Abstract
MCP-1, also known as CCL2, is a monocyte-attracting chemokine produced in lung epithelial cells. We previously reported an association of increased levels of plasma MCP-1 with primary graft dysfunction (PGD) after lung transplantation in a nested case control study of extreme phenotypes using a multiplex platform. In this study, we sought to evaluate the relationship between plasma MCP-1 levels as a biomarker across the full spectrum of PGD. We performed a prospective cohort study of 108 lung transplant recipients within the Lung Transplant Outcomes Group cohort. Plasma MCP-1 levels were measured pre-transplantation, 6 and 24 hours after transplantation. The primary outcome was development of grade 3 PGD within 72 hours of transplant, with secondary analyses at the 72- hour timepoint. Multivariable logistic regression was used to evaluate confounding. 30 subjects (28%) developed PGD. Median MCP-1 measured at 24 hours post transplant was elevated in subjects with PGD (167.95 vs. 103.5 pg/mL p=0.04). MCP-1 levels at 24 hours were associated with increased odds of grade 3 PGD after lung transplantation (OR for each 100 pg/mL 1.24, 95% CI 1.00, 1.53), and with grade 3 PGD present at the 72-hour timepoint (OR for each 100 pg/mL 1.57, 95% CI 1.18, 2.08), independent of confounding variables in multivariable analyses. MCP-1 levels measured pre-operatively and 6 hours after transplant were not significantly associated with PGD. Persistent elevations in MCP-1 levels at 24 hours are a biomarker of grade 3 PGD post-transplantation. Monocyte chemotaxis may play a role in the pathogenesis of PGD.
Keywords: Primary Graft Dysfunction, Lung transplantation, Monocyte Chemotactic Protein-1, Acute Lung Injury, Biomarker
Introduction
Lung transplantation is the only life-prolonging therapy for many end stage lung diseases [1]. It has become an increasingly common procedure, with 3,272 lung transplants reported in 2009 [2]. However, lung transplantation has consistently lagged behind other organs in survival rates, with a median survival of only 5.3 years [3–5].
PGD is a form of acute lung injury that develops within 72 hours of transplant. It affects 10–30% of all subjects receiving lung transplantation [4–6], and is associated with an increased risk of bronchiolitis obliterans syndrome (BOS), prolonged hospitalization, and increased short- and long-term mortality [7, 8]. The exact pathogenesis of PGD is unclear, however, ischemia reperfusion injury (IRI) is thought to be a driving force in its development [9].
Monocyte Chemotactic Protein-1 (MCP-1), also known as Chemokine (C-C motif) Ligand 2 (CCL2), is a monocyte attracting chemokine produced by many cell types, including endothelial, fibroblast, dendritic, and epithelial cells [10]. MCP-1 is important in mediating the early injury response by recruitment of monocytes, memory T cells, and NK cells [11]. Elevation in MCP-1 levels after lung ischemia reperfusion injury (IRI) has been established in a rat model [12]. In addition, MCP-1 has been identified as a promoter of systemic inflammation in response to alveolar hypoxia [13]. Furthermore, animal research has demonstrated that blockade of MCP-1 attenuates IRI in pulmonary and renal models [12, 14]. Based on the relationship of MCP-1 and IRI, we hypothesized that MCP-1 may be related to the development of PGD. We previously reported an association of increased levels of plasma MCP-1 among other mediators with PGD using a multiplex immunoassay platform in a nested case control study of grade 3 PGD compared with no PGD (grade 0) [15].
In the current study, we tested the hypothesis that plasma MCP-1 is a biomarker of PGD within 72 hours after transplantation, using a prospective cohort study including all grades of PGD, a monoplex assay specific to MCP-1, and multivariable adjustment of important confounding variables.
Materials and Methods
Selection of Subjects
The Lung Transplant Outcomes Group (LTOG) is a multi-center, prospective cohort study of lung transplant recipients that has been previously described [16–18]. Our prospective cohort study included all subjects enrolled between October 2006 and May 2008 at the University of Pennsylvania and Columbia University. Two centers were selected to reduce variability secondary to center effects, and these centers have the highest volume of lung transplants. Sample size was determined based on results of our prior case-control study of MCP-1 [15]. Plasma samples were prospectively collected pre-transplant, 6 and 24 hours after allograft reperfusion. These timepoints were chosen to reflect immediate post-transplant lung injury and lung injury that is contemporaneous with measurement of MCP-1. Samples were centrifuged within 60 minutes and then stored at −80°C for subsequent analysis. Clinical data were collected prospectively for all subjects as described in detail elsewhere [4]. The Institutional Review Boards at each site approved our study. Informed consent was obtained from each subject enrolled in the cohort. This study was conducted in compliance with the Declaration of Helsinki of 1975 as revised in 1996.
