Abstract
It has been known for nearly a half century that human tumors, including those derived from the nervous system such as glioblastomas, medulloblastoma, and neuroblastomas are much more sensitive than normal tissues to l-Met starvation. More recently, systemic l-Met depletion by administration of Pseudomonas putida methionine-γ-lyase (MGL) could effectively inhibit human tumors xenografted in mice. However, bacterial-derived MGLs are unstable in serum (t1/2 =1.9 ±0.2 hr) and highly immunogenic in primates. Since the human genome does not encode a human MGL enzyme, we created de novo a methionine degrading enzyme by reengineering the structurally homologous pyridoxal phosphate-dependent human enzyme cystathionine-γ-lyase (hCGL). hCGL degrades l-cystathionine but displays no promiscuous activity towards l-Met. Rational design and scanning saturation mutagenesis led to the generation of a variant containing three amino acid substitutions (hCGL-NLV) that degraded L-Met with a kcat/KM of 5.6×102 M−1s−1 and displayed a serum deactivation t1/2 =78 ± 5 hr (non-PEGylated). In vitro, the cytotoxicity of hCGL-NLV towards 14 neuroblastoma cell lines was essentially indistinguishable from that of the P. putida MGL. Intravenous administration of PEGylated hCGL-NLV in mice reduced serum l-Met from 123 μM to <5 μM for over 30 hours. Importantly, treatment of neuroblastoma mouse xenografts with PEGylated hCGL-NLV resulted in near complete cessation of tumor growth. Since the mode of action of hCGL-NLV does not require breaching the blood-brain barrier this enzyme may have potential application for sensitive tumors that arise from or metastasize to the central nervous system.
INTRODUCTION
L-methionine (L-Met) depletion has long been studied as a potential treatment for cancer, as many malignant human cell lines and tumors have a substantially higher requirement for l-Met than normal cells and tissues(1-5). Depletion of any essential amino will disrupt protein synthesis, but l-Met is also required for polyamine synthesis a known contributor to tumorigenesis (6-8); furthermore it is the major methylation source for DNA and other molecules (9). l-methionine-dependent tumor cell lines display an abnormally high demand for that amino acid and additionally, can show defects in the expression of methionine synthase that recycles L-homocysteine to l-Met and of methylthioadenosine phosphorylase (MTAP) which cleaves methylthioadenosine to 5-methylthioribose-1-phosphate that is further metabolized to l-Met (5, 10-17). Most non-malignant cells can grow on homocysteine/homocystine, whereas malignant cells must scavenge l-Met directly from their extracellular environment. When l-Met levels decrease from the normal human serum concentration of ~ 30 μM (18) to a threshold around ~ 5 μM these tumors are unable to survive (19). In vivo, a significant decrease in serum l-Met can be achieved by the systemic application of methionine-γ-lyase from Pseudomonas putida (pMGL) that degrades l-Met to α-ketobutyrate, methane thiol, and NH3 resulting in drastic retardation of tumor growth in a variety of animal models and has been shown to have synergistic effects in combination with chemotherapeutic agents such as 5-fluorouracil and vincristine (20-27). However, pMGL is rapidly inactivated in vitro (This work) and in vivo (28, 29) and has proven to be highly immunogenic in primate models (20).
To circumvent the significant limitations of bacterial MGLs for human therapy, we developed a human methionine-γ-lyase by protein engineering. Cystathionine-γ-lyase (CGL) is the last enzyme of the mammalian transsulfuration pathway for the conversion of l-methionine to l-cysteine (30). The human CGL (hCGL) displays 61% amino acid similarity with the P. putida MGL and 62 % similarity to the MGL from Trichomonas. These enzymes are structural homologues (Figure 1a, structural alignment of hCGL and MGL from Trichomonas) and belong to the γ-family of pyridoxal phosphate (PLP) dependent enzymes. Although MGL and CGL are related in their reaction chemistry (Figure 1b), CGL catalyzes the α,γ-elimination of l-cystathionine to l-cysteine, α-ketobutyrate, and NH3 but shows no detectable catalytic activity with l-Met. Using rational design and scanning saturation mutagenesis, coupled with a novel high throughput screen for methionine-γ-lyase activity, we engineered an hCGL variant containing 3 amino acid substitutions (hCGL-NLV) that displayed therapeutically significant rates of l-Met degradation. Biochemical analysis suggested possible mechanisms for the change in the selectivity of the enzyme from, l-cystathionine to l-Met. hCGL-NLV was shown to be much more stable to deactivation in serum relative to the P. putida MGL and exhibited favorable in vitro cytotoxicity towards a large panel of human neuroblastoma cell lines. We show that, administration of PEGylated hCGL-NLV into mouse xenografts bearing neuroblastoma tumors resulted in near complete cessation of tumor growth indicating that the engineered enzyme holds promise for cancer therapy.
Figure 1.
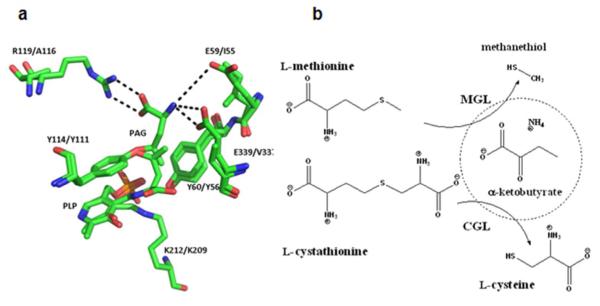
(a) Structural overlay of human CGL and MGL from Trichomonas vaginalis (PDB 3COG:1E5E) with the inhibitor propargylglycine (PAG) bound in two different orientations. (b) Reactions catalyzed by CGL and MGL.
Among enzyme therapies in cancer, asparaginase is one of oldest and most successful. Its inclusion has led to a >90% cure rate for standard risk Childhood ALL (31, 32). Currently, only bacterial enzymes derived from E. coli and Erwinia chrysanthemi (synonymous with Erwinia carotovora) are available. Pharmacokinetic studies have shown that asparaginase activity ≤ 0.4 U/mL provided insufficient deamination of ASN, whereas >0.4-0.7 U/mL was required for optimal (90%) ASN and glutamine deamination (33), both important predictors of improved survival. Since bacterial asparaginase is highly immunogenic, antibody response in patients inactivates the enzyme, shortens its half life and induces anaphylaxis. Despite clinical success, asparaginase therapy remains limited by its immunogenicity (34).
