Abstract
Objectives
Angiotensinogen in the kidneys is formed primarily in the proximal tubule cells and is secreted into the tubular fluid. Structurally, proximal tubules can be divided into three segments. The first segment, segment 1 (S1) is mainly confined to the pars convoluta, the second segment, segment 2 (S2) comprises the end of pars convoluta, and the third segment, segment 3 (S3) includes the major part of the pars recta. There are some reports describing angiotensinogen localization in kidneys; however, it remains uncertain which proximal tubule segments express angiotensinogen. To determine the detailed localization of angiotensinogen in the three proximal tubule segments, we established multistaining methods using segment-specific protein markers.
Methods
Using kidneys from Wistar–Kyoto rats, we performed immunohistochemistry and double or triple staining by fluorescence in-situ hybridization and/or immunofluorescence.
Results
Our results show that angiotensinogen mRNA and protein are expressed in the cortex and outer medulla of the normal rat kidney. Angiotensinogen mRNA was hardly detected in S1, detected weakly in S2 and strongly in S3 segments. In contrast, angiotensinogen protein was detected in S1 at high levels and less in S2 and S3 segments.
Conclusion
These data indicate divergence of angiotensinogen mRNA transcription and angiotensinogen protein synthesis and metabolism in different segments of the normal rat proximal tubules.
Keywords: angiotensinogen, fluorescence in-situ hybridization, immunofluorescence, proximal convoluted tubules, proximal straight tubules
INTRODUCTION
The importance of the renin–angiotensin system (RAS) in the regulation of blood pressure (BP) and fluid and electrolyte homeostasis is well recognized [1]. Recently, the focus of interest on the RAS has shifted towards understanding the local RAS in specific tissues [1–4]. In particular, the renal RAS is unique because all of the components necessary to generate intrarenal angiotensin II are present along the nephron in both interstitial and intratubular compartments [1]. The functions of angiotensin II as a sodium-retaining and water-retaining hormone in proximal tubules include proximal volume, salt and bicarbonate reabsorption, as well as activation of sodium-coupled transporters [5–7].
Angiotensinogen (AGT) is the only known substrate for renin, which is the rate-limiting enzyme of the RAS [4,8]. Because the level of AGT is close to the Michaelis–Menten constant for renin, not only renin levels but also AGT levels can control the activity of the RAS, and upregulation of AGT levels may lead to increased formation of angiotensin peptides [9,10]. Intrarenal AGT is formed primarily in the proximal tubule cells and is secreted into the tubular fluid [11]. Inappropriate activation of AGT may lead to the development and maintenance of hypertension, and when sustained, may contribute to progressive renal injury [1,4].
Structurally, the proximal tubule consists of three segments in a nephron unit [12,13]. Segment 1 (S1) is mainly confined to the beginning of the convoluted tubules, segment 2 (S2) comprises the end of the convoluted tubules, and segment 3 (S3) includes the major straight part of the tubules. Although it is recognized that AGT is present in the proximal tubules [14–18], it is controversial as to which proximal tubule segments express AGT mRNA and protein.
This study was performed to determine the detailed AGT localization in proximal tubule segments of normal rat kidney. We utilized a new technique of multiple staining using segment-specific protein markers and examined the colocalization of AGT mRNA as well as AGT protein using these markers.
MATERIALS AND METHODS
Animals and tissue preparation
All procedures and protocols used in this study were approved by the Institutional Animal Care and Use Committee of the Tulane University Health Sciences Center. Male 9-week-old Wistar–Kyoto (WKY) rats (Charles River Breeding Laboratories, Inc., Wilmington, Massachusetts, USA) were sacrificed using a guillotine, and kidneys were immediately harvested to prepare tissue sections [19]. All kidney samples were immersion-fixed in buffered-formalin (Anatech, Battle Creek, Michigan, USA) immediately after removal, embedded in paraffin, and 3-μm thick tissue sections were prepared by an out-sourcing company (Mass Histology Service, Worcester, Massachusetts, USA).
