Abstract
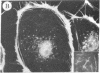
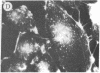
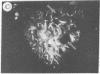
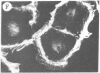
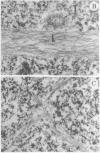
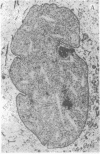
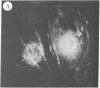
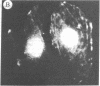
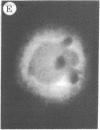
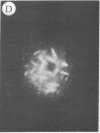
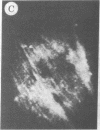
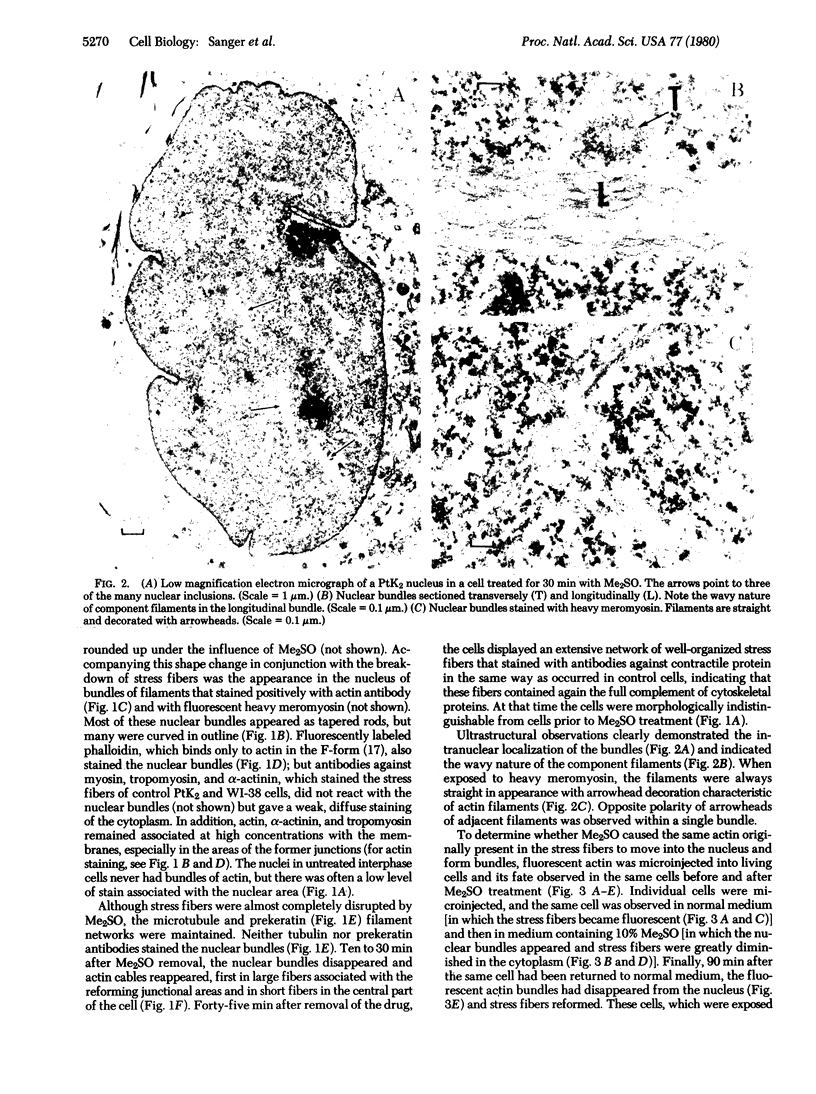
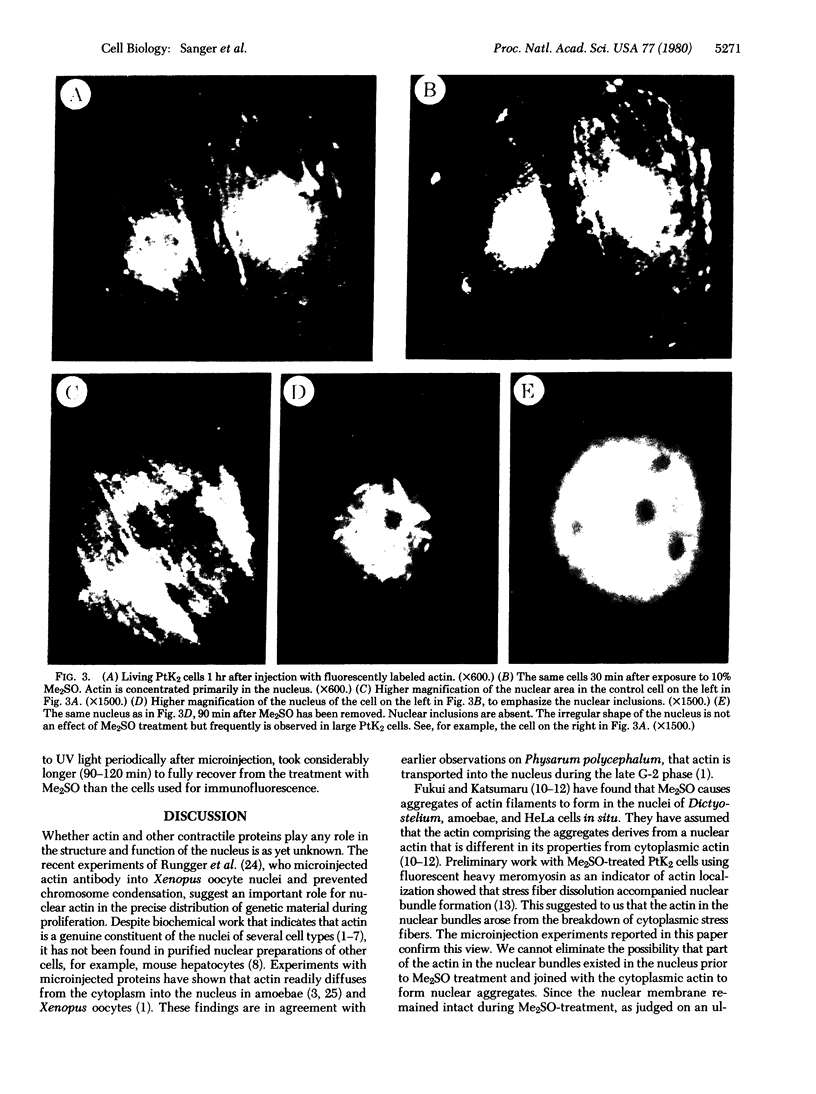
The addition of 10% dimethyl sulfoxide (Me2SO) to PtK2 and WI-38 cells caused stress fibers to disappear from the cytoplasm and numerous elongated inclusions to appear in the nucleus. When Me2SO was removed, the stress fibers reformed and the nuclear inclusions disappeared. These nuclear inclusions reacted with fluorescent heavy meromyosin, phalloidin, and actin antibody. In the electron microscope, needle-like structures were seen to be composed of wavy filaments that bound heavy meromyosin. Antibodies against other components of stress fibers--tropomyosin, alpha-actinin, and myosin--did not react with the inclusions. When fluorescently labeled actin was microinjected into living PtK2 and WI-38 cells, the fluorescent actin was incorporated into stress fibers. Subsequent exposure of the same cells to Me2SO led to breakdown of the fluorescent stress fibers and the appearance of fluorescent inclusions in the nucleus. Removal of Me2SO caused reversion to the normal interphase structure. These results indicate that under the influence of Me2SO, dissolution of stress fiber releases actin in a form which allows it to diffuse into the nucleus where it then becomes organized into filamentous bundles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg D. A., Rodewald R., Rebhun L. I. The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J Cell Biol. 1978 Dec;79(3):846–852. doi: 10.1083/jcb.79.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T. G., Merriam R. W. Diffusible and bound actin nuclei of Xenopus laevis oocytes. Cell. 1977 Dec;12(4):883–891. doi: 10.1016/0092-8674(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Harris D. C. Nuclear proteins. II. Similarity of nonhistone proteins in nuclear sap and chromatin, and essential absence of contractile proteins from mouse liver nuclei. J Cell Biol. 1976 Aug;70(2 Pt 1):440–452. doi: 10.1083/jcb.70.