Abstract
In recent years it has been recognized that the development of cancer involves a series of not only genetic but epigenetic changes across the genome. At the same time, connections between epigenetic regulation, chromatin packaging, and overall nuclear architecture are increasingly appreciated. The cell-type specific organization of heterochromatin, established upon cell differentiation, is responsible for maintaining much of the genome in a repressed state, within a highly compartmentalized nucleus. This review focuses on recent evidence that in cancer the normal packaging and higher organization of heterochromatin is often compromised. Gross changes in nuclear morphology have long been a criterion for pathologic diagnosis of many cancers, but the specific nuclear components impacted, the mechanisms involved, and the implications for cancer progression have barely begun to emerge. We discuss recent findings regarding distinct heterochromatin types, including the inactive X chromosome, constitutive heterochromatin of peri/centric satellites, and the peripheral heterochromatic compartment (PHC). A theme developed here is that the higher-order organization of satellites and the peripheral heterochromatic compartment may be tightly linked, and that compromise of this organization may promote broad epigenomic imbalance in cancer. Recent studies into the potential role(s) of the breast cancer tumor suppressor, BRCA1, in maintaining heterochromatin will be highlighted. Many questions remain about this new area of cancer epigenetics, which is likely more important in cancer development and progression than widely appreciated. We propose that broad, stochastic compromise in heterochromatin maintenance would create a diversity of expression profiles, and thus a rich opportunity for one or more cells to emerge with a selective growth advantage and potential for neoplasia.
Keywords: heterochromatin, XIST, cancer, satellite DNA, epigenetics, BRCA1
Our review of heterochromatin instability in cancer will begin by placing the “heterochromatic compartment” in the context of overall nuclear organization. Genomic DNA exhibits a higher-order organization within a complex, compartmentalized nuclear structure; this in turn is intimately associated with a series of epigenetic modifications that distinguish heterochromatin from euchromatin. In mammalian cells, much of our knowledge of how heterochromatin is formed and maintained comes from studies of X-chromosome inactivation in female cells, where it forms the condensed Barr body. Pathologists have long noted cancer-associated changes in gross nuclear appearance (reviewed in [1, 2]) but in recent years it was the loss of the heterochromatic Barr body, noted commonly in aggressive breast cancers, that prompted a more in-depth investigation into heterochromatin loss in cancer (reviewed in [3]). We will consider the nature and regulation of heterochromatin in normal mammalian cells, as understood from X-chromosome silencing, and how this led to a role for BRCA1 in satellite heterochromatin. A series of findings contribute to an exciting but still very much emerging story, which begins with the disappearing Barr body and leads to the misregulation of centromere-associated satellite heterochromatin and the peripheral heterochromatic compartment in cancer. Our emphasis is primarily on the heterochromatic compartment, yet we will consider that disruption of heterochromatin can alter the organized euchromatin and nuclear environment more generally.
Nuclear heterochromatic versus euchromatic compartments in normal somatic cells
Folding of the genome above nucleosome packaging of the 30nm fiber remains in many respects a puzzle, but in recent years great advances have been made in our understanding of the higher-order organization of the genome and nuclear structure. To facilitate the coordinated functions of DNA replication, different types of RNA transcription, splicing and export, the nucleus must act as a highly efficient factory, and we now understand it has specialized domains delegated to its multitude of distinct tasks. Evidence for this functional compartmentalization has been revealed by in situ techniques examining specific DNA or RNA sequences and different types of metabolic complexes directly within the nuclear environment. Chromatin segregates into two distinct but broad nuclear compartments: much of the repressed, densely-packaged heterochromatin clusters around the nuclear periphery or nucleolus, to form the “peripheral heterochromatic compartment” (PHC), whereas loosely packaged, active euchromatin fills most of the internal nucleoplasm (Figure 1A). The pattern of the PHC is cell-type specific and we suggest reflects a higher-order “blueprint” of the epigenome in that cell type (Figure 1B). This will be an important concept as we consider the degree to which the PHC may be disrupted in cancer.
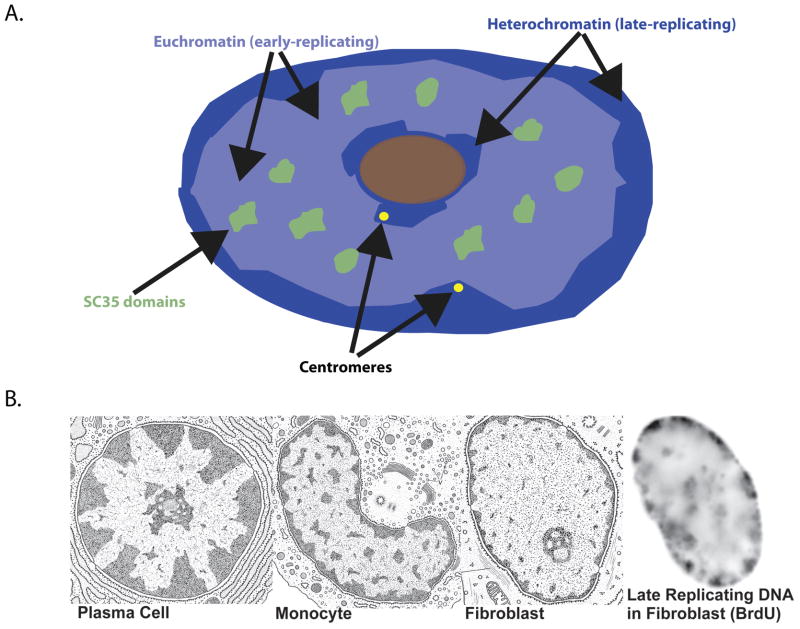
Figure 1.
A) Diagram depicting the separation of euchromatin (light blue) and heterochromatin (dark blue) domains in a normal human fibroblast nucleus. SC35 domains (green) rich in pre-mRNA metabolic factors reside within an internal euchromatic compartment. B) Patterns of heterochromatin (darkly-staining material) in the nucleus vary depending on cell type (reproduced from Thomas L. Lentz, M.D., Cell Fine Structure: An Atlas of Drawings of Whole-Cell Structure, Philadelphia, W.B. Saunders Publishing Company, 1971). This largely peripheral heterochromatin is also late-replicating as shown by Brdu labeling on far right in a human fibroblast nucleus.
The large euchromatic compartment is further delineated by a number of distinct sub-compartments and bodies. Nucleoli have long been recognized as prominent structures devoted to the efficient expression of rRNA and ribosomal assembly and other functions [4, 5]. Fluorescence techniques have revealed the presence of at least several other major sub-structures, not visible by phase microscopy, such as SC35 domains (speckles), PML bodies and Cajal Bodies, as reviewed elsewhere [6, 7]. Numerous SC35 domains facilitate pre-mRNA metabolism by congregating factors that form splicing and export complexes [8]. These are spatially associated with many highly active genes which cluster in the domain periphery [9, 10] (Figure 2A). On a smaller scale, there may also be some clustering of genes at up to 2000 tiny sites enriched for polymerase II, referred to as “transcription factories” [11] that are prevalent at the periphery of SC35 domains and throughout the nucleoplasm. It has been proposed that transcription factories may mediate the preferential association of genes from separate chromosomes [12]. While some evidence suggests that specific genes can interact with each other in a dynamic nucleus, other evidence suggests that much gene interaction is likely due to their affinity for a common structure [13–17]. What is clear is that there is a highly coordinated sequestration of genomic regions, with general segregation of inactive from active chromatin, and clustering of active genes at sites concentrated with RNA metabolic factors.