Fluorescence in-situ hybridization
Biotinylated locked nucleic acid (LNA)-modified DNA oligonucleotides (Exicon, Woburn, Massachusetts, USA) of AGT were used for fluorescence in-situ hybridization (FISH) [20,21]. LNA probes of AGT are designed by antisense and sense sequences (antisense: 5′-biotin-GGA TGG CCC GAG GAG G-3′, Tm = 77.0°C; sense: 5′-biotin-CCT CCT CGG GCC ATC C-3′, Tm = 75.7°C). Slide sections were deparaffinized through a series of xylene and ethanol baths. To permeabilize tissues, sections were treated with 1 μg/ml proteinase K in PBS for 30 min at 37°C and rinsed briefly in PBS containing 2 mg/ml glycine. Sections were prehybridized for 1 h and then hybridized for 18 h (Tm = 22°C) with 10 nmol/l of either antisense or sense (for negative control) 5′-biotin-labeled LNA probes. To block background signals, we used endogenous Biotin-Blocking Kit (Invitrogen, Carlsbad, California, USA). Sections were then incubated in blocking buffer (Roche Diagnostics, Indianapolis, Indiana, USA) for 1 h at room temperature. Sections were subsequently incubated with 2 μg/ml Streptavidin, Alexa Fluor 488 conjugate (Molecular Probes, Eugene, Oregon, USA). Conventional fluorescence images were observed with an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, Nebraska, USA) or fluorescence microscopy (BX51; Olympus, Tokyo, Japan).
Double and triple staining by immunofluorescence and/or fluorescence in-situ hybridization
Immunofluorescence double staining [22,23] was performed using anti-AGT antibody [1 : 500; rabbit polyclonal antibody was obtained from Immuno-Biological Laboratories Co. Ltd., Gunma, Japan] with the [S1-specific goat polyclonal antisodium glucose cotransporter 2 (SGLT2); apical brush border Na+-coupled glucose transporter] [24] antibody (1 : 100; Santa Cruz Biotechnology, Santa Cruz, California, USA), the S2-specific goat polyclonal anticarbonic anhydrase IV (CA IV) (apical brush border zinc metalloenzyme) [24,25] antibody (1 : 100; Santa Cruz Biotechnology), or the S3-specific mouse monoclonal antiecto-adenosine triphosphatase (ecto-ATPase; apical brush border enzyme) [24,26] antibody (1 : 100; Beckton, Dickinson Biosciences, Franklin Lakes, New Jersey, USA). Sections were covered with normal serum in PBS for 10 min and incubated with primary antibodies for 1 h at room temperature. Sections were washed, then, incubated with secondary antibodies for 1 h at room temperature. For secondary antibodies, 2 μg/ml Alexa Fluor 488 (donkey antigoat IgG for S1 segments/donkey antirabbit IgG for S2 segments/goat antirabbit IgG for S3 segments) for the detection of AGT protein and 2 μg/ml Alexa Fluor 594 (donkey antigoat IgG for S1 and S2 segments/goat antimouse IgG for S3 segments) for the detection of S1–S3-specific markers from Molecular Probes were used.
We also performed double staining using FISH and immunofluorescence methods [27–29]. Before the incubation with Streptavidin, Alexa Fluor 488 conjugate in the FISH method, the sections were incubated with rabbit polyclonal anti-SGLT2 antibody (1 : 100; Santa Cruz Bio-technology), goat polyclonal anti-CA IV antibody (1 : 100), or rabbit polyclonal antiecto-ATPase antibody (1 : 100; Santa Cruz Biotechnology) for 1 h at room temperature. After washing, the sections were incubated with 2 μg/ml Streptavidin, Alexa Fluor 488 conjugate for the detection of AGT mRNA and 2 μg/ml Alexa Fluor 594 (goat antirabbit IgG for S1 and S3 segments/donkey antigoat IgG for S2 segments) for the detection of S1–S3-specific markers for 1 h at room temperature.