Outcome Definition
PGD grade was determined using the consensus definition of the International Society for Heart and Lung Transplantation [19]. Radiographs and arterial blood gases were assessed at the time of admission to the ICU after transplantation (T0), and at 24, 48, and 72 hours after transplantation. Two blinded physicians examined chest radiographs, which qualified for PGD if they had diffuse infiltrates. The severity of PGD was graded by the PaO2/FiO2 ratio, with PaO2/FiO2 ratio less than 200 defining grade 3 PGD [4, 16, 19]. The primary end point was the development of grade 3 PGD within 72 hours (PGD) after transplantation. This definition has good discriminant validity for survival, and is thought to be an acceptable dichotomous outcome for studies evaluating the mechanism of PGD [20]. Sensitivity analyses were performed using grade 3 PGD present at 72 hours after transplantation (Persistent PGD) versus other grades at T72; grade 3 PGD present at 24 hours, and evaluating highest grade of PGD within 72 hours as an ordinal variable. We assessed for interaction by cardiopulmonary bypass status, pulmonary hypertension (defined as mean PAP ≥ 25 at the time of transplant) [21], and diagnosis (limited to our three largest diagnosis categories: COPD, IPF, and CF) given data identifying a link between MCP-1 levels and pulmonary vascular disease [22] and a relationship between elevated MCP-1 levels and interstitial lung disease [23]. Subjects with secondary pulmonary fibrosis were not labeled as IPF. Further analyses were performed evaluating the relationship of plasma MCP-1 level with survival to hospital discharge and ventilator-free days during the first 28 days of hospitalization, time to first episode of acute cellular rejection, defined as grade A2 or higher, [24] and time to bronchiolitis obliterans syndrome (BOS), defined according to ISHLT guidelines [25], and time to death or re-transplantation, using all-cause mortality. ACR information was only available from the University of Pennsylvania.
Measurement of MCP-1 levels
Plasma MCP-1 concentration was measured using a commercially available ELISA platform (R&D Systems, Minneapolis, MN). The intra-assay coefficient of variation was 7.6%. The lower limit of detection was 0.61 pg/mL.
Statistical Analysis
Continuous variables were analyzed using two sample t-tests or Wilcoxon rank sum tests, as appropriate. Longitudinal analyses using generalized estimating equations were performed to assess for a relationship in trend of MCP-1 levels over time with PGD. For multivariable models, the relationship of MCP-1 measured at each time-point with PGD was evaluated using logistic regression. Odds ratios were calculated per one standard deviation (100 pg/mL) change in MCP-1 level. We assessed recipient race, diagnosis, gender and age; donor race, gender and age; use of cardiopulmonary bypass and bypass time; transfusion of packed red blood cells, intra-operative saline infusion, and pulmonary artery systolic pressure individually as potential confounders. We used a bi-variate selection strategy, and confounding was defined as a greater than 20% change in odds ratio between MCP-1 level and PGD upon addition of a covariate to the model [26]. Based on the number of events in our cohort, a maximum of three covariates could be included in the model [27]. We analyzed the relationship between MCP-1 levels and worst PGD grade within 72 hours as an ordered variable (0,1,2,3) using ordinal logistic regression [28]. We generated Kaplan meier curves to evaluate the association of MCP-1 with longitudinal outcomes, using log rank test to calculate p-values. For all of the statistical analyses, a p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using STATA 11.1 (STATA Corp., College Station, TX).
Results
Study Population
Of the 108 subjects, 30 (28%) developed grade 3 PGD within 72 hours (PGD), 16 (15%) grade 2; 51 (47%) grade 1; and 11 (10%) subjects had no evidence of PGD (grade 0) within 72 hours. 14 (13%) subjects had grade 3 PGD at the 72 hour timepoint (Persistent PGD). Table 1 lists donor and recipient characteristics by PGD status. There were 63 subjects with blood samples available prior to transplant and 92 subjects with samples available at 6 and 24 hours after transplant. Subjects with data at all three timepoints (n=62) did not differ from subjects with missing samples on any major demographic characteristic. There were no values under the lower limit of detection.
Table 1.