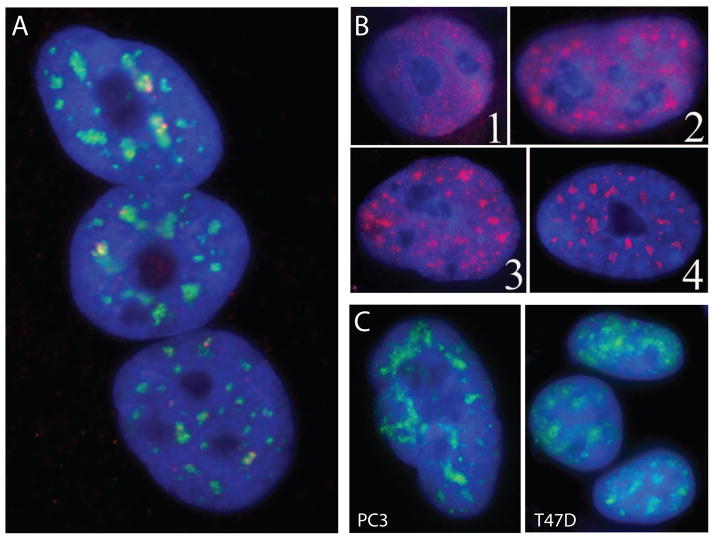
Figure 2.
A) SC35 domains (green) in normal human skeletal in the euchromatin compartment are associated at their edges with many specific active genes (red), for example the DMPK gene. B) SC35 domains in pluripotent (1) hESCs are diffuse and begin to form only upon differentiation (2, 3) and finally exhibit the normal somatic distribution in fully differentiated cells (4) (Reprinted from [40]). C) SC35 domains in cancer cells (PC3, T47D) display a diffuse pattern of SC35 localization with less defined accumulations compared to normal cells, more similar to earlier differentiating ES cells (shown in B).
How is this spatial separation maintained within the nuclear environment? It is known that nuclear positioning including proximity to the lamina and nuclear envelope strongly correlates with transcriptional activity, and studies into the functional basis for this have gained momentum. Lamin associated domains (LADs) are enriched for gene poor sequences, suggesting a repressive chromatin environment within these domains [18]. There is some evidence that targeting to the nuclear lamina can lead to gene silencing as it does in yeast [19], although this is not always the case in mammalian cells [20]. Further indication that sequences within LADs may often be hypomethylated in cancer suggests the regulation of this higher-order structure may be markedly altered in the cancer nuclear environment [21].
In our view, nuclear organization can be thought of as a paradoxical combination of the largely stable, higher-order organization (of nuclear sub-domains and genomic regions) with a highly dynamic shuffling of metabolic factors at the molecular level. We suggest the former reflects the heritable epigenetic program of the cell-type and the latter reflects the constant modulation of function in response to cellular cues. For example, it has been shown that the mobility of factors within SC-35 domains can be quite high [22, 23], even though the positioning of these domains in the overall nuclear space is often quite stable (discussed in[24]). In addition, in some cases specific genes show a very constant (near 100%) position adjacent to the domain, suggesting a stable structural relationship [9, 15, 16, 25, 26]. FRAP measurement of GFP-tagged proteins introduced into cells suggests what was initially a surprisingly high mobility of most nuclear factors, including most chromatin associated proteins (discussed in [27]). Providing that the tag or over-expression of proteins does not effect the exchange rates, then many endogenous factors in native context may show an equally high mobility. This highly dynamic molecular mileu is juxtaposed to a clearly higher-order structural arrangement of many nuclear components in a cell-type specific pattern that is stably inherited in a cell lineage.
One component that may set forth the framework for this structural organization is the potential association of specific genomic sequences with the nuclear periphery and lamina, as discussed above. Another component is the potential of certain factors to self-assemble into sub-nuclear domains, in some cases linked to sites of function or nucleated at sites of high gene expression (reviewed in [9, 28–30]). Some protein or RNA hallmarks of these nuclear domains and bodies clearly remain in the nuclear framework after extensive extraction and DNAse digestion to remove 90–95% of nuclear proteins and DNA. The complex structural components that resist extensive biochemical extraction have been collectively referred to as the nuclear “matrix” or “scaffold”, which may include a meta-stable “meshwork” of RNA and proteins, such as as lamins and actin (reviewed in [31]). Within a chromosomal region, the precise tethering of higher-order chromatin loops has long been described [32–34], and there is evidence this involves association with components of the insoluble matrix/scaffold [35–37], particularly in AT-rich Scaffold/Matrix attachment regions (S/MARs) some of which are cell-type dependent [38]. Furthermore, organization of chromatin at this level can be altered in cancer and other diseases, as well illustrated by the MAR-binding protein SATB1. SATB1 preferentially binds AT-rich sequences to organize chromatin loops, and it is overexpressed in aggressive breast cancers, where it impacts chromatin remodeling [39], as reviewed elsewhere in this volume.
While the compartmentalization of euchromatic and heterochromatic regions likely involves the combination of many factors, both at the chromatin level and higher-order nuclear domain level, it is clear that the organization and separation of these regions is constrained in a cell-type specific manner in somatic cells. Yet, this pattern is not as well established in early development and a less defined nuclear landscape can also be mirrored within the cancer cell nucleus.
Pluripotent stem cells have less defined nuclear sub-structure
It is important to note that the nuclear organization of pluripotent embryonic stem (ES) cells is unlike that of somatic cells in that ES cells lack a highly-ordered nuclear sub-structure [40], consistent with the observation of increased chromatin mobility in ES cell nuclei [41]. For example, SC35 domains are absent in pluripotent human ES cells (Figure 2B), suggesting that overall genomic organization (including heterochromatic and euchromatic compartments) has not yet been established or is not well defined. Mirroring the distribution in ES cells, the concentration of factors in SC35 domains is also often much more poorly defined in cancer cell lines (Figure 2C), although this remains to be systematically investigated in a broad set of tumors. Similarly, PML bodies, defined in normal nuclei by the promyelocytic leukemia (PML) protein and enriched with a host of transcriptional regulators, are often not present in pluripotent stem cells [40], but upon differentiation form discrete round bodies in the euchromatin compartment. While not our focus here, PML bodies are notable in the context of cancer as they can no longer form in specific types of leukemia [42]. While many functions related to transcriptional regulation have been proposed for PML bodies, some evidence suggests they may play a role in re-establishing the heterochromatic state in late-replicating DNA [43]. If so, this function could ultimately prove relevant to other changes in heterochromatin structure that will be discussed below. Given other parallels between ES cells and cancer cells, it is instructive to keep in mind that self-renewing stem cell nuclei are structurally different from somatic nuclei.
We next consider a well-studied model of how heterochromatin is initially formed in early embryonic cells and is then maintained in somatic cells, in order to understand the mechanism, and impact, of compromised somatic heterochromatin maintenance in cancer.
