Abstract
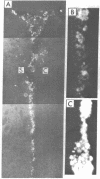
Dispersed, cultured bovine adrenal chromaffin cells transplanted into the cerebral ventricles of neonatal and adult rats survived at least 2 mo without evidence of immunological rejection. The cells can be identified by their strong yellow fluorescent reaction with glyoxylic acid, which suggests that they maintain intact the capability of synthesizing and storing catecholamines. The cells did not show sprouting or process formation and appeared to be free in the ventricle or aggregated in clusters. This shows that cells from different animal species and from different tissue origins can be transplanted and can survive in the cerebral ventricles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker C. F., Billingham R. E. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979 Nov 30;177(3):555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- Carruba M., Ceccarelli B., Clementi F., Mantegazza P. Selectivity in the reinnervation of iris and adrenal medulla by superior cervical ganglion after transplantation under the kidney capsule. Brain Res. 1974 Aug 30;77(1):39–53. doi: 10.1016/0006-8993(74)90803-8. [DOI] [PubMed] [Google Scholar]
- ERANKO O. Adrenaline and noradrenaline in adrenal autografts. Nature. 1956 Sep 15;178(4533):603–603. doi: 10.1038/178603a0. [DOI] [PubMed] [Google Scholar]
- Hervonen A., Hervonen H., Rechardt L. Axonal growth from the primitive sympathetic elements of human fetal adrenal medulla. Experientia. 1972 Feb 15;28(2):178–179. doi: 10.1007/BF01935742. [DOI] [PubMed] [Google Scholar]
- Jacobs B. B. Ovarian allograft survival. Prolongation after passage in vitro. Transplantation. 1974 Nov;18(5):454–457. doi: 10.1097/00007890-197411000-00012. [DOI] [PubMed] [Google Scholar]
- Kaplan H. J., Streilein J. W. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977 Mar;118(3):809–814. [PubMed] [Google Scholar]
- Kedinger M., Haffen K., Grenier J., Eloy R. In vitro culture reduces immunogenicity of pancreatic endocrine islets. Nature. 1977 Dec 22;270(5639):736–738. doi: 10.1038/270736a0. [DOI] [PubMed] [Google Scholar]
- Kondo H. Reinnervation of the rat adrenal medulla transplanted in the anterior eye chamber. J Anat. 1978 Oct;127(Pt 2):323–331. [PMC free article] [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- LONGMIRE W. P., Jr, SMITH S. W. Homologous transplantation of tissues. AMA Arch Surg. 1951 Mar;62(3):443–454. doi: 10.1001/archsurg.1951.01250030449013. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Davie J. M., Finke E. H. Prolongation of islet allograft survival following in vitro culture (24 degrees C) and a single injection of ALS. Science. 1979 Apr 20;204(4390):312–313. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Cooley M. A., Woolnough J., Walker K. Z. Thyroid allograft immunogenicity is reduced after a period in organ culture. Science. 1975 Apr 18;188(4185):259–261. doi: 10.1126/science.1118726. [DOI] [PubMed] [Google Scholar]
- Lueker D. C., Sharpton T. R. Survival of ovarian allografts following maintenance in organ culture. Transplantation. 1974 Nov;18(5):457–458. doi: 10.1097/00007890-197411000-00013. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hamberger B., Schultzberg M., Hökfelt T., Granberg P. O., Efendić S., Terenius L., Goldstein M., Luft R. Enkephalin- and somatostatin-like immunoreactivities in human adrenal medulla and pheochromocytoma. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4079–4083. doi: 10.1073/pnas.76.8.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERWIN R. M., HILL E. L. Fate of vascularized and nonvascularized subcutaneous homografts in mice. J Natl Cancer Inst. 1954 Feb;14(4):819–839. [PubMed] [Google Scholar]
- Olson L. Fluorescence histochemical evidence for axonal growth and secretion from transplanted adrenal medullary tissue. Histochemie. 1970;22(1):1–7. doi: 10.1007/BF00310543. [DOI] [PubMed] [Google Scholar]