Donor and recipient characteristics by Primary Graft Dysfunction Status.
| PGD (n=30) | No PGD (n=78) | P Value | |
|---|---|---|---|
| Diagnosis | 0.02 | ||
| COPD | 8 (27%) | 28 (35%) | |
| CF | 3 (7%) | 17 (22%) | |
| IPF | 11 (43%) | 30 (38%) | |
| PPH | 2 (7%) | 1 (1%) | |
| Sarcoid | 4 (13%) | 1 (1%) | |
| CHD | 1 (3%) | 0 (0%) | |
| Other | 1 (3%) | 1 (4%) | |
| Recipient Race | 0.03 | ||
| Caucasian | 22 (73%) | 69 (88%) | |
| African American | 7 (23%) | 6 (8%) | |
| Hispanic | 0 (0%) | 3 (4%) | |
| Asian American | 1 (3%) | 0 (0%) | |
| Female Recipient | 12 (40%) | 40 (53%) | 0.24 |
| Recipient Age | 53 ± 11 | 52 ± 13 | 0.64 |
| Donor Race | 0.82 | ||
| Caucasian | 20 (67%) | 42 (56%) | |
| African American | 5 (17%) | 20 (27%) | |
| Hispanic | 3 (10%) | 7 (9%) | |
| Asian American | 1 (3%) | 4 (5%) | |
| Other | 1 (3%) | 2 (3%) | |
| Female Donor | 13 (41%) | 40 (52%) | 0.28 |
| Donor Age | 38 ± 14 | 36 ± 14 | 0.48 |
| Donor Smoking | 9 (30%) | 14 (20%) | 0.29 |
| Donor PaO2 | 316 ± 131 | 320 ± 118 | 0.86 |
| Collection Site | 0.50 | ||
| Columbia | 16 (50%) | 36 (46%) | |
| Penn | 14 (47%) | 42 (54%) | |
| Bilateral Transplant | 24 (80%) | 48 (62%) | 0.07 |
| Use of CBP | 19 (63%) | 30 (38%) | 0.02 |
| CBP TIME (min) | 218 ± 65 | 228 ± 55 | 0.60 |
| Mean PAP | 32 ± 12 | 27 ± 8 | 0.07 |
| Median MCP-1 (pg/mL) | |||
| Pre-transplant | 266 (111, 409) | 173 (101, 411) | 0.71 |
| 6 hours post transplant | 505 (370, 984) | 383 (207, 883) | 0.06 |
| 24 hours post transplant | 168 (99, 351) | 104 (51, 225) | 0.04 |
PGD represents all subjects who developed grade 3 PGD within 72 hours.
COPD=Chronic Obstructive Lung Disease, IPF=Idiopathic Pulmonary Fibrosis, CF=cystic fibrosis, CHD=congenital heart disease, PaO2=partial pressure of oxygen
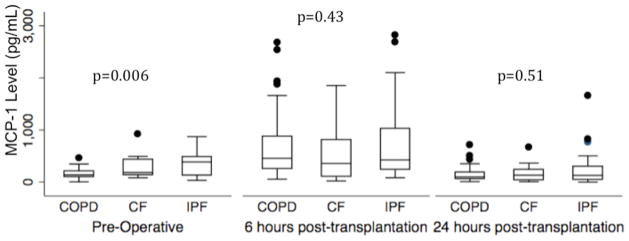
Pre-operative MCP-1 levels were higher in IPF compared to COPD and CF (Kruskal Wallis p value=0.006). Median pre–operative MCP1 level was 385 pg/mL in IPF (IQR 130–489) 138 pg/mL in COPD (IQR 98–212) and 184 pg/mL in CF (IQR 135–439) (Figure 1). Plasma MCP-1 levels at 6 and 24 hours did not differ significantly by diagnosis.
Figure 1.
Plasma MCP-1 concentration pre-transplant, 6 hours post-transplant, and postoperative day 1 stratified by diagnosis in subjects with our top three diagnoses, COPD (Chronic Obstructive Pulmonary Disease), CF (Cystic Fibrosis), and IPF (Idiopathic Pulmonary Fibrosis). P-values reported are for Wilcoxon rank sum test.
Horizontal line indicates median concentration. The upper and lower limits of the box indicate the inter-quartile range.
There was no significant interaction between MCP-1 level and diagnosis (COPD, CF, and IPF) on PGD risk (p for interaction at pre-operative time-point=0.44, at 6 hours p=0.40, at 24 hours p=0.40).