Lessons from X chromosome inactivation: heterochromatin is controlled at multiple levels
The epigenetic and chromatin remodeling events that occur during X chromosome inactivation provide a framework for understanding the multiple levels of control necessary for proper maintenance of condensed, silent heterochromatin. X-inactivation is the pre-eminent model for the formation and maintenance of facultative heterochromatin. Failure to properly silence one X chromosome results in embryonic lethality [44], as this event is required to compensate imbalanced gene expression between males (XY) and females (XX). Random inactivation of one X chromosome in female cells initiates early in development when the non-coding RNA XIST is expressed from and coats one X chromosome (Xi) in cis [45–47]. Euchromatic histone marks are quickly replaced by marks associated with inactive heterochromatin including H3K27me3, H3K9me2, and UbH2A, (reviewed in [48]) and individual gene promoters become methylated (reviewed in [49]). This is followed by a transformation in the chromosome’s overall architecture to condensed, tightly packaged chromatin which moves into the peripheral heterochromatin compartment, excluding access to transcriptional machinery within the nuclear interior (reviewed in [50]). This dense heterochromatin structure, termed the Barr Body, is easily visualized by light microscopy in normal cells, and is frequently lost in cancer (reviewed in [3]), an important observation further discussed below.
Studies aimed at reversing X inactivation in terminally differentiated cells highlight the redundancy of repressive marks that collectively maintain the integrity of heterochromatin. Upon loss of Xist, genes on the inactivated X remain repressed by multiple, synergistic epigenetic modifications [51]. Thus, while XIST is necessary for initiation of X inactivation, it is not required for overall maintenance of the inactive state [52, 53] Over time, however, loss of XIST results in leaky stochastic gene expression and loss of DNA methylation, suggesting that reactivation of a previously silent chromosome would involve a suite of epigenetic changes impacting both broad chromatin structure and local regulation at the individual gene level.
Revisiting the Disappearing Barr body
Over 50 years ago, pathologists first noted that the Barr body, characterized by late-replicating, condensed heterochromatin, is frequently lost in breast cancers [54, 55], and other recent observations link this to more aggressive tumors [56, 57]. Independently, studies surveying gene expression in breast and ovarian cancers report over-expression of a limited number of X-linked genes in specific tumor sub-types [56, 58] suggesting the possibility that one or more of these misregulated X chromosomal genes contributes to tumor development. Furthermore, several studies have found that certain primary breast and ovarian cancer cells which lack an inactivated X chromosome (Xi) retain at least 2 active X (Xa) chromosomes [56, 57, 59, 60]. Two plausible mechanisms for loss of the Barr body have been proposed. The first involves decondensation of the heterochromatic Barr body associated with loss of the XIST transcript, followed by gene activation. Evidence to support the mechanism that XIST RNA mislocalizes in cancer comes from in situ studies in breast carcinoma cells [3, 61, 62]. The prevailing consensus, however, is that loss of the Xi in female cancers is facilitated by mitotic error and duplication of Xa, resulting in isodisomy of the active X chromosome [3, 56, 59, 62, 63]. While there is evidence for both models of Xi loss, each would result in a “double dose” of some or all X-linked genes. Whereas the latter is lethal in early development, overexpression of a subset of X-linked genes could lead to selection for expression profiles in somatic cells that promote abnormal growth and the potential for neoplasia.
One study [64] implicated that loss of the Barr body in cancer was attributed to loss of BRCA1, a tumor suppressor linked to several fundamental cell regulatory processes and widely implicated in development of breast and ovarian cancers [65, 66]. The apparent cytological overlap reported in this study between BRCA1 and XIST RNA across the Xi raised the important possibility of a direct role of BRCA1 in localizing XIST [64]. However, further study revealed that BRCA1 does not structurally paint the Xi or XIST territory, as do markers of Xi heterochromatin, nor does BRCA1 status correlate with XIST localization [61]. Instead, BRCA1 overexpression correlates with enhanced XIST expression, suggesting BRCA1 may impact XIST transcription [61, 62] which may be due, directly or indirectly, to BRCA1’s function as a transcription factor [67].
Irrespective of the precise targets of BRCA1, there is substantial evidence that there is a propensity for X chromosome “misbehavior” in certain aggressive breast cancers which often involves over-representation or overexpression of X-linked genes (reviewed in [3]). We suggest regulatory factors, possibly including BRCA1, exert global effects on chromatin that can indirectly impact XIST RNA and the Xi. For example, recent data indicates that Aurora B Kinase (AURKB), a broad chromatin regulator commonly overexpressed in cancer [68], regulates the association of XIST with Xi [69]. Thus broad defects in chromatin regulation can impact XIST RNA and, potentially, other non-coding RNAs involved in maintaining heterochromatin.
A role for BRCA1 with constitutive satellite heterochromatin
Among the many reported functions of the BRCA1 ubiquitin ligase, it was well established that it localizes to sites of DNA repair in damaged cells (reviewed in [70]), but its localization in normal cells was not well studied. While BRCA1 does not coat the Xi or localize XIST RNA [61, 63, 71], BRCA1 foci were discovered, unexpectedly, to associate with constitutive satellite heterochromatin on the X and other chromosomes [72]. Moreover, this occurs specifically during normal replication of peri/centric heterochromatin within chromocenters [72] (Figure 3). The direct overlap of BRCA1 with the outer edge of replicating satellite DNA foci complements data indicating DNA replication occurs at the periphery of mouse chromocenters [73]. Further, BRCA1 was found to colocalize with Topoisomerase II at sites of replicating satellite heterochromatin [72], consistent with evidence for a role of BRCA1 in ubiquitylation of Topoisomerase II during replication [74]. Evidence linking misregulation of satellite DNA with genomic instability was also found as cells lacking BRCA1 were found to have an increased frequency of aberrant chromosome segregation including lagging chromosomes and DNA bridges. Intriguingly, a large fraction of these DNA bridges contained satellite DNA [72], consistent with the possibility that defective satellite DNA replication may contribute to mitotic instability.
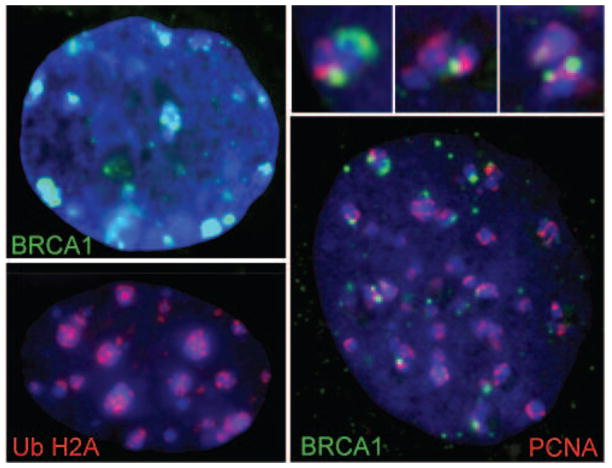
Figure 3.
BRCA1 (top left, green) and UbH2A (bottom left, red) associate with mouse chromocenters in a subset of cells. BRCA1 association is coincident with satellite DNA replication, as indicated by co-labeling with PCNA (red, right). Adapted from [72]. Enlargement of BRCA1/PCNA over chromocenters is shown above, right.