As hCGL-NLV is an engineered human protein, it is expected to be subject to immune tolerance, and moreover, computational predictions indicate that the 3 amino acid substitutions are unlikely to generate a T cell neo-epitope. While, as with any protein therapeutic candidate, it is impossible to rule out the possibility that hCGL-NLV could elicit antibody responses in some patients, the extensive clinical data with approved engineered antibodies (35, 36) supports the notion that the introduction of a small, carefully selected number of amino acid substitutions into engineered human proteins does not generally result in an adverse immunogenicity profile.
RESULTS AND DISCUSSION
(See Supporting Information for additional Results of: Construction of Synthetic Genes, Protein Expression and Purification, Detection limits of DTNB assay.)
In addition to their native substrates, both hCGL and pMGL exhibit appreciable activity towards l-cysteine and dl-homocysteine (Table 1). However, l-Met is not a substrate of hCGL and likewise, pMGL has no activity with l-cystathionine as substrate, within the detection limit of our assay (see supporting results) (Table 1). Thus, hCGL does not exhibit promiscuous activity towards substrates with a methylthioethane side chain (37). Structural overlays of hCGL and an MGL from Trichomonas revealed that in hCGL the side-chains of residues E59, R119, and E339 are important for the orientation of the cysteine terminus of its substrate, l-cystathionine, while in MGL the corresponding positions are occupied by amino acids with hydrophobic side chains, namely I55, A116, and V337 (Figure 1a). Positions 59, 119 and 339 in hCGL were subjected to combinatorial pairwise saturation mutagenesis and the resulting clones were screened for methionine-γ-lyase activity using a novel high throughput plate assay that relies on the colorimetric detection of the α-ketobutyrate product of the reaction using 3-methylbenzothiazolin-2-one hydrazone (MBTH)(38). Clones expressing mutagenized hCGL were grown in microtiter well plates to mid-log phase, the cells were lysed chemically, incubated with 5 mM l-Met at pH 7.3 and 37 °C followed by MBTH and the Abs320 was determined spectrophotometrically. Screening of >2,000 colonies (2x theoretical diversity) from a saturation library (library A) at positions 119 and 339 gave 14 unique clones (Supporting Table S1, Library A) capable of producing α-ketobutyrate from l-Met. The active clones encoded hCGL with Pro or Val substitutions at pos. 339 with the latter resulting in higher product formation. The clone with the highest activity, hCGL(R119L, E339V), was used as a template to randomize the E59/R119L codons. Screening of an additional 2,000 clones led to the isolation of 31 clones exhibiting greater α-ketobutyrate formation than the hCGL(R119L, E339V) (Table S2, Library B). The respective enzymes were produced and purified by IMAC in small scale and then rank-ordered with respect to their relative 2nd order rate constants (apparent kcat/KM when [S] is << KM) in a reaction with 0.1 mM l-Met and continuous monitoring of methane thiol formation with 5,5′-dithiobis-2-nitrobenzoic acid (DTNB, Table S3). The highest apparent kcat/KM was obtained with hCGL (E59N, R119L, E339V) designated hCGL-NLV. Steady state Michaelis-Menten parameters for pMGL, hCGL, and hCGL-NLV with l-Met, l-cystathionine, l-cysteine or dl-homocysteine were determined in 100 mM phosphate buffer, pH 7.3 at 37 °C (Table 1). hCGL-NLV displayed significant methionine-γ-lyase activity as well as increased activity towards l-Cys and dl-homocysteine. However, this enzyme had approximately 15-fold lower kcat/KM for l-cystathionine, the physiological substrate of hCGL, suggesting that its methionine-γ-lyase activity is not simply a consequence of broader substrate specificity, a rather common outcome in enzyme engineering. For comparison, the kinetics of hCGL-E339V, hCGL-E339P, hCGL-R119Q-E339V, hCGL-E339V-R119L and hCGL-E59A-R119S-E339V (hCGL-ASV) for the degradation of l-Met and l-cystathionine were also determined (Supporting Table S4). Consistent with the results of the screening assay, hCGL-NLV displayed better kinetics than either the single or the double mutant enzymes above.
Table 1.
| Michaelis-Menten Kinetics |
P. putida methionine-γ-lyase | ||
|---|---|---|---|
|
| |||
| Substrate | kcat ( s−1) | KM (mM) | kcat/KM (s−1 mM−1) |
| l-methionine | 20 ± 0.4 | 0.34 ± 0.03 | 59 ± 6 |
| l-cystathionine | nd | nd | nd |
| l-cysteine | 6.5 ± 0.2 | 1.1 ± 0.1 | 6 ± 0.7 |
| dl-homocysteine | 86 ± 3 | 2.9 ± 0.2 | 30 ± 3 |
|
| |||
| Substrate | Human cystathionine-γ-lyase | ||
|
| |||
| l-methionine | nd | nd | nd |
| l-cystathionine | 3.7 ± 0.2 | 0.40 ± 0.07 | 9 ± 2 |
| l-cysteine | 0.15 ± 0.02 | 0.79 ± 0.30 | 0.2 ± 0.1 |
| dl-homocysteine | 0.35 ± 0.02 | 1.7 ± 0.30 | 0.21 ± 0.04 |
|
| |||
| Substrate | Human cystathionine-γ-lyase-NLV | ||
|
| |||
| l-methionine | 7.9 ± 0.4 (≥106 *) |
14 ± 1.5 | 0.56 ± 0.07 |
| l-cystathionine | 0.54 ± 0.02 | 0.83 ± 0.1 | 0.65 ± 0.07 (-14) |
| l-cysteine | 2.7 ± 0.1 | 0.84 ± 0.1 | 3.2 ± 0.3 (16) |
| dl-homocysteine | 2.6 ± 0.1 | 0.9 ± 0.1 | 2.8 ± 0.3 (13) |
nd = not detected, ( ) = fold change from hCGL.
estimated from DTNB assay limits. All reactions were performed at 37 °C with the MBTH assay.
Of importance to therapeutic applications, (non-PEGylated) hCGL-NLV displayed substantially greater stability in pooled human serum at 37 °C relative to the P. putida MGL with t1/2 deactivation of 78 ± 5 hr in contrast to 1.9 ± 0.2 hr for the bacterial enzyme (Supporting Figure S1). Although hCGL-NLV has activity in vitro with other plasma metabolites (eg l-cystathionine, l-Cys, and l-homocysteine) it is not expected that this will greatly affect their homeostasis in vivo. Plasma l-Cystathionine is excreted in the urine at a rate of 38.5 μM/day and maintained in the serum at concentration of 0.24 ± 0.06 μM (39) at which concentrations hCGL-NLV would have the negligible turnover rate of 1.5 × 10−4 s−1 on its degradation. In humans plasma l-Cys, and l-homocysteine exist either as the free amino acids, the (mixed) disulfide form, or bound to serum proteins at concentrations around 170 μM (54% free) and 6 μM (31% free) respectively (40). Consistent with the existence of a protected fraction of l-Cys and l-Hcys, mice injected with pMGL were shown to have a 90 % reduction in l-Met, but only a 46 % reduction in l-Hcys and no effect on l-Cys levels(41). hCGL-NLV is 2–and 10-fold less active towards l-Cys and l-homocysteine respectively relative to pMGL and therefore its effect on these metabolites would be expected to be significantly lower.
