Abstract
The stress-related neuropeptide, corticotropin-releasing factor (CRF), is prominent in neurons of the pontine micturition center, Barrington’s nucleus. These neurons co-innervate spinal preganglionic neurons that control the bladder and locus coeruleus (LC) neurons that provide norepinephrine innervation throughout the brain. Adeno-associated viral (AAV) vector-mediated transfer of CRF cDNA was used to increase CRF expression in Barrington’s nucleus neurons and investigate the impact of a gain of function in Barrington’s nucleus spinal and LC projections. AAV transfer of the reverse CRF cDNA sequence served as the control. Bladder urodynamics and behavior were assessed four weeks after vector injection into Barrington’s nucleus. Rats with bilateral injections of AAV-CRF cDNA into Barrington’s nucleus had immunohistochemical evidence of CRF overexpression in neurons and transport to the spinal cord and LC. The bladder: body weight ratio was greater and micturition pressure was less in these rats compared to controls, consistent with an inhibitory influence on bladder function. Other indices of urodynamic function were not altered. CRF innervation of the LC was increased in rats with bilateral Barrington’s nucleus injections of AAV-CRF cDNA and this was associated with increased burying behavior, an endpoint of LC activation by CRF. The results provide immunohistochemical evidence for viral vector-induced CRF overexpression in Barrington’s nucleus neurons and underscore the ability of AAV vector-mediated transfer to increase CRF function in selective circuits. The findings support an inhibitory influence of CRF in Barrington’s nucleus regulation of the bladder and an excitatory influence on the brain norepinephrine system that translates to behavioral activation.
Keywords: cystometry, rat, urinary, locus coeruleus, spinal cord
Corticotropin-releasing factor (CRF) is a hypothalamic neurohormone that is released into the median eminence to initiate adrenocorticotropin secretion in response to stress (Vale et al., 1981). CRF is also present in brain circuits where it modulates neuronal activity to regulate behavioral and autonomic responses to stressors. Coordinated neurohormone and neuromodulator actions of CRF are thought to integrate different aspects of the stress response (Valentino & Van Bockstaele, 2002; Bale & Vale, 2004). CRF-containing forebrain nuclei include the paraventricular hypothalamic nucleus, the central amgydaloid nucleus and the bed nucleus of the stria terminalis (Swanson et al., 1983; Sakanaka et al., 1987). Additionally, clusters of CRF-expressing neurons exist in the hindbrain in regions implicated in autonomic function (Swanson et al., 1983). Most prominent of these is Barrington’s nucleus, the pontine micturition center.
Barrington’s nucleus regulates the descending limb of the micturition reflex through its projections to preganglionic parasympathetic neurons of the lumbosacral spinal cord (de Groat, 1981; de Groat et al., 1993; Matsumoto et al., 1995b; a; de Groat, 2006). These neurons are activated by increases in bladder pressure and initiate detrusor muscle contraction when bladder pressure reaches the micturition threshold (de Groat et al., 1993; Rouzade-Dominguez et al., 2003). Many of these same spinal-projecting neurons also innervate the norepinephrine nucleus, locus coeruleus (LC), which initiates forebrain electroencephalographic (EEG) activation, an arousal response to increases in bladder pressure and a central limb of the micturition reflex (Page et al., 1992; Valentino et al., 1996). Within Barrington’s nucleus spinal projections, excitatory amino acids mediate the activation of parasympathetic neurons and bladder contraction, whereas CRF has an inhibitory influence (Matsumoto et al., 1995b; a; Pavcovich & Valentino, 1995). In contrast, CRF is excitatory to LC neurons (Curtis et al., 1997). Simultaneous CRF release in both spinal and LC projections from Barrington’s nucleus neurons could promote continence by inhibiting or delaying bladder contraction until an arousal response has occurred.
Excessive CRF activity has been implicated in the pathophysiology of stress-related diseases, including irritable bowel syndrome, post-traumatic stress disorder and depression and CRF antagonists have been proposed as treatments for these disorders (Chrousos & Gold, 1992; Bremner et al., 1997; Holsboer, 1999; Wong et al., 2000; Gold & Chrousos, 2002; Martinez & Tache, 2006). We recently demonstrated that repeated social stress increases CRF mRNA and protein in Barrington’s nucleus neurons and produces urinary retention and a dysfunctional urodynamic profile, suggesting that the social stress-induced bladder dysfunction resulted from excessive CRF inhibition in efferents regulating the bladder (Wood et al., 2009). Nonetheless, the function of CRF in Barrington’s nucleus projections and the consequences of CRF overexpression in these projections have not been clarified. Notably, evidence also exists for an excitatory influence of CRF on bladder urodynamics (Klausner et al., 2005).
This study used adeno-associated viral (AAV) mediated transfer of CRF cDNA to produce CRF overexpression selectively in Barrington’s nucleus neurons. The consequences of the resulting gain of CRF function in Barrington’s nucleus neuronal projections on bladder urodynamics and on behavior associated with LC activation were examined.
