Abstract
Study Design
This study implemented immunohistochemistry to assay prostaglandin E2 (PGE2) receptor EP2 expression in the dorsal root ganglion (DRG) of rats after painful cervical facet joint injury.
Objective
The objective of this study was to identify if inflammatory cascades are induced in association with cervical facet joint distraction-induced pain by investigating the time course of EP2 expression in the DRG.
Summary of Background Data
The cervical facet joint is a common source of neck pain and non-physiological stretch of the facet capsular ligament can initiate pain from the facet joint via mechanical injury. PGE2 levels are elevated in painful inflamed and arthritic joints, and PGE2 sensitizes joint afferents to mechanical stimulation. Although in vitro studies suggest the EP2 receptor subtype contributes to painful joint disease the EP2 response has not been investigated for any association with painful mechanical joint injury.
Methods
Separate groups of male Holtzman rats underwent either a painful cervical facet joint distraction injury or sham procedure. Bilateral forepaw mechanical allodynia was assessed, and immunohistochemical techniques were used to quantify EP2 expression in the DRG at days 1 and 7.
Results
Facet joint distraction induced mechanical allodynia that was significant (p<0.024) at all time points. Painful joint injury also significantly elevated total EP2 expression in the DRG at day 1 (p=0.009), which was maintained also at day 7 (p<0.001). Neuronal expression of EP2 in the DRG was only increased over sham levels at day 1 (p=0.013).
Conclusions
Painful cervical facet joint distraction induces an immediate and sustained increase of EP2 expression in the DRG, implicating peripheral inflammation in the initiation and maintenance of facet joint pain. The transient increase in neuronal EP2 suggests, as in other painful joint conditions, that after joint injury non-neuronal cells may migrate to the DRG, some of which likely express EP2.
Keywords: facet, pain, EP2, neck, joint, inflammation
INTRODUCTION
Back and neck pain represent widespread debilitating conditions afflicting greater than 30% of the adults in the United States [1]. The facet joint has been identified as the source of neck pain in 25–65% of patients [2], with mounting evidence that mechanical injury of this joint can initiate pain, particularly in the cervical spine [3,4,5,6,7,8,9]. Both mechanical injury and inflammation of the facet joint have been shown to produce persistent pain in otherwise normal rats [10,11,12]. Further, mechanical injury of the facet joint increases cytokine mRNA in the DRG [11] and intra-articular injection of a non-steroidal anti-inflammatory agent alleviates injury-induced pain in that same model [13], suggesting that inflammation has a role in the pain response following a mechanical joint insult. Although there are increasing suggestions that mechanical joint injury can initiate inflammatory responses in the context of pain, the molecular mechanisms of facet joint injury-induced pain remain poorly defined.
Inflammatory mediators, such as cytokines, prostaglandins, and neuropeptides, increase within the joint and the DRG in joint inflammation and arthritis [12,14,15,16,17]. In particular, prostaglandin E2 (PGE2) has been identified as a key mediator of inflammation-induced behavioral sensitivity and increased neuronal excitability [18,19,20,21]. PGE2 produced in inflamed tissue sensitizes afferents by binding one of four possible G-protein-coupled receptors (EP1–EP4) [22,23], all of which are expressed in the DRG [24]. Of these receptors, in vitro studies have suggested that EP2 is involved in the pathophysiology of painful joint conditions [25]. Separately, blockade of this receptor relieves pain after spinal cord injury [26], implicating it in injury-induced pain. Despite the suggested relationship between PGE2 and its receptor EP2 in joint pain from inflammation, no study has evaluated the EP2 response in the DRG after a mechanically induced painful joint injury.