L-Met Inhibition of hCGL
It was surprising that we could not detect any l-Met hydrolysis with hCGL at any concentration despite its smaller size compared to l-cystathionine. dl-homocysteine is also smaller than l-cystathionine yet it is degraded by hCGL, though with a 40-fold lower kcat/KM. We examined the hypothesis that dl-homocysteine is a substrate of hCGL whereas l-Met is not due to leaving group effects. Degradation of homocysteine leads to H2S (pKa = 7)(42) whereas l-Met degradation leads to methanethiol (pKa = 10.3 (42)). The possibility that l-Met binds to the active site but the γ-lyase activity is disfavored by the high pKa of methanethiol could be ruled out since we found that even 20 mM l-Met did not inhibit l-cystathionine degradation by hCGL. This observation raised the possibility that the relatively hydrophobic l-Met molecule is unable to partition into the hydrophilic active site of hCGL.
Hydropathy Studies
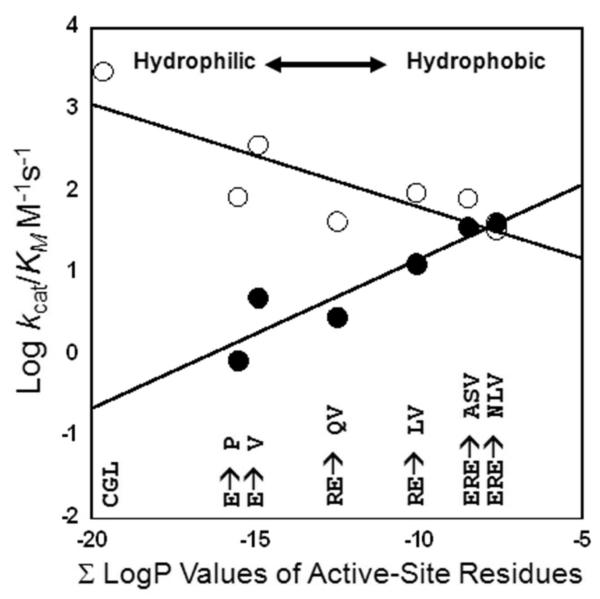
The LogP values of the active-site residues unique to hCGL were summed (∑ LogP) to yield a value = -19.7 (hydrophilic) while the ∑ LogP value of the pMGL unique active-site is -2.87 (hydrophobic). These values correlate well with the LogP values of their respective substrates (L-cystathionine = -5.82, l-Met = -2.19). Consistent with this hypothesis, comparison of the active-site hydropathy values (∑ LogP) of hCGL, hCGL-E339V, hCGL-E339P, hCGL-E339Q-R119L, hCGL-E339V-R119L, hCGL-ASV and hCGL-NLV with the Log kcat/KM values (log10 value of kcat divided by KM values found from fits to the Michaelis-Menten equation) for l-Met and l-cystathionine hydrolysis revealed a positive linear relationship for the turnover of l-Met and a negative linear relationship for the turnover of l-cystathionine (Figure 2). This appears to hold true for dl-homocysteine and l-Cys as well which have intermediate hydropathy values, LogP = -2.58, and -2.79 respectively. dl-homocysteine and l-Cys are substrates that can partition into the hCGL active-site and hCGL-NLV with a more hydrophobic active site (∑ LogP = -7.64) increases the kcat/KM for these substrates 13 and 6-fold respectively. It should be noted that in previous studies , when E339 of hCGL was mutated to either Lys, Ala or Tyr the kcat/KM for l-Cys increased by 2-, 3-, and 7-fold respectively, results that are also consistent with the effect of active site hydrophobicity as a key determinant of CGL substrate specificity (43).
Figure 2.
Plot of the sum of the calculated LogP values of residues in the active sites of hCGL, hCGL-E339P, hCGL-E339V, hCGL-R119L-E339Q, hCGL-R119L-E339V, hCGL-ASV and hCGL-NLV versus their Log kcat/KM values for the hydrolysis of l-Met (•) and l-Cystathionine (○).
Immunogenicity Assessment
To assess the likelihood that the three point mutations (E59N-R119L-E339V) in hCGL could generate a novel T cell epitope (i.e. generate a peptide fragment with affinity to MHC II) we compared the computationally predicted MHC(II) binding scores (consensus method) for peptides containing each mutated residue relative to the corresponding wt sequence for 8 of the most common HLA alleles that collectively cover nearly 95% of the human population (44) The consensus percentile rank (CPR) score ratios indicated no significant change in predicted MHCII binding suggesting that the E59N-R119L-E339V mutations are unlikely to introduce neo-epitopes (Table S5).