Materials and Methods
Animals
Adult Sprague-Dawley male rats (250g–300 g, Charles River, Wilmington, MA) were singly housed upon arrival and throughout the experiment in a climate-controlled environment and kept on a 12-h light-dark cycle (7:00 am, lights on). Rats had free access to food and water. Surgery for vector injections was done at least 7 days after arrival. Appropriate measures were taken to minimize pain and discomfort. Studies were carried out in accordance with the National Institutes of Health guidelines for the care and the use of laboratory animals and were approved by the Children’s Hospital Institutional Animal Care and Use Committee.
Vector Design and Packaging
Adeno-associated viral vectors (AAV2/1) were produced in order to express CRF or enhanced green fluorescent protein (GFP). A mouse cDNA clone of CRF was obtained from Open Biosystems (GenBank Accession number BC119036.1). The amino acid sequences of mouse and rat (NM_031019) mature CRF peptides are identical. Flanking EcoRI sites were used to isolate the CRF cDNA fragment, which was then ligated into the AAV2 vector plasmid pZAC 2.1. Clones were selected with the insert oriented in forward and reverse (as a non-coding control) direction. Transgene expression is driven by the constitutively active EF1α promoter and both vectors include an SV40 intron, WPRE post-transcriptional enhancer, and a BGH poly-A signal. The packaging, purification, and determination of vector titers were performed by the University of Pennsylvania Vector Core, as previously described (Passini et al., 2003). Briefly, recombinant AAV2/1 vectors were generated using a triple transfection approach and purified using the CsCl sedimentation method. Genome vector copy titers were determined by real-time PCR (TaqMan Universal Master Mix, Applied Biosystems). Injection titers were between 1.2 and 1.3 × 1013 GC/ml. Aliquots were kept at −70°C until use.
Surgery and Vector Injection
Rats were prepared for stereotaxic surgery and neuronal recording with simultaneous microinjection as previously described (Kreibich et al., 2008). Briefly, rats were anesthetized with a 2% mixture of isofluorane-in-air, positioned in a stereotaxic and surgically prepared for placement of a double barrel glass micropipette into Barrington’s nucleus. The center barrel was filled with 2% pontamine sky blue in 0.5 M sodium acetate and served as the recording pipette. The ejection barrel was filled with a solution containing a mixture (1:1) of AAV-GFP and either AAV containing CRF cDNA inserted in the forward direction (AAV-CRFF) or AAV containing CRF cDNA inserted in the reverse direction (AAV-CRFR). Barrington’s nucleus was localized through electrophysiological recordings as previously described (Rouzade-Dominguez et al., 2003). Once the pipette was positioned into the region considered to be Barrington’s nucleus, 100nl of the vector solution was microinfused by repeated application of pressure (20–40 psi, 30 msec pulses) using a picospritzer. The ejection pipette was calibrated to deliver known volumes (Kreibich et al., 2008). Bilateral injections were done in every animal. After the skin over the skull was sutured, rats were observed until ambulatory and placed back into their home cage.
Behavior
At 28 days after the injection, behavior of some rats was examined in a novel cage environment. Rats were placed into a novel cage, identical to the home cage, but filled with 5 cm of bedding (Bed-O’cobs®). Behavior was videotaped for 60 min. Behavior was quantified as the time spent grooming or burying/digging and incidence of rearing by an individual blind to the experimental groups.
Cystometry
Bladder catheters were implanted under isofluorane anesthesia 29 days after vector injection as previously described (Kiddoo et al., 2006). On the following day, rats were placed in the cystometry chamber and habituated for 15-min with the catheter not attached to the perfusion pump. Urodynamic function was recorded in the unanesthetized state 24 h later for 60-min using cystometry equipment and software (Medical Associates, St. Albans, VT). Saline was infused into the bladder (100 μl/min) while bladder pressure, capacity, void volume and intermicturition interval were constantly monitored. After the 60 min recording, rats were deeply anesthetized with isofluorane, the bladders dissected and the rats were transcardially perfused with 4% paraformaldahyde. The brain and lumbosacral section of the spinal cord were dissected for immunohistochemical studies.
Immunofluoresence
Brains and spinal cords were post-fixed overnight at 4°C and stored in a 20% sucrose solution in phosphate buffer (PB) containing 0.1% sodium azide at 4°C for at least 24 h. Frozen sections (30μm-thick) were cut on a cryostat and collected in a series of 4 wells containing PB. CRF immunoreactivity was visualized using rabbit anti-CRF (C-70, 1:2,000, Wylie Vale, Salk Institute) and rhodamine conjugated donkey anti-rabbit antisera (1:200, Jackson ImmunoResearch, West Grove, PA) as previously described (Wood et al., 2009). Tyrosine hydroxylase (TH) immunoreactivity was visualized in some of the same sections by incubating in a cocktail of rabbit anti-CRF and mouse anti-TH (1:5,000, Immunostar, lot 22941, Hudson, WI) for 48 h at 4°C. AMCA-conjugated donkey anti-mouse (1:200; Jackson ImmunoResearch) was used as the secondary antibody to visualize TH-immunoreactivity. Sections were rinsed in PB, mounted and coverslipped with fluoromount. The characterization and specificity of the anti-CRF antibody has been previously described (Sawchenko et al., 1984; Foote & Cha, 1988; Van Bockstaele et al., 1996). This antiserum is preabsorbed with a melanocyte-stimulating hormone αMSH;1 mg/ml), a peptide with which it has been reported to cross-react (Nahon et al., 1989). Additionally, we previously demonstrated robust reactivity of a 1:4000 dilution of the antibody with CRF (≥8 ng) and a lack of cross-reactivity with up to 1 mg of either melanin-concentrating hormone or MSH using immunodot blot (Van Bockstaele et al., 1996). Sections incubated in the absence of antibody or with the antibody preabsorbed with CRF (1 mg/ml) do not show immuno labeling (Van Bockstaele et al., 1996).