Relationship of MCP-1 Plasma Levels with Grade 3 PGD within 72 hours (PGD)
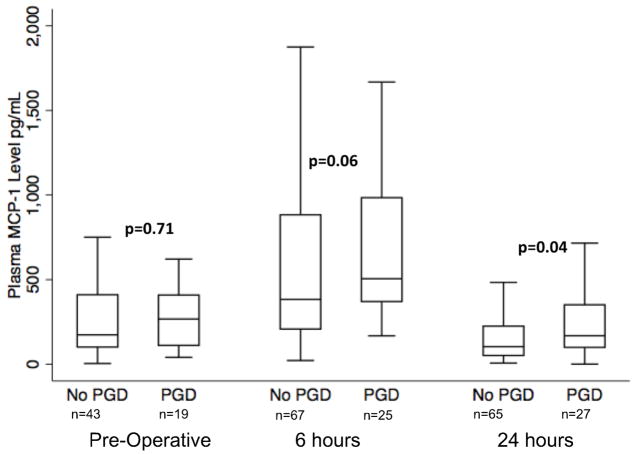
Median MCP-1 levels measured at 24 hours post-transplantation were higher in subjects with PGD (168 pg/mL; IQR 99–351) than in those without PGD (104 pg/mL; IQR 51–225) (p=0.04) (Figure 2). MCP-1 levels also tended to be higher in subjects with PGD at 6 hours post transplantation compared to those without (Table 1), although this did not reach statistical significance. There was no significant difference in pre-operative median MCP-1 levels between subjects with PGD within 72 hours (266 pg/mL; IQR 111–409) and without (173 pg/mL; IQR 101–411) (p=0.71) and without (Table 1) In the entire cohort, MCP-1 levels were elevated pre-operatively, nearly doubled at 6 hours, and then decreased to below pre-operative levels by 24 hours after transplant (Figure 2). However, MCP-1 levels at 24 hours remained higher in the PGD group compared to the non-PGD group.
Figure 2.
Plasma MCP-1 concentration in subjects without grade 3 PGD within 72 hours and with grade 3 PGD within 72 hours at each timepoint. P-values reported are for Wilcoxon rank sum test at each time point. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the inter-quartile range.
The OR for PGD for each 100 pg/mL increment in MCP-1 level was 1.24 (95% CI 1.00,1.53), p=0.048. Bivariable adjustment did not demonstrate significant confounding of the relationship between MCP-1 levels at 24 hours and PGD by clinical variable, as defined by a change in OR by 20% or more on adjustment [27], although inclusion of some variables produced confidence intervals for the OR crossing 1 (Table 2). Therefore, a summary model was not constructed [29–31]. Longitudinal GEE analysis of MCP-1 levels demonstrated a borderline association between changes in MCP-1 level across time and grade 3 PGD within 72 hours (OR per 100pg/mL increment 1.06 95% CI: 1.00,1.12 p=0.06).
Table 2.
Bivariable analysis of MCP-1 measured at 24 hours and PGD
| Variable | Odds Ratio (95% CI) PGD |
p value | Odds Ratio (95% CI) Persistent PGD |
p value |
|---|---|---|---|---|
| MCP-1 per 100 pg/mL (n=92) | 1.24 (1.00, 1.53) | 0.048 | 1.57 (1.18, 2.09) | 0.002 |
| Adjusted for CBP | 1.23 (1.00, 1.53) | 0.05 | 1.55 (1.17, 2.07) | 0.003 |
| Recipient Race | 1.24 (0.98, 1.56) | 0.07 | 1.58 (1.18, 2.11) | 0.002 |
| Recipient Diagnosis | 1.24 (0.99, 1.54) | 0.06 | 1.59 (1.18, 2.13) | 0.002 |
| Recipient Sex | 1.27 (1.02, 1.58) | 0.03 | 1.59 (1.19, 2.14) | 0.002 |
| Recipient Age | 1.24 (1.00, 1.53) | 0.05 | 1.59 (1.18, 2.14) | 0.002 |
| Donor Sex | 1.30 (1.02, 1.65) | 0.03 | 1.75 (1.24, 2.46) | 0.001 |
| Donor Age | 1.24 (0.99, 1.55) | 0.06 | 1.59 (1.18, 2.15) | 0.002 |
| Donor Race | 1.24 (0.99, 1.54) | 0.05 | 1.57 (1.18, 2.09) | 0.002 |
| Donor Smoking | 1.22 (0.98, 1.52) | 0.07 | 1.62 (1.18, 2.23) | 0.003 |
| Donor PaO2 | 1.25 (0.99, 1.58) | 0.06 | 1.62 (1.19, 2.20) | 0.002 |
| Blood (ml) during first 24 hrs | 1.25 (1.00, 1.55) | 0.05 | 1.56 (1.17, 2.08) | 0.002 |