Immunofluorescence triple staining was performed using segment-specific antibodies [23]. After the blocking, sections were incubated with primary antibodies (rabbit polyclonal anti-SGLT2 antibody, goat polyclonal anti-CA IV antibody, and mouse monoclonal anti-ecto-ATPase antibody) diluted in blocking reagent including normal serum for 1 h at room temperature. After washing, the sections were incubated with 2 μg/ml Alexa Fluor 488 donkey antirabbit IgG, 2 μg/ml Alexa Fluor 594 donkey antigoat IgG, and 2 μg/ml Alexa Fluor 350 donkey antimouse IgG for the detection of S1, S2, and S3-specific markers, respectively, for 1 h at room temperature.
In all staining methods, we confirmed that no staining was seen in negative control. Renal localization of target mRNA and protein was observed with fluorescence microscopy (BX51).
Immunohistochemistry
AGT and SGLT2 expressions were examined by immuno-histochemistry (IHC) [30,31]. IHC was performed by an automated system (Autostainer; Dako, Carpinteria, California, USA) and samples were counterstained with hematoxylin. The concentrations of primary antibodies against AGT and SGLT2 were 1 : 500 and 1 : 100, respectively. Conventional images were observed with phase contrast microscopy (BX41; Olympus, Tokyo, Japan).
RESULTS
In-situ hybridization histochemistry with biotinylated probes to angiotensinogen mRNA and immunostaining for angiotensinogen protein in normal rat kidney sections
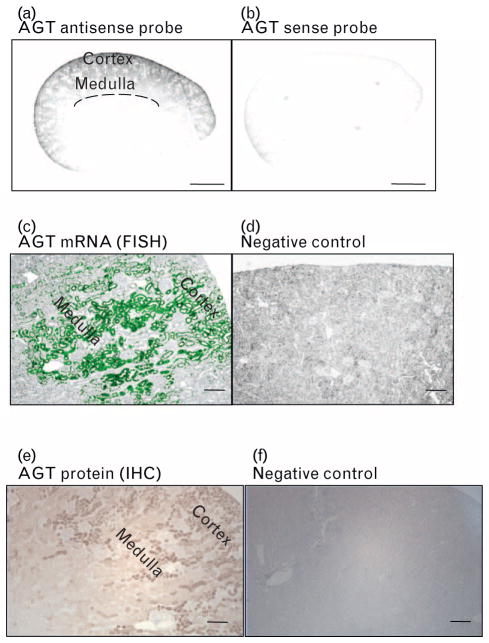
The detection of AGT mRNA in a whole kidney section was confirmed by low magnification using an Odyssey Infrared Imaging System (Fig. 1a). Sense probe (negative control) showed no signal (Fig. 1b).
FIGURE 1.
High magnification of normal rat kidney showing the pattern of in-situ hybridization of angiotensinogen (AGT) antisense probe (a) and control (b). Scale bar, 2 mm. Representative images of AGT mRNA by FISH: (antisense probe: (c), sense probe: (d)) and AGT protein by immunohistochemistry (IHC); (IHC staining: (e), negative control staining: (f)) in normal rat kidney sections are detected over tubules in cortex and outer stripe of outer medulla. Magnification, × 100 (scale bar, 200 μm). FISH, fluorescence in-situ hybridization.
AGT mRNA by the FISH method was recognized by strong positive staining in the proximal tubules in the cortex and outer stripe of outer medulla regions under fluorescence microscopy (Fig. 1c). Sense probe was not hybridized with AGT mRNA (Fig. 1d).
AGT protein by the IHC method was also recognized by strong positive staining in the proximal tubules in the cortex and outer stripe of outer medulla regions under phase contrast microscopy (Fig. 1e). No staining was seen in negative control (Fig. 1f).