Further insight into the normal role of BRCA1 in pericentric heterochromatin, and its significant links to cancer, has very recently been gained. Zhu et al. [75], recently identified histone H2A as a specific substrate of BRCA1 within pericentric satellite DNA in both mouse and human. This study reported that depletion of BRCA1, by RNAi or in Brca1 mutant brains, results in overexpression of satellite DNA and abnormal heterochromatin structure, presumably through decreased levels of monoubiquitylated histone H2A (UbH2A). Supporting this, rescue by re-expression of BRCA1 or a functional UbH2A mimic was reported to restore normal silencing of pericentric satellites [75]. However, it is important to consider the collective chromatin environment, as cancer cells may well lack multiple normal epigenetic modifications to heterochromatin. If multiple levels of control are lost in cancer, restoring just one (e.g. BRCA1 or UbH2A) could plausibly restore silencing of satellite repeats. It is unknown what other marks are also perturbed in epigenetically unstable cancer cells and how they may contribute to loss of satellite repression. It is curious that, as shown in Figure 3, we find accumulation of UbH2A on mouse chromocenters, but only in a small subset (<5%) of cells, suggesting this mark may be transient or inaccessible to antibody detection during much of the cell cycle. Further work will be required to understand the role of this repressive histone mark within normal satellite DNA.
The functional consequence of satellite de-repression was addressed in this same study by lentiviral over-expression of satellite DNA in normal human mammary cells [75], which led to frequent abnormal mitoses, including lagging chromosomes and DNA bridges, consistent with mitotic defects and centrosome amplification observed in BRCA1 −/ − cells [72, 76]. While this was interpreted to be an effect of satellite RNA on mitotic stability, it cannot be ruled out that the presence of exogenous satellite DNA could induce widespread genomic instability, independent of transcription. Furthermore, aberrant expression of satellite DNA from ectopic locations cannot be equated to overexpression of endogenous satellite RNA within pericentric heterochromatin loci, as in BRCA1 null cells.
There are likely multiple mechanisms that can lead to genomic instability [77], however epigenetic defects in peri/centric regions surrounding centromeres is likely be particularly detrimental to proper chromosome segregation [78]. Further study will be necessary to determine whether loss of UbH2A through BRCA1 depletion at endogenous pericentric heterochromatin loci, and the associated expression of satellite RNA, is indeed the mechanism contributing to mitotic defects in BRCA−/ −cancer cells. As there are likely many layers of epigenetic control responsible for the silencing of peri/centric heterochromatin, as discussed above, BRCA1-mediated ubiquitylation of histone H2A may be just one of these players. What is clear is that many hallmarks of heterochromatin, including DNA methylation and numerous histone modifications, are often progressively compromised in cancer.
BRCA1-specific or broad misregulation of peri/centric satellites in cancer?
Hypomethylation of repetitive elements, particularly satellite DNA, is common to many diverse types of cancer (reviewed in [79]. Areas of hypomethylation are often associated with rearrangements, amplifications and chromosome destabilization events in cancer and other genetic disorders [80]. However, not all DNA is equal in regards to its level of demethylation nor in its response to demethylating agents [81]. In particular, pericentric satellites, which can occupy large, megabase-scale blocks of the genome, are highly susceptible to demethylation and evidence suggests that loss of methylation induced by demethylating agents within pericentric satellites may have profound impact on the stability of the rest of the genome, including chromosome segregation [82]. Evidence for increased susceptibility to demethylation is particularly well-studied in the case of the 6Mb block of Satellite II on chromosome 1. Hypomethylation of satellite II, reported for several types of tumors, and rearrangements involving the pericentromere of chromosome 1 is the diagnostic tool for ICF (immunodeficiency, centromeric region instability, and facial anomalies) Syndrome, a disease resulting from lack of the DNA methyltransferase Dnmt3b [83]. And in a variety of cancers, pericentric rearrangements and changes in nuclear positioning of Chr1 Satellite II heterochromatin occur at an increased frequency compared to other pericentric regions [81, 84–86], suggesting a unique vulnerability of this large block of satellite DNA.
As heterochromatin is controlled at multiple levels, it is unclear that hypomethylation of repetitive elements directly results in aberrant expression of repeats. However, recent evidence suggests that overexpression of satellites and other repeats may be a more widespread phenomenon in cancer. While overexpression of alpha satellite repeats was attributed to loss of BRCA1 [75], there are multiple lines of evidence to support that overexpression of satellite and other repetitive elements may be widely prevalent in cancer [87, 88]. For example, a study by Ting et al. reported aberrant overexpression alpha sat and Satellite II, as studied primarily in pancreatic and a few epithelial cancers, suggesting this phenomenon may not be limited to BRCA1 null tumors. Moreover, satellite overexpression correlated with altered expression of a subset of genes suggesting a potential relationship between satellite misregulation and gene expression changes [87]. There remain many questions as to the nature and extent of satellite misregulation in cancer that will require distinctive approaches (Hall et al, submitted). For example, are all satellite loci equally misregulated within the nucleus and is hypomethylation of these sequences and/or loci directly responsible for the increased transcription? Furthermore, do satellite transcripts accumulate in the nucleus or cytoplasm and could they have a functional and possibly detrimental consequence? Finally, is loss of proper control of pericentric satellites strictly a result of or does it contribute to changes in the global nuclear environment?
Linking satellite heterochromatin to the overall framework of nuclear structure: a hypothesis
As discussed above, the regulation of heterochromatin occurs within a nucleus highly compartmentalized into specialized nuclear domains in normal cells. Therefore, misregulation of heterochromatic blocks such as peri/centric satellites may reflect or promote instability of overall nuclear organization. The organization and clustering of peri/centric heterochromatin sequences in normal cells may be thought of as providing anchor points for nuclear proteins and genomic elements. In support of this, several studies aimed at uncovering the sequence elements interacting with nuclear matrix/scaffold proteins have uncovered a preferential association with satellite and other repeat-rich sequences [89]. Matrix attachment regions, which comprise base unpairing regions (BURs) and associate with Topoisomerase II [90], are highly AT-rich, further bolstering a role for AT-rich satellite heterochromatin as an anchor to the nuclear scaffold (elements that behave as insoluble structures resistant to extraction). In particular, the presence of large blocks of satellite DNA (often up to several megabases) would provide an exceptionally tight structural foothold. This could provide a functional explanation for why large regions of repetitive DNA comprise such a large percentage of the mammalian genome.
If peri/centric heterochromatin provides anchor points for genomic organization, epigenetic alterations to these regions in cancer could impact other gene and nuclear structural interactions. Supporting this, pathologists often define irregularity in nuclear lamina structure coupled with anomalies in heterochromatin structure as a hallmark of specific cancers [2]. It is not too far afield to speculate that epigenetic changes to peri/centric heterochromatin (such as hypomethylation or histone deacetylation) may impact the ability for these sequences to interact with the larger nuclear framework. This reduced binding could then allow for access of these sequences to transcriptional machinery, resulting in aberrant expression of satellite sequences. While we point out that overexpression of satellite RNA itself may well have a functional consequence, loss of interaction between nuclear structural proteins and peri/centric heterochromatin could further affect the global nuclear environment with potentially greater impact.