Pharmacological Optimization, in vitro Cytotoxicity, and in vivo Half Life of PEG-hCGL-NLV
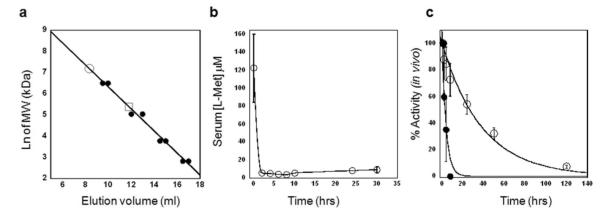
To investigate the therapeutic efficacy of hCGL-NLV in vivo, the enzyme was PEGylated by conjugation to methoxy PEG succinimidyl carboxymethyl ester, MW 5,000 Da at an 80:1molar ratio (80 PEGs per subunit). Initial tests using PEG:enzyme ratios of 10:1, 20:1, 40:1, and 80:1 showed the greatest homogeneity at an 80:1 ratio (Figure S2). PEGylation has been extensively exploited to increase the hydrodynamic radius of proteins, preventing renal filtration and in turn markedly increasing circulation persistence; note that PEGylation significantly increased the circulation t1/2 of the structurally homologous pMGL in mice and primates (20). To prevent inactivation during PEGylation it was essential to pre-incubate the enzyme with 10 mM pyridoxal phosphate (PLP), to avoid conjugation of K212 to the PEG succinimidyl ester. Analytical size exclusion chromatography of the resulting PEG-hCGL-NLV gave a single homogeneous peak with an apparent MW of 1,300 KDa per tetramer, a 6-fold increase from unmodified tetramer (Figure 3a). PEG-hCGL-NLV displayed l-Met degradation kinetics identical to those of the unmodified hCGL-NLV with kcat = 7.8 ± 0.4 s−1, KM = 13.5 ± 1.5 mM and a t1/2 = 39 ± 2 hrs in pooled human serum (Figure S3). The specific activity of PEG-hCGL-NLV was 10 U/mg protein at 37 °C and pH 7.3 and had an endotoxin level of ≤ 12.5 EU/mg as assessed by Limulus Amebocyte Lysate assay (The endotoxin received by the mice was >> 2 orders of magnitude fold lower than doses showing no observed effect in mice (45, 46)). The cytotoxicity of PEG-hCGL-NLV towards14 neuroblastoma (NB) lines established to be l-Met dependent (25) was compared to that of pMGL (The myelocytomatosis viral related oncogene, neuroblastoma derived (MYCN) amplification(47) status, anaplastic lymphoma kinase (ALK) mutation(48) status, and α-thalassemia/mental-retardation-syndrome-X-linked (ATRX) mutation(49) status and IC50 values are summarized in Table S6). While PEG-hCGL-NLV has a lower kcat/KM than pMGL for l-Met, its much higher stability in serum and in growth media led to nearly identical IC50 values with an average of 0.091 ± 0.043 U and 0.086 ± 0.037 U, respectively. In Balb/c mice (n=5) maintained on a l-Met(-) Homocysteine(-)Choline(-) {Met(-)Hcyss(-)Chl(-)} diet, tail vein administration of 200 U PEG-hCGL-NLV led to a decrease in the serum l-Met concentration from 124 ± 37 μM (pretreatment) to 3.9 ± 0.7 μM at 8 hr post-injection. Maintaining the animals on a Met(-)Hcyss(-)Chl(-) diet during treatment is necessary due to the high metabolic rate of mice which leads to rapid replenishment of the serum l-Met pool following depletion(19, 25). (It is not yet known if l-Met depletion therapy in humans will necessitate that the patients are placed on L-Met-deficient diet. The serum L-Met replenishment rate in man is estimated to be roughly 2 μM/hr (est. from Ref (24)) compared to ~ 4 μM/hr in mice (est. from Ref (50)) . An l-Met deficient diet would likely augment a treatment regimen but may not be necessary with an enzyme displaying a good PK/PD profile). In mice treated with PEG-hCGL-NLV, the serum l-Met was maintained at a therapeutically relevant level for at least 30 hr (Figure 3b). This is on par with various formulations of PEGylated-pMGL which have been reported to maintain serum l-Met levels in mice at <5 μM for 8 hrs in one study (50) to 48 hrs in an earlier report (28). The PEG-hCGL-NLV activity in the blood of the injected mice decreased with a t1/2 of 30 ± 3 hr (Figure 3c), much longer than non-PEGylated hCGL-NLV (t1/2 = 1.8 ± 0.7 hr).
Figure 3.
(a) Analytical size exclusion chromatography of MW standards (•), hCGL-NLV (□) and PEGylated hCGL-NLV (○). (b) Pharmacodynamics of PEG-hCGL-NLV in balb/c mice maintained on a Met(-)Hcyss(-)Chl(-) diet. Mice (n=5) were treated with 200 U PEG-hCGL-NLV administered by tail-vein injection and blood methionine level (○) was monitored by HPLC. (c) In vivo activity of PEG-hCGL-NLV (○) and unPEGylated hCGL-NLV (•) in serum as a function of time following tail-vein injection of 100U PEG-hCGL-NLV in athymic mice (n=5).
Therapy of established NB Xenografts
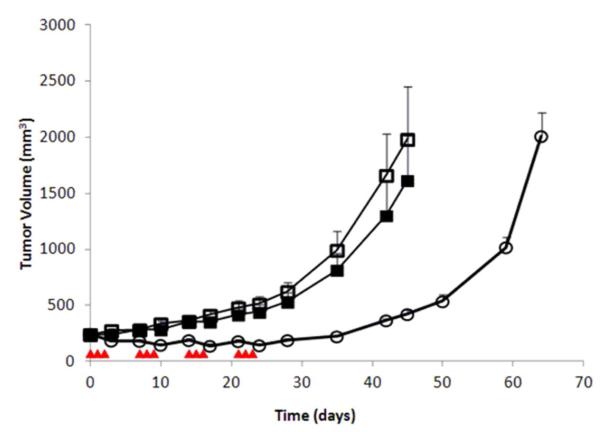
Athymic mice bearing LAN-1 neuroblastoma tumor xenografts were treated with 100 Units PEG-hCGL-NLV, administered via tail vein injection once daily for 3 times a week for 4 weeks. Met(-)Hcyss(-)Chl(-) diet was fed to the animals on the days of PEG-hCGL-NLV administration. Xenografted control mice were either fed a constant normal diet or a Met(-)Hcyss(-)Chl(-) diet 3 times a week for 4 weeks using an identical schedule as in the treatment group. 24 hr after the third PEG-hCGL-NLV injection during the last (4th) week of treatment, plasma methionine concentration was 6 ± 2 μM among PEG-hCGL-NLV-treated mice fed Met(-)Hcyss(-)Chl(-) diet, in contrast to 124 ± 37 μM before treatment (n=10). Mice fed Met(-)Hcyss(-)Chl(-) diet exhibited a 10 - 15% reversible weight loss (Figure S4). While control mice on Met(-)Hcyss(-)Chl(-) diet only had no significant reduction in tumor growth, the administration of PEG-hCGL-NLV completely inhibited the growth of the LAN-1 tumors during the treatment period (p<0.01, Figure 4). Even three weeks after the last round of treatment with PEG-hCGL-NLV, rebound tumor volume was small (0.5 fold increase), whereas in the control groups tumor growth increased 3- and 4-fold in mice maintained on Met(-)Hcyss(-)Chl(-) or on normal diet, respectively.
Figure 4.
Evaluation of PEG-hCGL-NLV in athymic mice bearing LAN-1 xenografts. (□) Control animal group maintained on normal diet (N=10); (■) Animals maintained on Met(-)Hcyss(-)Chl(-) diet (N=10); (○) animals treated with 100 U PEG-hCGL-NLV in combination with Met(-)Hcyss(-)Chl(-) mouse feed (N=10). Treatment days are designated by ( ). Tumor growth rate was expressed as mean ± SEM (standard error of the mean) for each group. * p<0.01 for the PEG-hCGL-NLV treated group relative to the two untreated groups.