We previously developed a model of cervical facet joint injury that uses mechanical loading via joint distraction to produce pain in the rat [3,10,27]. That model induces pain in the back of the neck and forepaw that develops within a day after joint injury and is sustained for nearly four weeks [4,11,28]. In addition, although spinal inflammation has been shown to relate to the severity of joint injury and pain in that model [11], local inflammatory responses have not been characterized. Although inhibition of cyclooxygenase (COX) activity, which diminishes prostaglandin synthesis, has been shown to alleviate joint pain [13,29,30], no study has defined the specific receptor components of the prostaglandin signaling pathway that may be involved in facet joint-mediated pain. Therefore, this study tested the hypothesis that painful facet joint distraction is associated with increased expression of the PGE2 receptor EP2 in the DRG.
MATERIALS AND METHODS
Male Holtzman rats (Harlan Sprague-Dawley, Indianapolis, IN) (408±26g) were housed under USDA- and AAALAC-compliant conditions with a 12-12 hour light-dark cycle and free access to food and water. All experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain [31].
General Surgical Procedures
All surgical procedures were performed under inhalation isoflurane anesthesia (4% for induction, 2.5% for maintenance). A cervical facet joint distraction was applied across the bilateral C6–C7 facet joints in the rat, as previously described [10,11,32]. Briefly, a midline incision was made along the back of the neck, and the C6–C7 facet joints and their capsules were exposed and cleared of soft tissues and muscle. The interspinous ligaments from C5-T1 were transected, and the C6 and C7 laminae were attached to a loading device via microforceps. For the injury group (n=9), the C6 vertebra was displaced rostrally and C7 was held stationary, distracting the bilateral C6–C7 facet joints in a controlled manner known to reliably induce persistent pain symptoms [3,10,11,32]. Markers were attached to the C6 and C7 laminae and tracked by a camera mounted to the surgical dissecting scope during the distraction in order to quantify the magnitude of each distraction applied to the joint [4,32]. Separate groups of rats undergoing joint distraction injury were monitored post-operatively for 1 (n=5) and 7 days (n=4) at which point DRG tissue was harvested. Additional groups of rats underwent sham surgical procedures with no applied joint distraction (n=4 day 1; n=5 day 7). Following surgery, wounds were closed using 3-0 polyester suture and surgical staples, and rats were recovered in room air with controlled temperature and humidity. The magnitude of applied joint distraction was compared between the day 1 and day 7 injury groups using a t-test with significance at p<0.05.
Behavioral Assessment
Bilateral forepaw mechanical allodynia was evaluated in all rats following surgical procedures on days 1, 3, 5, and 7 or until the time of tissue harvest. Baseline measurements also were recorded for each rat prior to any procedure. Methods to quantify forepaw allodynia have been previously validated [10,33]. Briefly, rats were placed in cages with a wire mesh floor and acclimated to the testing environment for 15 minutes. Rats then underwent three rounds of 10 stimulations to the plantar surface of each forepaw using 2g and 4g von Frey filaments (Stoelting; Wood Dale, IL). During each round, the number of responses, defined as emphatic lifting and/or licking of the forepaw, to stimulation with each filament was recorded separately for the right and left forepaws. The number of responses elicited during all three rounds was recorded. Because of the bilateral nature of the applied injury, the responses of the right and left paws were averaged to obtain a withdrawal total for each rat. A repeated-measures ANOVA with Tukey's HSD test was used to compare responses to baseline for each group.
Tissue Harvest, Processing & Immunohistochemistry of EP2 Expression in the C6 DRG
On the day of tissue harvest, rats were given an overdose of sodium pentobarbital (65mg/kg) and perfused transcardially with 300mL of phosphate buffered saline (PBS) and 250mL of 4% paraformaldehyde in PBS (pH7.4). Naïve un-operated rats (n=3) were also included in these studies to serve as controls. The left C6 DRGs were harvested and post-fixed in the same fixative solution for 1 hour at room temperature. DRGs were then transferred to 50% ethanol overnight, dehydrated in a graded ethanol series, and embedded in paraffin. Serial transverse sections (10μm) were taken for immunohistochemistry (IHC) and mounted onto APES-coated slides. Sections were deparaffinized and rehydrated prior to incubation with rabbit anti-EP2 (1:1000; Cayman Chemicals; Ann Arbor, MI) and mouse anti-MAP2 (1:200; Covance; Emeryville, CA) primary antibodies overnight at 4°C. DRG tissue sections that were not incubated with primary antibodies were included as negative controls. The following day, sections were washed and incubated with goat anti-rabbit Alexa488 and goat anti-mouse Alexa546 (Invitrogen; Carlsbad, CA) secondary antibodies and cover-slipped using Fluoro-Gel (EMS; Hatfield, PA).
