Abstract
Objectives
Despite the well-recognized consequences of obstructive sleep apnea (OSA), its treatment remains unsatisfactory. Therapeutic strategies are complicated by often poor adherence in the case of continuous positive airway pressure or the highly variable efficacy in the case of many upper airway surgeries. Computational models of the upper airway using finite element analysis to simulate the effects of various anatomic and physiologic manipulations on pharyngeal mechanics could be helpful in predicting surgical success.
Study Design
Computational and physiologic study.
Methods
Using representative OSA magnetic resonance images and experimentally measured upper airway dilator muscle activities, we developed a working two-dimensional and a partial three-dimensional model of the upper airway.
Results
As predicted from experimental measurements, the OSA model airway has a closing pressure of −2 cm H2O. Manipulations such as palatal stiffening, palatal resection, and tongue stiffening all have demonstrable effects on pharyngeal mechanics. We have also developed a partial three-dimensional OSA model in which we simulate the mechanics of the pharyngeal airway in the mid-sagittal and parasagittal slices, spanning more than 1 inch in thickness. Using this model, we have observed important effects of tongue and palatal stiffening on anteroposterior collapse of the pharyngeal airway.
Conclusions
Our data suggest that computational modeling is feasible and can be used to generate hypotheses for subsequent clinical trials regarding anatomic manipulations in OSA. We further believe that the goal of individualizing OSA therapy on the basis of underlying mechanisms could be facilitated by computational modeling.
Keywords: Apnea, model, finite element, breathing, lung, sleep, upper airway
INTRODUCTION
Despite its high prevalence and well established sequelae, therapeutic strategies for obstructive sleep apnea (OSA) remain unsatisfactory.1 Although continuous positive airway pressure is the treatment of choice for OSA, it provides only modest benefits to patients because of highly variable adherence rates.2 As a result, new therapeutic strategies that avoid the need for patient adherence are actively being sought.
Despite highly variable efficacy (reportedly only 41% on average for uvulopalatopharyngoplasty [UPPP]), upper airway surgery is performed commonly for the treatment of snoring as well as sleep apnea.3 Because surgical techniques result in permanent anatomic changes, without any need for patient adherence once the procedure is completed, many investigators have sought methods to predict responsiveness to surgical therapy to maximize potential benefits on patient outcome.
We and others have been using the technique of finite element analysis (FEA) to model the upper airway to allow the prediction of changes in pharyngeal mechanics after anatomic and physiologic manipulations.4 FEA is a widely accepted numerical procedure for obtaining solutions to many of the problems encountered in engineering analyses. Recently, we have reported a two-dimensional (2D) working model of the normal human upper airway5–7 but have now expanded our findings to OSA patients in both the mid-sagittal and parasagittal planes. Thus, we report here a working 2D and partial three-dimensional (3D) model of the OSA patient airway during sleep and how it responds to a variety of manipulations. Because of the complexity of the human upper airway, the lack of suitable animal models, and the lack of feasibility of human experiments to manipulate anatomy, we believe that computer modeling may be the ideal technique with which to predict outcomes after upper airway manipulation.
Aim
Using a computational model of the OSA upper airway, we tested the impact of anatomic and physiologic manipulations on upper airway mechanics, with the ultimate goal of individualizing therapy on the basis of anatomic and physiologic measurements.
