Abstract
Phospholipase A2 (PLA2) was purified about 180,000 times compared with the starting soluble-protein extract from developing elm (Ulmus glabra) seeds. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis the purified fraction showed a single protein band with a mobility that corresponded to 15 kD, from which activity could be recovered. When analyzed by matrix-assisted laser-desorption ionization-time-of-flight mass spectrometry, the enzyme had a deduced mass of 13,900 D. A 53-amino acid-long N-terminal sequence was determined and aligned with other sequences, giving 62% identity to the deduced amino acid sequence of some rice (Oryza sativa) expressed sequence tag clones. The purified enzyme had an alkaline pH optimum and required Ca2+ for activity. It was unusually stable with regard to heat, acidity, and organic solvents but was sensitive to disulfide bond-reducing agents. The enzyme is a true PLA2, neither hydrolyzing the sn-1 position of phosphatidylcholine nor having any activity toward lysophosphatidylcholine or diacylglycerol. The biochemical data and amino acid sequence alignments indicate that the enzyme is related to the well-characterized family of animal secretory PLA2s and, to our knowledge, is the first plant enzyme of this type to be described.
PLA2 (phosphatide 2-acylhydrolase, EC 3.1.1.4) specifically hydrolyzes glycerophospholipids at the sn-2 position to yield free fatty acids and lysophospholipids. Little is known about plant PLA2s; no plant PLA2 has been purified to any extent and no plant gene or cDNA encoding such an enzyme has been identified. The animal PLA2s, however, are a diverse family of well-studied enzymes that are known to play important roles in a number of basic cellular processes (Waite, 1987; Dennis, 1994). They are divided into several distinct groups, groups I, II, III, and V, which belong to a class of small (13–18 kD), secretory, and extremely heat-stable enzymes having between five and eight disulfide bonds and requiring millimolar concentrations of Ca2+ for maximum activity. This type of PLA2 is best known from snake venom, bee venom, and pancreatic juice, where it occurs abundantly and has a digestive role. More recently, PLA2 has been found in high levels in rheumatoid arthritic synovial fluid (Vadas et al., 1981; Seilhamer et al., 1989) and also at low levels in many mammalian tissues (Tischfield, 1997). The group IV enzymes are intracellular, high-Mr PLA2s that are translocated to membranes in the presence of micromolar concentrations of Ca2+. This type of PLA2 has a preference for phospholipids, with arachidonate at the sn-2 position, and has been suggested to be involved in signal transduction and in the initiation of eicosanoid synthesis (Dennis, 1994). In addition to these groups there is also a group of 40kD, Ca2+-independent, intracellular PLA2s specific for arachidonyl-plasmenylcholine (Dennis, 1994).
In plant tissues phospholipid-degrading enzymes other than PLA2 have been studied in detail, such as phospholipase D (Pappan et al., 1997a, 1997b), phospholipase C (Yotsushima et al., 1993; Hirayama et al., 1995), and acyl hydrolases (Huang 1987; Sahsah et al., 1994; Teissère et al., 1995). With regard to PLA2 several studies of plant enzyme activities, in more or less crude preparations, have been reported (Moreau and Morgan, 1988; Kawakita et al., 1993; Kim et al., 1994; Roy et al., 1995). The two products of PLA2 activity, fatty acids and LPC, have also been shown to stimulate several plasma membrane enzymes, such as H+-ATPase (Palmgren et al., 1988), NADH oxidases (Brigthman et al., 1991) and protein kinases (Klucis and Polya, 1987; Martiny-Baron and Scherer, 1989). These have been proposed to serve as second messengers in signal transduction. Linolenic acid, the precursor of the plant-signal substance jasmonic acid (Creelman and Mullet, 1997), is thought to be liberated from phospholipids by phospholipase A or an acyl hydrolase for further metabolism into jasmonic acid. This is analogous to the synthesis of the structurally related prostaglandins from arachidonic acid in animals (Bergey et al., 1996).
Previously, we studied the formation of hydroxylated and epoxygenated fatty acids in microsomal preparations from developing oil seeds that accumulate these acids in their triacylglycerols. Both ricinoleic (12-hydroxy-octadeca-9-enoic) acid in castor bean (Ricinus communis) seeds and vernolic (12-epoxy-octadeca-9-enoic) acid in Euphorbia lagascae seeds were found to be synthesized by oxygenation of acyl chains attached to phospholipids, after which the oxygenated fatty acids were selectively removed by microsomal PLA2 activities (Bafor et al., 1991, 1993; Ståhl et al., 1995). It was further shown that microsomal fractions from developing seeds of Cuphea procumbens and elm (Ulmus glabra), which accumulate mainly capric (decanoic) acid in their triacylglycerols, had PLA2 activities with pronounced selectivities for C8–C12 fatty acids (Ståhl et al., 1995). The developing seed PLA2 activities were proposed to be involved in the channeling of these uncommon fatty acids from the membrane lipids into the storage lipids. We have also found PLA2 activities specific for phospholipids with oxygenated acyl groups in microsomal preparations from plant tissues that are not involved in the accumulation of such fatty acids, and we proposed that such activities are part of a general repair mechanism after oxidative damage to membranes (Banas et al., 1992; Bafor et al., 1993).
With the intention to characterize and purify one of the acyl-selective PLA2s in microsomal preparations of developing oil seeds that accumulate uncommon fatty acyl groups, we initiated the purification of the medium-chain-selective PLA2 activity found in microsomal fractions of developing elm seed. We achieved a 30,000-fold purification of the main PLA2 activity found in detergent-solubilized microsomes, as communicated in a preliminary report (Ståhl et al., 1997). However, the low abundance of the enzyme and limitations in the collection of material prevented us from further purification to homogeneity of this low Mr PLA2. From the present study we report the purification to homogeneity and the characterization of a biochemically related, soluble PLA2 from the same tissue and show that this enzyme is related to the low-Mr secretory PLA2s found in various animal tissues.