A Carl Zeiss LSM 510 microscope (Carl Zeiss LLC; Thornwood, NY) equipped with Argon, HeNe, and Coherent Chameleon fs-pulsed NIR lasers was used to image each DRG at 40× magnification. For each tissue section, two images were taken at similar anatomical locations in the DRG to avoid bias in image sampling. Metamorph 6.0 image analysis software (Universal Imaging Inc; Sunnyvale, CA) was used to quantify EP2 expression in the DRG. Positive EP2 signals were defined as pixel intensities that were greater than the positive threshold intensity determined using naïve tissue [34,35]. For each image, total EP2 was measured by the integrated pixel intensity for EP2, which is defined as the average positive pixel intensity multiplied by the area of positive pixels for EP2. Neuronal EP2 was measured as the amount of EP2 co-localized with neurons and quantified by the integrated pixel intensity of EP2 overlapping MAP2 positive pixels. Both average total EP2 and neuronal EP2 expression were determined relative to levels in the normal tissue. A two-way ANOVA, with time (day 1, day 7) and group (injury, sham) as the factors, and post-hoc Bonferroni correction compared the temporal changes in EP2 expression in the DRG.
RESULTS
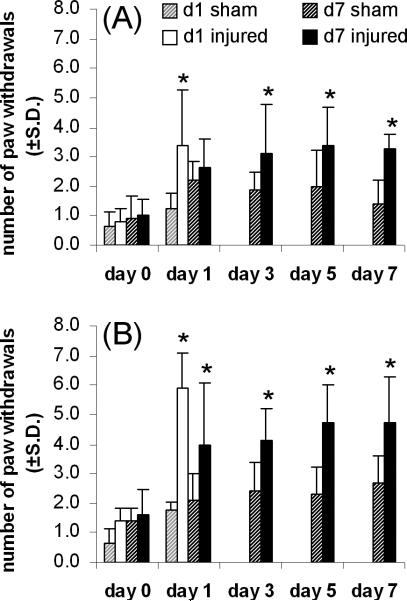
All rats in both of the injury groups received the same joint distraction regardless of the time of tissue harvest. The average applied joint distraction was 0.63±0.10 mm for the day 1 injury group and 0.66±0.01 mm for the day 7 group, and these distraction magnitudes were not different from each other. Mechanical allodynia was evident in all of the rats undergoing a facet joint distraction (Figure 1). Using the 2g von Frey filament, allodynia was significantly elevated over baseline values on day 1 for the day 1 injury group (p<0.003) and on days 3, 5, and 7 for the other group of injury rats (p<0.024) (Figure 1A). In addition, allodynia also was significantly elevated over baseline at all time points for the day 1 (p<0.001) and day 7 (p<0.012) injury groups using the 4g filament (Figure 1B). In contrast to injury responses, sham responses were not different from baseline at any time point for either of the sham groups, for stimulation by either the 2g or 4g filament (Figure 1).
Figure 1.
Mechanical allodynia in the forepaw as measured by the average number of responses to von Frey filament stimulation using 2g (A) and 4g (B) filaments. Forepaw allodynia is induced after facet joint distraction compared to baseline (*p<0.024) on the days after injury when tissue was harvested whereas sham responses are not changed from baseline.