Sacral sections of the spinal cord were blocked, placed in 1-inch by 1-inch tissue-tek mold and submerged in O.C.T compound (Tissue-Tek®, Torrance, CA). Sections (14μm) were cut and mounted onto charged slides (ProbeOnPlus, Fisher Scientific). A second ProbeOnPlus slide was positioned onto the first such that is was raised over the sections and a solution containing rabbit anti-CRF was injected in the small gap between the slides. Slides were then placed in a humidified chamber for 48 h at 4°C. Slides were rinsed in PBS and incubated with rhodamine-conjugated donkey anti-rabbit antibody (1:200) for 90 min at room temperature. After final washes in PB slides were coverslipped with fluoromount.
In-situ hybridization
Rats were decapitated and the brains were dissected and flash frozen in 2-methylbutane (Fisher Chemical, Waltham, MA) on a bed of dry ice. Frozen sections (14 μm thick) were cut on a cryostat and mounted onto charged slides (Colorfrost® Plus Microscope Slides, Erie Scientific). Slides were incubated in 10% formalin before being acetylated (0.25% acetic anhydride), and dehydrated in ascending ethanol concentrations (70–100%). The sections were hybridized using an antisense riboprobe to CRF mRNA (Dr. Audrey F. Seasholtz, University of Michigan) as previously described (Wood et al., 2009). Slides were coated with Kodak NTB2 liquid autoradiography emulsion (10-day exposure at 4° C) and then counterstained with cresyl violet.
Image analysis and quantification
Immunofluorescence was visualized using a Leica DMRXA microscope. Images were captured with a Hamamatsu ORCA-ER digital camera (Bridgewater, NJ, USA) using Open Laboratory software (Perkin Elmer). Images visualized with different fluorescent filters were pseudo-colored using Open Laboratory software. For quantification of CRF-immunoreactive cells in Barrington’s nucleus, sections were photographed at same exposure and the grayscale images were inverted using Image J (rsbweb.nih.gov/ij/). A background density value was determined by placing a region of interest lateral and ventral to the mesencephalic trigeminal nucleus. A threshold of 2x this background density was used as the criterion for CRF-immunolabeling. An oval region of interest was drawn to encompass Barrington’s nucleus at its largest extent and this was superimposed on all images. All neurons within this region of interest that exceeded the threshold were counted as CRF-immunoreactive neurons in Barrington’s nucleus. CRF cells were counted in 3 randomly chosen sections from an individual rat and averaged as the value for that rat. The mean determined from all individual rats was averaged for the group mean.
For quantification of CRF innervation of the LC, only sections that were dual labeled for TH and CRF and were at the level where the LC covered the greatest area (mid level) were used. Images to be compared were taken at the same exposure. The area containing TH-immunoreactive neurons was outlined. The grayscale image was inverted. The Image J Optical Density calibration was used to determine the density of CRF fibers in the area within the LC outline. For all density measurements, the mean density of a background region was subtracted from the mean density of the region of interest and the result was multiplied by the area of the region of interest to yield the integrated density measurement. Data from multiple sections of the same rat were averaged (2–6 sections per rat) to yield one determination per rat. Quantification was done by individuals that were blind to treatment.
Statistical analysis
Effects were statistically compared between groups by a one-way analysis of variance (ANOVA) using JMP 9.0 software (SAS Institute Inc.).
Results
AAV vector-mediated transduction of CRF in Barrington’s nucleus neurons
The placement and extent of the injections were indicated in sections by visualization of GFP. Of 25 rats injected bilaterally with AAV-CRFF, 14 had bilateral injections in Barrington’s nucleus as determined by GFP. In 6 rats the injection was only present in Barrington’s nucleus ipsilaterally and in 5 rats the injection was outside of Barrington’s nucleus on both sides. Figure 1 shows examples of CRF mRNA expression in Barrington’s nucleus revealed by in situ hybridization in sections from an uninjected rat and rats injected with AAV-CRFR and AAV-CRFF. CRF mRNA is endogenously expressed in Barrington’s nucleus as depicted in the uninjected control case and as previously reported (Imaki et al., 1991). CRF mRNA expression in Barrington’s nucleus in a rat that was injected with AAV-CRFR appeared comparable to the uninjected control. In contrast, the signal representing CRF mRNA was much greater in the subject that was injected with AAV-CRFF(Fig. 1).
Figure 1.
AAV vector-mediated overexpression of CRF mRNA in Barrington’s nucleus neurons. Photomicrographs of representative coronal sections through Barrington’s nucleus show the hybridization signal for CRF mRNA from an uninjected control rat (Control), a rat injected with AAV-CRFR and a rat injected with AAV-CRFF. The top and bottom panels show same sections in darkfield and brightfield illumination, respectively. Sections were counterstained with Cresyl violet. Sections were processed at the same time and images captured using the same exposure time. Asterisks indicate the fourth ventricle. Top is dorsal and right is medial. Bar indicates 100 μm.