MATERIALS AND METHODS
[1-14C]Palmitic acid, [1-14C]oleic acid, and 1,2-[1-14C]dipalmitoyl-PC were purchased from Amersham. [1-14C]Capric acid, 1-palmitoyl-LPC, palmitic acid, oleic acid, capric acid, PLA2 (from cobra [Naja naja kaouthia] venom), and phospholipase C (from Bacillus cereus) were obtained from Sigma. Lubrol PX (Thesit) was obtained from Boehringer Mannheim. Q-Sepharose Fast-Flow, Superose 12 (10/30 column) and μRPC SC C2/C18 (2.1/10 column) were from Pharmacia.
Radioactive fatty acids were adjusted to a specific radioactivity of 167 Bq/nmol by dilution with nonradioactive fatty acids before used in [14C]acyl-PC synthesis. 1-Palmitoyl-2-[14C]acyl-PC substrates were synthesized by acylating TFA anhydrides of the 14C-labeled fatty acids to palmitoyl-LPC by the method described by Kanda and Wells (1981). 1-Palmitoyl-2-[14C]palmitoyl-DAG was prepared by treatment of 1-palmitoyl-2-[14C]palmitoyl-PC with commercial phospholipase C. The products were purified on silica-gel TLC plates before used in assays.
Seeds from elm (Ulmus glabra) trees were collected from local trees at the mid to late stage of seed development (i.e. when the seed coats were partly filled and the seed wings were still green). The seed coats were removed and the white embryos were immediately frozen in liquid N2 and stored at −80°C.
Assays of PLA2 Activity
PLA2 assays were done using radioactive phospholipids dispersed in mixed micelles of the nonionic detergent lubrol PX as follows: 14C-Acyl substrate (1–50 nmol per assay) dissolved in chloroform was taken to dryness under a stream of N2 and solubilized in assay buffer (50 mm Tris-HCl, pH 8.0, 10 mm CaCl2, and 0.06% [w/v] lubrol PX) by incubation at 70°C for 5 to 10 min and mixed thoroughly. In standard assays, enzyme fractions (0.5–10 μL) were incubated at 30°C for 5 to 30 min with 5 or 10 nmol of 1-palmitoyl-2-[14C]palmitoyl-PC in a total volume of 50 μL. The reaction was stopped by the addition of 400 μL of chloroform:methanol:acetic acid (50:50:1, v/v) followed by 150 μL of water to yield a two-phase system, according to the method of Bligh and Dyer (1959). The samples were mixed thoroughly and centrifuged at 10,000g for 1 min.
Assays of PLA2 Activity from SDS-PAGE
Chromatographed SDS-PAGE (ExelGel 8–18%, Pharmacia) lanes (not fixed) were divided in 2- to 3-mm-wide pieces and placed in Eppendorf tubes. Proteins were eluted from the gel pieces in 400 μL of 20 mm Tris, pH 8.0, containing 0.5% (w/v) SDS by rotating the tubes end over end for 16 h at 37°C. The eluates were concentrated to 100 μL in a SpeedVac concentrator (Savant, Farmingdale, NY) and the proteins were precipitated with ethanol/chloroform (Wessel and Flügge, 1984) to remove the SDS. Air-dried pellets were solubilized in 150 μL of assay buffer, and assays were initiated by adding 5 nmol of 1-palmitoyl-2-[14C]palmitoyl-PC solubilized in 50 μL of assay buffer. The assays were performed for 3 h at 30°C and terminated and extracted as described above.
Analytical Procedures
The lipid-containing chloroform phases from PLA2 assays were applied to minicolumns of silica gel (about 40 mg of silica gel) and the columns were rinsed with an additional 400 μL of chloroform. The eluates from the silica columns, which contained the free 14C-fatty acids, were collected into scintillation vials. The radioactivity was determined by liquid-scintillation counting after addition of 6 mL of toluene:ethanol (2:1, v/v) containing 0.4% (w/v) of 2(1-butylphenyl)-5-(4-biphenyl)-1,3,4-oxadiazole as a scintillant.
When, in addition to free fatty acids, radioactive complex lipids were to be monitored, the chloroform-soluble lipids were separated on silica-gel TLC plates (Silica 60, Merck, Darmstadt, Germany). The plates were developed with chloroform:methanol:acetic acid:water (170:30:20:7, v/v) when PC or LPC was used as the substrate and with hexane:diethylether:acetic acid (70:30:1, v/v) when DAG was the substrate. Unsaturated lipids were located by staining with I2 vapor, whereas saturated lipids were visualized by spraying with water. Lipid areas and the rest of the lanes were removed from the plates and assayed for radioactivity by scintillation counting as described above.
Purification of the Elm PLA2
Frozen, developing elm embryos, 60 g, were homogenized with a mortal and pestle and subsequently with an Ultra-Turrax (Janke and Kunkel, Staufen, Germany) in 600 mL of ice-cold 100 mm KH2PO4 buffer, pH 7.2, containing 0.33 m Suc. The homogenate was filtered through two layers of Miracloth (Calbiochem) and centrifuged at 10,000g for 12 min. The supernatant was filtered through one layer of Miracloth and centrifuged at 100,000g for 90 min. The 100,000g supernatant was either used immediately or stored at −20°C.