Instability manifest by breakdown of the peripheral heterochromatin compartment
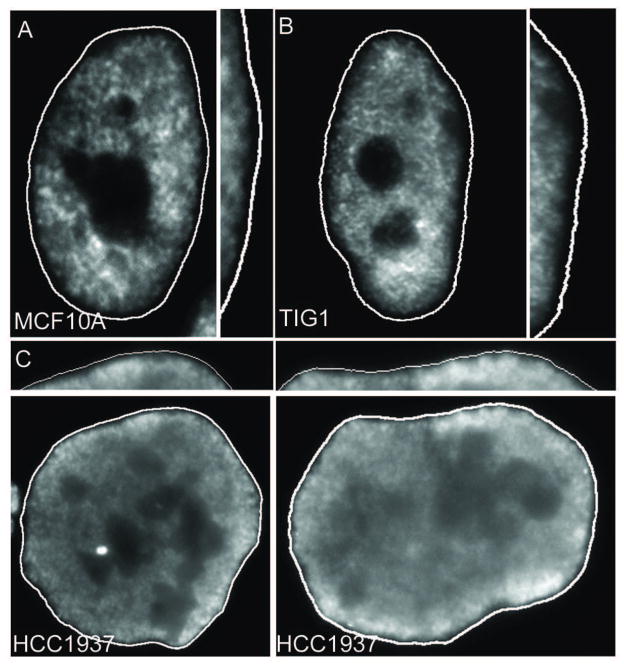
While loss of control within specific domains or types of heterochromatin (e.g. the inactive X and peri/centric heterochromatin) may arise via independent mechanisms (Figure 5), evidence for global dysregulation of heterochromatin in cancer is mounting. The apposition of the peripheral heterochromatin compartment (PHC) to the nuclear periphery and lamina may be a foundation to establish and maintain the overall repressive chromatin environment, sequestering satellites and other sequences away from the nuclear interior which contains active genes, “transcription factories” and SC35 domains. Among the many dynamic changes to the nuclear environment in cancer [36, 91], pathologists have long described changes to the overall pattern of chromatin organization observed by staining of tissue sections and tumor cells grown in culture, referred to as “chromatin texture” [2]. In particular, striking differences in the darkly-staining heterochromatin domains are especially prominent and are the basis for diagnosis of several cancer sub-types [2, 36, 92]. Specifically, heterochromatin can undergo drastic changes in localization including the formation of aberrant aggregates and, most often, an overall lack of condensed, darkly-staining material within cancer nuclei. This general loss of heterochromatin is common to many cancer types and is often associated with high malignancy and poor prognosis [2]. This implies that the presence of the repressed peripheral heterochromatin compartment may often be compromised in cancer, although this can be difficult to discern by light microscopy. We have recently developed a fluorescence microscopy assay which demonstrates loss of peripheral heterochromatin in cancer samples [3, 72]. This technique uses the COT-1 RNA FISH assay, originally developed to examine silencing of the inactive X chromosome [93], coupled with standard DNA staining, to highlight a peripheral nuclear region that lacks significant transcription in normal cells. COT-1 is composed of the repeat fraction of the genome [94] and is typically used to suppress non-specific hybridization to repetitive elements. When instead used as a labeled probe, it illuminates heterogeneous nuclear RNA (hnRNA) from transcriptionally active areas of the nucleus. In normal somatic cells, the silent peripheral heterochromatin compartment (including the heterochromatic Barr body) can be distinguished by lack of hybridization to a COT-1 probe under non-denaturing conditions (Figure 4). Strikingly, unlike in normal (MCF10A & Tig-1) cells, the COT-1 RNA signal in cancer cells extends into the normally silent peripheral compartment, indicating global de-repression of the sequences that normally occupy this domain (Figure 4). This new assay allows for a global “profile” of the integrity of the heterochromatin content and pattern within the nucleus that could be integrated along with standard pathology analyses to identify heterochromatin imbalance within a diverse population of cells. While specific mechanisms underlying global de-regulation of silenced heterochromatin are currently unknown, this understudied phenomenon typifies a variety of tumor types, and can have widespread implications for the cancer genome.
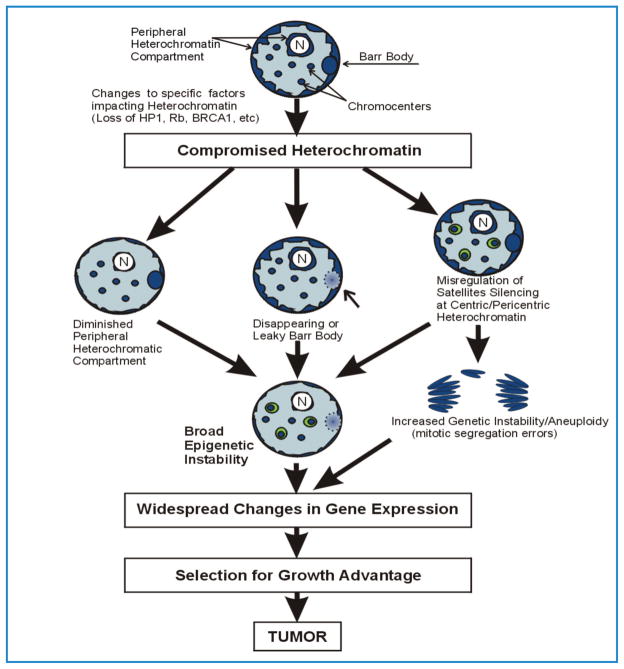
Figure 5.
Heterochromatin instability would generate a diversity of expression profiles. Compromise of heterochromatin could be manifest in several different ways including loss of the Barr body, misregulation of peri/centric satellites and compromise of the peripheral heterochromatin compartment. Changes to chromatin modifiers that impact heterochromatin maintenance (e.g. AURKB, BRCA1) have the potential to impact all types of heterochromatin more broadly. Epigenetic instability within peri/centric regions may also increase the potential for genetic instability through mitotic segregation errors. Loss of control of any or all heterochromatin types could lead to broad epigenetic instability with a potential to impact gene expression profiles and, ultimately, selection for abnormal growth. The heterochromatic regions of the nucleus are shown in dark blue. N= nucleolus. The Barr body is depicted as a dark blue oval at the nuclear periphery. The lighter oval indicates compromised Barr body silencing.
Figure 4.
In normal human nuclei (A, B), a prominent rim of DNA (dark rim) lacking COT-1 RNA (white nucleoplasmic signal) apposed to the edge of the nucleus (white line) demonstrates a normal peripheral heterochromatic compartment. The heterochromatic compartment is visualized by RNA hybridization using a Cot-1 probe. Some cancer cells (below) have a much less prominent rim, indicating a compromise of this peripheral compartment. C) The outer edge of each nucleus is enlarged for each cell type. Reprinted from [72].
Imbalanced epigenome in cancer: potential impact of heterochromatin instability
The epigenetic diversity in cancer is well highlighted by aberrations in methylation status across the genome [79]. However, a paradox is emerging wherein silencing of tumor suppressors via hypermethylation occurs in the context of widespread hypomethylation (particularly in heterochromatic regions). While evidence suggests hypomethylation likely occurs in large blocks of the genome [21], hypermethylation may occur either in similar large blocks, or as a gene-specific event [95]. These regions or individual genes that become hypermethylated may contain tumor suppressors, which would then be selected for on the basis of growth advantage (reviewed in [96]). A diversity of epigenetic and expression profiles between cancer types is appreciated, yet very recent evidence for intratumor heterogeneity in gene expression and chromosome aberrations sheds light on this diversity within a given tumor “landscape”, reflecting growth selection and evolution even within individual tumors [97]. While aberrant silencing of a small number of genes is likely to be selected for within individual tumors, global misregulation of broader classes of heterochromatin would result in epigenetically diverse profiles with the ability to impact a larger range of gene expression profiles. This diversity in gene expression would provide an even greater capacity for growth selection (Figure 5).