- Olson L., Malmfors T. Growth characteristics of adrenergic nerves in the adult rat. Fluorescence histochemical and 3H-noradrenaline uptake studies using tissue transplantations to the anterior chamber of the eye. Acta Physiol Scand Suppl. 1970;348:1–112. [PubMed] [Google Scholar]
- Perlow M. J., Freed W. J., Hoffer B. J., Seiger A., Olson L., Wyatt R. J. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979 May 11;204(4393):643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- Pohorecky L. A., Piezzi R. S., Wurtman R. J. Steroid induction of phenylethanolamine-N-methyl transferase in adrenomedullary explants: independence of adrenal innervation. Endocrinology. 1970 Jun;86(6):1466–1468. doi: 10.1210/endo-86-6-1466. [DOI] [PubMed] [Google Scholar]
- ROGERS B. O. The problem of skin homografts; a brief review. Plast Reconstr Surg (1946) 1950 Apr;5(4):269–282. doi: 10.1097/00006534-195004000-00001. [DOI] [PubMed] [Google Scholar]
- Raju S., Grogan J. B. Allograft implants in the anterior chamber of the eye of the rabbit. Early vascularization and sensitization of the host. Transplantation. 1969 Jun;7(6):475–483. doi: 10.1097/00007890-196906000-00004. [DOI] [PubMed] [Google Scholar]
- Raju S., Grogan J. B. Immunologic study of the brain as a privileged site. Transplant Proc. 1977 Mar;9(1):1187–1191. [PubMed] [Google Scholar]
- Raju S., Grogan J. B. Immunology of anterior chamber of the eye. Transplant Proc. 1971 Mar;3(1):605–608. [PubMed] [Google Scholar]
- Rao D. S., Grogan J. B. Orthotopic skin graft survival in rats that have harbored skin implants in the anterior chamber of the eye. Transplantation. 1977 Nov;24(5):377–381. doi: 10.1097/00007890-197711000-00010. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Lundberg J. M., Terenius L., Elfvin L. G., Elde R. Enkephalin-like immunoreactivity in nerve terminals in sympathetic ganglia and adrenal medulla and in adrenal medullary gland cells. Acta Physiol Scand. 1978 Aug;103(4):475–477. doi: 10.1111/j.1748-1716.1978.tb06243.x. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Sollinger H. W., Burkholder P. M., Rasmus W. R., Bach F. H. Prolonged survival of xenografts after organ culture. Surgery. 1977 Jan;81(1):74–79. [PubMed] [Google Scholar]
- Subba Rao D. S., Grogan J. B. Host response to tissues placed in the anterior chamber of the eye: demonstration of migration inhibition factor and serum blocking activity. Cell Immunol. 1977 Sep;33(1):125–133. doi: 10.1016/0008-8749(77)90140-x. [DOI] [PubMed] [Google Scholar]
- Svendgaard N. A., Björklund A., Hardebo J. E., Stenevi U. Axonal degeneration associated with a defective blood-brain barrier in cerebral implants. Nature. 1975 May 22;255(5506):334–336. doi: 10.1038/255334a0. [DOI] [PubMed] [Google Scholar]
- Tator C. H. Chemotherapy of brain tumors. Uptake of tritiated methotrexate by a transplantable intracerebral ependymoblastoma in mice. J Neurosurg. 1972 Jul;37(1):1–8. doi: 10.3171/jns.1972.37.1.0001. [DOI] [PubMed] [Google Scholar]
- Torre J. C., Surgeon J. W. A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: the SPG method. Histochemistry. 1976 Oct 22;49(2):81–93. doi: 10.1007/BF00495672. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Krisch B., Otten U., Thoenen H. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker K., Tschechne B., Tschechne D. Formation of cholinergic synapses on adrenal chromaffin cells in anterior eye chamber transplants. Brain Res. 1978 Aug 25;152(2):334–340. doi: 10.1016/0006-8993(78)90260-3. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Zwarg U., Habura O. Electron microscopic evidence for the formation of synapses and synaptoid contacts in adrenal medullary grafts. Brain Res. 1977 Jan 28;120(3):533–539. doi: 10.1016/0006-8993(77)90406-1. [DOI] [PubMed] [Google Scholar]
- Waggener J. D., Beggs J. L. Vasculature of Neural Neoplasms. Adv Neurol. 1976;15:27–49. [PubMed] [Google Scholar]
- Zalewski A. A., Goshgarian H. G., Silvers W. K. The fate of neurons and neurilemmal cells in allografts of ganglia in the spinal cord of normal and immunologically tolerant rats. Exp Neurol. 1978 Apr;59(2):322–330. doi: 10.1016/0014-4886(78)90160-7. [DOI] [PubMed] [Google Scholar]