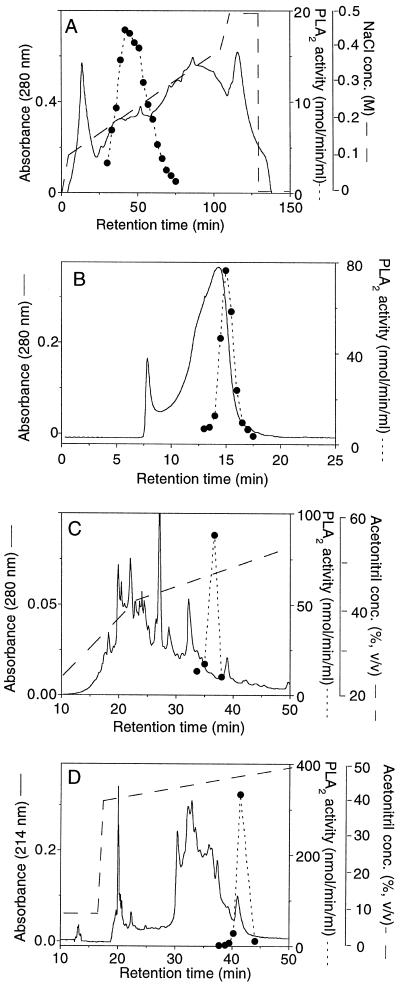
The 100,000g supernatant was brought to 55% (w/v) with (NH4)2SO4 and stirred at 4°C for 1 h. Precipitated proteins were pelleted by centrifugation at 10,000g for 10 min and resuspended in 130 mL of 50 mm diethanolamine buffer, pH 8.5. Ice-cold acetone was added to a final concentration of 45% (v/v) after which the extract was left at 4°C for 30 min. Precipitated proteins were removed by centrifugation for 10 min at 10,000g. The resulting supernatant was dialyzed against 20 volumes of 20 mm piperidine, pH 11.0, overnight and then applied to a Q-Sepharose Fast-Flow column (1 × 10 cm) equilibrated in 20 mm piperidine, pH 11.0. The column was eluted with a linear NaCl gradient from 100 to 500 mm in 20 mm piperidine, pH 11.0, at a flow rate of 2 mL/min. Three-milliliter fractions were collected and assayed for PLA2 activity. A single broad peak of activity was eluted at an NaCl concentration of 200 to 300 mm.
Peak fractions were pooled, concentrated on Centricon-10 to 0.6 mL, and chromatographed in three separate runs on a Superose 12 gel-filtration column (1.0 × 30.0 cm) in 20 mm Tris-HCl, pH 8.0, with 150 mm NaCl at a flow rate of 0.4 mL/min. Fractions (0.5 mL) were collected and assayed for PLA2 activity. Peak fractions from all three runs were pooled, and PLA2 was further purified using a C4 reverse-phase HPLC column (0.46 × 10.0 cm, Vydac, Hesperia, CA) equilibrated with 0.1% TFA. The column was developed at 0.4 mL/min with a 30-min gradient (40–55% of acetonitrile in 0.1% TFA) and peaks, monitored at 214 and 280 nm, were collected manually. Collected fractions from four separate runs were assayed for PLA2 activity. Peak fractions were pooled and the acetonitrile content was reduced by evaporation in a SpeedVac concentrator. The PLA2 was then purified to apparent homogeneity on a reverse-phase C2/C18 column (0.21 × 10.0 cm) equilibrated in 0.1% TFA and developed at 100 μL/min with a 60-min gradient (30–60% acetonitrile in 0.1% TFA) using a SMART system (Pharmacia). Peaks monitored at 214 nm were automatically collected and then assayed for PLA2 activity. The PLA2 activity eluted as a discrete peak in the gradient at about 48% acetonitrile.
SDS-PAGE
Protein fractions were, if necessary, concentrated by evaporation in a SpeedVac and precipitated with ethanol/chloroform according to the method of Wessel and Flügge (1984). Samples (final volume 20 μL) in 50 mm Tris-acetate, pH 7.5, containing 1% SDS (w/v), with or without 10 mm DTT, were heated to 95°C for 5 min, centrifuged for 5 min at 13,000g, and then loaded onto a horizontal 8 to 18% gradient polyacrylamide gel (ExelGel SDS, Pharmacia) with a 33-mm stacking zone and a 77-mm separating zone. Electrophoresis was performed in a Multiphor II unit (Pharmacia) at 15°C for 80 min at 600 V and stained with colloidal Coomassie blue (Neuhoff et al., 1988) or with silver according to the manufacturer's instructions.
Molecular Mass Determination by MALDI-TOF MS
Native or reduced and iodoacetamide-alkylated-purified PLA2 was mixed with matrix 2,5-dihydroxybenzoic acid (14 mg/mL) in a 1:1 ratio (v/v). From each sample 1-μL aliquots were deposited on gold-covered steel sample mesa and dried in a vacuum. The molecular masses of native or reduced and alkylated PLA2 were analyzed in the positive mode on an LDI-1700XP instrument (Linear Scientific, Inc., Reno, NV) equipped with an N2 laser (339 nm) using 7 μJ of energy.
N-Terminal Sequence Analysis and Similarity Searches in Databases
About 1.8 μg of purified PLA2 was reduced by incubation with 0.1 m Tris-HCl, pH 8.5, containing 8 m guanidine-HCL, 10 mm EDTA, and 20 mm DTT at 56°C for 30 min and then the Cys residues were alkylated with 20 mm iodoacetamide for 60 min at room temperature. Both reduction and alkylation were performed under an Ar atmosphere. The reduced and alkylated PLA2 was desalted on a reverse-phase C2/C18 column (0.21 × 10 cm) equilibrated with 0.1% TFA in water and eluted at 100 μL/min with a 30-min acetonitrile gradient (30–60%) using a SMART chromatographic station. Peaks were monitored at 214 and 280 nm, and automatic peak collection was used. Amino acid sequencing was performed on a sequencer (model ABI 476A, Applied Biosystems/Perkin-Elmer) according to the manufacturer's instructions. The N-terminal amino acid sequence was used as a query for the Basic Local Alignment Search Tool (BLAST) at the National Center for Biotechnology Information (Bethesda, MD), and the “blastp” search program was used against the Nonredundant GenBank CDS translations+PDB+SwissProt+SPupdate+PIR, and the “tblastn” search program against nonredundant GenBank+EMBL+DDBJ+ PDB sequences and the Nonredundant Database of GenBank EST Division.
Determination of Protein Concentration
The protein concentrations in fractions of the purification scheme were determined by the method of Bradford (1976), except in the fractions from the two last purification steps, the determination was done by comparing eluted peak areas monitored at 214 nm with peak areas of standard proteins (Buck et al., 1989), and the staining intensities of the sample protein band were compared with known standards in colloidal Coomassie blue-stained SDS-PAGE gels.