Triple immunofluorescence staining with antibodies against three proximal tubule segment-specific markers
Before the AGT localization study, separation of three proximal tubule segments using each specific marker was examined. The images of immunofluorescence (Fig. 2a) and phase contrast (Fig. 2b) are shown. Proximal tubule segments were discriminated visually by specific markers. S1 (green color) and S2 (red color) segments were located around the glomerulus and the S3 (blue color) segments were found in the corticomedullary region of the kidney. Under strict investigation by microscope images, the markers for S1 and S2 segments were colocalized, at least in part, therefore, we detected only strong positive fluorescence in the next localization study.
FIGURE 2.
Immunofluorescence triple staining with antibodies against three proximal tubule segment-specific markers (a) and phase contrast (b). Green indicates S1-specific marker, sodium glucose cotransporter 2 (SGLT2) staining, red indicates S2-specific marker, carbonic anhydrase IV (CA IV) staining, and blue indicates S3-specific marker, ecto-ATPase staining. Magnification, × 200 (scale bar, 100 μm). CA IV, carbonic anhydrase IV; DT, distal tubules; ecto-ATPase, ecto-adenosine triphosphatase; G, glomerulus; S, segment; SGLT2, sodium glucose cotransporter 2.
Angiotensinogen mRNA detection in proximal tubule segments
Double staining of AGT mRNA (by FISH) and each specific marker (by immunofluorescence) are shown in Fig. 3. Each pair of panels represents the same field. From the merged picture of AGT mRNA and specific marker, AGT mRNA localization was determined. Pictures were taken at two magnifications: × 100 (upper panels) and × 400 (lower panels). Left panel (green color) shows AGT mRNA detection by FISH (Fig. 3a, d, g, j, m, and p). Segment-specific markers were stained by red color. S1-specific marker, SGLT2, detection by immunofluorescence is shown in Fig. 3b and e. S2-specific marker, CA IV, detection by immunofluorescence is shown in Fig. 3h and k. S3-specific marker, ecto-ATPase, detection by immunofluorescence is shown in Fig. 3n and q.
FIGURE 3.
Angiotensinogen AGT mRNA expression in the S1, S2, and S3 segments. AGT mRNA in the S1 segments (a–f). AGT mRNA in the S2 segments (g–l). AGT mRNA in the S3 segments (m–r). Green indicates AGT mRNA (a, d, g, j, m and p). Red indicates segment-specific markers, SGLT2 (b and e), CA IV (h and k), and ecto-ATPase (n and q). Merged images of AGT mRNA and each specific marker are shown in c, f, i, l, o and r. Arrows indicate merged areas. The images at the lower left show AGT mRNA and specific-markers are expressed strongly in the brush border. Dotted lines indicate the edge of the tissue. Magnification of a, b, c, g, h, i, m n and o, × 100 (scale bar, 200 μm). Magnification of d, e, f, j, k, l, p, q, and r, × 400 (scale bar, 50 μm). AGT, angiotensinogen; CA IV, carbonic anhydrase IV; CO; cortex; ecto-ATPase, ecto-adenosine triphosphatase; S, segment; SGLT2, sodium glucose cotransporter 2.
From the merged image of AGT mRNA and SGLT2, AGT mRNA detection was little perceptible in S1 segments (Fig. 3c and f). This result was confirmed by another method using two consecutive sections (Fig. 4). The staining of AGT mRNA in the proximal tubules by the FISH method (Fig. 4a) and SGLT2 staining of proximal tubules by the IHC method (Fig. 4b) in the same field of tissue sections was performed. These data for staining of two consecutive sections reinforced the conclusion that AGT mRNA detection was not detected much in S1 segments.
FIGURE 4.
Angiotensinogen (AGT) mRNA expression in the S1 segments. The AGT mRNA staining in proximal tubules by FISH method (a) and SGLT2 staining proximal tubules by IHC method (b) in the same field of consecutive tissue sections was investigated. In consecutive section-2, positive staining for SGLT2 was numbered from 1 to 19. Same tubules in consecutive section-1 were also numbered. These two consecutive sections staining data reinforce that AGT mRNA expression is not detected much in S1 segments. The images at the lower left show AGT mRNA and SGLT2 are expressed strongly in the brush border. AGT, angiotensinogen; FISH, fluorescence in-situ hybridization; G, glomeruli; IHC, immunohistochemistry; S, segment; SGLT2, sodium glucose cotransporter 2. Magnification, × 200 (scale bar, 100 μm).