). Tumor growth rate was expressed as mean ± SEM (standard error of the mean) for each group. * p<0.01 for the PEG-hCGL-NLV treated group relative to the two untreated groups.
SUMMARY
The differential susceptibility of malignant and non-malignant cells to l-Met starvation provides a useful therapeutic window for cancer therapy. Even partial depletion solely through l-Met omission in a parenteral nutrition (TPN) regimen has been shown to result in tumor reductions in gastric cancer in both the rat model and in humans (51-53). However, dietary restriction alone only results in ~ 40 % decrease in serum l-Met levels (54). A much more significant reduction in the serum l-Met concentration, resulting in drastic inhibition of tumor growth in a number of animal models (27, 41, 55, 56), has been achieved by the administration of MGLs. Humans do not encode an MGL and consequently, bacterial enzymes have been investigated as agents for l-Met depletion therapy. In particular, the P. putida MGL was found to display the most favorable catalytic properties, having a low KM and a relatively high kcat (57, 58). Administration of pMGL was shown to drastically affect the growth of human neuroblastoma, lung, colon, and brain tumors in mouse xenografts maintained on L-Met restricted diet (21, 22, 25, 59). Unfortunately, both in mice and in the non-human primate model, pMGL was shown to be deactivated very rapidly with a half-life of approximately 2 hr due to the loss of the PLP cofactor(26). Nonetheless, pMGL given at a 90 mg/kg dose every 8 hr for 2 weeks resulted in a steady-state depletion of plasma methionine to less than 2 μM in animals maintained on l-Met restricted diet. Only mild toxicities were observed, manifested as decreased appetite and slight weight loss. However, anti-MGL antibodies were detected after administration and an additional round of treatment with pMGL resulted in anaphylactic shock and death in some animals(26). Yang et al. sought to increase the pharmacokinetic half-life and reduce the immunogenicity of MGL by amine conjugation to polyethylene glycol (PEGylation). PEGylation of pMGL drastically improved circulatory persistence (≥ 36 fold) in primates and attenuated severe immunological responses (with anti-MGL antibody titers increasing upon repeated treatments), however only a marginal improvement in pharmacodynamics was observed (serum l-Met was maintained at <5 μM for 12 hrs vs 8 hrs for the unPEGylated enzyme) (20, 29). PEGylation of bacterial and even of mammalian proteins appears to delay but not eliminate immune responses as evident in clinical studies of PEGylated M. arginii arginine deiminase (60) or pegloticase (61).
To augment the anti-neogenic potential of systemic l-Met depletion and overcome the disadvantages observed with pMGL we engineered hCGL into a pharmacologically efficacious human methionine γ-lyase. Although the overall structures of hCGL and pMGL align with a RMS deviation of 0.8 Å they only share a 61 % aa sequence similarity. Many of the differences are seen in the degree of hydrophobicity in the active sites which correlates with the relative hydrophobicities of their substrates. The hCGL-NLV is engineered from a native human enzyme and thus it is likely to be much less immunogenic than pMGL circumventing problems with adverse immune responses, including neutralizing antibodies and anaphylactic reactions observed in earlier studies. l-Met depletion may be particularly attractive for the treatment of brain tumors, a hypothesis we plan to test in future studies using orthotopic glioblastoma mouse models (62). The paucity of chemotherapeutics that can target tumors from across the blood-brain barrier makes systemic l-Met depletion an appealing strategy for the treatment of tumors that arise from or metastasize to the central nervous system, both significant challenges in cancer treatment.
MATERIALS AND METHODS
(See Supporting Information for additional Materials and Methods of: Construction of Synthetic Genes; Saturation Mutagenesis Libraries; Construction of hCGL-E339V variant; Protein Expression and Purification; Kinetic Assays; 96-well plate screen; L-Met Inhibition of hCGL; Immunogenicity Calculations; WST8 Assay for NB Cell Proliferation; in vitro half-lives; Serum Stability; Pharmacological Optimization of hCGL Variants; Methionine Depletion in mouse plasma; Mouse Feed.)
Hydropathy Calculations
Using structural alignments of hCGL (3COG) and pMGL (2O7C) as a guide we identified residues in the active site within 5-6 Å of a covalently bound inactivator, propargylglycine. We found that F58, L62, C116, A119, and V339 were unique to the pMGL active-site whereas in hCGL these residues correspond to E59, S63, G116, R119, and E339 respectively. By convention, the relative hydropathy of a solute is described by the logarithm of its octanol water partition coefficient (LogP). Using ChemAxon software we calculated the LogP values of each of the aforementioned residues with their α amino and carboxy termini in the amide form (63). (We used this method because the LogP values are in good agreement with available experimental values and allowed us to estimate LogP values for compounds without known reference values like L-cystathionine) We also calculated the active site LogP values for three hCGL variants, hCGL-E339V, hCGL-R119L -E339V, and hCGL-E59N-R119L-E339V - (hCGL-NLV). These LogP values were summed for each enzyme to calculate an overall hydropathy value for each active site and plotted against the Log kcat/KM values for each enzymes ability to hydrolyze L-Met and L-cystathionine (This is similar to an analysis of lipase performed by Hirata et.al. (64)). We also calculated the LogP values for the substrates L-Met, l-homocysteine, L-Cys, and L-cystathionine.
Tumor Cell Lines
NB cell line LAN-1 was obtained from Dr. Robert Seeger (Children’s Hospital of Los Angeles; Los Angeles, CA), NB cell line NMB-7 from Dr. Liao of McMaster University (Hamilton, ON, Canada). SK-N-BE(1)N, SK-N-BE(2)C, SK-N-BE(2)N, SK-N-BE(2)S, LAI-5S and SH-EP-1, 55N and 66N were kindly provided by Dr. Robert Ross, Fordham University (New York, NY). NB cell lines SK-N-LD and SK-N-MM were established at Memorial Sloan-Kettering Cancer Center (MSKCC, New York, NY). IMR-32 and CHP-212 were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI with 10% fetal calf serum (F10) as previously described (65).
In vivo half-lives
hCGL-NLV and PEG-hCGL-NLV activity was assayed on sera following injection of 50 U of enzyme in nude mice. Serum samples were obtained at 1, 2, 4, 8, 24, 50, and 120 hours after injection. Half lives of enzymatic activity was calculated by SigmaPlot using the exponential decay equation (y = ae-bx).
