Ancoli-Israel S, Kripke DF, Mason W, Kaplan OJ. Sleep apnea and periodic movements in an aging sample. J Gerontol. 1985;40:419–425. doi: 10.1093/geronj/40.4.419. [DOI] [PubMed] [Google Scholar]
- 89.Krieger J, Turlot JC, Mangin P, Kurtz D. Breathing during sleep in normal young and elderly subjects: hypopneas, apneas, and correlated factors. Sleep. 1983;6:108–120. doi: 10.1093/sleep/6.2.108. [DOI] [PubMed] [Google Scholar]
- 90.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 91.Browne HA, Adams L, Simonds AK, Morrell MJ. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J. 2003;21:523–529. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- 92.Mendez R, De Oca MM, Rassulo J, Celli B. Effects of age on ventilatory drive response to CO2. Chest. 1996;110:61S. [Google Scholar]
- 93.Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- 94.Naifeh KH, Severinghaus JW, Kamiya J, Krafft M. Effect of aging on estimates of hypercapnic ventilatory response during sleep. J Appl Physiol. 1989;66:1956–1964. doi: 10.1152/jappl.1989.66.4.1956. [DOI] [PubMed] [Google Scholar]
- 95.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol. 2007;581(Pt 1):291–298. doi: 10.1113/jphysiol.2006.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roth T, Roehrs T, Rosenthal L. Hypersomnolence and neurocognitive performance in sleep apnea. Curr Opin Pulm Med. 1995;1:488–490. doi: 10.1097/00063198-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 97.Nowak M, Kornhuber J, Meyrer R. Daytime impairment and neurodegeneration in OSAS. Sleep. 2006;29:1521–1530. doi: 10.1093/sleep/29.12.1521. [DOI] [PubMed] [Google Scholar]
- 98.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7:161–166. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 99.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 100.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 101.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neubauer JA. Invited review: physiological and pathophysiological responses to intermittent hypoxia. J Appl Physiol. 2001;90:1593–1599. doi: 10.1152/jappl.2001.90.4.1593. [DOI] [PubMed] [Google Scholar]
- 103.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 104.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]
- 105.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 106.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study, I. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 107.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 108.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 109.Bliwise DL. Is sleep apnea a cause of reversible dementia in old age? J Am Geriatr Soc. 1996;44:1407–1409. doi: 10.1111/j.1532-5415.1996.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 110.Janssens JP, Pautex S, illeret H, Michel JP. Sleep disordered breathing in the elderly. Aging (Milano) 2000;12:417–429. doi: 10.1007/BF03339872. [DOI] [PubMed] [Google Scholar]
- 111.Bliwise DL. Neuropsychological function and sleep. Clin Geriatr Med. 1989;5:381–394. [PubMed] [Google Scholar]
- 112.Dealberto MJ, Pajot N, Courbon D, Alpérovitch A. Breathing disorders during sleep and cognitive performance in an older community sample: the EVA Study. J Am Geriatr Soc. 1996;44:1287–1294. doi: 10.1111/j.1532-5415.1996.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 113.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26:596–599. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 114.Cohen-Zion M, Stepnowsky C, MarlerQ13 , Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc. 2001;49:1622–1627. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 115.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 116.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–668. doi: 10.1212/01.wnl.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 117.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 118.Ancoli-Israel S, Gehrman P, Kripke DF, et al. Long-term follow-up of sleep disordered breathing in older adults. Sleep Med. 2001;2:511–516. doi: 10.1016/s1389-9457(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 119.Sezginsoy B, Ross K, Wright JE, Bernard MA. Obesity in the elderly: survival of the fit or fat. J Okla State Med Assoc. 2004;97:437–439. quiz 440-441. [PubMed] [Google Scholar]
- 120.Salihu HM, Bonnema SM, Alio AP. Obesity: what is an elderly population growing into? Maturitas. 2009;63:7–12. doi: 10.1016/j.maturitas.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 121.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