From the merged image of AGT mRNA and CA IV, AGT mRNA was detected apparently in some areas of the S2 segments (Fig. 3i and l). In the high magnification (× 400) picture, AGT mRNA localization is shown by arrows (Fig. 3l). The image at the lower left shows AGT mRNA and specific-marker are expressed strongly in the brush border (Fig. 3l).
From the merged image of AGT mRNA and ecto-ATPase, AGT mRNA was detected predominantly in the S3 segments (Fig. 3o and r). The image at the lower left shows AGT mRNA and specific-marker are expressed strongly in the brush border (Fig. 3r).
Angiotensinogen protein detection in proximal tubule segments
Double staining of AGT protein (by immunofluorescence) and each specific marker (by immunofluorescence) are shown in Fig. 5. Each pair of panels represents the same field. From the merged picture of AGT protein and specific marker, AGT protein localization was determined. Pictures were taken at two magnifications: × 100 (upper panels) and × 400 (lower panels). Left panel (green color) shows AGT protein detection by immunofluorescence (Fig. 5a, d, g, j, m, and p). Segment-specific markers were stained by red color. SGLT2 detection by immunofluorescence is shown in Fig. 5b and e. CA IV detection by immunofluorescence is shown in Fig. 5h and k. Ecto-ATPase detection by immunofluorescence is shown in Fig. 5n and q.
FIGURE 5.
Angiotensinogen (AGT) protein expression in the S1, S2, and S3 segments. AGT protein in the S1 segments (a–f). AGT protein in the S2 segments (g–l). AGT protein in the S3 segments (m–r). Green indicates AGT protein (a, d, g, j, m and p). Red indicates segment-specific markers, SGLT2 (b and e), CA IV (h and k), and ecto-ATPase (n and q). Merged images of AGT protein and each specific-marker are shown in c, f, i, l, o and r. Arrows indicate merged areas. The images at the lower left show AGT protein and specific-markers are expressed strongly in the brush border. Dotted lines indicate the edge of the tissue. Magnification of a, b, c, g, h, i, m, n and o, × 100 (scale bar, 200 μm). Magnification of d, e, f, j, k, l, p, q, and r, × 400 (scale bar, 50 μm). AGT, angiotensinogen; CA IV, ecto-ATPase, ecto-adenosine triphosphatase, carbonic anhydrase IV; CO; cortex; S, segment; SGLT2, sodium glucose cotransporter 2.
From the merged image of AGT protein and SGLT2, strong AGT protein was detected in the S1 segments (Fig. 5c and f). The image at the lower left shows AGT protein and specific-marker are expressed strongly in the brush border (Fig. 5f).
From the merged image of AGT protein and CA IV, AGT protein was detected clearly in some areas of the S2 segments (Fig. 5i and l). In the high magnification (× 400) picture, AGT protein localization is shown by arrows. The image at the lower left shows AGT protein and specific-marker are expressed strongly in the brush border (Fig. 5l).
From the merged image of AGT protein and ecto-ATPase, AGT protein was also recognized in the S3 segments (Fig. 5o and r). In the high magnification (× 400) picture, AGT protein localization is shown by arrows. The image at the lower left shows AGT protein and specific-marker are expressed strongly in the brush border (Fig. 5r).