AAV-CRFF injection into Barrington’s nucleus resulted in intense CRF immunolabeling of neurons (Fig. 2). Most of these neurons were double labeled for GFP and CRF although some single labeled GFP neurons were observed. The extent of GFP labeled cells was similar in rats that received AAV-CRFR and colocalization of GFP- and CRF-immunoreactivity was apparent in some Barrington’s nucleus neurons (Fig. 2 arrowheads). However, CRF-immunoreactive neurons were fewer and immunolabeling was lighter in AAV-CRFR cases. To be able to compare CRF-immunolabeling between both groups the exposure times were matched and set at the longest duration at which CRF-immunolabeled neurons of AAV-CRFF rats could still be distinctly visualized. At this exposure, only occasional Barrington’s nucleus neurons of AAV-CRFR rats met the criterion of 2 times the background that defined cell immunolabeling. In rats injected with AAV-CRFF, the mean number of CRF-immunolabeled neurons/section ranged from 8–69 cells/section with a mean of 29±5 cells/section (n=14 rats). In rats injected with AAV-CRFR the range was 0–2 with a mean of 0.2±0.1 cells/section (n=14 rats). A one way ANOVA revealed a statistically significant difference between groups (F(1,26)=33; p<0.0001). AAV-CRFF rats having at least 14 (half the mean) CRF-immunolabeled neurons in Barrington’s nucleus were used in the cystometry and behavioral analysis described below.
Figure 2.
AAV vector-mediated overexpression of CRF protein in Barrington’s nucleus neurons. Shown are fluorescent photomicrographs of representative sections through Barrington’s nucleus from rats that were injected with AAV-CRFF (top panels) and AAV-CRFR (bottom panels). The left panels show GFP labeled neurons and indicate the extent of the injection. The center panels show CRF immunoreactivity. Sections were processed at the same time and images captured using the same exposure. Note that endogenous CRF labeling in neurons in the CRFR case is much lighter than CRF labeling in neurons from the rat injected with AAV-CRFF. The panels on the right show the merged images and indicate co-localization of GFP and CRF in neurons. The arrowheads point to the same cells in all figures and show GFP labeling in the CRF containing neurons in the AAV-CRFR case. The arrow points to a region of CRF-immunoreactive fibers that is typically seen and derives from the central nucleus of the amygdala (Van Bockstaele et al., 1998). Asterisks indicate the fourth ventricle. Top is dorsal and right is medial. Bar indicates 100 μm.
CRF axonal transport from AAV-transduced Barrington’s nucleus neurons
Barrington’s nucleus neurons project to the lumbosacral spinal cord where they densely innervate the preganlionic parasympathetic neurons projecting to the bladder (Kuru & Yamamoto, 1964; Satoh et al., 1978; Vincent & Satoh, 1984). Evidence for GFP transport to the spinal cord was apparent in both AAV-CRFF and AAV-CRFR cases where it colocalized within CRF-immunoreactive fibers (Fig. 3). The density of spinal CRF-immunolabeling was not quantified for comparison because endogenous CRF is high in this region and it is unlikely that immunostaining would be sufficiently sensitive to reveal any further increase.
Figure 3.
Transport of GFP protein to the spinal cord. Shown are fluorescent photomicrographs of coronal sections at the level of the lumbosacral spinal cord showing GFP and CRF immunoreactivity and the merged image from rats injected with AAV-CRFF (top) and AAV-CRFR (bottom). Arrowheads point to the same fiber that is dual labeled for GFP and CRF. Bar indicates 100 μm.
Barrington’s nucleus is also a source of CRF in the LC (Valentino et al., 1996). Figure 4 shows examples of CRF innervation of the LC of AAV-CRFF and AAV-CRFR injected rats. Quantification confirmed a higher density of CRF-immunoreactive fibers within the LC at a middle level in AAV-CRFF rats compared to AAV-CRFR rats (F(1,20)=5.9, P=0.03) (Fig. 6).
Figure 4.
AAV-CRFF injection results in CRF overexpression in fibers innervating the LC. Shown are fluorescent photomicrographs of sections through the LC showing CRF (red) and tyrosine hydroxylase (blue) immunolabeling in a rat injected with AAV-CRFR (top) and AAV-CRFF (bottom). Sections were processed at the same time and images taken at the same exposure time. Asterisks indicate the fourth ventricle. Top is dorsal and right is medial. Bar indicates 100 μm.
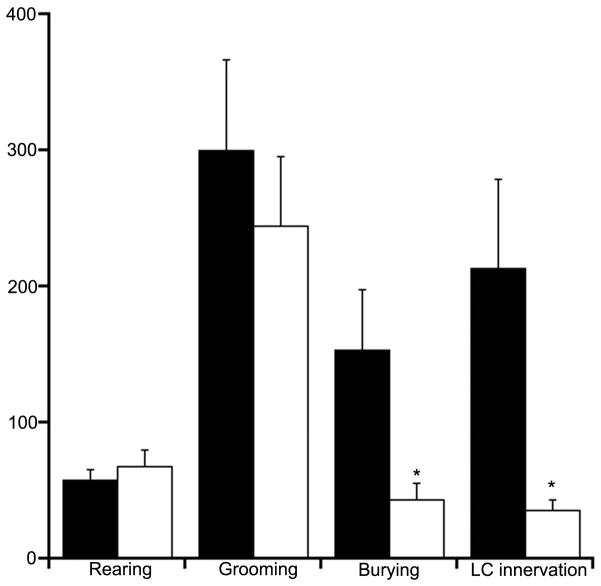
Figure 6.