| Intra-operative crystalloid (ml) | 1.25 (0.98, 1.60) | 0.07 | 1.58 (1.18, 2.13) | 0.002 |
| Transplant type | 1.24 (0.99, 1.55) | 0.06 | 1.59 (1.18, 2.13) | 0.002 |
| PASP at transplantation | 1.25 (0.99, 1.55) | 0.05 | 1.57 (1.18, 2.09) | 0.002 |
PaO2=partial pressure of oxygen, PASP=pulmonary artery systolic pressure
Sensitivity Analysis with Full Spectrum of PGD
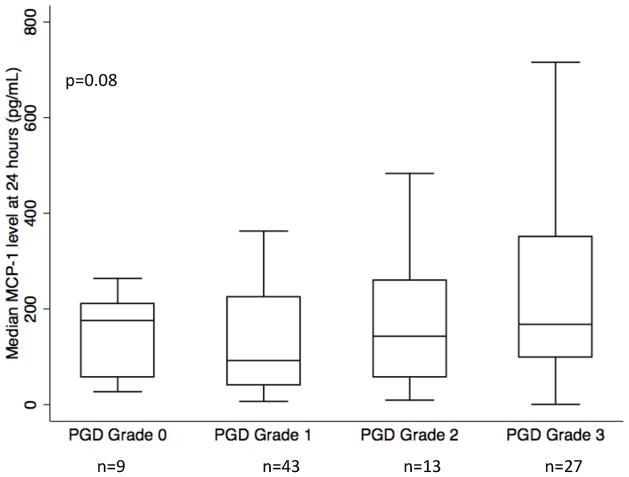
Ordinal logistic regression was used to test the relationship of plasma MCP-1 level 24 hours after transplant with the highest grade of PGD within 72 hours (0, 1, 2, 3). The OR (per 100 pg/mL increment) was 1.21 (95% CI: 1.00, 1.47 p=0.05) for each one unit increase in grade. As shown in Figure 3, median MCP-1 levels increased with each PGD grade, though the trend test was of borderline significance (p=0.08), perhaps due to small numbers in some individual categories.
Figure 3.
Plasma MCP-1 concentration at the 24-hour time-point by highest grade of PGD within 72 hours. P value is reported for the non-parametric test for trend. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the inter-quartile range. Number of subjects in each category is listed below grade (of 92 subjects with MCP-1 level measured at 24 hours).
Relationship of MCP-1 with Grade 3 PGD at 72 hours (Persistent PGD)
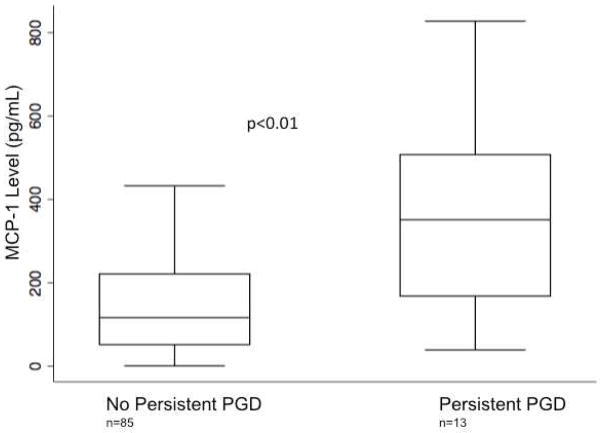
In sensitivity analyses, median MCP-1 levels at the 24-hour time point were higher in the persistent PGD group (351 pg/mL IQR 168–508), compared to all other grades (116 pg/mL IQR 51–221) (p=0.01) (Figure 4). There was no significant difference between median MCP-1 levels in subjects with persistent PGD and those without and the pre-operative and 6-hour timepoints and those who did not have grade 3 PGD within 72 hours at the pre-operative (312 pg/mL vs. 173 pg/mL p=0.37) and 6 hour time points (616 pg/mL vs. 498 pg/mL p=0.17). The corresponding OR for each 100 pg/mL increase in plasma MCP-1 level at 24 hours with grade 3 PGD present at the 72-hour time point was 1.57 (95% CI 1.18, 2.08 p<0.01). Bivariable logistic regression did not demonstrate significant confounding of the relationship between MCP-1 levels at 24 hours and grade 3 PGD at 72 hours by any covariates (Table 2). Median MCP-1 levels differed across grades of PGD measured at 24 hours, with the highest levels in grade 2 and grade 3 (p=0.02) (Supplemental Figure 1).
Figure 4.
Plasma MCP-1 concentration in subjects with grade 3 PGD at 72 hours (persistent PGD) compared to subjects without grade 3 PGD at 72 hours. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the interquartile range. P-value reported is for Wilcoxon rank sum test.
Association of MCP-1 with Other Clinical Outcomes
There was no association between plasma MCP-1 level at 24 hours and ventilator free days (Spearman’s rho −0.20, p=0.23). There was no association between plasma MCP-1 level at 24 hours and survival to hospital discharge (OR 0.98, 95% CI 0.67,1.44 p=0.93).