MATERIALS AND METHODS
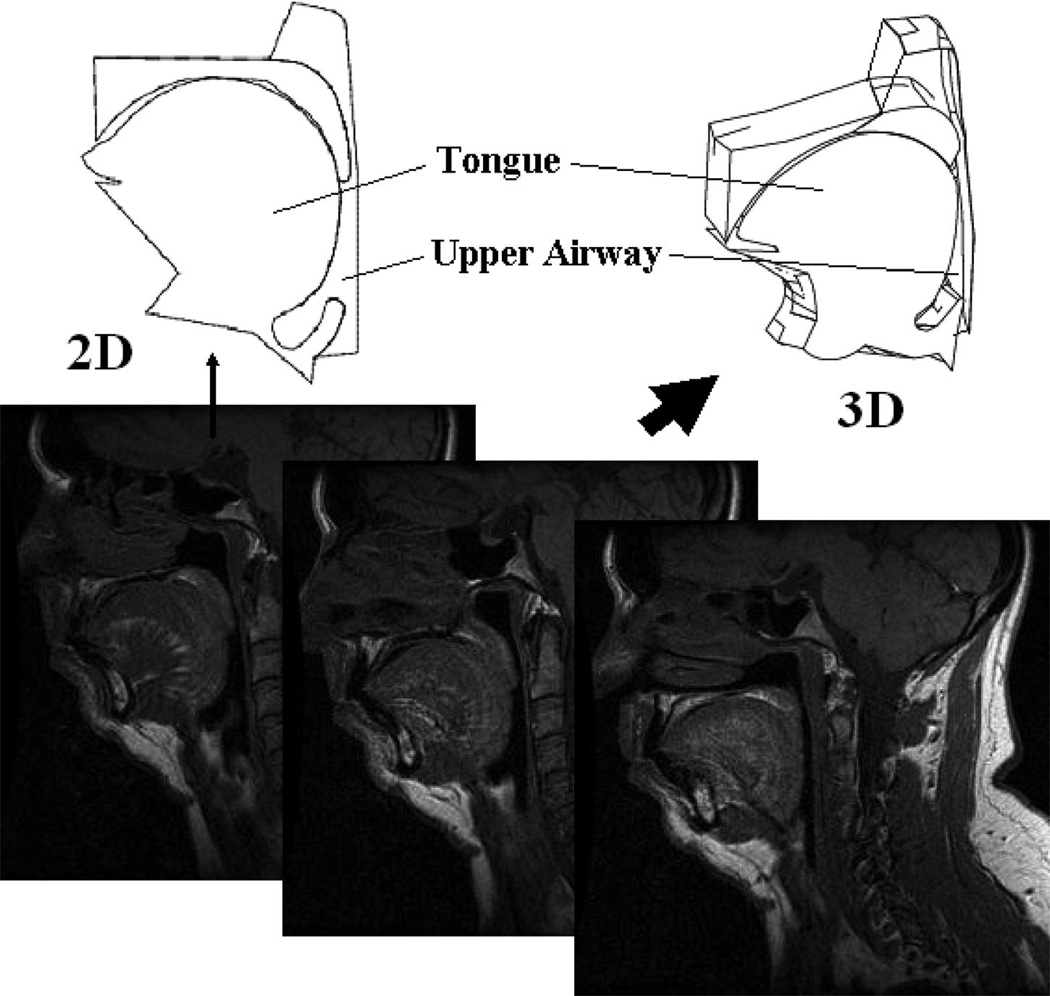
We have developed both a 2D and a partial 3D anatomic model of the pharyngeal airway on the basis of sagittal plane magnetic resonance images (MRIs) from representative OSA patient images (institutional review board approved) (Fig. 1). We define key points along the boundaries of all structures deemed essential for the model (tongue, mandible, hard palate, soft palate, uvula, hyoid bone, epiglottis, etc.). The pharyngeal airway includes the anterior wall, the posterior wall, and two lateral walls (in the 3D model). The anterior wall, which is composed of the soft palate, uvula, tongue, and epiglottis, is fully deformable under loads. The soft palate and the tongue have sliding movement on their contact surfaces during deformations. Because the posterior pharyngeal wall is thin and attached to the vertebral bodies, we modeled it as a rigid structure. Our major purpose is to test the effects of UPPP, tongue tissue stiffening, and palatal implants (Restore Medical, St. Paul, MN) on upper airway collapsibility in the anteroposterior plane using the 2D or 3D model. For the 3D model, we assume anatomic symmetry about the midline (Fig. 2). We used multiple parasagittal MRI slices in addition to the mid-sagittal slice to define the necessary anatomy for the 3D model. The distance from the midsagittal plane to the lateral wall is 1.35 cm, which means the full width of the upper airway in our model spans 2.7 cm.
Fig. 1.
Mid-sagittal and parasagittal slices from magnetic resonance image for representative sleep apnea patient and resulting two-dimensional and partial three-dimensional structures that were generated.
Fig. 2.
Mesh that was used in creating finite element model for partial three-dimensional sleep apnea model.