Effects of CRF overexpression in Barrington’s nucleus neurons on behavior. Bars indicate the mean incidence of rearing, time (s) spent grooming or burying, and density of CRF innervation of the LC. Solid bars represent the means of rats injected with AAV-CRFF and open bars represent the mean of rats injected with AAV-CRFR; n=9 for both groups for behavioral studies; For LC innervation n=12 for AAV-CRFF and 10 for AAV-CRFR. *p<0.05.
Effects of CRF overexpression in Barrington’s nucleus on bladder urodynamics and behavior
CRF within Barrington’ nucleus spinal projections has been reported to have an inhibitory influence on bladder urodynamics (Pavcovich & Valentino, 1995). In vivo cystometry of AAV injected rats at 31 days after the injection revealed that micturition pressure was lower in AAV-CRFF rats (F(1,24)=4.6, p<0.05) and there was a tendency for bladder capacity to be increased but this did not reach statistical significance (F(1,24)=2.3, p=0.1) (Fig. 5). However, intermicturition interval, micturition volume, and micturition threshold and resting pressure were comparable between AAV-CRFF and AAV-CRFR rats. The bladder: body weight ratio was greater in AAV-CRFF rats compared to AAV-CRFR rats (F(1,27)=6.6, p<0.02), consistent with urinary retention (Fig. 5). Body weights were comparable between groups (430±12 g vs. 428±13 g, AAV-CRFF and AAV-CRFR, respectively, F(1,27)=0.01; p=0.9) Interestingly, the bladder: body weight ratio of rats with only a single injection of AAV-CRFF in Barrington’s nucleus also tended to be greater than those that received AAV-CRFR (0.61±0.40, F(1,22)=3.9, p=0.06).
Figure 5.
Effect of CRF overexpression in Barrington’s nucleus neurons on urodynamics. The bar graph on the left compares the mean bladder: body weight ratio (Ratio ×1000), intermicturition interval (IMI, s), micturition volume (MV, μl) and bladder capacity (BC, μl). The bar graph on the right compares resting pressure, micturition threshold and micturition pressure. For urodynamic measures, bars represent the mean of 11 rats injected with AAV-CRFF (solid bars) and 15 rats injected with AAV-CRFR (open bars). For the bladder: body weight ratio bars represent the mean of 11 rats injected with AAV-CRFF (solid bars) and 18 rats injected with AAV-CRFR (open bars). Vertical lines represent S.E.M. *p<0.05.
Central CRF administration elicits certain unconditioned behaviors in rats in a novel environment (Howard et al., 2008). Therefore, behaviors of AAV-CRFF and AAV-CRFR rats were quantified over a 60 min period during which rats were in a novel environment. Rearing and grooming were comparable between groups (Fig. 6). However, burying behavior, which is associated with activation of the LC-norepinephrine system (Howard et al., 2008), was substantially elevated in AAV-CRFF rats (F(1,16)=5.6, p=0.03) (Fig. 6). Rats that had only one accurately placed injection of AAV-CRF in Barrington’s nucleus had durations of burying that resembled those administered AAV-CRFR (51±20 s).
Discussion
The present study provided immunohistochemical evidence of regionally selective CRF overexpression in Barrington’s nucleus neurons and their projections using viral gene transfer. Local injection of AAV-CRFF increased CRF mRNA and protein in Barrington’s nucleus neurons. Detection of GFP in the regions of lumbosacral spinal parasympathetic neurons provided evidence for distant protein transport. Taken with CRF upregulation in LC projections, this is consistent with previous anatomical evidence for collateral projections of Barrington’s nucleus neurons to the spinal cord and LC (Valentino et al., 1996). Increased CRF expression in Barrington’s nucleus spinal projections was associated with a decrease in micturition pressure and increased bladder mass, supporting the inhibitory influence of CRF in this circuit that was suggested by previous pharmacological studies (Pavcovich & Valentino, 1995; Kiddoo et al., 2006). Viral-induced CRF overexpression in Barrington’s nucleus neuronal projections to the LC increased burying behavior in a novel environment, an active coping behavior that is elicited by CRF-induced activation of the LC-norepinephrine system (Howard et al., 2008). Together, the results underscore the ability of AAV transduction to manipulate CRF protein expression in specific neuronal circuits in brain and translate this to a gain in function that has physiological and behavioral consequences. Moreover, they suggest that biological conditions that result in CRF overexpression in Barrington’s nucleus neurons will have similar urological and/or behavioral consequences.