RESULTS
Purification of PLA2
Eighty percent of the total PLA2 activity in a crude extract from developing seed embryos was recovered in the 100,000g supernatant after centrifugation (data not shown). This fraction was used for further purification according to the procedure summarized in Table I. The procedure included (NH4)2SO4 fractionation, acetone precipitation, and column chromatography on Q-Sepharose Fast Flow (anion-exchange), Superose 12 (gel filtration), and C4 and C2/C18 reverse-phase columns (Fig. 1). Several of the purification steps were achievable because of the extreme stability of the PLA2 in organic solvents at a low pH. Both the precipitation with acetone 45% (v/v) and reverse-phase chromatography with 0.1% (v/v) TFA and elution with an acetonitrile gradient are extremely harsh conditions that denature most enzymes. These steps were very useful for the PLA2 purification, giving a high degree of purification and little loss in activity. The final purification, to apparent homogeneity, was achieved with a C2/C18 column; PLA2 activity coincided with a discrete protein peak eluted at a acetonitrile concentration of about 48% (Fig. 1D). Based on specific activity the PLA2 was purified about 180,000-fold compared with the 100,000g supernatant, with a total recovery of 16% (Table I).
Table I.
Purification of soluble PLA2 from developing elm seeds
| Purification Step | Protein | Total Activity | Specific Activity | Purification | Yield |
|---|---|---|---|---|---|
| mg | nmol/min | nmol min−1 mg−1 | -fold | % | |
| 100,000g Supernatant | 3340 | 833 | 0.25 | 1 | 100 |
| (NH4)2SO4 Pellet | 1060 | 563 | 0.53 | 2 | 68 |
| Acetone supernatant | 150 | 780 | 5.2 | 21 | 94 |
| Q-Sepharose Fast Flow | 24 | 420 | 17.5 | 70 | 50 |
| Gel filtration | 3.8 | 263 | 69.2 | 277 | 32 |
| C4-HPLC | 0.09 | 173 | 1,922 | 7,690 | 21 |
| C2/C18-SMART | 0.003 | 133 | 44,330 | 177,300 | 16 |
Figure 1.
Elution profiles of protein (——) and PLA2 activity (- - - - -) from the different chromatographic steps in the purification of the elm PLA2: Q-Sepharose Fast Flow (A), Superose 12 (B), C4 reverse-phase HPLC (C), and C2/C18 reverse-phase SMART (D).
Molecular Mass of Purified PLA2
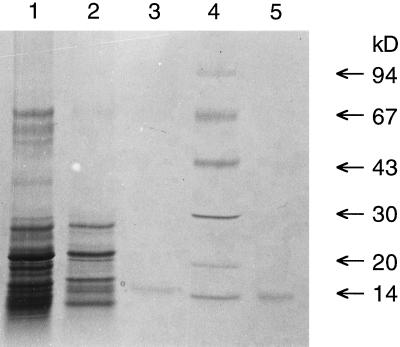
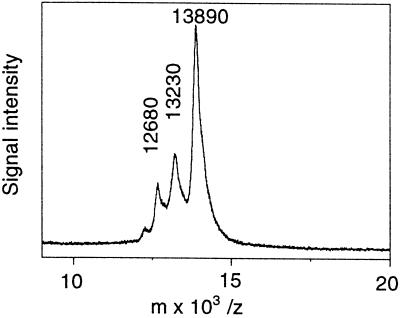
The purified PLA2 fraction showed one band with a molecular mass of about 15 kD when subjected to SDS-PAGE on an 8 to 18% gradient gel under reducing conditions and stained with colloidal Coomassie blue (Fig. 2). After electrophoresis and under nonreducing conditions, PLA2 activity could be recovered and was found to coincide with the 15-kD band (Fig. 3). A similar amount of snake venom PLA2 was included in adjacent lanes for a comparison and gave about the same recovery of activity (Fig. 3). For a more precise molecular mass determination a MALDI-TOF MS analysis was performed. The purified fraction gave a major peak with a molecular mass of 13,900 D and two minor peaks with masses of 13,200 and 12,700 D (Fig. 4). With alkylation of Cys residues in the purified fraction all three peaks gained about 700 to 800 mass units, indicating the presence of 12 to 14 Cys residues (data not shown).
Figure 2.
SDS-PAGE analysis of fractions from the three last purification steps of the elm PLA2 purification, along with a commercial PLA2 from cobra venom. Lane 1, Twenty-five micrograms of Superose 12 eluate; lane 2, 2 μg of C4-reverse phase eluate; lane 3, 0.1 μg of C2/C18 reverse-phase eluate; lane 4, molecular size markers; and lane 5, 0.1 μg of cobra venom PLA2. Samples were reduced with DTT and separated on an 8 to 18% gradient gel.
Figure 3.
Recovery of activity from elm and cobra PLA2 from a SDS-PAGE gel. Duplicate samples of 50 ng of PLA2 from cobra venom and of purified elm-seed PLA2, unreduced, were separated on an 8 to 18% gradient gel. One lane with cobra venom and one lane with elm PLA2 were immediately sliced into 2- to 3-mm-wide pieces, from which proteins were eluted, precipitated, and assayed for PLA2 activity, as described in Methods. Remaining lanes were stained overnight with colloidal Coomassie blue. Lane 1, Cobra venom PLA2; lane 2, elm seed PLA2.
Figure 4.
Molecular mass determination of purified PLA2 fraction by MALDI-TOF MS. Purified elm PLA2 (1–2 pmol/μL) was analyzed in a MALDI-TOF MS instrument, as described in Methods.
Characterization of Purified PLA2 Activity
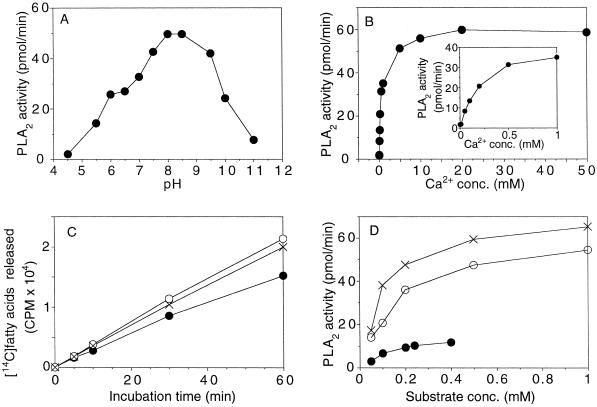
The enzyme exhibited optimal activity at a pH between 8.0 and 9.0 and had no activity below pH 5.0 (Fig. 5A). EGTA (1 mm) totally abolished activity (data not shown), indicating an absolute requirement for Ca2+. Optimal concentration of CaCl2 for activity was as high as 10 to 15 mm, although 0.5 mm was sufficient to achieve 50% of maximal activity (Fig. 5B).