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- Douvas A. S., Harrington C. A., Bonner J. Major nonhistone proteins of rat liver chromatin: preliminary identification of myosin, actin, tubulin, and tropomyosin. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3902–3906. doi: 10.1073/pnas.72.10.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Osborn M., Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978 Aug;17(2):365–391. [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fukui Y. Intranuclear actin bundles induced by dimethyl sulfoxide in interphase nucleus of Dictyostelium. J Cell Biol. 1978 Jan;76(1):146–157. doi: 10.1083/jcb.76.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Katsumaru H. Dynamics of nuclear actin bundle induction by dimethyl sulfoxide and factors affecting its development. J Cell Biol. 1980 Jan;84(1):131–140. doi: 10.1083/jcb.84.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Katsumaru H. Nuclear actin bundles in Amoeba, Dictyostelium and human HeLa cells induced by dimethyl sulfoxide. Exp Cell Res. 1979 May;120(2):451–455. doi: 10.1016/0014-4827(79)90412-9. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Rubin R., Ko C. The presence of actin in nuclei: a critical appraisal. Cell. 1977 Nov;12(3):601–608. doi: 10.1016/0092-8674(77)90260-4. [DOI] [PubMed] [Google Scholar]
- Hoessli D., Rungger-Brändle E., Jockusch B. M., Gabbiani G. Lymphocyte alpha-actinin. Relationship to cell membrane and co-capping with surface receptors. J Cell Biol. 1980 Feb;84(2):305–314. doi: 10.1083/jcb.84.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB S. W., BISCHEL M., HERSCHLER R. J. DIMETHYL SULFOXIDE: EFFECTS ON THE PERMEABILITY OF BIOLOGIC MEMBRANES (PRELIMINARY REPORT). Curr Ther Res Clin Exp. 1964 Mar;6:193–198. [PubMed] [Google Scholar]
- Jockusch B. M., Brown D. F., Rusch H. P. Synthesis and some properties of an actin-like nuclear protein in the slime mold Physarum polycephalum. J Bacteriol. 1971 Nov;108(2):705–714. doi: 10.1128/jb.108.2.705-714.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Kelley K. H., Meyer R. K., Burger M. M. An efficient method to produce specific anti-actin. Histochemistry. 1978 Apr 4;55(3):177–184. doi: 10.1007/BF00495757. [DOI] [PubMed] [Google Scholar]