AAV vector mediated CRF overexpression
Because excessive CRF is thought to underlie symptoms of stress-related disorders, including depression and post-traumatic stress disorder, diverse approaches have been used to mimic increased CRF activity in brain or within specific brain circuits. This condition has been modeled with CRF overexpressing mice and with the addition of the Cre/LoxP system, a certain degree of regional and temporal selectivity to CRF overexpression can be added (Stenzel-Poore et al., 1994; van Gaalen et al., 2002; Lu et al., 2008). However, even in this model, the restriction is not localized to a single CRF-containing nucleus. Viral vector mediated gene transfer allows for more precision in localizing gene manipulations to specific brain nuclei. Early studies generated recombinant herpes simplex virus that expressed prepro CRF in neuronal, glial and epithelia cell lines and post-mitotic cells in primary culture (Tomasec et al., 1999). The mature peptide was processed and secreted and shown to be functionally active. More recently, injection of a lentiviral vectors containing the rat CRF gene have been used to overexpress CRF in the central amygdalar nucleus and/or the bed nucleus of the stria terminalis and determine the effects of this manipulation on behavior (Keen-Rhinehart et al., 2009; Regev et al., 2011; Flandreau et al., 2012). The present study is unique in using AAV and focusing on Barrington’s nucleus, a prominent CRF-containing nucleus related to autonomic function. Moreover, it provided anatomical evidence for axonal transport of the overexpressed protein to projection sites. Unlike the lentivirus vector used in a previous study, the AAV vector could not accommodate both CRF and GFP cDNA. To be able to determine both the extent of the injection and CRF overexpression, a mixture of vectors was injected. Although an alternative approach would be to fuse smaller tags to the CRF sequence, this could affect the functionality of CRF.
Impact of CRF overexpression in Barrington’s nucleus neurons on bladder function
Early studies demonstrated that dorsal pontine lesions, including the LC disrupt micturition (Barrington, 1925; Osumi et al., 1975). However, anatomical tracing and mapping of sites where chemical stimulation elicits bladder contraction shave more precisely localized the pontine micturition center to Barrington’s nucleus (Loewy et al., 1979; Hida & Shimazu, 1982; Willette et al., 1988; Noto et al., 1989; Pavcovich & Valentino, 1995; Blok & Holstege, 1997). Barrington’s nucleus neurons are activated as bladder pressure increases and through their innervation and activation of the preganglionic parasympathetic neurons in the lumbosacral spinal cord they initiate detrusor contraction when pressure reaches the micturition threshold (Rouzade-Dominguez et al., 2003; de Groat, 2006). The activation of spinal preganglionic neurons by Barrington’s nucleus projections is excitatory amino acid mediated (Matsumoto et al., 1995b; a). CRF is prominently expressed in Barrington’s nucleus neurons of both rats and primates (Swanson et al., 1983; Austin et al., 1995; Valentino et al., 1995). In contrast to excitatory amino acid neurotransmission, CRF in this pathway is inhibitory because bladder contractions elicited by discrete chemical stimulation of Barrington’s nucleus are enhanced by intrathecal administration of CRF antagonists and inhibited by intrathecal CRF (Pavcovich & Valentino, 1995). Additionally, intrathecal CRF increased intermicturition interval and bladder capacity, whereas intrathecal administration of CRF antagonists had opposite effects in urodynamic studies in unanesthetized rats (Kiddoo et al., 2006). However, another cystometry study using female Wistar-Kyoto rats reported excitatory effects of CRF (Klausner et al., 2005).
The inhibitory influence of CRF in Barrington’s nucleus spinal projections is hypothesized to play a role in social stress-induced bladder dysfunction. Rats and mice that become subordinate in social stress models develop urinary retention that leads to bladder hypertrophy and can result in death (Desjardins et al., 1973; Henry et al., 1982; Chang et al., 2009; Wood et al., 2009). Rats and mice exposed to social stress using the resident-intruder model exhibit urinary retention, non-micturition bladder contractions, increased intermicturition interval, increased bladder capacity and micturition volume and increased bladder mass (Chang et al., 2009; Wood et al., 2009). This was associated with enhanced CRF mRNA and protein expression in Barrington’s nucleus neurons (Wood et al., 2009). Notably, this stress did not increase CRF mRNA in the parventricular hypothalamic nucleus (Wood et al., 2010). Moreover, repeated restraint stress, which did not increase CRF mRNA in Barrington’s nucleus neurons, did not alter the urodynamic profile or bladder mass (Wood et al., 2009). Together, the findings suggested that CRF upregulation in Barrington’s nucleus spinal projections is responsible for functional and structural social stress-induced bladder changes (Wood et al., 2009). The present findings provide partial support for that hypothesis. CRF overexpression in Barrington’s nucleus neurons by AAV vector transduction reproduced the increased bladder mass seen in the social stress model, consistent with a degree of urinary retention. Additionally, the decreased micturition pressure could result from an increased inhibitory influence of CRF overexpression. However, although the magnitude of CRF overexpression produced by viral transduction was more pronounced than that seen in the social stress model, this did not reproduce the identical urodynamic profile. The inability of CRF overexpression to reproduce all of the urodynamic changes associated with social stress may be related to differences in the magnitude and duration of CRF overexpression in the two conditions. In the viral transduction study the degree of CRF overexpression is greater and probably of a longer duration, being 4 weeks after AAV-CRFF injection compared to 72 h after the seven daily exposures to stress. It is possible that compensatory mechanisms, such as receptor downregulation, have developed in the viral vector model of CRF overexpression to protect spinal neurons against excess CRF. Another consideration is a potential impact on other neuromediators within Barrington’s nucleus neurons and their projections. This may include decreased excitatory amino acid tone to spinal neurons. With the exception of CRF and excitatory amino acid neurotransmission, little is known of the neurochemical signaling of Barrington’s nucleus neurons.