Figure 5.
A, The effect of pH. Incubations were carried out with 1 ng of purified PLA2, 0.2 mm 1-palmitoyl-2-[14C]palmitoyl-PC, 1 mm lubrol PX, and 10 mm CaCl2 in 75 mm buffer at 30°C for 15 min in a final volume of 50 μL. Buffers used were: acetic acid, pH 4.5 to 5.5; Mes, pH 5.5 to 6.5; Bis-Tris propane, pH 6.5 to 9.5, and Caps, pH 9.5 to 11. Bis-Tris propane, 1,3-bis(Tris[hydroxymethyl]methylamino)propane; Caps, 3-(cyclohexylamino)-1-propanesulfonic acid. B, The effect of Ca2+ concentration on PLA2 activity. Incubations were carried out with 1 ng of purified PLA2 in 50 mm Tris-HCl buffer, pH 8.0, in the presence of 0.2 mm 1-palmitoyl-2-[14C]palmitoyl-PC, 1 mm lubrol PX, and with Ca2+ concentrations as indicated in the figure in a final volume of 50 μL at 30°C for 15 min. C, Time-course incubations of purified PLA2 with and without BSA. Purified PLA2, 0.5 ng, was incubated in the presence of 0.2 mm 1-palmitoyl-2-[14C]palmitoyl-PC, 1 mm lubrol PX, 10 mm CaCl2, 50 mm Tris-HCl, pH 8.0, in the absence of BSA (•), with 50 μg of BSA (○), or with 250 μg of BSA (×), in a final volume of 50 μL for the indicated times at 30°C. D, Effect of substrate concentration at various lubrol PX/phosphatidylcholine molar ratios. Purified PLA2, 1 ng, was incubated in the presence of 10 mm CaCl2, 50 mm Tris-HCl, pH 8.0, 0.02 to 1 mm 1-palmitoyl-2-[14C]palmitoyl-PC without detergent (•) or with a lubrol PX/phosphatidylcholine molar ratio of 2 (○) or 6 (×) in a final volume of 50 μL for 10 min at 30°C. conc., Concentration.
The purified enzyme was tested for its specificity with respect to the sn position in PC by incubation with 1-palmitoyl-2-[14C]palmitoyl-PC and 1,2-[14C]dipalmitoyl-PC for an extended incubation time, followed by an analysis of 14C-labeled hydrolysis products on TLC plates. No radioactive LPC was formed when the enzyme was incubated with 1-palmitoyl-2-[14C]palmitoyl-PC, indicating that the enzyme was not active against the sn-1 position of PC (Table II). With 1,2-[14C]dipalmitoyl-PC as a substrate about equimolar amounts of radioactive fatty acids and LPC were formed (Table II), confirming that the purified enzyme does not possess phospholipase B activity. The purified PLA2 had no activity toward the neutral acyl lipid 1-palmitoyl-2-[14C]palmitoyl-DAG or the lysophospholipid 1-[14C]oleoyl-LPC (Table II).
Table II.
Specificity of purified elm seed PLA2
| Substrate | Recovered
14C Radioactivity
|
|
|---|---|---|
| Fatty acids | LPC | |
| % of total | ||
| 1-Palmitoyl-2-[14C]palmitoyl-PC | 48 | 0.3 |
| 1,2-[14C]Dipalmitoyl-PC | 34 | 30 |
| 1-Palmitoyl-2-[14C]palmitoyl-DAG | 0 | – |
| 1-[14C]Oleoyl-LPC | 0.2 | 99.8 |
Purified elm PLA2, 2 ng, was incubated for 30 min with 5 nmol of radioactive substrate under standard assay conditions.
The acyl preferences for the purified elm PLA2 were analyzed by measuring activity with 1-palmitoyl-2-14C-acyl-PC, with caproyl, palmitoyl, and oleoyl groups at the sn-2 position. Similar assays with cobra PLA2 were included in this experiment for a comparison. The elm enzyme was found to hydrolyze the caproyl and oleoyl groups at about twice the rate of the palmitoyl groups, whereas the cobra enzyme hydrolyzed all three acyl groups at about the same rate (Table III). The specific activity of the purified elm enzyme with the preferred substrate 1-palmitoyl-2-[14C]caproyl-PC was found to be 90 μmol min−1 mg−1.
Table III.
Acyl specificities of the purified elm seed and cobra PLA2s
| Substrate | PLA2 Activity
|
|
|---|---|---|
| Elm PLA2 | Cobra PLA2 | |
| pmol/min | ||
| 1-Palmitoyl-2-[14C]palmitoyl-PC | 39 | 48 |
| 1-Palmitoyl-2-[14C]oleoyl-PC | 77 | 40 |
| 1-Palmitoyl-2-[14C]caproyl-PC | 93 | 51 |
Purified elm or cobra PLA2, 1 ng, was incubated for 10 min with 25 nmol of radioactive substrate under standard assay conditions.
The elm PLA2 was stable both in organic solvents and at extreme pH conditions (see above) and was also extremely heat stable. Incubations with the purified enzyme at 100°C for 5 min did not affect activity, whereas a 30-min incubation at this temperature caused only a 40% decrease in activity (results not shown). DTT (5 mm) or 1% (v/v) β-mercaptoethanol were, however, found to completely abolish activity (results not shown), indicating the likely presence of structurally important disulfide bonds.