- 123.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 124.Yim S, Malhotra A, Veves A. Antioxidants and CVD in diabetes: where dowe stand now? Curr Diab Rep. 2007;7:8–13. doi: 10.1007/s11892-007-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carlson JT, Hedner JA, Ejnell H, Peterson LE. High prevalence of hypertension in sleep apnea patients independent of obesity. Am J Respir Crit Care Med. 1994;150:72–77. doi: 10.1164/ajrccm.150.1.8025776. [DOI] [PubMed] [Google Scholar]
- 126.Worsnop CJ, Naughton MT, Barter CE, Morgan TO, Anderson AI, Pierce RJ. The prevalence of obstructive sleep apnea in hypertensives. Am J Respir Crit Care Med. 1998;157:111–115. doi: 10.1164/ajrccm.157.1.9609063. [DOI] [PubMed] [Google Scholar]
- 127.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 128.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 129.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/ diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111:614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 130.Goff EA, O’Driscoll DM, Simonds AK, Trinder J, Morrell MJ. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep. 2008;31:1009–1017. [PMC free article] [PubMed] [Google Scholar]
- 131.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 132.Chan J, Sanderson J, Chan W, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. 1997;111:1488–1493. doi: 10.1378/chest.111.6.1488. [DOI] [PubMed] [Google Scholar]
- 133.Munoz R, Duran-Cantolla J, Martínez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 134.Hodkinson HM, Pomerance A. The clinical pathology of heart failure and atrial fibrillation in old age. Postgrad Med J. 1979;55:251–254. doi: 10.1136/pgmj.55.642.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 136.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 137.Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165:1245–1250. doi: 10.1164/rccm.200110-022OC. [DOI] [PubMed] [Google Scholar]
- 138.Bradley TD, Floras JS. Sleep apnea and heart failure, II: Central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 139.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 140.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure: types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 141.Naughton MT, Lorenzi-Filho G. Sleep in heart failure. Prog Cardiovasc Dis. 2009;51:339–349. doi: 10.1016/j.pcad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 142.Cherniack NS, Longobardo G, Evangelista CJ. Causes of Cheyne-Stokes respiration. Neurocrit Care. 2005;3:271–279. doi: 10.1385/NCC:3:3:271. [DOI] [PubMed] [Google Scholar]
- 143.Cherniack NS, Longobardo GS. Cheyne-Stokes breathing: an instability in physiologic control. N Engl J Med. 1973;288:952–957. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- 144.Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 145.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 146.Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position reduces severity of central sleep apnea/Cheyne-Stokes respiration. Sleep. 2006;29:1045–1051. doi: 10.1093/sleep/29.8.1045. [DOI] [PubMed] [Google Scholar]
- 147.Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure: relationship to arterial PCO2. Chest. 1993;104:1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- 148.Yumino D, Bradley TD. Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc. 2008;5:226–236. doi: 10.1513/pats.200708-129MG. [DOI] [PubMed] [Google Scholar]
- 149.Cody RJ, Torre S, Clark M, Pondolfino K. Age-related hemodynamic, renal, and hormonal differences among patients with congestive heart failure. Arch Intern Med. 1989;149:1023–1028. [PubMed] [Google Scholar]
- 150.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 151.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 152.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 153.Doerr C, Mcleland J, Kampelman J, Boero J, Duntley S. Objective CPAP compliance in the older adult population [abstract] Sleep. 2007;30:A183. [Google Scholar]
- 154.Chong MS, Ayalon L, Marler M, et al. Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer’s disease with sleep disordered breathing. J Am Geriatr Soc. 2006;54:777–781. doi: 10.1111/j.1532-5415.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 155.Berry RB, Patel PB. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep. 2006;29:1052–1056. doi: 10.1093/sleep/29.8.1052. [DOI] [PubMed] [Google Scholar]
- 156.Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 157.Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 158.Tamura A, Kawano Y, Naono S, Kotoku M, Kadota J. Relationship between beta-blocker treatment and the severity of central sleep apnea in chronic heart failure. Chest. 2007;131:130–135. doi: 10.1378/chest.06-0919. [DOI] [PubMed] [Google Scholar]
- 159.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]