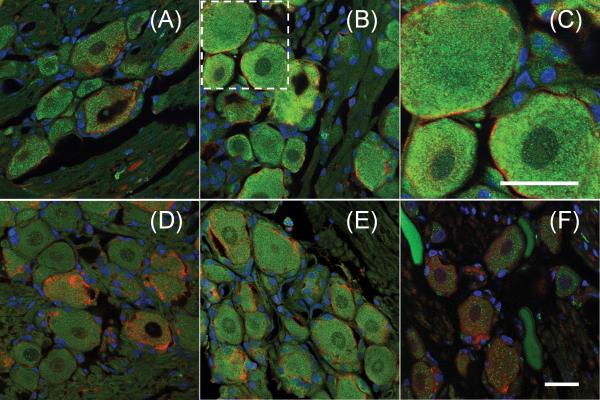
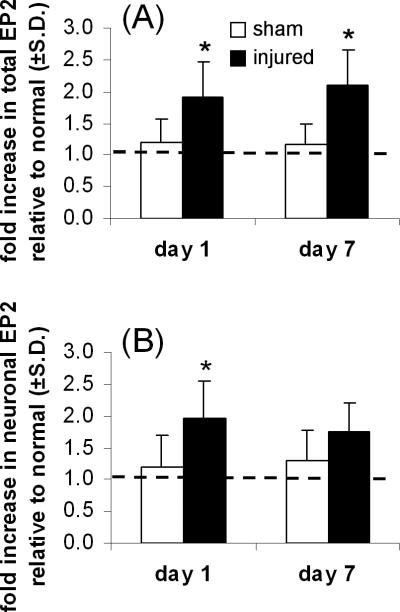
EP2 was detected in the DRGs of all rats, and its expression was identified primarily in neurons (Figure 2). Painful facet joint distraction induced an increase in EP2 expression in the DRG at both day 1 and day 7 (Figure 2). In fact, total normalized EP2 expression was significantly increased (p=0.009) in the day 1 injury group compared to sham levels at the same time point (Figure 3A). Total EP2 in the DRG remained significantly (p<0.001) elevated in the injury group over sham responses at day 7 (Figure 3A). On average, 93±10% of the EP2 that was detected in the DRG was localized to neurons (Figure 2). Neuronal expression of EP2 was significantly increased (p=0.013) after painful injury at day 1 (Figure 3B). Yet, by day 7 neuronal EP2 expression in the injury group was no longer different from sham (Figure 3B).
Figure 2.
Expression of the EP2 receptor (green) in the DRG. On day 1, co-labeling for EP2 and neurons (MAP2; red) demonstrates that EP2 expression after sham (A) is less than after painful joint injury (B) and that most EP2 is found in neurons. The boxed area in (B) is magnified in (C) to highlight the neuronal expression of EP2. Similarly, on day 7, EP2 expression after sham (D) is less than after joint injury (E). EP2 expressed in the DRG of naïve rats served for comparison (F). Scale bars in (C) and (F) are 20μm. Scale bar in (F) applies to panels A–B and D–F. Nuclei are shown in blue.
Figure 3.
Quantification of total (A) and neuronal (B) expression of EP2 in the DRG. (A) After painful joint injury, total EP2 is increased at day 1 (*p=0.009) and day 7 (*p<0.001). (B) Neuronal EP2 is increased at only day 1 (*p=0.013). The dashed line indicates EP2 expression in the DRG of normal rats.
DISCUSSION
These findings demonstrate that facet joint loading sufficient to induce behavioral sensitivity also increases expression of the PGE2 receptor EP2 in the DRG (Figures 1–3). In particular, increased EP2 expression is evident immediately after painful facet joint distraction and is maintained at this elevated level through day 7, mimicking the pattern of behavioral sensitivity (Figures 1 and 3). Despite the increase in EP2 at days 1 and 7, neuron-specific expression of EP2 is only increased on day 1 after injury (Figure 3). Although several studies identify roles for the PGE2 receptors in joint inflammation and arthritis [16,22,23,25,36], this is the first study to demonstrate an association between mechanical joint injury-induced pain and EP2 upregulation. PGE2 increases in painful inflamed and arthritic joints [15,16], and intra-arterial application of PGE2 sensitizes joint afferents to mechanical stimulation [37,38]. Yet, those studies only identify PGE2 as a likely mediator of joint pain and do not identify which receptor(s) are responsible for the effects of PGE2 on sensitivity. The current study demonstrates that EP2 expression increases in the DRG for at least one week in response to a painful joint injury. Taken together with the literature, these data implicate EP2 in joint-mediated pain.