Therapy of established NB Xenografts
All animal experiments were carried out according to an Institutional Animal Care and Use Committee (IACUC) approved protocol at Memorial Sloan-Kettering Cancer Center, following institutional guidelines for the proper and humane use of animals in research. Athymic female nude mice were purchased from the National Cancer Institute. Mice with 5-10 mm established tumors were randomly separated into groups of 10 mice each. In mice xenografted with LAN-1 neuroblastoma, 100 Units of PEG-hCGL-NLV was given iv three times a week for 4 weeks. 0.5 cm -1 cm tumors are generally regarded as standard for tumor therapy studies since Institutional Animal Care and Use Committee (IACUC) requirement stipulates that mice with tumor that is >15% body weight (or 2 cm maximal tumor diameter) causes undue distress and animals have to be sacrificed. Tumor volume was calculated as (a2b)/2 where a is the width of the tumor (small diameter), and b the length (large diameter), both in millimeters (66). All of the tumors were growing at the time when the treatment was started and all of the mice finally died of tumor when the treatment was stopped. There were no spontaneous regressions in this experiment and in this tumor model. Met(-)Hcyss(-)Chl(-) diet was fed to the animals on the days of PEG-hCGL-NLV administration. Mice were bled 24 hr after the third PEG-hCGL-NLV injection during the last week of treatment. The weight of mice was measured twice weekly and normalized to the weight at day 0 (start of experiment) and used as an index of toxicity. A 20% maximum weight loss was used as a guideline in designing PEG-hCGL-NLV dosing, and none of the mice required sacrificing because of toxicity. Tumor size was measured twice per week and expressed as mean ± SEM (standard error of the mean) for each group. Mice were sacrificed when their tumor sizes exceeded 20 mm in diameter.
Supplementary Material
ACKNOWLEDGEMENTS
Funding: This work was supported by grants 1 R01 CA154754 and R01 CA139059 from NIH and by the Cancer Prevention and Research Initiative of Texas (CPRIT) grant RP100890. We would also like to thank S. Cramer for her help with kinetics experiments.
Footnotes
Conflict of interest statement:
Supporting Information This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Kreis W, Goodenow M. Methionine requirement and replacement by homocysteine in tissue cultures of selected rodent and human malignant and normal cells. Cancer research. 1978;38:2259. [PubMed] [Google Scholar]
- 2.Breillout F, Antoine E, Poupon MF. Methionine dependency of malignant tumors: a possible approach for therapy. © Oxford University Press; 1990. pp. 1628–1632. [DOI] [PubMed] [Google Scholar]
- 3.Kreis W, Baker A, Ryan V, Bertasso A. Effect of nutritional and enzymatic methionine deprivation upon human normal and malignant cells in tissue culture. Cancer research. 1980;40:634. [PubMed] [Google Scholar]
- 4.Kreis W. Tumor therapy by deprivation of L-methionine: rationale and results. Cancer treatment reports. 1979;63:1069. [PubMed] [Google Scholar]
- 5.Halpern BC, Clark BR, Hardy DN, Halpern RM, Smith RA. The effect of replacement of methionine by homocystine on survival of malignant and normal adult mammalian cells in culture. Proceedings of the National Academy of Sciences. 1974;71:1133. doi: 10.1073/pnas.71.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas T, Thomas TJ. Polyamine metabolism and cancer. Journal of Cellular and Molecular Medicine. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Megosh L, Gilmour SK, Rosson D, Soler AP, Blessing M, Sawicki JA, O’Brien TG. Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer research. 1995;55:4205–4209. [PubMed] [Google Scholar]
- 8.Auvinen M, Paasinen A, Andersson LC, Hölttä E. Ornithine decarboxylase activity is critical for cell transformation. 1992. [DOI] [PubMed]
- 9.Lu S, Epner DE. Molecular mechanisms of cell cycle block by methionine restriction in human prostate cancer cells. Nutrition and cancer. 2000;38:123–130. doi: 10.1207/S15327914NC381_17. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman RM, Jacobsen SJ. Reversible growth arrest in simian virus 40-transformed human fibroblasts. Proceedings of the National Academy of Sciences. 1980;77:7306. doi: 10.1073/pnas.77.12.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman RM, Jacobsen SJ, Erbe RW. Reversion to methionine independence in simian virus 40-transformed human and malignant rat fibroblasts is associated with altered ploidy and altered properties of transformation. Proceedings of the National Academy of Sciences. 1979;76:1313. doi: 10.1073/pnas.76.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman RM, Erbe RW. High in vivo rates of methionine biosynthesis in transformed human and malignant rat cells auxotrophic for methionine. Proceedings of the National Academy of Sciences. 1976;73:1523. doi: 10.1073/pnas.73.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern PH, Mecham JO, Wallace CD, Hoffman RM. Reduced free-methionine in methionine-dependent SV40-transformed human fibroblasts synthesizing apparently normal amounts of methionine. Journal of cellular physiology. 1983;117:9–14. doi: 10.1002/jcp.1041170103. [DOI] [PubMed] [Google Scholar]
- 14.Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro Cellular & Developmental Biology-Plant. 1984;20:663–670. doi: 10.1007/BF02619617. [DOI] [PubMed] [Google Scholar]
- 15.Backlund PS, Chang CP, Smith RA. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5′-methylthioadenosine. Journal of Biological Chemistry. 1982;257:4196. [PubMed] [Google Scholar]
- 16.Backlund PS, Smith RA. Methionine synthesis from 5′-methylthioadenosine in rat liver. Journal of Biological Chemistry. 1981;256:1533. [PubMed] [Google Scholar]
- 17.Ashe H, Clark BR, Chu F, Hardy DN, Halpern BC, Halpern RM, Smith RA. N5-methyltetrahydrofolate: homocysteine methyltransferase activity in extracts from normal, malignant and embryonic tissue culture cells. Biochemical and biophysical research communications. 1974;57:417. doi: 10.1016/0006-291x(74)90947-4. [DOI] [PubMed] [Google Scholar]