Time-course incubation of about 1 ng of purified PLA2 with 1-palmitoyl-2-[14C]palmitoyl-PC at a concentration range of 0.02 to 1 mm (with 1 mm lubrol PX) showed linear activity for about 10 to 15 min (data not shown). The inclusion of 50 or 250 μg of BSA in the assay cocktail prolonged the linearity of time-course incubations substantially (Fig. 5C), possibly indicating a decreased product inhibition because of the scavenging of the produced free fatty acids. Substrate-saturation curves of the purified PLA2 without detergent present, and at two fixed detergent/substrate molar ratios (2 and 6), showed that the activity is greatly increased by the dispersion of the substrate in mixed micelles and that the detergent/PC molar ratio strongly affects activity (Fig. 5D). Under the assay conditions used, the enzyme is saturated with about 1 mm substrate.
N-Terminal Sequence Analysis
About 1.8 μg of purified PLA2 (130 pmol) was reduced and alkylated with iodoacetamide and the N-terminal sequence was determined by automatic Edman degradation. Fifty-seven cycles were run, with an initial yield of approximately 17% and a repetitive yield of 94.3% for the 36 first cycles. The 53 first cycles gave a clear, single first-choice amino acid, and the amino acid sequence was read manually to be: 1—LNVGVQATGT—SISVGKGCSR—KCESEFCSVP—PFLRYGKYCG—LLYSGCPGEK—PCD—53. A low, double-background signal (about 10% of the main signal) could be read partly at about 25 cycles and was likely to be two degradation products of the main sequence; the two cleavage sites in the native protein were inferred from this signal to be between residues 7 and 8 and between residues 13 and 14 (data not shown). The main N-terminal amino acid sequence was used in a BLAST search (see Methods) and the best aligned sequences found were EST-sequences from rice (Oryza sativa; accession nos. C27540, D47320, D47653, and D47724). The C27540 sequence originated from a callus mRNA and the others originated from 8-d-old shoots. All four rice clones have nearly the same nucleotide sequences. An alignment of the N-terminal amino acid sequence of the elm PLA2 with the deduced amino acid sequence from C27540 and D47653 is shown in Figure 6. The overall identity is 62% and it is noteworthy that the positions of all six Cys residues in the elm enzyme sequence are conserved in the rice EST clones.
Figure 6.
Alignment of the N-terminal amino acid sequence of the purified elm PLA2 with the deduced amino acid sequences of the rice EST sequences D47653 and C27540 (accession nos.) and the 49 first N-terminal amino acids of animal secretory group I (cobra) and group II (human synovial fluid) PLA2. The last amino acid in the predicted signal peptide (certainty 0.76 by PSORT World Wide Web server) of the D47653 sequence is underlined. The consensus line shows identical amino acids for the three plant sequences. Stars denote amino acid residues that are conserved among all animal secretory PLA2s (Chen et al., 1994b).
The PSORT World Wide Web server predicted a 25-amino acid-long signal peptide for export through the Golgi in the rice-shoot EST sequences. The predicted cleavage site between Gly-25 and Leu-26 coincides with the start of the aligned elm PLA2 N-terminal amino acid sequence (Fig. 6). The rice callus EST sequence starts in the middle of the predicted signal peptide. A region in the amino acid sequence of the animal secretory PLA2s, called the Ca2+-binding loop, is found in the N-terminal sequences of both the elm PLA2 and the putative rice PLA2s (Fig. 6). The active-site region of animal secretory PLA2s was also found in the putative rice PLA2s (Fig. 6) but is downstream of the elm N-terminal sequence. Of the 17 highly conserved amino acids that are common to all animal secretory PLA2s (Chen et al., 1994b), 10 are present in the N-terminal part of the group I and II PLA2s, which has been aligned in Figure 6. Of these 10, 9 are found in the putative rice PLA2 sequences.
DISCUSSION
The purification to homogeneity of a soluble PLA2 from developing elm seeds is, to our knowledge, the first purified PLA2 enzyme from a plant. The amount of this enzyme is extremely low in the plant tissue and a purification factor of 180,000-fold was needed to obtain a homogenous protein, as judged by SDS-PAGE. A crucial part of the purification protocol was the use of reverse-phase chromatography, which has also been used successfully in the purification of several secretory PLA2s from animal tissues (Tojo et al., 1984; Forst et al., 1986; Kramer et al., 1989).
The purified protein appeared homogeneous when analyzed by SDS-PAGE, and activity recovered from unreduced samples coincided with a single protein band with a molecular mass of 15 kD. A positive control of PLA2 from cobra venom showed that the method of recovering PLA2 activity from unreduced SDS-PAGE gels also functioned well for animal secretory PLA2, with a comparable recovery of activity. When analyzed by MALDI-TOF MS, the purified fraction gave a single major peak of 13,900 D and two minor peaks of 13,200 and 12,700 D. All three proteins gained about the same amount of mass after alkylation of Cys residues, indicating 12 to 14 Cys residues in each protein. The fact that all three proteins have a similarly high number of Cys residues suggests that they are related, which was also strongly supported by the N-terminal amino acid sequencing, in which a background signal gave a sequence implying that these minor proteins were proteolytic products of the major protein. The calculated reduction in molecular mass from the native protein caused by these cleavages corresponded to about 690 and 1230 mass units, respectively. These figures matched the molecular mass differences between the major and the two minor peaks obtained by MALDI-TOF MS of the purified fraction. The main signal in the N-terminal amino acid sequencing of the reduced and alkylated purified protein yielded a 53-residue-long sequence. When used as a query for the BLAST program at the National Center for Biotechnology Information, the N-terminal amino acid sequence gave best matches with the deduced amino acid sequences of some rice EST clones. The general identity score obtained was 62%, indicating that they are closely related. The general identity is highest from residue 32 of the elm N-terminal sequence and downstream. Only 2 of the 21 last amino acid residues in the elm N-terminal sequence are different in the rice sequences. This highly conserved area in the elm and putative rice PLA2s includes the conserved Ca2+-binding loop motif of animal secretory PLA2s and a short stretch between the Ca2+-binding loop and the active-site region of animal secretory PLA2s. Furthermore, all six Cys residues that were found in the elm N-terminal sequence are conserved in the rice sequences. In animal secretory PLA2s, the Cys residues, in addition to the Ca2+-binding loop and the active-site region, are known to be conserved within the different subgroups (Dennis, 1994).