The upregulation of EP2 protein expression in the DRG in response to a painful facet joint distraction is consistent with reports of activation of other inflammatory and nociceptive responses in this type of painful injury. The joint distraction magnitude imposed in this study has been shown to immediately activate capsule nociceptors, and also to modulate the cellular stress response and regulate mRNA levels of cytokines in the DRG at later times [10,11,39,40]. Accordingly, it is not surprising that EP2 is also upregulated in the DRG at both days 1 and 7 (Figure 3A). However, this study did not quantify expression of the other PGE2 receptors; both EP3 and EP4 have been show to be altered in the DRG in response to peripheral inflammation, and selective antagonism of EP4 alleviates pain from ankle inflammation [22,23]. Additional studies are needed to evaluate the other PGE2 receptors and PGE2 itself in order to develop a complete picture of the inflammatory response induced following painful facet joint loading. However, inhibition of COX activity, and thus diminished prostaglandin synthesis, alleviates facet joint pain in this same injury model [13], suggesting that prostaglandins such as PGE2 contribute to mechanically-induced joint pain. Furthermore, the current study identifies EP2 as an important target for future studies to block receptor-specific prostaglandin activity, refining the understanding of those cellular responses relevant to injury-induced joint pain. Nonetheless, the functional role of specific prostaglandin receptors in facet-mediated pain is still unknown.
Neurons constitute the main source of EP2 in the DRG, and neuronal expression of EP2 is significantly upregulated immediately after painful facet joint injury, in parallel with total EP2 expression (Figures 2 and 3). Despite the persistent upregulation of total EP2 expression evident at day 7, neuronal expression of this receptor is no longer different from sham at this time point (Figure 3B), which indicates that non-neuronal cells may also be expressing EP2 after facet joint injury. Macrophages expressing EP2 have been identified in the synovium of arthritic rats [41]; following sciatic nerve injury, expression of EP2 is upregulated in the nerve most likely by non-resident cells [42]. Furthermore, macrophages have been reported to infiltrate the DRG within three days of painful antigen-induced arthritis [43]. Taken together with findings in the current study, these reports suggest that inflammatory cells may infiltrate and contribute to the increased EP2 expression in the DRG on day 7 after joint distraction. However, although there is a subtle increase in neuronal EP2 at day 7 after painful injury, this was not significant, which may be an artifact of the small sample size of the groups in that time point and not actually indicate a real shift in EP2 expression to non-neuronal sources. Additional studies using larger group sizes are needed to verify that neuronal EP2 is unchanged after injury and to identify any non-neuronal sources of EP2 in the DRG in this model.
Although EP2 expression increases in the DRG after a painful facet joint injury (Figures 2 and 3), this study does not characterize EP2 expression in the spinal cord, where prostaglandins, specifically PGE2, have been shown to contribute to hyperexcitability in response to peripheral inflammation [21]. Painful facet joint injury is associated with spinal inflammation [11,13] and induces increased neuronal firing in the spinal cord dorsal horn [32]. Additionally, blockade of spinal EP2 alleviates pain after a spinal cord injury [26], implicating spinal EP2 in injury-induced pain. This collection of findings suggests that spinal PGE2 may contribute to facet joint injury-mediated spinal hyperexcitability and pain. Because spinal PGE2 and EP2 are associated with spinal neuronal hyperexcitability and pain, both of which are induced by facet joint injury, further studies characterizing spinal EP2 expression after joint injury will provide insight into its role in facet joint-mediated pain.