We highlight the importance of understanding the mechanisms responsible for phenotypic variability in cancer in the context of global aberrations within the nuclear environment. Much research now provides evidence that compartmentalization of the nucleus is necessary for large-scale regulation involving spatial separation of euchromatic versus heterochromatic domains. The broad epigenetic and genetic impact of instability within heterochromatin domains is an understudied, yet widespread phenomenon in cancer. We suggest that heterochromatic domains are integral to the proper positioning of sequences within the nuclear environment and highlight that loss of compartmentalization of heterochromatin is likely to destabilize the epigenome more generally. Genomic instability is important in cancer, but defects in the maintenance of heterochromatin, including loss of Xi, peri/centric heterochromatin and the peripheral heterochromatic compartment, could result in an analogous broad epigenetic instability. Improper regulation of any of these heterochromatic regions could lead to an overall restructuring of the nuclear neighborhood with profound impact on gene expression and the propensity for generating growth advantage phenotypes.
Acknowledgments
Research support to JBL is provided by NIH grant GM053234 from the Institute of General Medicinal Sciences. D.C. is supported by NIH National Research Service Award 1F32CA154086 from the National Cancer Institute. We thank Heather Kolpa for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal isclaimers that apply to the journal pertain.
References
- 1.Pienta KJ, Partin AW, Coffey DS. Cancer as a disease of DNA organization and dynamic cell structure. Cancer Research. 1989;49:2525–2532. [PubMed] [Google Scholar]
- 2.Fischer AH, Zhao C, Li QK, Gustafson KS, Eltoum I-E, Tambouret R, Benstein B, Savaloja LC, Kulesza P. The cytologic criteria of malignancy. J Cell Biochem. 2010;110:795–811. doi: 10.1002/jcb.22585. [DOI] [PubMed] [Google Scholar]
- 3.Pageau GJ, Hall LL, Ganesan S, Livingston DM, Lawrence JB. The disappearing Barr body in breast and ovarian cancers. Nature Reviews Cancer. 2007 doi: 10.1038/nrc2172. [DOI] [PubMed] [Google Scholar]
- 4.Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nature Reviews Molecular Cell Biology. 2007;8:574–585. doi: 10.1038/nrm2184. [DOI] [PubMed] [Google Scholar]
- 5.Pederson T, Tsai RYL. In search of nonribosomal nucleolar protein function and regulation. The Journal of Cell Biology. 2009;184:771–776. doi: 10.1083/jcb.200812014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spector D. Nuclear bodies. Cell 2006 [Google Scholar]
- 7.Dundr M. Nuclear bodies: multifunctional companions of the genome. Curr Opin Cell Biol. 2012 doi: 10.1016/j.ceb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nature Reviews Molecular Cell Biology. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- 9.Hall LL, Smith KP, Byron M, Lawrence JB. Molecular anatomy of a speckle. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:664–675. doi: 10.1002/ar.a.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing Y, Johnson CV, Dobner PR, Lawrence JB. Higher level organization of individual gene transcription and RNA splicing. Science. 1993;259:1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- 11.Jackson DA. The amazing complexity of transcription factories. Briefings in Functional Genomics and Proteomics. 2005;4:143–157. doi: 10.1093/bfgp/4.2.143. [DOI] [PubMed] [Google Scholar]
- 12.Osborne CS, Chakalova L, Mitchell JA, Horton A, Wood AL, Bolland DJ, Corcoran AE, Fraser P. Myc dynamically and preferentially relocates to a transcription factory occupied by Igh. PLoS Biol. 2007;5:e192. doi: 10.1371/journal.pbio.0050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Göndör A, Ohlsson R. Chromosome crosstalk in three dimensions. Nature. 2009;461:212–217. doi: 10.1038/nature08453. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence JB, Clemson CM. Gene associations: true romance or chance meeting in a nuclear neighborhood? The Journal of Cell Biology. 2008;182:1035–1038. doi: 10.1083/jcb.200808121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JM. Coregulated human globin genes are frequently in spatial proximity when active. The Journal of Cell Biology. 2006;172:177–187. doi: 10.1083/jcb.200507073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shopland LS, Johnson CV, Byron M, McNeil J, Lawrence JB. Clustering of multiple specific genes and gene-rich R-bands around SC-35 domains: evidence for local euchromatic neighborhoods. Journal of Cell Biology. 2003;162:981–990. doi: 10.1083/jcb.200303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang CH, Belmont AS. Close encounters between active genes in the nucleus. Genome Biol. 2005;6:237. doi: 10.1186/gb-2005-6-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 19.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 20.Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. The Journal of Cell Biology. 2008;180:51–65. doi: 10.1083/jcb.200706060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, Mcdonald OG, Wen B, Wu H, Liu Y, Diep D, et al. Increased methylation variation in epigenetic domains across cancer types. Nat Genet. 2011;43:768–775. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruhlak MJ, Lever MA, Fischle W, Verdin E, Bazett-Jones DP, Hendzel MJ. Reduced mobility of the alternate splicing factor (ASF) through the nucleoplasm and steady state speckle compartments. Journal of Cell Biology. 2000;150:41–51. doi: 10.1083/jcb.150.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phair RD, Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- 24.Shopland LS. Seeking Common Ground in Nuclear Complexity. The Journal of Cell Biology. 2000;150:1F–4. doi: 10.1083/jcb.150.1.f1. [DOI] [PubMed] [Google Scholar]
- 25.Moen PT, Johnson CV, Byron M, Shopland LS, la Serna, de IL, Imbalzano AN, Lawrence JB. Repositioning of muscle-specific genes relative to the periphery of SC-35 domains during skeletal myogenesis. Molecular Biology of the Cell. 2004;15:197–206. doi: 10.1091/mbc.E03-06-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Plutz M, Belmont AS. Hsp70 gene association with nuclear speckles is Hsp70 promoter specific. The Journal of Cell Biology. 2010;191:711–719. doi: 10.1083/jcb.201004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hager GL, Nagaich AK, Johnson TA, Walker DA, John S. Dynamics of nuclear receptor movement and transcription. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression. 2004;1677:46–51. doi: 10.1016/j.bbaexp.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Moen P, Smith K, Lawrence J. Compartmentalization of specific pre-mRNA metabolism: an emerging view. Hum Mol Genet. 1995;4(Spec No):1779–89. doi: 10.1093/hmg/4.suppl_1.1779. [DOI] [PubMed] [Google Scholar]
- 29.Misteli T. The concept of self-organization in cellular architecture. The Journal of Cell Biology. 2001;155:181–186. doi: 10.1083/jcb.200108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajendra TK, Praveen K, Matera AG. Genetic Analysis of Nuclear Bodies: From Nondeterministic Chaos to Deterministic Order. Cold Spring Harb Symp Quant Biol. 2011;75:365–374. doi: 10.1101/sqb.2010.75.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickerson J. Experimental observations of a nuclear matrix. J Cell Sci. 2001 doi: 10.1242/jcs.114.3.463. [DOI] [PubMed] [Google Scholar]