The human pharyngeal airway is considered a mechanical system that can be described mathematically on the basis of solid mechanics and fluid mechanics theory.7 Because the strain of the tongue and other pharyngeal tissues is small during the upper airway collapse, the passive tongue and all other pharyngeal tissues or bones are assumed to be linear elastic materials. For the genioglossus, the major upper airway dilator muscle, we developed previously a muscle contraction model based on the microstructure of skeletal muscle to simulate its nonlinear behavior during the contraction along its fiber’s axial direction.7 This contraction model directly builds a bridge between the measured electromyogram (EMG) signals and the contractile force. Therefore, the measured EMG signal corresponding to each upper airway pressure is used as the model input to determine the value of the contractile force generated in the muscle fiber under such a situation, as previously reported.7
On the basis of prior studies in OSA patients, we modified our previously reported negative pressure-EMG curve for the normal upper airway model into one for the apnea patient model. For the sleeping airway, we estimate, on the basis of our experimental studies, that both tonic and phasic genioglossal muscle activities decrease by 20% to 30% for sleep apnea patients during breathing8 and that the phasic activation increases slowly as upper airway pressure becomes more negative because of a substantial reduction but not complete loss of the negative-pressure reflex mechanisms. A nadir epiglottic pressure of −6 cm H2O is used in our sleeping upper airway simulation.
Because the pressure drop across the upper airway is small and the flow velocity is generally much smaller than the speed of sound, we have assumed that air in the pharyngeal airway is essentially incompressible. As we did in our previously reported 2D simulation,6,7 we used a laminar flow model to describe the flow in the upper airway. Our previous estimates of the effect of turbulent flow on our model indicate it to be minor, in keeping with clinical observations using variable gas densities. As boundary conditions, the pressures at the entrance and the exit to the upper airway are selected before each simulation. Using such a flow model, we simulate the air flow and pressure distribution along the upper airway. The local pressure distribution is then used as the load on surfaces of the tongue, soft palate, and uvula to calculate their deformations.
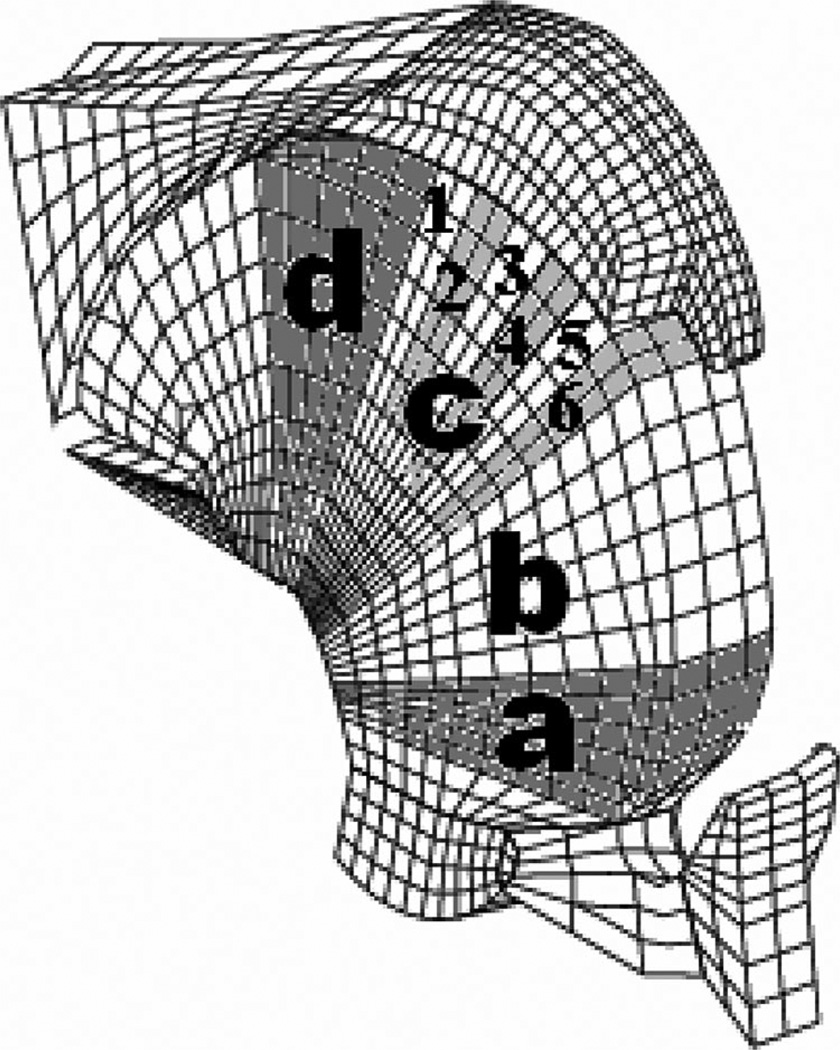
Using our 2D and 3D model, we simulate upper airway collapse during sleep under various conditions by calculating the closing pressure (measure of pharyngeal collapsibility, i.e., more positive is more collapsible, more negative is less collapsible). 9 We examined the model under several conditions. Closing pressure was first determined in the OSA model under normal sleeping conditions without airway manipulation. Second, three palatal implants (Restore Medical, St. Paul, MN) 2.5 cm in length and 2 mm in diameter are inserted in parallel into the soft palate such that we can simulate the effects of the palatal implants on upper airway mechanics using our 3D model. Finally, we also tested the effects of the tongue stiffening in different regions with and without palatal implants on upper airway collapsibility because this may represent a future method of treating OSA (either alone or in combination with palatal procedures such as UPPP or palatal implants) (Fig. 3). Tongue stiffening could be achieved by inserting implants or by creating scar tissue to raise the tissue stiffness in a tongue region of interest.