Although painful facet joint injury induces an upregulation of EP2 expression, these results reflect only a single injury modality, specifically tensile stretch of the facet joint capsule. Facet-mediated pain can also arise from other mechanical loading conditions, such as joint compression and shear that can occur during whiplash [5, 44], or with joint degeneration from osteoarthritis [45]. It is not known if the results from the current study would be conserved under these different conditions. In addition, the current study only identifies an upregulation of EP2 expression in the DRG after injury and does not investigate its functional role in injury-induced or other joint-mediated pain responses. Further studies utilizing intra-articular application of a previously validated synthetic antagonist of EP2 [26] are needed to identify the functional role of increased EP2 in the DRG in order to fully understand the contribution of this receptor to facet-mediated pain. Lastly, as with findings from any animal model, it is important to consider these findings in the context of the model itself when extrapolating any potential implications to the human condition [46]. Specifically, it is important to consider whether the model and its findings simulate the injury or the symptoms, and to recognize that cellular responses in the absence of any pharmacological intervention are only associative and may not be causal.
Despite some limitations, the data reported in this study support the role of EP2 in both the induction and maintenance of pain after mechanical facet joint injury. A host of responses involving neurotrophins, neuropeptides, and other inflammatory mediators likely contributes to facet-mediated pain. However, the relief of pain through intra-articular application of the anti-inflammatory drug ketorolac supports an important role of prostaglandin signaling in this painful injury [13]. In addition, ketorolac administration is sufficient to prevent the upregulation of a mediator of the integrated stress response in the DRG [13]. Because facet joint injury upregulates inflammatory mediators in the DRG [11] and local anti-inflammatory treatment is able to prevent injury-induced changes in the DRG [13], there is a strong link between painful mechanical joint injury, inflammation, and neuronal plasticity. Despite this relationship, it is still unknown how EP2 contributes to joint pain. Nevertheless, by identifying increased expression of EP2, this study provides a basis for a peripheral role of PGE2 in facet joint pain and suggests that mechanical joint injury may induce local inflammation despite not having an inflammatory insult.
KEY POINTS
Painful cervical facet joint injury induces increased EP2 expression in the DRG that is sustained for at least 7 days.
EP2 expression in the DRG is primarily increased in neurons early after the injury but is transient.
Findings suggest that pain after a mechanical joint injury may be due to peripheral inflammatory responses, particularly those involving prostaglandins in the DRG.
Acknowledgments
The National Institutes of Health/National Institute of Arthritis, Musculoskeletal and Skin Diseases (#AR056288) funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis Rheum. 2007;57:656–65. doi: 10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- 2.van Eerd M, Patijn J, Lataster A, et al. Cervical facet pain. Pain Pract. 2010;10:113–23. doi: 10.1111/j.1533-2500.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- 3.Dong L, Winkelstein BA. Simulated whiplash modulates expression of the glutamatergic system in the spinal cord suggesting spinal plasticity is associated with painful dynamic cervical facet loading. J Neurotrauma. 2010;27:163–74. doi: 10.1089/neu.2009.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10:436–45. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Panjabi MM, Cholewicki J, Nibu K, et al. Capsular ligament stretches during in vitro whiplash simulations. J Spinal Disord. 1998;11:227–32. [PubMed] [Google Scholar]
- 6.Siegmund GP, Myers BS, Davis MB, et al. Mechanical evidence of cervical facet capsule injury during whiplash. Spine. 2001;26:2095–101. doi: 10.1097/00007632-200110010-00010. [DOI] [PubMed] [Google Scholar]