- Jockusch H., Jockusch B. M., Burger M. M. Nerve fibers in culture and their interactions with non-neural cells visualized by immunofluorescence. J Cell Biol. 1979 Mar;80(3):629–641. doi: 10.1083/jcb.80.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Winterhalter K. H., Birchmeier W. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3814–3818. doi: 10.1073/pnas.76.8.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake C. D., Rosenbaum E. E., Jacob S. W. Summary of the New York Academy of Sciences symposium on the "biological actions of dimethyl sulfoxide". Ann N Y Acad Sci. 1967 Mar 15;141(1):670–671. doi: 10.1111/j.1749-6632.1967.tb34939.x. [DOI] [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Peterson J. L., McConkey E. H. Non-histone chromosomal proteins from HeLa cells. A survey by high resolution, two-dimensional electrophoresis. J Biol Chem. 1976 Jan 25;251(2):548–554. [PubMed] [Google Scholar]
- Rubin R. W., Goldstein L., Ko C. Differences between nucleus and cytoplasm in the degree of actin polymerization. J Cell Biol. 1978 Jun;77(3):698–701. doi: 10.1083/jcb.77.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger D., Rungger-Brändle E., Chaponnier C., Gabbiani G. Intranuclear injection of anti-actin antibodies into Xenopus oocytes blocks chromosome condensation. Nature. 1979 Nov 15;282(5736):320–321. doi: 10.1038/282320a0. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W. Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol. 1980 Aug;86(2):568–575. doi: 10.1083/jcb.86.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W. Changing patterns of actin localization during cell division. Proc Natl Acad Sci U S A. 1975 May;72(5):1913–1916. doi: 10.1073/pnas.72.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Gwinn J., Sanger J. M. Dissolution of cytoplasmic actin bundles and the induction of nuclear actin bundles by dimethyl sulfoxide. J Exp Zool. 1980 Aug;213(2):227–230. doi: 10.1002/jez.1402130210. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Intracellular localization of actin with fluorescently labelled heavy meromyosin. Cell Tissue Res. 1975 Aug 27;161(4):431–434. doi: 10.1007/BF00224134. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Presence of actin during chromosomal movement. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2451–2455. doi: 10.1073/pnas.72.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M. Intermediate filament aggregation: effect on cell topology, cell spreading and surface transport. Eur J Cell Biol. 1980 Apr;21(1):67–71. [PubMed] [Google Scholar]
- Sanger J. W., Sanger J. M. The cytoskeleton and cell division. Methods Achiev Exp Pathol. 1979;8:110–142. [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]