Fig. 3.
Various regions of tongue that were delineated for tongue stiffening manipulations. Also illustrates various regions within “c” region of interest to isolate area of tongue where stiffening had maximum impact on airway mechanics.
The governing equations and boundary conditions for air flow and tissue deformation are solved numerically using the FEA method.8,10 A nonlinear dynamic analysis software, ADINA (ADINA R&D, Watertown, MA), together with our own software, is used to make this simulation of air flow, tissue deformation, and airway collapse. Eight-node rectangular solid elements and three-node triangular fluid elements are used in the 2D simulation. Twenty-seven-node 3D solid elements and four-node 3D fluid elements are used in the 3D simulation.
RESULTS
On the basis of the parameters outlined in Methods, we have performed a number of simulations to predict the mechanics of the pharyngeal airway. In the 2D model, we observed a closing pressure of −2 cm H2O under sleeping condition, which is consistent with the values observed experimentally. To assess the role of UPPP, we simulated graded tissue resections of the soft palate (Fig. 4). As can be seen in Figure 4, there are incremental improvements in closing pressure with increasing tissue resection. Once sufficient palate has been resected, occlusion occurs behind the tongue, with no further improvements in pharyngeal mechanics occurring with greater palate resection.
Fig. 4.
Progressive improvements in pharyngeal mechanics that occur with ongoing resections of uvula and soft palate. Once occlusion occurs behind tongue, further palatal resection is ineffective.