- 7.Weisshaar CL, Dong L, Bowman AS, et al. Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J Neurotrauma. 2010;27:2261–71. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 8.Winkelstein BA, Nightingale RW, Richardson WJ, et al. The cervical facet capsule and its role in whiplash injury. Spine. 2000;25:1238–46. doi: 10.1097/00007632-200005150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Yoganandan N, Cusick JF, Pintar Fa, et al. Whiplash injury determination with conventional spine imaging and cryomicrotomy. Spine. 2001;26:2443–8. doi: 10.1097/00007632-200111150-00010. [DOI] [PubMed] [Google Scholar]
- 10.Dong L, Odeleye AO, Jordan-Sciutto KL, et al. Painful facet joint injury induces neuronal stress activation in the DRG: implications for cellular mechanisms of pain. Neurosci Lett. 2008;443:90–4. doi: 10.1016/j.neulet.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KE, Davis MB, Winkelstein BA. Capsular ligament involvement in the development of mechanical hyperalgesia after facet joint loading: behavioral and inflammatory outcomes in a rodent model of pain. J Neurotrauma. 2008;25:1383–93. doi: 10.1089/neu.2008.0700. [DOI] [PubMed] [Google Scholar]
- 12.Tachihara H, Kikuchi S, Konno S, et al. Does facet joint inflammation induce radiculopathy?: an investigation using a rat model of lumbar facet joint inflammation. Spine. 2007;32:406–12. doi: 10.1097/01.brs.0000255094.08805.2f. [DOI] [PubMed] [Google Scholar]
- 13.Dong L, Guarino BB, Jordan-Sciutto KL, et al. Activating transcription factor 4, a mediator of the integrated stress response, is increased in the dorsal root ganglia following painful facet joint distraction. Neuroscience. 2011;193:377–86. doi: 10.1016/j.neuroscience.2011.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd BL, Morris VH, Urban L. Pathophysiology of joint pain. Ann Rheum Dis. 1996;55:276–83. doi: 10.1136/ard.55.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn JH, Bazan NG. Identification of prostaglandin E2 and leukotriene B4 in the synovial fluid of painful, dysfunctional temporomandibular joints. J Oral Maxillofac Surg. 1990;48:968–71. doi: 10.1016/0278-2391(90)90011-p. [DOI] [PubMed] [Google Scholar]
- 16.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- 17.Schaible HG, Schmelz M, Tegeder I. Pathophysiology and treatment of pain in joint disease. Adv Drug Deliv Rev. 2006;58:323–42. doi: 10.1016/j.addr.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Bär K, Natura G, Telleria-Diaz A, et al. Changes in the effect of spinal prostaglandin E2 during inflammation: prostaglandin E (EP1-EP4) receptors in spinal nociceptive processing of input from the normal or inflamed knee joint. J Neurosci. 2004;24:642–51. doi: 10.1523/JNEUROSCI.0882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubb BD, Birrell GJ, McQueen DS, et al. The role of PGE2 in the sensitization of mechanoreceptors in normal and inflamed ankle joints of the rat. Exp Brain Res. 1991;84:383–92. doi: 10.1007/BF00231460. [DOI] [PubMed] [Google Scholar]
- 20.Lin C, Amaya F, Barrett L, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther. 2006;319:1096–103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 21.Vasquez E, Bär K, Ebersberger A, et al. Spinal prostaglandins are involved in the development but not the maintenance of inflammation-induced spinal hyperexcitability. J Neurosci. 2001;21:9001–8. doi: 10.1523/JNEUROSCI.21-22-09001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donaldson LF, Humphrey PS, Oldfield S, et al. Expression and regulation of prostaglandin E receptor subtype mRNAs in rat sensory ganglia and spinal cord in response to peripheral inflammation. Prostaglandins Other Lipid Mediat. 2001;63:109–22. doi: 10.1016/s0090-6980(00)00101-5. [DOI] [PubMed] [Google Scholar]