DISCUSSION
The RAS serves to maintain extracellular volume homeostasis, a task that is primarily achieved by the kidney [32]. Based on the notion that significant amounts of angiotensin II are formed locally, elements of an intrarenal, self-contained RAS have been investigated [4,33]. These have been assigned chiefly to the proximal tubule in which AGT, renin, angiotensin, angiotensin-converting enzyme (ACE), basolateral and brush border membrane angiotensin receptors, and high concentrations of angiotensin II have been localized [1,34]. Both renin and AGT localize in proximal tubule intracellular vesicles. Renin synthesized by the juxtaglomerular apparatus cells is the primary source of both circulating and intrarenal levels. AGT is formed primarily in the proximal tubule cells, and has been found near or in the brush borders of the proximal tubules [4,8]. However, the detailed segments for AGT mRNA transcription and AGT protein translation in the proximal tubules and its circulation is still unclear. In this study, we focused on the detailed regions of AGT expression.
Structurally, the proximal tubule can be subdivided into three segments in the cortical labyrinth and the medullary rays. Yanagawa et al. [15] reported that AGT was detected in the culture medium obtained from both proximal convoluted tubule cells and proximal straight tubule cells. Darby et al. [17] reported that immunostaining for AGT was present in the proximal convoluted tubules of the kidney. Also, nonisotopic hybridization histochemistry using oligo-deoxynucleotide probes showed AGT mRNA expression in proximal convoluted tubules of the rat renal cortex. However, the latest study by Pohl et al. [18] reported that AGT mRNA signal was restricted primarily to the proximal straight tubules. In contrast, AGT protein was localized to subapical compartments of the proximal convoluted tubules [18]. Thus, it remains unclear as to which proximal tubule segments (convoluted, straight, or both) express AGT message and protein. The discrepancy concerning the expression in the proximal tubules may be caused by difficulty of the separation of proximal tubule segments.
To specify the AGT localization in the cortex and outer medulla regions (Fig. 1), we used three proximal tubule segment-specific markers, which are expressed in proximal tubule brush borders. Because the three segments can be specifically recognized by immunofluorescence triple staining (Fig. 2), it was decided that the three markers were very useful tools to study AGT localization. Using double staining approaches, we determined that AGT mRNA was expressed primarily in the S3 segments with weak AGT mRNA expression in the S2 segments (Fig. 3). In contrast, AGT protein was strongly expressed in the S1 segments, and was also present in the S2 and S3 segments (Fig. 5).
Our data clearly show that the main localization of AGT mRNA and AGT protein are different. Recently, we also established a primary culture of proximal tubule segments from normal mouse kidneys, and we reported that S2 and S3 segments contain AGT mRNA expression and all segments, S1, S2, and S3 contain AGT protein [35]. The present study and our recent study demonstrated noticeably similar results using different methods/species; AGT mRNA transcription and AGT protein synthesis and metabolism are performed in different proximal tubule segments. Intrarenal AGT metabolism is extremely complex, and the details have not been fully elucidated. However, the present data show that AGT mRNA is transcribed primarily in the S3 segments and weakly in the S2 segments. Then, translation of AGT protein may be performed and secreted into the tubular fluid. Because there is no evidence about AGT transport into proximal tubule cells of S3 segments through membrane receptors, we suggest that excreted AGT protein into urine is caused mainly by secreted AGT protein in S3 segments.
Pohl et al. [18] reported that AGT protein is a ligand of megalin, which is expressed in the S1 segments, and, indeed, AGT protein was detected in the S1 segments in this study. From these results, the binding of AGT protein to specific membrane receptors, such as megalin and cubilin, and endocytosis of these components in S1 segments is important for AGT metabolism. However, the data have not yet settled the question regarding the source of AGT protein in the S1 segments. We previously observed that AGT mRNA and protein are expressed not only in proximal tubules but also in the glomeruli [36,37], especially in mesangial cells (Supplemental Figure S1, http://links.lww.com/HJH/A201) [37]. A possible explanation to support these results is that the secreted AGT from glomeruli binds to specific membrane receptors like megalin and cubilin, and endocytosis of these components may take place in S1 segments. Because AGT is a large molecule (50–60 kDa), AGT secreted in the liver cannot filter across the glomeruli [38]; however, on the contrary, there is a report that even larger protein, albumin is partially filtered [39]. Also, Matsusaka et al. showed almost abolished plasma and renal AGT protein in liver-specific AGT knockout mice [40]. Therefore, further investigation is still needed on the complete metabolism of AGT.