- 32.Cook PR, Brazell IA. Supercoils in human DNA. J Cell Sci. 1975;19:261–279. doi: 10.1242/jcs.19.2.261. [DOI] [PubMed] [Google Scholar]
- 33.Vogelstein B, Pardoll DM, Coffey DS. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980;22:79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- 34.Gerdes MG, Carter KC, Moen PT, Lawrence JB. Dynamic changes in the higher-level chromatin organization of specific sequences revealed by in situ hybridization to nuclear halos. The Journal of Cell Biology. 1994;126:289–304. doi: 10.1083/jcb.126.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linnemann AK, Krawetz SA. Maintenance of a functional higher order chromatin structure: The role of the nuclear matrix in normal and disease states. Gene therapy & molecular biology. 2009;13:231. [PMC free article] [PubMed] [Google Scholar]
- 36.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nature Reviews Cancer. 2004;4:677. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 37.Heng HHQ, Goetze S, Ye CJ, Liu G, Stevens JB, Bremer SW, Wykes SM, Bode J, Krawetz SA. Chromatin loops are selectively anchored using scaffold/matrix-attachment regions. J Cell Sci. 2004;117:999–1008. doi: 10.1242/jcs.00976. [DOI] [PubMed] [Google Scholar]
- 38.Linnemann AK, Krawetz SA. Silencing by nuclear matrix attachment distinguishes cell-type specificity: association with increased proliferation capacity. Nucleic Acids Research. 2009;37:2779–2788. doi: 10.1093/nar/gkp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 40.Butler JT, Hall LL, Smith KP, Lawrence JB. Changing nuclear landscape and unique PML structures during early epigenetic transitions of human embryonic stem cells. J Cell Biochem. 2009;107:609–621. doi: 10.1002/jcb.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Developmental Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeler JS, Dejean A. The PML nuclear bodies: actors or extras? Curr Opin Genet Dev. 1999;9:362–367. doi: 10.1016/s0959-437x(99)80054-9. [DOI] [PubMed] [Google Scholar]
- 43.Luciani JJ, Depetris D, Usson Y, Metzler-Guillemain C, Mignon-Ravix C, Mitchell MJ, Megarbane A, Sarda P, Sirma H, Moncla A, et al. PML nuclear bodies are highly organised DNA-protein structures with a function in heterochromatin remodelling at the G2 phase. J Cell Sci. 2006;119:2518–2531. doi: 10.1242/jcs.02965. [DOI] [PubMed] [Google Scholar]
- 44.Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes & Development. 1997;11:156–166. doi: 10.1101/gad.11.2.156. [DOI] [PubMed] [Google Scholar]
- 45.Clemson CM, McNeil JA, Willard HF, Lawrence JB. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. Journal of Cell Biology. 1996;132:259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown C, Hendrich B, Rupert J, Lafreniere R. The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 doi: 10.1016/0092–8674(92)90520-M. Cell | ScienceDirect.com. [DOI] [PubMed] [Google Scholar]
- 47.Brockdorff N, Ashworth A, Kay GF, McCabe VM, Norris DP, Cooper PJ, Swift S, Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71:515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- 48.Heard E. Delving into the diversity of facultative heterochromatin: the epigenetics of the inactive X chromosome. Curr Opin Genet Dev. 2005;15:482–489. doi: 10.1016/j.gde.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Boumil RM, Lee JT. Forty years of decoding the silence in X-chromosome inactivation. Human Molecular Genetics. 2001;10:2225–2232. doi: 10.1093/hmg/10.20.2225. [DOI] [PubMed] [Google Scholar]
- 50.Hall LL, Lawrence JB. XIST RNA and architecture of the inactive X chromosome: implications for the repeat genome. Cold Spring Harb Symp Quant Biol. 2010;75:345–356. doi: 10.1101/sqb.2010.75.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Csankovszki G, Nagy A, Jaenisch R. Synergism of Xist Rna, DNA Methylation, and Histone Hypoacetylation in Maintaining X Chromosome Inactivation. The Journal of Cell Biology. 2001;153:773–784. doi: 10.1083/jcb.153.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall LL, Clemson CM, Byron M, Wydner K, Lawrence JB. Unbalanced X;autosome translocations provide evidence for sequence specificity in the association of XIST RNA with chromatin. Human Molecular Genetics. 2002;11:3157–3165. doi: 10.1093/hmg/11.25.3157. [DOI] [PubMed] [Google Scholar]
- 53.Brown CJ, Willard HF. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature. 1994;368:154–156. doi: 10.1038/368154a0. [DOI] [PubMed] [Google Scholar]
- 54.Barr M. Chromosomes, sex chromatin, and cancer. Proc Can Cancer Conf. 1957;1957 -PubMed - NCBI. In. [PubMed] [Google Scholar]
- 55.Borah V, Shah PN, Ghosh SN, Sampat MB, Jussawalla DJ. Further studies on the prognostic importance of Barr body frequency in human breast cancer: with discussion on its probable mechanism. J Surg Oncol. 1980;13:1–7. doi: 10.1002/jso.2930130102. [DOI] [PubMed] [Google Scholar]
- 56.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Kawakami T, Zhang C, Taniguchi T, Kim CJ, Okada Y, Sugihara H, Hattori T, Reeve AE, Ogawa O, Okamoto K. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23:6163–6169. doi: 10.1038/sj.onc.1207808. [DOI] [PubMed] [Google Scholar]
- 58.Jazaeri AA, Yee CJ, Sotiriou C, Brantley KR, Boyd J, Liu ET. Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers. J Natl Cancer Inst. 2002;94:990–1000. doi: 10.1093/jnci/94.13.990. [DOI] [PubMed] [Google Scholar]
- 59.Sirchia SM. Loss of the Inactive X Chromosome and Replication of the Active X in BRCA1-Defective and Wild-type Breast Cancer Cells. Cancer Research. 2005;65:2139–2146. doi: 10.1158/0008-5472.CAN-04-3465. [DOI] [PubMed] [Google Scholar]
- 60.Benoît M, Hudson T, Maire G, Squire J, Arcand S, Provencher D, Mes-Masson A, Tonin P. Global analysis of chromosome X gene expression in primary cultures of normal ovarian surface epithelial cells and epithelial ovarian cancer cell lines. Int J Oncol. 2007;30:5–17. [PubMed] [Google Scholar]
- 61.Pageau GJ, Hall LL, Lawrence JB. BRCA1 does not paint the inactive X to localize XIST RNA but may contribute to broad changes in cancer that impact XIST and Xi heterochromatin. J Cell Biochem. 2007;100:835–850. doi: 10.1002/jcb.21188. [DOI] [PubMed] [Google Scholar]
- 62.Sirchia S, Tabano S, Monti L, Recalcati M. Misbehaviour of XIST RNA in Breast Cancer Cells. PLoS ONE. 2009 doi: 10.1371/journal.pone.0005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent-Salomon A, Ganem-Elbaz C, Manié E, Raynal V, Sastre-Garau X, Stoppa-Lyonnet D, Stern MH, Heard E. X inactive-specific transcript RNA coating and genetic instability of the X chromosome in BRCA1 breast tumors. Cancer Research. 2007;67:5134–5140. doi: 10.1158/0008-5472.CAN-07-0465. [DOI] [PubMed] [Google Scholar]
- 64.Ganesan S, Silver DP, Greenberg RA, Avni D, Drapkin R, Miron A, Mok SC, Randrianarison V, Brodie S, Salstrom J, et al. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell. 2002;111:393–405. doi: 10.1016/s0092-8674(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 65.Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25:5864–5874. doi: 10.1038/sj.onc.1209874. [DOI] [PubMed] [Google Scholar]