Immunohistochemical studies provide evidence for expression of somatostatin and atrial naturietic factor in Barrington’s nucleus neurons, although their physiological functions in this pathway have not been demonstrated (Sutin & Jacobowitz, 1988; Ryan & Gundlach, 1995). Nonetheless, it is conceivable that altered CRF expression in Barrington’s nucleus neurons induced by viral transduction indirectly alters urodynamic function by effects on other neuromediators within these neurons and their spinal projections. Likewise, social stress may have a differential effect on non-CRF neurotransmitters in these projections, although evidence for this is lacking at this time.
Impact of CRF overexpression in Barrington’s nucleus neurons on LC innervation and behavior
Barrington’s nucleus is a source of CRF in the LC, the major norepinephrine-containing nucleus in the brain (Valentino et al., 1996). CRF increases LC neuronal discharge rate and norepinephrine release in forebrain targets of the LC and this is associated with forebrain electroencephalographic activation, indicative of arousal (Curtis et al., 1997; Page & Abercrombie, 1999). CRF also changes the pattern of LC firing to one that would promote cognitive flexibility and attentional set shifting studies in rats show that local CRF within the LC increases cognitive flexibility (Snyder et al., 2012). Electrophysiological and neurochemical evidence for stress-elicited CRF release in the LC suggest that this mediates behavioral arousal aspects of the stress response (Valentino et al., 1991; Kawahara et al., 2000). A major source of CRF that impacts on the LC during stress is the central nucleus of the amygdala, which supplies CRF axons that terminate in the dorsolateral peri-LC (Curtis et al., 2002). In contrast, Barrington’s nucleus neurons innervate the nuclear aspect of the LC where cell bodies are clustered (Valentino et al., 1996). Many of the CRF-immunoreactive Barrington’s nucleus neurons that project to the LC collateralize to the spinal cord (Valentino et al., 1996). The LC branches may promote forebrain arousal and facilitate attentional shifts towards bladder or other pelvic-related stimuli in an effort to promote motor behaviors that are compatible with the visceral response of elimination (Valentino et al., 2011).
One behavioral endpoint of CRF activation of the LC is burying behavior, an active stress coping response. We previously demonstrated that centrally administered CRF dose-dependently elicits burying, grooming and head shakes in rats and that CRF-elicited burying, but not other behaviors are mediated by LC norepinephrine projections to the forebrain (Howard et al., 2008). The current finding that AAV-CRFF upregulated CRF expression in Barrington’s nucleus projections to the LC and increased burying behavior, is consistent with the idea that CRF overexpression in this projection promotes LC-mediated active coping responses. It is also possible that enhanced CRF expression in Barrington’s nucleus neurons affects a non-CRF containing projection to the LC, although there is currently no evidence for such non-CRF projections.
Summary
The present study demonstrated the ability of AAV vector mediated gene transfer to upregulate CRF mRNA and protein in Barrington’s nucleus and produce a gain of CRF function in its spinal and LC projections. Using this method, the results suggested that CRF overexpression in these circuits alters bladder function and promotes active coping behavior.
Acknowledgments
This work was supported by grants from the NIH-NIDDK to RV (Project 1, 5P50-DK0532620) and JHW (3P50-DK0532620-12S1) and a Molecular Therapy Center grant (P30-DK47757) from the NIDDK (JHW); and R01-NS038690 from NINDS (JHW). TAG was supported in part by a training grant from the NIDDK (T32-DK007748). The authors thank Sandra Luz and Andrew Truong Ho for technical assistance.
Footnotes
None of the authors have a conflict of interest to disclose.