Others and we have reported that intrarenal AGT expression and urinary AGT excretion are increased in various models of hypertension including angiotensin II-dependent hypertensive rats [38,41–43], Dahl salt-sensitive hypertensive rats [44,45], and spontaneously hypertensive rats [46,47], as well as diabetic nephropathy [48–52], immunoglobulin A nephropathy [30], and radiation nephropathy [53]. Moreover, transgenic mice overexpressing rat Agt gene in renal proximal tubule cells developed hypertension and renal injury, which were prevented or reversed by treatment with angiotensin II type 1 (AT1) receptor blockers [54], indicating the importance of intrarenal AGT in the mechanism of hypertension and kidney diseases. In future, the role of receptor-mediated endocytosis of AGT protein in S1 segments, and the relationship between intrarenal AGT expression in S3 segments and urinary excretion volume will bring a useful result to elucidate the mechanism of hypertension. Our staining tools using segment-specific markers will be helpful in assessing the changes of AGT localization in the development and progression of hypertension, diabetes, and other renal diseases, and the role of the specified proximal tubule segments will develop new insight.
Acknowledgments
The authors acknowledge critical discussion and/or excellent technical assistances from Maki Urushihara, MD, PhD and Salem I. Elkhayat, MS (Tulane University).
Information about previous presentation: All authors have read and approved the submission of the article; the article has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract.
This study was supported by a grant from National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408), a COBRE grant from National Center for Research Resources (P20RR017659), and a grant from National Heart, Lung, and Blood Institute (R01HL026371).
Abbreviations
- ACE
angiotensin-converting enzyme
- AGT
angiotensinogen
- AT1
angiotensin II type 1
- BP
blood pressure
- CA IV
carbonic anhydrase IV
- ecto-ATPase
ecto-adenosine triphosphatase
- FISH
fluorescence in-situ hybridization
- LNA
locked nucleic acid
- RAS
renin–angiotensin system
- SGLT2
sodium glucose cotransporter 2
- WKY
Wistar–Kyoto
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine: a paradigm shift? Circulation. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 3.Danser AH, Admiraal PJ, Derkx FH, Schalekamp MA. Angiotensin I-to-II conversion in the human renal vascular bed. J Hypertens. 1998;16 (12 Pt 2):2051–2056. doi: 10.1097/00004872-199816121-00029. [DOI] [PubMed] [Google Scholar]
- 4.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39 (2 Pt 2):316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum M, Quigley R, Quan A. Effect of luminal angiotensin II on rabbit proximal convoluted tubule bicarbonate absorption. Am J Physiol. 1997;273 (4 Pt 2):F595–F600. doi: 10.1152/ajprenal.1997.273.4.F595. [DOI] [PubMed] [Google Scholar]
- 6.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riquier-Brison AD, Leong PK, Pihakaski-Maunsbach K, McDonough AA. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am J Physiol Renal Physiol. 2010;298:F177–F186. doi: 10.1152/ajprenal.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 9.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 10.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27 (3 Pt 2):465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 11.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helbert MJ, Dauwe SE, Van der Biest I, Nouwen EJ, De Broe ME. Immunodissection of the human proximal nephron: flow sorting of S1S2S3, S1S2 and S3 proximal tubular cells. Kidney Int. 1997;52:414–428. doi: 10.1038/ki.1997.348. [DOI] [PubMed] [Google Scholar]
- 13.Cristofori P, Zanetti E, Fregona D, Piaia A, Trevisan A. Renal proximal tubule segment-specific nephrotoxicity: an overview on biomarkers and histopathology. Toxicol Pathol. 2007;35:270–275. doi: 10.1080/01926230601187430. [DOI] [PubMed] [Google Scholar]