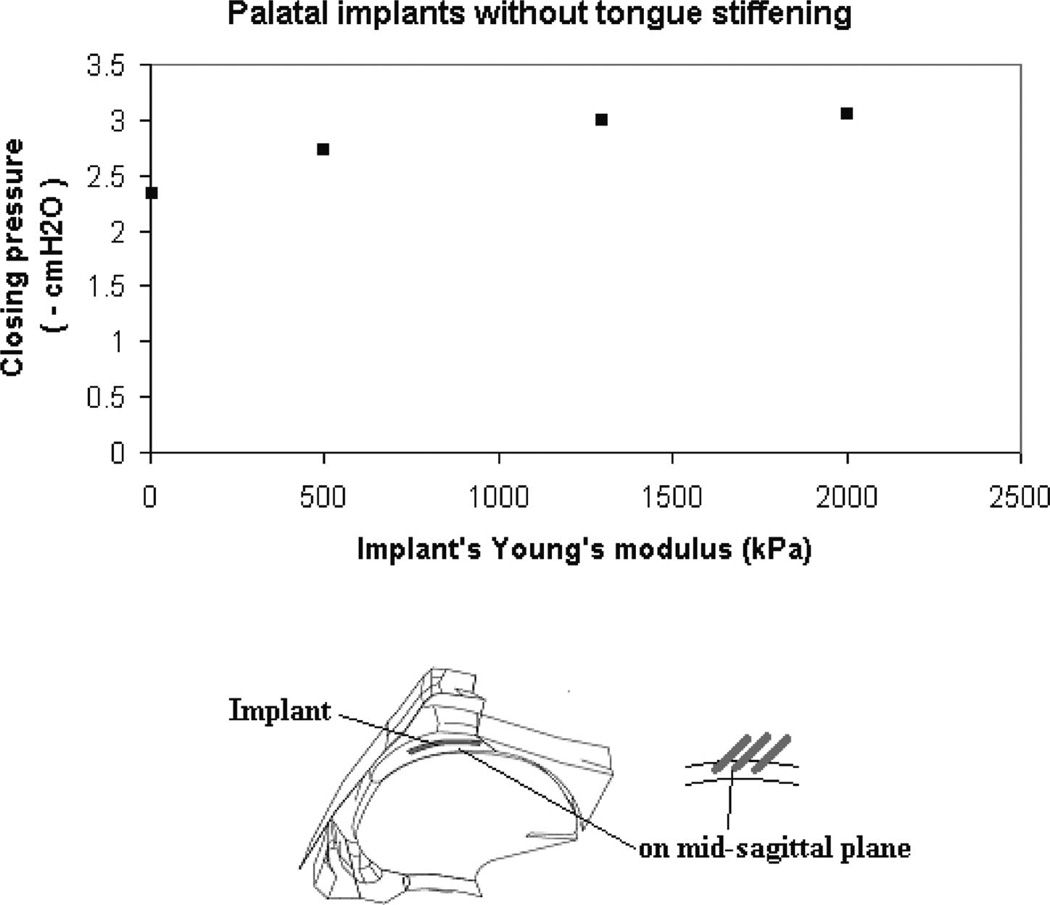
In Figure 5, we show the impact of three palatal implants in isolation on the closing pressure of our sleep apnea 3D airway model. With increasing implant stiffness, only modest improvements in closing pressure are observed. The effects of combined palatal implants and tongue stiffening were also modeled. Figure 6 shows results from 2D simulations. We observed airway closure at −2 cm H2O for the sleep apnea airway before manipulations and only small improvements (more negative closing pressure) with tongue and palatal implants. The tongue implants appear to be more effective when placed parallel to the genioglossal muscle fibers as compared with perpendicular; however, neither orientation was highly effective.
Fig. 5.
The effect of palatal implants (without tongue stiffening) on the closing pressure. With increasing implant stiffness, there are only modest improvements in closing pressure.
Fig. 6.
Palatal and tongue implants have only modest effects at locations illustrated.
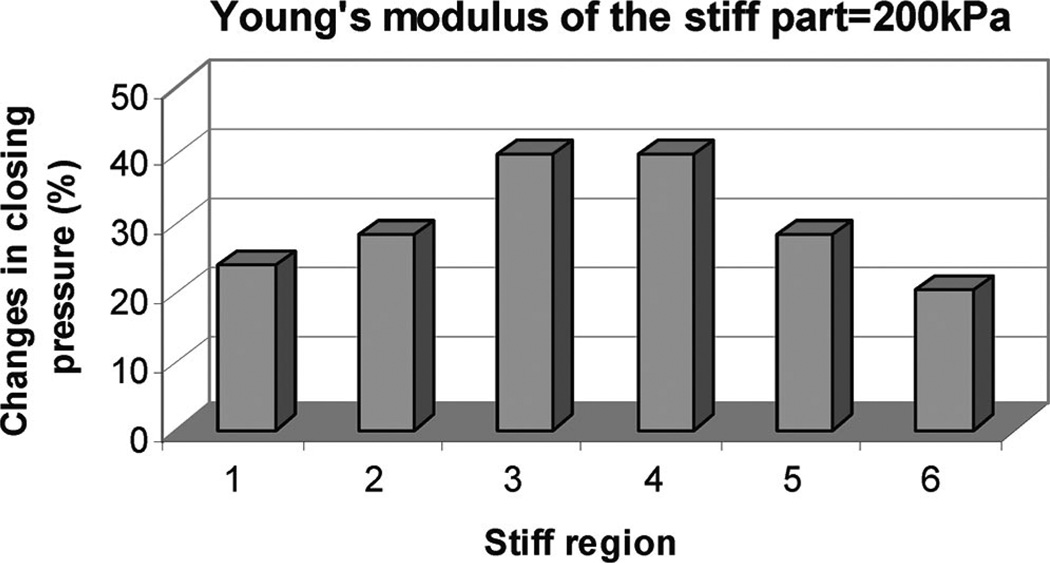
We further examined the effects of tongue stiffening using the 3D model shown in Figures 7 and 8. We assessed the role of tongue stiffening (without palatal implants) in various regions and observed the greatest effects when the tongue was stiffened in the “c” region, that is, the portion of the tongue that is adjacent to the soft palate. In Figure 8, we explored which portion of the c region was most critical to these mechanical effects. These data could help identify the most effective position when tongue implants are used to make the tongue somewhat stiffer in a very limited area.