We were unable to detect an interaction between MCP-1 and either cardiopulmonary bypass (p=0.3) or pulmonary hypertension (p=0.65). Median pre-operative levels did not differ between subjects with cardiopulmonary bypass (238 pg/mL IQR: 96, 541) and those without (230 pg/mL IQR: 115, 385) (p=0.69) There were 61 subjects with pulmonary hypertension, who did not have higher levels of MCP-1 than those without pulmonary hypertension (179 pg/mL vs. 166 pg/mL, p=0.80).
Of the 49 subjects with information on ACR, 22 had at least one episode of grade A2 rejection or greater (45%). We were unable to examine the association between pre-operative MCP-1 levels and time to ACR due to smaller number of subjects at this timepoint. There was no association between MCP-1 levels at 6 or 24 hour timepoint with time to first episode of ACR (Supplemental Figure 2). There were 31 of 99 subjects (31%) with BOS and 22 of 99 subjects (22%) who died. There was no association between MCP-1 levels at any timepoint and time to BOS (Supplemental Figure 3) or time to death (Supplemental Figure 4).
Discussion
We report an association between plasma MCP-1 levels measured 24 hours after transplantation and increased odds of PGD. The association of plasma MCP-1 levels with PGD was significant across PGD grades and was strongest with grade 3 PGD at 72 hours, a phenotype of persistent PGD. MCP-1 levels at 24 hours were also higher in concurrent PGD, measured at 24 hours. Trend analyses indicated that plasma MCP-1 levels peak 6 hours after transplantation, and then decrease to below pre-operative levels at 24 hours. Despite the decrease between 6 and 24 hours, plasma MCP-1 levels were persistently elevated in the PGD group at the 24-hour time point. This study builds on our prior work analyzing MCP-1 in extreme phenotypes of PGD by demonstrating the relationship between the full spectrum of PGD using an ordinal analysis and demonstrating that the major differences appear at later time points, indicating that plasma MCP-1 levels at 24 hours may be used as a biomarker of PGD.
Murine models of ischemia reperfusion injury indicate that MCP-1 levels peak between 5–8 hours after injury [32], which is consistent with our finding of elevated MCP-1 in all subjects at 6 hours, regardless of PGD. This characteristic may partly explain the lack of association with PGD at this timepoint in our study. MCP-1 is thought to be a key activator of pro-inflammatory macrophages, while other mediators, such as IL-4 and IL-13, activate the alternative macrophage phenotype, which is important in clearance of apoptotic cells and epithelial cell repair [33]. In vitro, MCP-1 secretion is inhibited by cytokines promoting the alternative macrophage phenotype [34]. Therefore, the lack of decrease in plasma levels of MCP-1 at 24 hours in subjects with PGD may be a marker of dysregulation of the repair process, indicating either an inability to shift to the alternative or anti-inflammatory macrophage phenotype or a persistent pro-inflammatory signal inhibiting a shift to an anti-inflammatory state. Interestingly, MCP-1 levels were not associated with concurrent grade 3 PGD at 24 hours; however, MCP-1 levels were highest in those subjects who had persistent grade 3 PGD at 72 hours. This supports our hypothesis that elevations in MCP-1 levels persisting after 24 hours are a biomarker for ongoing inflammation and lack of injury resolution, leading to the association at 72 hours.
Pre-operative MCP-1 levels were elevated in IPF compared to other diagnoses, consistent with prior studies in IPF [23, 35, 36]. Experimental lung models demonstrated that MCP-1 receptor blockade decreased fibrosis and epithelial inflammation and remodeling [37]. The pro-fibrotic properties of MCP-1 may further explain the stronger association between MCP-1 and grade 3 PGD at 72 hours, indicating in those subjects, there may be prolonged postoperative inflammation leading to fibrosis.
MCP-1 is thought to play a role in allograft rejection, and is increased during acute rejection episodes of other solid organ transplants [38]. MCP-1 levels in bronchoalveolar lavage (BAL) are elevated in acute cellular rejection (ACR) and BOS [39]. Although we did not find an association between early MCP-1 levels and later outcomes; further studies should focus on longitudinal measurements of MCP-1 to clarify the role of MCP-1 in both early and late allograft dysfunction.