- 66.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–5863. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 67.Gorski JJ, Savage KI, Mulligan JM, McDade SS, Blayney JK, Ge Z, Harkin DP. Profiling of the BRCA1 transcriptome through microarray and ChIP-chip analysis. Nucleic Acids Research. 2011;39:9536–9548. doi: 10.1093/nar/gkr679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meraldi P, Honda R, Nigg E. Aurora kinases link chromosome segregation and cell division to cancer susceptibility. Curr Opin Genet Dev. 2004 doi: 10.1016/j.gde.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Hall LL, Byron M, Pageau G, Lawrence JB. AURKB-mediated effects on chromatin regulate binding versus release of XIST RNA to the inactive chromosome. The Journal of Cell Biology. 2009;186:491–507. doi: 10.1083/jcb.200811143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huen MSY, Sy SMH, Chen J. BRCA1 and its toolbox for the maintenance of genome integrity. Nature Reviews Molecular Cell Biology. 2010;11:138–148. doi: 10.1038/nrm2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao C, Sharp J, Kawahara M, Davalos A. The XIST noncoding RNA functions independently of BRCA1 in X inactivation. Cell. 2007 doi: 10.1016/j.cell.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 72.Pageau GJ, Lawrence JB. BRCA1 foci in normal S-phase nuclei are linked to interphase centromeres and replication of pericentric heterochromatin. Journal of Cell Biology. 2006;175:693–701. doi: 10.1083/jcb.200602055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quivy JP, Roche D, Kirschner D, Tagami H, Nakatani Y, Almouzni G. A CAF-1 dependent pool of HP1 during heterochromatin duplication. The EMBO Journal. 2004;23:3516–3526. doi: 10.1038/sj.emboj.7600362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lou Z, Minter-Dykhouse K, Chen J. BRCA1 participates in DNA decatenation. Nat Struct Mol Biol. 2005;12:589–593. doi: 10.1038/nsmb953. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Q, Pao GM, Huynh AM, Suh H, Tonnu N, Nederlof PM, Gage FH, Verma IM. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature. 2011;477:179–184. doi: 10.1038/nature10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Molecular and Cellular Biology. 2005;25:8656–8668. doi: 10.1128/MCB.25.19.8656-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285–95. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slee RB, Steiner CM, Herbert B-S, Vance GH, Hickey RJ, Schwarz T, Christan S, Radovich M, Schneider BP, Schindelhauer D, et al. Cancer-associated alteration of pericentromeric heterochromatin may contribute to chromosome instability. Oncogene. 2011 doi: 10.1038/onc.2011.502. [DOI] [PubMed] [Google Scholar]
- 79.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Narayan A, Ji W, Zhang XY, Marrogi A, Graff JR, Baylin SB, Ehrlich M. Hypomethylation of pericentromeric DNA in breast adenocarcinomas. Int J Cancer. 1998;77:833–838. doi: 10.1002/(sici)1097-0215(19980911)77:6<833::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 81.Ji W, Hernandez R, Zhang XY, Qu GZ, Frady A, Varela M, Ehrlich M. DNA demethylation and pericentromeric rearrangements of chromosome 1. Mutat Res. 1997;379:33–41. doi: 10.1016/s0027-5107(97)00088-2. [DOI] [PubMed] [Google Scholar]
- 82.Prada D, González R, Sánchez L, Castro C, Fabián E, Herrera LA. Satellite 2 demethylation induced by 5-azacytidine is associated with missegregation of chromosomes 1 and 16 in human somatic cells. Mutat Res. 2012;729:100–105. doi: 10.1016/j.mrfmmm.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Hansen RS. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proceedings of the National Academy of Sciences. 1999;96:14412–14417. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barki-Celli L, Lefebvre C, Le Baccon P, Nadeau G, Bonnefoix T, Usson Y, Vourc’h C, Khochbin S, Leroux D, Callanan M. Differences in nuclear positioning of 1q12 pericentric heterochromatin in normal and tumor B lymphocytes with 1q rearrangements. Genes Chromosom. Cancer. 2005;43:339–349. doi: 10.1002/gcc.20179. [DOI] [PubMed] [Google Scholar]
- 85.Pandis N, Teixeira MR, Gerdes AM, Limon J, Bardi G, Andersen JA, Idvall I, Mandahl N, Mitelman F, Heim S. Chromosome abnormalities in bilateral breast carcinomas. Cytogenetic evaluation of the clonal origin of multiple primary tumors. Cancer. 1995;76:250–258. doi: 10.1002/1097-0142(19950715)76:2<250::aid-cncr2820760215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 86.Mertens F, Johansson B, Höglund M, Mitelman F. Chromosomal imbalance maps of malignant solid tumors: a cytogenetic survey of 3185 neoplasms. Cancer Research. 1997;57:2765–2780. [PubMed] [Google Scholar]
- 87.Ting DT, Lipson D, Paul S, Brannigan BW, Akhavanfard S, Coffman EJ, Contino G, Deshpande V, Iafrate AJ, Letovsky S, et al. Aberrant Overexpression of Satellite Repeats in Pancreatic and Other Epithelial Cancers. Science. 2011 doi: 10.1126/science.1200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eymery A, Horard B, El Atifi-Borel M, Fourel G, Berger F, Vitte A-L, Van den Broeck A, Brambilla E, Fournier A, Callanan M, et al. A transcriptomic analysis of human centromeric and pericentric sequences in normal and tumor cells. Nucleic Acids Research. 2009;37:6340–6354. doi: 10.1093/nar/gkp639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Small D, Nelkin B, Vogelstein B. Nonrandom distribution of repeated DNA sequences with respect to supercoiled loops and the nuclear matrix. Proc Natl Acad Sci USA. 1982;79:5911–5915. doi: 10.1073/pnas.79.19.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Platts AE, Quayle AK, Krawetz SA. In-silico prediction and observations of nuclear matrix attachment. Cell Mol Biol Lett. 2006;11:191–213. doi: 10.2478/s11658-006-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zaidi SK, Young DW, Javed A, Pratap J, Montecino M, van Wijnen A, Lian JB, Stein JL, Stein GS. Nuclear microenvironments in biological control and cancer. Nature Reviews Cancer. 2007;7:454–463. doi: 10.1038/nrc2149. [DOI] [PubMed] [Google Scholar]
- 92.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 93.Hall LL, Byron M, Sakai K, Carrel L, Willard HF, Lawrence JB. An ectopic human XIST gene can induce chromosome inactivation in postdifferentiation human HT-1080 cells. Proc Natl Acad Sci USA. 2002;99:8677–8682. doi: 10.1073/pnas.132468999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Britten R, Kohne D. Repeated Sequences in DNA. Science. 1968;161:529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- 95.Easwaran HP, Van Neste L, Cope L, Sen S, Mohammad HP, Pageau GJ, Lawrence JB, Herman JG, Schuebel KE, Baylin SB. Aberrant Silencing of Cancer-Related Genes by CpG Hypermethylation Occurs Independently of Their Spatial Organization in the Nucleus. Cancer Research. 2010;70:8015–8024. doi: 10.1158/0008-5472.CAN-10-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. New England Journal of Medicine. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]