- 23.Omote K, Kawamata T, Nakayama Y, et al. Effects of a novel selective agonist for prostaglandin receptor subtype EP4 on hyperalgesia and inflammation in monoarthritic model. Anesthesiology. 2002;97:170–6. doi: 10.1097/00000542-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Cruz Duarte P, St-Jacques B, Ma W. Prostaglandin E2 contributes to the synthesis of brain-derived neurotrophic factor in primary sensory neuron in ganglion explant cultures and in a neuropathic pain model. Exp Neurol. 2012;234:466–81. doi: 10.1016/j.expneurol.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Ellman M, Muddasani P, et al. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum. 2009;60:513–23. doi: 10.1002/art.24258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KE, Thinnes JH, Gokhin DS, et al. A novel rodent neck pain model of facet-mediated behavioral hypersensitivity: implications for persistent pain and whiplash injury. J Neurosci Meth. 2004;137:151–9. doi: 10.1016/j.jneumeth.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Rothman SM, Hubbard RD, Lee Ke, et al. Detection, transmission, and perception of pain. In: Slipman C, Simeone F, Derby R, editors. Interventional Spine: An Algorithmic Approach. Elsevier; 2007. pp. 29–37. [Google Scholar]
- 29.Davies P, Bailey PJ, Goldenberg MM. The role of arachidonic acid oxygenation products in pain and inflammation. Ann Rev Immunol. 1984;2:335–57. doi: 10.1146/annurev.iy.02.040184.002003. [DOI] [PubMed] [Google Scholar]
- 30.Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygnease-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–5. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 32.Quinn KP, Dong L, Golder FJ, et al. Neuronal hyperexcitability in the dorsal horn after painful facet joint injury. Pain. 2010;151:414–21. doi: 10.1016/j.pain.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubbard RD, Winkelstein BA. Transient cervical nerve root compression in the rat induces bilateral forepaw allodynia and spinal glial activation: mechanical factors in painful neck injuries. Spine. 2005;30:1924–32. doi: 10.1097/01.brs.0000176239.72928.00. [DOI] [PubMed] [Google Scholar]
- 34.Abbadie C, Brown JL, Mantyh PW, et al. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–9. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- 35.Rothman SM, Winkelstein BA. Chemical and mechanical nerve root insults induce differential behavioral sensitivity and glial activation that are enhanced in combination. Brain Res. 2007;1181:30–43. doi: 10.1016/j.brainres.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem. 2001;275:16083–91. doi: 10.1074/jbc.M011408200. [DOI] [PubMed] [Google Scholar]
- 37.Schaible HG, Schmidt RF. Discharge characteristics of receptors with fine afferents from normal and inflamed joints: influence of analgesics and prostglandins. Agents Actions Suppl. 1986;19:99–117. [PubMed] [Google Scholar]
- 38.Schepelmann K, Messlinger K, Schaible HG, et al. Inflammatory mediators and nociception in the joint: excitation and sensitization of slowly conducting afferent fibers of cat's knee by prostaglandin I2. Neuroscience. 1992;50:237–47. doi: 10.1016/0306-4522(92)90395-i. [DOI] [PubMed] [Google Scholar]
- 39.Lee KE, Davis MB, Mejilla RM, et al. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car Crash J. 2004;48:373–95. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Chen C, Kallakuri S, et al. Neurophysiological and biomechanical characterization of goat cervical facet joint capsules. J Orthop Res. 2005;23:779–87. doi: 10.1016/j.orthres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Kurihara Y, Endo H, Akahoshi T, et al. Up-regulation of prostaglandin E receptor EP2 and EP4 subtypes in rat synovial tissues with adjuvant arthritis. Clin Exp Immunol. 2001;123:323–30. doi: 10.1046/j.1365-2249.2001.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma W, Eisenach JC. Four PGE2 EP receptors are up-regulated in injured nerve following partial sciatic nerve ligation. Exp Neurol. 2003;183:581–92. doi: 10.1016/s0014-4886(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 43.Schaible HG, von Banchet GS, Boettger MK, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann NY Acad Sci. 2010;1193:60–9. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 44.Pearson AM, Ivancic PC, Ito S, et al. Facet joint kinematics and injury mechanisms during simulated whiplash. Spine. 2004;29:390–7. doi: 10.1097/01.brs.0000090836.50508.f7. [DOI] [PubMed] [Google Scholar]
- 45.Kirpalani D, Mitra R. Cervical facet joint dysfunction: a review. Arch Phys Med Rahabil. 2008;89:770–4. doi: 10.1016/j.apmr.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Winkelstein BA. How can animal models inform on the transition to chronic symptoms in whiplash? Spine. 2011;36:S218–25. doi: 10.1097/BRS.0b013e3182387f96. [DOI] [PMC free article] [PubMed] [Google Scholar]