There are several limitations of our study. Plasma samples were not available at every time point for each subject, with 92 measurements at the 6 and 24-hour timepoints, and 63 measurements pre-operatively. Missing values at the pre-operative timepoint were secondary to logistical issues with obtaining sample prior to transplantation. At the 6 and 24-hour timepoints, missing values were secondary to failure of the assay or insufficient sample for assay. Subjects with a complete set of data did not differ from subjects with incomplete data in significant demographic characteristics, including recipient diagnosis and use of cardiopulmonary bypass; making informative censoring unlikely. We do not have plasma measurements at 72 hours after transplantation, therefore, we cannot evaluate the association of concurrent MCP-1 levels with PGD at 72 hours. Additionally, BAL samples were not available, and we were unable to test associations between the plasma and lung compartment. Our cohort is not powered to study diagnosis-specific associations in all categories of end stage lung disease; therefore we limited our disease-specific analysis to COPD, IPF, and CF. We did not account for the possibility of concomitant infection causing elevations in MCP-1, as these data were not collected [40]. However, any resulting misclassification bias would likely be non-differential and thus bias our PGD results towards the null.
In conclusion, we report that persistent elevation of the biomarker, MCP-1, at 24 hours after transplant is present in subjects with PGD. Further research may focus on validating this association in a larger, more heterogeneous population and elucidating the role of activated macrophages in the development of PGD, as well as evaluating later time points in connection with ACR and BOS. In particular, determining the predominant macrophage phenotype at the 24-hour time point in subjects with PGD may provide important pathophysiologic insights in future studies.
Supplementary Material
Supplemental Figure 1: Plasma MCP-1 concentration at the 24-hour time-point by grade of PGD at 24 hours. P value is reported for the non-parametric test for trend. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the inter-quartile range. Number of subjects in each category is listed below grade (of 92 subjects with MCP-1 level measured at 24 hours).
Supplemental Figure 2a: Association of plasma MCP-1 levels at the 6-hour timepoint with time to ACR.
Supplemental Figure 2b: Association of plasma MCP-1 levels at the 24-hour timepoint with time to ACR.
Supplemental Figure 3a: Association of plasma MCP-1 levels at the pre-operative timepoint with time to BOS.
Supplemental Figure 3b: Association of plasma MCP-1 levels at the 6-hour timepoint with time to BOS.
Supplemental Figure 3c: Association of plasma MCP-1 levels at the 24-hour timepoint with time to BOS.
Supplemental Figure 4a: Association of plasma MCP-1 levels at the pre-operative timepoint with time to death
Supplemental Figure 4b: Association of plasma MCP-1 levels at the 6-hour timepoint with time to death.
Supplemental Figure 4c: Association of plasma MCP-1 levels at the 24-hour timepoint with time to death.
ABBREVIATIONS
- PGD
Primary graft dysfunction
- CCL2
Chemokine ligand 2
- MCP-1
Monocyte Chemotactic Protein-1
- ALI
Acute lung injury
- LTOG
Lung Transplant Outcomes Group
- IPF
Idiopathic pulmonary fibrosis
- COPD
Chronic obstructive pulmonary disease
- CF
Cystic fibrosis
- ACR
Acute cellular rejection
- BOS
Bronchiolitis obliterans syndrome
- PASP
Pulmonary Arterial Systolic Pressure
- IPAH
Idiopathic Pulmonary Arterial Hypertension
- ABG
Arterial Blood Gas
- PASP
Pulmonary Arterial Systolic Pressure
Footnotes
AUTHOR CONTRIBUTIONS:
Dr. R. Shah conducted all statistical analyses and primarily wrote the manuscript. Drs. Arcasoy, Cantu, Lee, Lederer, Kohl, and Sonnett, assisted with subject recruitment and supervision of clinical assessments, sample collection and editing the manuscript. Mrs. Demissie assisted with data collection and coordination across study centers. Drs. Diamond, Kawut, and Lederer edited the manuscript. Dr. Christie supervised the data collection and the statistical analyses, assisted with interpretation of results, provided funding and edited the manuscript. Dr. Ware coordinated the performance of all plasma MCP-1 assays and edited the manuscript.