When the elm and putative rice PLA2 sequences are compared with sequences of animal secretory PLA2s, the general identity scores are not very high. However, the catalytically important motifs of animal secretory PLA2s, the Ca2+-binding loop and the active-site motifs, are both found in the rice sequences. The active site is positioned downstream of the elm N-terminal sequence. Only one of the Cys residues found outside of the catalytic motif in the elm enzyme is conserved in animal PLA2 sequences. This might indicate that, although the catalytic areas of both plant and animal plant PLA2s are conserved, the overall three-dimensional structure and the disulfide arrangement outside of these areas might be quite different. This has been shown to be the case for the structural relationship between bee venom and animal secretory group I and II PLA2s (Scott et al., 1990).
The biochemical and catalytic properties of the elm PLA2 are similar to those of secretory PLA2s found in animal tissues. Well-established properties of secretory PLA2s are the following: molecular mass of 13 to 15 kD; absolute requirement for Ca2+ for activity with an optimal concentration in the millimolar range; stability to heat, acid conditions, and organic solvents but sensitivity to disulfide-reducing agents such as DTT and β-mercaptoethanol; and specificity for the sn-2 position of phospholipids with no activity toward neutral lipids. The animal secretory PLA2s are one of the best-studied classes of enzymes, and the basis of their catalytic activities has been explained in great detail. Ca2+, which is bound to a conserved amino acid region called the “Ca2+-binding loop,” has been shown to participate in the catalytic site by stabilizing the transition state (Scott et al., 1990). The extreme stability of the enzyme is due to the high number of disulfide bonds giving it a cross-linked architecture. The N-terminal sequence of the elm PLA2 is rich in Cys residues, and the MS data indicate the likely presence of six disulfide bridges in the entire protein. Most of the animal secretory PLA2s have seven disulfide bonds, although recent findings have shown PLA2s with six or eight (Chen et al., 1994a, 1994b).
The biochemical similarity between the elm PLA2 and the animal PLA2s (the latter being extracellular enzymes), as well as the homology of the elm enzyme with the rice putative PLA2s (which have putative signal peptide sequences for secretion), indicate that the elm enzyme is likely to be a secreted protein. Our original suggestion for the physiological role of PLA2 activities in developing seeds was the removal of unusual fatty acids from membrane lipids for their subsequent incorporation into triacylglycerols (Ståhl et al., 1995). The triacylglycerol synthesis is believed to take place on the cytosolic side of ER membranes, and an involvement of PLA2 in this metabolism would require a cytosolic or ER membrane-bound localization. However, disulfide bonds are not expected to occur in a protein that is fully exposed to the reducing conditions of the cytosol (Davidson and Dennis, 1990). Furthermore, the pronounced acyl specificity that we found for the PLA2 activity in microsomal preparations of the developing elm seeds (Ståhl et al., 1995) was not observed for the purified enzyme. The purified elm PLA2 hydrolyzes PC with caproyl groups only 2 times faster than with palmitoyl groups.
The secretion of a protein out to the cell wall would be a translocation to an acidic environment with a pH of 5.0 to 6.0. The purified elm PLA2, like most animal secretory PLA2s, has an alkaline pH optimum and minimal activity below pH 6.0. The elm enzyme, if it was secreted, would most likely be inactive in the cell wall. It is therefore difficult to speculate about the function of this type of enzyme in plant tissues. In animal tissues secretory, nonpancreatic PLA2s have been studied intensively during the last decade, but their functions have not been conclusively established. Nevertheless, the identification of plant PLA2 enzymes and genes, and studies of their patterns of occurrence and expression, will yield new information that certainly will help us to understand their function in plant tissues.
ACKNOWLEDGMENTS
Professor Lennart Kenne, Dr. Suresh Gohil, and Susanna Broberg (Department of Chemistry, Swedish University of Agricultural Sciences) are acknowledged for help with MALDI-TOF MS analysis.
Abbreviations:
- DAG
1,2-diacyl-sn-glycerol
- EST
expressed sequence tag
- LPC
lysophosphatidylcholine
- MALDI-TOF
matrix-assisted laser-desorption ionization-time-of-flight
- PC
3-snphosphatidylcholine
- PLA2
phospholipase A2
- TFA
trifluoroacetic acid
Footnotes
This research was supported by the Swedish Agricultural and Forestry Research Council, the Swedish Foundation for Strategic Research, the Swedish Farmers Research Foundation, and Metapontum Agrobios (Metaponto, Italy).