- 18.Stabler SP, Marcell PD, Podell ER, Allen RH. Quantitation of total homocysteine, total cysteine, and methionine in normal serum and urine using capillary gas chromatography-mass spectrometry. Analytical biochemistry. 1987;162:185–196. doi: 10.1016/0003-2697(87)90026-1. [DOI] [PubMed] [Google Scholar]
- 19.Kokkinakis DM, Von Wronski MA, Vuong TH, Brent TP, Schold SC., Jr Regulation of O6-methylguanine-DNA methyltransferase by methionine in human tumour cells. British journal of cancer. 1997;75:779. doi: 10.1038/bjc.1997.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, Wang J, Lu Q, Xu J, Kobayashi Y, Takakura T, Takimoto A, Yoshioka T, Lian C, Chen C, Zhang D, Zhang Y, Li S, Sun X, Tan Y, Yagi S, Frenkel EP, Hoffman RM. PEGylation confers greatly extended half-life and attenuated immunogenicity to recombinant methioninase in primates. Cancer Research. 2004:6673–6678. doi: 10.1158/0008-5472.CAN-04-1822. [DOI] [PubMed] [Google Scholar]
- 21.Tan Y, Xu M, Tan X, Wang X, Saikawa Y, Nagahama T, Sun X, Lenz M, Hoffman RM. Overexpression and large-scale production of recombinant L-methionine-a-deamino-g-mercaptomethane-lyase for novel anticancer therapy. Protein Expression and Purification. 1997;9:233–245. doi: 10.1006/prep.1996.0700. [DOI] [PubMed] [Google Scholar]
- 22.Tan Y, Xu M, Guo H, Sun X, Kubota T, Hoffman RM. Anticancer efficacy of methioninase in vivo. Anticancer research. 1996;16:3931–3936. [PubMed] [Google Scholar]
- 23.Lishko VK, Lishko OV, Hoffman RM. Depletion of serum methionine by methioninase in mice. Anticancer research. 1993;13:1465. [PubMed] [Google Scholar]
- 24.Tan Y, Zavala JSR, Han Q, Xu M, Sun X, Tan X, Magana R, Geller J, Hoffman RM. Recombinant methioninase infusion reduces the biochemical endpoint of serum methionine with minimal toxicity in high-stage cancer patients. Anticancer research. 1997;17:3857–3860. [PubMed] [Google Scholar]
- 25.Hu J, Cheung NK. Methionine depletion with recombinant methioninase: in vitro and in vivo efficacy against neuroblastoma and its synergism with chemotherapeutic drugs. International journal of cancer. 2009;124:1700–1706. doi: 10.1002/ijc.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Wang J, Yoshioka T, Li B, Lu Q, Li S, Sun X, Tan Y, Yagi S, Frenkel EP, Hoffman RM. Pharmacokinetics, methionine depletion, and antigenicity of recombinant methioninase in primates. Clinical Cancer Research. 2004;10:2131–2138. doi: 10.1158/1078-0432.ccr-03-0068. [DOI] [PubMed] [Google Scholar]
- 27.Yoshioka T, Wada T, Uchida N, Maki H, Yoshida H, Ide N, Kasai H, Hojo K, Shono K, Maekawa R, Yagi S, Hoffman RM, Sugita K. Anticancer efficacy in vivo and in vitro, synergy with 5-fluorouracil, and safety of recombinant methioninase. Cancer Research. 1998;58:2583–2587. [PubMed] [Google Scholar]
- 28.Sun X, Yang Z, Li S, Tan Y, Zhang N, Wang X, Yagi S, Yoshioka T, Takimoto A, Mitsushima K, Suginaka A, Frenkel EP, Hoffman RM. In vivo efficacy of recombinant methioninase is enhanced by the combination of polyethylene glycol conjugation and pyridoxal 5′-phosphate supplementation. Cancer Research. 2003;63:8377. [PubMed] [Google Scholar]
- 29.Yang Z, Sun X, Li S, Tan Y, Wang X, Zhang N, Yagi S, Takakura T, Kobayashi Y, Takimoto A, Yoshioka T, Suginaka A, Frenkel EP, Hoffman RM. Circulating half-life of PEGylated recombinant methioninase holoenzyme is highly dose dependent on cofactor pyridoxal-5′-phosphate. Cancer Research. 2004;64:5775. doi: 10.1158/0008-5472.CAN-04-1406. [DOI] [PubMed] [Google Scholar]
- 30.Rao AM, Drake MR, Stipanuk MH. Role of the Transsulfuration Pathway and of {gamma}-Cystathionase Activity in the Formation of Cysteine and Sulfate from Methionine in Rat Hepatocytes. Journal of Nutrition. 1990;120:837. doi: 10.1093/jn/120.8.837. [DOI] [PubMed] [Google Scholar]
- 31.Pui CH. Toward a total cure for acute lymphoblastic leukemia. J Clin Oncol. 2009;27:5121–5123. doi: 10.1200/JCO.2009.24.8518. [DOI] [PubMed] [Google Scholar]
- 32.Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet. 2005;44:367–393. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- 34.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL) Oncologist. 2007;12:991–998. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 35.Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Magdelaine-Beuzelin C, Vermeire S, Goodall M, Baert F, Noman M, Assche GV, Ohresser M, Degenne D, Dugoujon JM, Jefferis R. IgG1 heavy chain-coding gene polymorphism (G1m allotypes) and development of antibodies-to-infliximab. Pharmacogenetics and genomics. 2009;19:383. doi: 10.1097/FPC.0b013e32832a06bf. [DOI] [PubMed] [Google Scholar]
- 37.Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 38.Takakura T, Mitsushima K, Yagi S, Inagaki K, Tanaka H, Esaki N, Soda K, Takimoto A. Assay method for antitumor L-methionine -lyase: comprehensive kinetic analysis of the complex reaction with L-methionine. Analytical biochemistry. 2004;327:233–240. doi: 10.1016/j.ab.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Ditscheid B, Fünfstück R, Busch M, Schubert R, Gerth J, Jahreis G. Effect of L-methionine supplementation on plasma homocysteine and other free amino acids: a placebo-controlled double-blind cross-over study. European journal of clinical nutrition. 2005;59:768–775. doi: 10.1038/sj.ejcn.1602138. [DOI] [PubMed] [Google Scholar]
- 40.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. Journal of Chromatography: Biomedical Applications. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 41.Kokkinakis DM, Schold SC, Hori H, Nobori T. Effect of long-term depletion of plasma methionine on the growth and survival of human brain tumor xenografts in athymic mice. Nutrition and cancer. 1997;29:195–204. doi: 10.1080/01635589709514624. [DOI] [PubMed] [Google Scholar]
- 42.Serjeant EP, Dempsey B. Ionization constants of organic acids in solution. Pergamon Press; Oxford (UK): 1979. (IUPAC Chemical Data Series No. 23). [Google Scholar]