DISCLOSURES:
All authors have read the Translational Research policy on disclosure of potential conflicts of interest. Drs. R. Shah, Diamond, Cantu, Christie, Kawut, Lee, Sonnett, Ware, and Ms. Demissie have no financial or personal relationships that could impact the described research to disclose. Dr. Arcasoy has received grants from APT and Alynlam. Dr. Lederer is on the steering committee for a clinical trial sponsored by Intermune, has received institutional research funding from Boehringer-Ingelheim and Gilead, has served on an advisory board for Gilead, and as a consultant for Gilead and ImmuneWorks, has pending institutional research funding from ImmuneWorks, and has research funding from the NIH. This study was funded by the National Institutes of Health [Grants HL087115, HL0861619, HL103836 and HL088263].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340(14):1081–91. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011;30(10):1104–22. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010;29(10):1104–18. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–41. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312–6. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JC, Christie JD, Keshavjee S. Primary Graft Dysfunction: Definition, Risk Factors, Short- and Long-Term Outcomes. Semin Respir Crit Care Med. 2010;31(02):161, 171. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 7.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of Immediate Primary Lung Allograft Dysfunction on Bronchiolitis Obliterans Syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 8.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2005;24(10):1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 9.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 10.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244(2):487–93. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 12.Eppinger MJ, Deeb GM, Bolling SF, Ward PA. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol. 1997;150(5):1773–84. [PMC free article] [PubMed] [Google Scholar]
- 13.Chao J, Wood JG, Gonzalez NC. Alveolar macrophages initiate the systemic microvascular inflammatory response to alveolar hypoxia. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, et al. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J Am Soc Nephrol. 2003;14(4):1066–71. doi: 10.1097/01.asn.0000059339.14780.e4. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman SA, Wang L, Shah CV, Ahya VN, Pochettino A, Olthoff K, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9(2):389–96. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180(10):1010–5. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covarrubias M, Ware LB, Kawut SM, De Andrade J, Milstone A, Weinacker A, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7(11):2573–8. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 19.Christie JD, Van Raemdonck D, de Perrot M, Barr M, Keshavjee S, Arcasoy S, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24(10):1451–3. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29(11):1231–9. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonneau G, I, Robbins M, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, et al. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2007;176(10):1041–7. doi: 10.1164/rccm.200610-1559OC. [DOI] [PubMed] [Google Scholar]
- 23.Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J. 1999;14(2):376–82. doi: 10.1034/j.1399-3003.1999.14b23.x. [DOI] [PubMed] [Google Scholar]
- 24.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 25.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 26.Maldonado G, Greenland S. Interpreting model coefficients when the true model form is unknown. Epidemiology. 1993;4(4):310–8. doi: 10.1097/00001648-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 28.Bender R, Grouven U. Ordinal logistic regression in medical research. J R Coll Physicians Lond. 1997;31(5):546–51. [PMC free article] [PubMed] [Google Scholar]
- 29.Steyerberg EW, Eijkemans MJ, Habbema JD. Stepwise selection in small data sets: a simulation study of bias in logistic regression analysis. J Clin Epidemiol. 1999;52(10):935–42. doi: 10.1016/s0895-4356(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 30.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer DLS. Applied Logistic Regression. New York: John Wiley and Sons; 2000. [Google Scholar]
- 32.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, et al. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int. 2005;68(2):611–22. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175(1):342–9. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 35.Shinoda H, Tasaka S, Fujishima S, Yamasawa W, Miyamoto K, Nakano Y, et al. Elevated CC chemokine level in bronchoalveolar lavage fluid is predictive of a poor outcome of idiopathic pulmonary fibrosis. Respiration. 2009;78(3):285–92. doi: 10.1159/000207617. [DOI] [PubMed] [Google Scholar]
- 36.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165(3):378–81. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 37.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, et al. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004;204(5):594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 38.Grandaliano G, Gesualdo L, Ranieri E, Monno R, Stallone G, Schena FP. Monocyte chemotactic peptide-1 expression and monocyte infiltration in acute renal transplant rejection. Transplantation. 1997;63(3):414–20. doi: 10.1097/00007890-199702150-00015. [DOI] [PubMed] [Google Scholar]
- 39.Belperio JA, Keane MP, Burdick MD, Lynch JP, Xue YY, Berlin A, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. The Journal of Clinical Investigation. 2001;108(4):547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7(3):311–7. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Plasma MCP-1 concentration at the 24-hour time-point by grade of PGD at 24 hours. P value is reported for the non-parametric test for trend. Horizontal line indicates median concentration. The upper and lower limits of the box indicate the inter-quartile range. Number of subjects in each category is listed below grade (of 92 subjects with MCP-1 level measured at 24 hours).
Supplemental Figure 2a: Association of plasma MCP-1 levels at the 6-hour timepoint with time to ACR.
Supplemental Figure 2b: Association of plasma MCP-1 levels at the 24-hour timepoint with time to ACR.
Supplemental Figure 3a: Association of plasma MCP-1 levels at the pre-operative timepoint with time to BOS.
Supplemental Figure 3b: Association of plasma MCP-1 levels at the 6-hour timepoint with time to BOS.
Supplemental Figure 3c: Association of plasma MCP-1 levels at the 24-hour timepoint with time to BOS.
Supplemental Figure 4a: Association of plasma MCP-1 levels at the pre-operative timepoint with time to death
Supplemental Figure 4b: Association of plasma MCP-1 levels at the 6-hour timepoint with time to death.
Supplemental Figure 4c: Association of plasma MCP-1 levels at the 24-hour timepoint with time to death.