LITERATURE CITED
- Bafor M, Smith MA, Jonsson L, Stobrt AK, Stymne S. Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean endosperm. Biochem J. 1991;280:507–514. doi: 10.1042/bj2800507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafor M, Smith MA, Jonsson L, Stobart AK, Stymne S. Biosynthesis of vernoleate (cis-12-epoxyoctadeca-cis-9-enoate) in microsomal preparations from developing endosperm of Euphorbia lagascae. Arch Biochem Biophys. 1993;303:145–151. doi: 10.1006/abbi.1993.1265. [DOI] [PubMed] [Google Scholar]
- Banas A, Johansson I, Stymne S. Plant microsomal phospholipases exhibit preference for phosphatidylcholine with oxygenated acyl groups. Plant Sci. 1992;84:137–144. [Google Scholar]
- Bergey DR, Howe GA, Ryan CA. Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brigthman AO, Zhu XZ, Morrè DJ. Activation of plasma membrane NADH oxidase activity by products of phospholipase A. Plant Physiol. 1991;96:1314–1320. doi: 10.1104/pp.96.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MA, Olah TA, Weitzman CJ, Cooperman BS. Protein estimation by the product of integrated peak area and flow rate. Anal Biochem. 1989;182:295–299. doi: 10.1016/0003-2697(89)90597-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Engle SJ, Seilhamer JJ, Tischfield JA. Cloning and recombinant expression of a novel human low molecular weight Ca2+-dependent phospholipase A2. J Biol Chem. 1994a;269:2365–2368. [PubMed] [Google Scholar]
- Chen J, Engle SJ, Seilhamer JJ, Tischfield JA. Cloning and characterization of novel rat and mouse low molecular weight Ca2+-dependent phospholipase A2s containing 16 cysteines. J Biol Chem. 1994b;269:23018–23024. [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Davidson FF, Dennis EA. Evolutionary relationships and implications for the regulation of phospholipase A2 from snake venom to human secreted forms. J Mol Evol. 1990;31:228–238. doi: 10.1007/BF02109500. [DOI] [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- Forst S, Weiss J, Elsbach P. Structural and functional properties of a phospholipase A2 purified from an inflammatory exudate. Biochemistry. 1986;25:8381–8385. doi: 10.1021/bi00374a008. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Ohto C, Mizoguchi T, Shinozaki K. A gene encoding a phosphatidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1995;92:3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AHC (1987) Lipases. In PK Stumpf, ed, The Biochemistry of Plants, Vol 9. Academic Press, New York, pp 91–119
- Kanda P, Wells MA. Facile acylation of glycerophosphocholine catalyzed by trifluoroacetic anhydride. J Lipid Res. 1981;22:877–879. [PubMed] [Google Scholar]
- Kawakita K, Senda K, Doke N. Factors, affecting in vitro activation of potato phospholipase A2. Plant Sci. 1993;92:183–190. [Google Scholar]
- Kim DK, Lee HJ, Lee Y. Detection of two phospholipase A2 (PLA2) activities in leaves of higher plant Vicia faba and comparison with mammalian PLA2's. FEBS Lett. 1994;343:213–218. doi: 10.1016/0014-5793(94)80558-x. [DOI] [PubMed] [Google Scholar]
- Klucis E, Polya GM. Calcium-independent activation of two plant leaf calcium-regulated protein kinases by unsaturated fatty acids. Biochem Biophys Res Commun. 1987;147:1041–1047. doi: 10.1016/s0006-291x(87)80175-4. [DOI] [PubMed] [Google Scholar]
- Kramer RM, Hession C, Johansen B, Hayes G, MacGray P, Chow EP, Tizard R, Pepinsky RB. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989;264:5768–5775. [PubMed] [Google Scholar]
- Martiny-Baron G, Scherer GFE. Phospholipid-stimulated protein kinase in plants. J Biol Chem. 1989;264:18052–18059. [PubMed] [Google Scholar]
- Moreau RA, Morgan CP. Proteolytic activation of a lipolytic enzyme activity in potato leaves. Plant Sci. 1988;55:205–211. [Google Scholar]
- Neuhoff V, Arold N, Taube D, Ehrhart W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Sommarin M, Ulvskog P, Jørgensen PL. Modulation of plasma membrane H+-ATPase from oat roots by lysophosphatidylcholine, free fatty acids and phospholipase A2. Physiol Plant. 1988;74:11–19. [Google Scholar]
- Pappan K, Qin W, Dyer JH, Zheng L, Wang X. Molecular cloning and functional analysis of polyphosphoinositide-dependent phospholipase D, PLDbeta, from Arabidopsis. J Biol Chem. 1997a;272:7055–7061. doi: 10.1074/jbc.272.11.7055. [DOI] [PubMed] [Google Scholar]
- Pappan K, Zheng S, Wang X. J Biol Chem. 1997b;272:7048–7054. doi: 10.1074/jbc.272.11.7048. [DOI] [PubMed] [Google Scholar]
- Roy S, Pouénat ML, Caumont C, Cariven C, Prévost MC, Esquerré-Tugayé MT. Phospholipase activity and phospholipid patterns in tobacco cells treated with fungal elicitor. Plant Sci. 1995;107:17–25. [Google Scholar]
- Sahsah Y, Thi ATP, Roy-Macauley H, d'Arcy-Lameta A, Repellin A, Zuily-Fodil Y. Purification and characterization of a soluble lipolytic acylhydrolase from cowpea (Vigna unguiculata L.) leaves. Biochim Biophys Acta. 1994;1215:66–73. doi: 10.1016/0005-2760(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Scott DL, White SP, Otwinowski Z, Yuan W, Gelb MH, Sigler PB. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990;250:1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, Kloss J, Johnson LK. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- Ståhl U, Banas A, Stymne S. Plant microsomal phospholipid acyl hydrolases have selectivities for uncommon fatty acids. Plant Physiol. 1995;107:953–962. doi: 10.1104/pp.107.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl U, Ek B, Banas A, Lenman M, Sjödahl S, Stymne S. Purification and characterization of a microsomal phospholipase A2 from developing elm seeds. In: Williams JP, Khan MU, Lem NW, editors. Physiology, Biochemistry and Molecular Biology of Plant Lipids. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 244–246. [Google Scholar]
- Teissère M, Borel M, Cailoll B, Nari J, Gardies AM, Noat G. Purification and characterization of a fatty acyl-ester hydrolase from post-germinated sunflower seeds. Biochim Biophys Acta. 1995;1255:105–112. doi: 10.1016/0005-2760(94)00222-k. [DOI] [PubMed] [Google Scholar]
- Tischfield JA. A reassessment of the low molecular weight phospholipase A2 gene family in mammals. J Biol Chem. 1997;272:17247–17250. doi: 10.1074/jbc.272.28.17247. [DOI] [PubMed] [Google Scholar]
- Tojo H, Teramoto T, Yamano T, Okamoto M. Purification of intracellular phospholipase A2 from rat spleen supernatant by reverse-phase high-performance liquid chromatography. Anal Biochem. 1984;137:533–537. doi: 10.1016/0003-2697(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Vadas P, Wasi S, Movat HZ, Hay JB. Extracellular phospholipase A2 mediates inflammatory hyperaemia. Nature. 1981;293:583–585. doi: 10.1038/293583a0. [DOI] [PubMed] [Google Scholar]
- Waite M (1987) The phospholipases. In DJ Hanahan, ed, Handbook of Lipid Research, Vol 5. Plenum Press, New York, pp 111–133
- Wessel D, Flügge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Yotsushima K, Mitsui T, Takaoka T, Hayakawa T, Igaue I. Purification and characterization of membrane-bound inositol-specific phospholipase C from suspension-cultured rice (Oryza sativa L.) cells. Plant Physiol. 1993;102:165–172. doi: 10.1104/pp.102.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]