References
- Austin MC, Rice PM, Mann JJ, Arango V. Localization of corticotropin-releasing hormone in the human locus coeruleus and pedunculopontine tegmental nucleus: an immunocytochemical and in situ hybridization study. Neuroscience. 1995;64:713–727. doi: 10.1016/0306-4522(94)00420-a. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barrington FJT. The effect of lesion of the hind- abnd mid-brain on micturition in the cat. Quart J Exp Physiol. 1925;15:81–102. [Google Scholar]
- Blok BFM, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett. 1997;222:195–198. doi: 10.1016/s0304-3940(97)13384-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol. 2009;297:F1101–1108. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connally KR, Valentino RJ. Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J Neuroendocrinol. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- de Groat W. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147(Suppl 2):S25–40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System. Harwood Academic Publishers; London: 1993. pp. 227–290. [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Autonomic Nervous System. 1981;3:135. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Cha CI. Distribution of corticotropin-releasing factor-like immunoreactivity in brainstem of two monkey species (saimiri sciureus and macaca fasicularis): an immunohistochemical study. J Comp Neurol. 1988;276:239–264. doi: 10.1002/cne.902760208. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psych. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Henry JP, Meehan WP, Stephens PM. Role of subordination in nephritis of socially stressed mice. Contr Nephrol. 1982;30:38–42. doi: 10.1159/000406416. [DOI] [PubMed] [Google Scholar]
- Hida T, Shimazu N. The interrelation between the laterdorsal tegmental area and lumbosacral segments of rats as studied by HRP method. Arch Histol Jap. 1982;45:495–504. doi: 10.1679/aohc.45.495. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Sawachenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neuroscience. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiddoo DA, Valentino RJ, Zderic S, Ganesh A, Leiser SC, Hale L, Grigoriadis DE. mpact of the State of Arousal and Stress Neuropeptides on Urodynamic Function in the Freely Moving Rat. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00742.2005. [DOI] [PubMed] [Google Scholar]
- Klausner AP, Streng T, Na YG, Raju J, Batts TW, Tuttle JB, Andersson KE, Steers WD. The role of corticotropin releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci. 2005;123:26–35. doi: 10.1016/j.autneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Yamamoto H. Fiber connections of the pontine detrusor nucleus (Barrington) J Comp Neurol. 1964;123:161–186. doi: 10.1002/cne.901230203. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533–538. doi: 10.1016/0006-8993(79)90584-5. [DOI] [PubMed] [Google Scholar]
- Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M, Refojo D, Ekker M, Rubenstein JL, Stalla GK, Singewald N, Holsboer F, Wotjak CT, Wurst W, Deussing JM. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des. 2006;12:4071–4088. doi: 10.2174/138161206778743637. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Hisamitsu T, de Groat WC. Non-NMDA glutamatergic excitatory transmission in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Brain Res. 1995a;693:246–250. doi: 10.1016/0006-8993(95)00738-c. [DOI] [PubMed] [Google Scholar]
- Matsumoto G, Hisamitsu T, de Groat WC. Role of glutamate and NMDA receptors in the descending limb of the spinobulbospinal micturition reflex pathway of the rat. Neurosci Lett. 1995b;183:58–61. doi: 10.1016/0304-3940(94)11114-x. [DOI] [PubMed] [Google Scholar]
- Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- Noto H, Roppolo JR, Steers WD, De Groat WC. Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res. 1989;492:99–115. doi: 10.1016/0006-8993(89)90893-7. [DOI] [PubMed] [Google Scholar]
- Osumi Y, Fujiwara H, Oishi R, Takaori S. Central cholinergic activation by chlorfenvinphos, and organophosphate, in the rat. Jpn J Pharmacol. 1975;25:47–54. doi: 10.1254/jjp.25.47. [DOI] [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Page ME, Akaoka H, Aston-Jones G, Valentino RJ. Bladder distention activates locus coeruleus neurons by an excitatory amino acid mechanism. Neuroscience. 1992;51:555–563. doi: 10.1016/0306-4522(92)90295-d. [DOI] [PubMed] [Google Scholar]
- Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat by corticotropin-releasing hormone from Barrington’s nucleus. Neurosci Lett. 1995;196:185–188. doi: 10.1016/0304-3940(95)11873-u. [DOI] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington’s nucleus neurons to pelvic visceral stimuli: a juxtacellular labeling study. Eur J Neurosci. 2003;18:3325–3334. doi: 10.1111/j.1460-9568.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Gundlach AL. Anatomical localisation of preproatrial natriuretic peptide mRNA in the rat brain by in situ hybridisation histochemistry: novel identification in olfactory regions. J Comp Neurol. 1995;356:168–182. doi: 10.1002/cne.903560204. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederes K. Corticotropin-releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxide-diaminobenzidene method. J Comp Neurol. 1987;260:256–298. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Satoh K, Shimizu N, Tohyama M, Toshiro M. Localization of the micturition reflex center at dorsolateral pontine tegmentum of the rat. Neurosci Lett. 1978;8:27–33. doi: 10.1016/0304-3940(78)90092-7. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretry neurons of the adrenalectomized rat. Proc Natl Acad Sci USA. 1984;81:1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin EL, Jacobowitz DM. Immunocytochemical localization of peptides and other neurochemicals in the rat laterodorsal tegmental nucleus and adjacent area. J Comp Neurol. 1988;270:243–270. doi: 10.1002/cne.902700206. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Tomasec P, Preston CM, Linton EA, Ahmed I, Lowenstein PR, Castro MG. Generation of a recombinant herpes simplex virus type 1 expressing the rat corticotropin-releasing hormone precursor: endoproteolytic processing, intracellular targeting and biological activity. Neuroendocrinology. 1999;70:439–450. doi: 10.1159/000054506. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington’s nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Pavcovich LA, Hirata H. Evidence for corticotropin-releasing hormone projections from Barrington’s nucleus to the periaqueductal gray region and dorsal motor nucleus of the vagus in the rat. J Comp Neurol. 1995;363:402–422. doi: 10.1002/cne.903630306. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ. Corticotropin-releasing factor: putative neurotransmitter actions of a neurohormone. In: Pfaff D, AA, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 81–102. [Google Scholar]
- Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol. 2011;8:19–28. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Satoh K. Corticotropin-releasing factor (CRF) immunoreactivity in the dorsolateral pontine tegmentum: further studies on the micturition reflex system. Brain Res. 1984;308:387–391. doi: 10.1016/0006-8993(84)91085-0. [DOI] [PubMed] [Google Scholar]
- Willette RN, Morrison S, Sapru HN, Reis DJ. Stimulation of opiate receptors in the dorsal pontine tegmentum inhibits reflex contraction of the urinary bladder. J Pharmacol Exp Ther. 1988;244:403–409. [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1671–1678. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]