- 43.Huang S, Chua JH, Yew WS, Sivaraman J, Moore PK, Tan CH, Deng LW. Site-Directed Mutagenesis on Human Cystathionine-[gamma]-Lyase Reveals Insights into the Modulation of H2S Production. Journal of molecular biology. 2010;396:708–718. doi: 10.1016/j.jmb.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 44.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, Kubo RT, Chesnut RW, Grey HM, Sette A. Several common HLA-DR types share largely overlapping peptide binding repertoires. The Journal of Immunology. 1998;160:3363. [PubMed] [Google Scholar]
- 45.Purswani MU, Eckert SJ, Arora HK, Noel GJ. Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. Journal of Antimicrobial Chemotherapy. 2002;50:51–58. doi: 10.1093/jac/dkf091. [DOI] [PubMed] [Google Scholar]
- 46.Deb K, Chaturvedi MM, Jaiswal YK. Gram-negative bacterial LPS induced poor uterine receptivity and implantation failure in mouse: Alterations in IL-1b expression in the preimplantation embryo and uterine horns. Infectious diseases in Obstetrics and Gynecology. 2005;13:125–134. doi: 10.1080/10647440500147885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. The New England journal of medicine. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 48.Azarova AM, Gautam G, George RE. Emerging importance of ALK in neuroblastoma. Seminars in cancer biology. 2011;21:267–275. doi: 10.1016/j.semcancer.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung NK, Zhang J, Lu C, Parker M, Bahrami A, Tickoo SK, Heguy A, Pappo AS, Federico S, Dalton J, Cheung IY, Ding L, Fulton R, Wang J, Chen X, Becksfort J, Wu J, Billups CA, Ellison D, Mardis ER, Wilson RK, Downing JR, Dyer MA, St Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome, P Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA : the journal of the American Medical Association. 2012;307:1062–1071. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takakura T, Takimoto A, Notsu Y, Yoshida H, Ito T, Nagatome H, Ohno M, Kobayashi Y, Yoshioka T, Inagaki K, Yagi S, Hoffman RM, Esaki N. Physicochemical and pharmacokinetic characterization of highly potent recombinant L-methionine gamma-lyase conjugated with polyethylene glycol as an antitumor agent. Cancer Research. 2006;66:2807. doi: 10.1158/0008-5472.CAN-05-3910. [DOI] [PubMed] [Google Scholar]
- 51.Goseki N, Yamazaki S, Shimojyu K, Kando F, Maruyama M, Endo M, Koike M, Takahashi H. Synergistic effect of methionine-depleting total parenteral nutrition with 5-fluorouracil on human gastric cancer: a randomized, prospective clinical trial. Jpn J Cancer Res. 1995;86:484–489. doi: 10.1111/j.1349-7006.1995.tb03082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epner DE. Can dietary methionine restriction increase the effectiveness of chemotherapy in treatment of advanced cancer? Journal of the American College of Nutrition. 2001;20:443. doi: 10.1080/07315724.2001.10719183. [DOI] [PubMed] [Google Scholar]
- 53.Cao H, Lin H, Ye SH. Influence of L-methionine-deprived total parenteral nutrition with 5-fluorouracil on gastric cancer and host metabolism. 2001;7 doi: 10.3748/wjg.v7.i5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durando X, Thivat E, Farges MC, Cellarier E, D’Incan M, Demidem A, Vasson MP, Barthomeuf C, Chollet P. Optimal methionine-free diet duration for nitrourea treatment: a Phase I clinical trial. Nutrition and cancer. 2008;60:23. doi: 10.1080/01635580701525877. [DOI] [PubMed] [Google Scholar]
- 55.Tan Y, Sun X, Xu M, Tan X, Sasson A, Rashidi B, Han Q, Wang X, An Z, Sun F, Hoffman RM. Efficacy of recombinant methioninase in combination with cisplatin on human colon tumors in nude mice. Clinical Cancer Research. 1999;5:2157–2163. [PubMed] [Google Scholar]
- 56.Kokkinakis DM, Wick JB, Zhou QX. Metabolic response of normal and malignant tissue to acute and chronic methionine stress in athymic mice bearing human glial tumor xenografts. Chemical research in toxicology. 2002;15:1472–1479. doi: 10.1021/tx020033n. [DOI] [PubMed] [Google Scholar]
- 57.Esaki N, Soda K. L-methionine gamma-lyase from Pseudomonas putida and Aeromonas. Methods in enzymology. 1987;143:459. doi: 10.1016/0076-6879(87)43081-4. [DOI] [PubMed] [Google Scholar]
- 58.Ito S, Nakamura T, Eguchi Y. Purification and characterization of methioninase from Pseudomonas putida. Journal of Biochemistry. 1976;79:1263. doi: 10.1093/oxfordjournals.jbchem.a131180. [DOI] [PubMed] [Google Scholar]
- 59.Kokkinakis DM, Hoffman RM, Frenkel EP, Wick JB, Han Q, Xu M, Tan Y, Schold SC. Synergy between methionine stress and chemotherapy in the treatment of brain tumor xenografts in athymic mice. Cancer research. 2001;61:4017. [PubMed] [Google Scholar]
- 60.Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V, Petrillo A, Ascierto PA, Curley SA, Izzo F. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. Journal of Clinical Oncology. 2010;28:2220. doi: 10.1200/JCO.2009.26.7765. [DOI] [PubMed] [Google Scholar]
- 61.Reinders MK, Tim L. New advances in the treatment of gout: review of pegloticase. Therapeutics and Clinical Risk Management. 2010;6:543. doi: 10.2147/TCRM.S6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Investigational new drugs. 1999;17:343–360. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- 63.MarvinSketch ChemAxon. 5.5.0.1 edn 2011.
- 64.Hirata H, Kawanishi M, Iwata Y, Sakaki K, Yanagishita H. Kinetic studies on lipase-catalyzed acetylation of 2-alkanol with vinyl acetate in organic solvent. Journal of Oleo Science. 2007;56:309–317. doi: 10.5650/jos.56.309. [DOI] [PubMed] [Google Scholar]
- 65.Cheung NKV, Guo HF, Modak S, Cheung IY. Anti-idiotypic antibody facilitates scFv chimeric immune receptor gene transduction and clonal expansion of human lymphocytes for tumor therapy. Hybridoma and hybridomics. 2003;22:209–218. doi: 10.1089/153685903322328938. [DOI] [PubMed] [Google Scholar]
- 66.Sanceau J, Poupon MF, Delattre O, Sastre-Garau X, Wietzerbin J. Strong inhibition of Ewing tumor xenograft growth by combination of human interferon-alpha or interferon-beta with ifosfamide. Oncogene. 2002;21:7700–7709. doi: 10.1038/sj.onc.1205881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.