Fig. 7.
Marked improvement in efficacy of tongue stiffening when “c” region is targeted as opposed to “b” region.
Fig. 8.
The “3” and “4” regions within “c” region are most effective locations for tongue stiffening.
We have also assessed the impact of tongue stiffening, with and without palatal implants in place. With 1,000 KPa tongue stiffening in region c in addition to palatal implants having 1300 KPa Young’s modulus, the closing pressure becomes more negative than −7 cm H2O (Fig. 9). This value reflects an airway less collapsible than most snorers and could therefore be expected to provide major benefits to airway stability.
Fig. 9.
Increasing stiffness of tongue leads to improvements in closing pressures, particularly with concomitant palatal implants.
DISCUSSION
This study leads to a number of important insights. First, a partial 3D model of the upper airway based on imaging from OSA patients can be developed. Second, this model can be used to perform simulations of interest using computational methods (i.e., FEA). Third, the closing pressure, an outcome measure for the pharyngeal model of OSA, was −2 cmH2O, which is similar to that of a patient with mild to moderate OSA based on experimental measurements. Fourth, by including parasagittal slices, we now have a more complete model of the pharyngeal airway that simulates mechanical properties, at least in part in the lateral dimension. Thus, we believe that the present study provides proof of concept that upper airway surgical therapy can be individualized using available imaging and computational techniques. This strategy may be useful in the future to optimize therapeutic strategies but will obviously have to be tested in real patients undergoing operations.
The reported success rates from various surgical interventions have been quite variable in the OSA arena. Randomized trials are quite challenging in this area because many patients and clinicians are unwilling to conduct such studies. Moreover, the variability in operative techniques between various surgeons makes it difficult to perform comparative studies. Finally, because blinding is difficult to achieve in such studies, there may be enrollment bias in favor of a patient group judged more likely to respond to a particular intervention. Thus, despite excellent attempts at clinical research in the area of upper airway surgery, progress has been somewhat slow.
We believe that computational modeling is a viable strategy with which to assess the impact of upper airway anatomic interventions. Although further model validation is clearly required and is ongoing, there are a number of observations that suggest that the results from our model do indeed predict reality. First, we predicted, on the basis of our finite element model of the normal female upper airway, a passive collapsing pressure of −7 cm H2O. This matches precisely what Isono et al.11 had experimentally measured. Second, after inputting anatomic and physiologic data from our experimental measurements, we observed high-frequency oscillations in our pharyngeal airway model during inspiration, which were consistent with snoring. This snoring behavior represented a result from our model rather than an input variable, again suggesting the model reflects reality. Third, because we were initially lacking data on the Young’s modulus of tongue tissue, we back calculated what Young’s modulus would be required in our model to yield a critical closing pressure of −5 cm H2O for the normal male’s passive airway (as observed experimentally).11 This calculation revealed a value of 6,000 Pa Young’s modulus for the passive tongue. We subsequently found a report in the literature documenting a 6,200 Pa Young’s modulus for passive skeletal muscle,12 suggesting internal validity for our model. Fourth and finally, in our normal airway model, we assessed the effects of palatal implants on pharyngeal mechanics and predicted changes of similar magnitude to those seen for other commonly performed upper airway procedures. Although published comparative trials are somewhat sparse, our findings are again in keeping with the available clinical data.13 Thus, we believe that our model is effective in predicting reality but acknowledge that ongoing efforts are needed in this area.
The strategy of tongue stiffening to treat sleep apnea has received minimal attention in the literature. Some authors have argued that the ineffectiveness of isolated palatal procedures may reflect ongoing compromise in the retroglossal airway.14 Similarly, isolated tongue procedures are unlikely to work if anatomic compromise persists in the velopharynx. In fact, one report suggests that the technique of mandibular advancement may be particularly effective among patients who have failed to benefit from UPPP.15 Thus, as also suggested by our model, strategies that address both retroglossal and retropalatal compromise by tissue stiffening or other means deserve further investigation.
Despite its strengths, this study has a number of limitations. Because the results are based primarily on FEA, we cannot draw any conclusions regarding actual patient care. Thus, we support ongoing clinical trials in this area. As with any model, a number of assumptions (e.g., laminar flow) and parameters are required to obtain any quantitative results. One of the potential strengths of the model is that we can modify these parameters to assess their relative importance. On the other hand, one could argue that the output from the model is only as good as the assumptions that are made in defining the input parameters. Although we accept this potential criticism, we would point out that for within subject comparisons (e.g., pre- and post-tongue stiffening), any errors introduced by these assumptions (e.g., only partial 3D) are likely to be self-canceling. In addition, our assumptions are unlikely to be grossly incorrect because they are all in keeping with available data from the literature. Similarly, we have only modeled effectively the behavior of one muscle (the genioglossus) under the assumption that it is representative of multiple phasic muscles important in upper airway mechanics. Despite these recognized limitations, we believe that our methods are valid and that interesting hypotheses can be generated for subsequent clinical trials.
CONCLUSIONS
We believe that FEA is a viable approach to stratifying the likelihood of surgical success for the treatment of sleep apnea. Our model suggests that strategies to improve the mechanical functions of the pharyngeal airway will be most successful if they target both the velopharynx and the retroglossal airway. Computational modeling may play a role in hypothesis generation for subsequent randomized trials.
Acknowledgments
This work was funded in part by Restore Medical and supported by NIA Beeson Award (AG024837), RO1-HL73146, and SCOR Neurobiology of Sleep and Sleep Apnea Project 1. Dr. Malhotra serves a consultant to Restore Medical.
BIBLIOGRAPHY
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 3.Woodson BT, Naganuma H. Comparison of methods of airway evaluation in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 1999;120:460–463. [PubMed] [Google Scholar]
- 4.Mansour KF, Rowley JA, Badr MS. Measurement of pharyngeal cross-sectional area by finite element analysis. J Appl Physiol. 2006;100:294–303. doi: 10.1152/japplphysiol.00364.2005. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: the importance of airway length. Am J Resp Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, White DP, Malhotra A. The impact of anatomic manipulations on pharyngeal collapse: results from a computational model of the normal human upper airway. Chest. 2005;128:1324–1330. doi: 10.1378/chest.128.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y, Malhotra A, White DP. Computational simulation of human upper airway collapse using a pressure-/state-dependent model of genioglossal muscle contraction under laminar flow conditions. J Appl Physiol. 2005;99:1138–1148. doi: 10.1152/japplphysiol.00668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangel D, Mezzanotte WS, White DP. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol. 1991;70:2574–2581. doi: 10.1152/jappl.1991.70.6.2574. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AR, Eisele DW, Smith PL. Pharyngeal airway obstruction in obstructive sleep apnea: pathophysiology and clinical implications. Otolaryngol Clin North Am. 1998;31:911–918. doi: 10.1016/s0030-6665(05)70098-0. [DOI] [PubMed] [Google Scholar]
- 10.Bathe KJ. Finite Element Procedures. Englewood Cliffs, NJ: Prentice Hall; 1996. [Google Scholar]
- 11.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 12.Duck F. Physical Properties Of Tissue: A Comprehensive Reference Book. London: Academic Press; 1990. [Google Scholar]
- 13.Romanow JH, Catalano PJ. Initial U.S. pilot study: palatal implants for the treatment of snoring. Otolaryngol Head Neck Surg. 2006;134:551–557. doi: 10.1016/j.otohns.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Schwab RJ. Imaging for the snoring and sleep apnea patient. Dent Clin North Am. 2001;45:759–796. [PubMed] [Google Scholar]
- 15.Millman RP, Rosenberg CL, Carlisle CC, Kramer NR, Kahn DM, Bonitati AE. The efficacy of oral appliances in the treatment of persistent sleep apnea after uvulopalatopharyngoplasty. Chest. 1998;113:992–996. doi: 10.1378/chest.113.4.992. [DOI] [PubMed] [Google Scholar]