- 14.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 15.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–941. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 16.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 17.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 18.Pohl M, Kaminski H, Castrop H, Bader M, Himmerkus N, Bleich M, et al. Intrarenal renin angiotensin system revisited: role of megalin-dependent endocytosis along the proximal nephron. J Biol Chem. 2010;285:41935–41946. doi: 10.1074/jbc.M110.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urushihara M, Ohashi N, Miyata K, Satou R, Acres OW, Kobori H. Addition of angiotensin II type 1 receptor blocker to CCR2 antagonist markedly attenuates crescentic glomerulonephritis. Hypertension. 2011;57:586–593. doi: 10.1161/HYPERTENSIONAHA.110.165704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson KL, Thach DC. LNA flow-FISH: a flow cytometry-fluorescence in situ hybridization method to detect messenger RNA using locked nucleic acid probes. Anal Biochem. 2009;390:109–114. doi: 10.1016/j.ab.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen R, Nielsen PS, Jensen TH. Dramatically improved RNA in situ hybridization signals using LNA-modified probes. RNA. 2005;11:1745–1748. doi: 10.1261/rna.2139705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, et al. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261–269. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 23.Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 24.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93:397–404. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown D, Zhu XL, Sly WS. Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. Proc Natl Acad Sci U S A. 1990;87:7457–7461. doi: 10.1073/pnas.87.19.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai Y, Stelzner MG, Lee WS, Wells RG, Brown D, Hediger MA. Expression of mRNA (D2) encoding a protein involved in amino acid transport in S3 proximal tubule. Am J Physiol. 1992;263 (6 Pt 2):F1087–F1092. doi: 10.1152/ajprenal.1992.263.6.F1087. [DOI] [PubMed] [Google Scholar]
- 27.Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, et al. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci. 2000;20:9224–9234. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pineau I, Barrette B, Vallieres N, Lacroix S. A novel method for multiple labeling combining in situ hybridization with immunofluorescence. J Histochem Cytochem. 2006;54:1303–1313. doi: 10.1369/jhc.6A7022.2006. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Hioki H, Konno M, Pan S, Nakamura H, Nakamura KC, et al. Expression of gap junction protein connexin36 in multiple subtypes of GABAergic neurons in adult rat somatosensory cortex. Cereb Cortex. 2011;21:2639–2649. doi: 10.1093/cercor/bhr051. [DOI] [PubMed] [Google Scholar]
- 30.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, et al. Intrarenal RAS activity and urinary angiotensinogen excretion in antithymocyte serum nephritis rats. Am J Physiol Renal Physiol. 2008;295:F1512–F1518. doi: 10.1152/ajprenal.00058.2008. [DOI] [PubMed] [Google Scholar]
- 32.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 33.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382–F1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamiyama M, Garner MK, Farragut KM, Kobori H. The establishment of a primary culture system of proximal tubule segments using specific markers from normal mouse kidneys. Int J Mol Sci. 2012;13:5098–5111. doi: 10.3390/ijms13045098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takamatsu M, Urushihara M, Kondo S, Shimizu M, Morioka T, Oite T, et al. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008;23:1257–1267. doi: 10.1007/s00467-008-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species—ERK/JNK pathways. Hypertens Res. 2010;33:1174–1181. doi: 10.1038/hr.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richoux JP, Cordonnier JL, Bouhnik J, Clauser E, Corvol P, Menard J, et al. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res. 1983;233:439–451. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 40.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, et al. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263 (5 Pt 1):E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 43.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung S, Park CW, Shin SJ, Lim JH, Chung HW, Youn DY, et al. Tempol or candesartan prevents high-fat diet-induced hypertension and renal damage in spontaneously hypertensive rats. Nephrol Dial Transplant. 2010;25:389–399. doi: 10.1093/ndt/gfp472. [DOI] [PubMed] [Google Scholar]
- 47.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int J Biol Sci. 2007;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–927. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobori H, Katsurada A, Miyata K, Ohashi N, Satou R, Saito T, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294:F1257–F1263. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S, Bivona BJ, Kobori H, Seth DM, Chappell MC, Lazartigues E, et al. Major role for ACE-independent intrarenal ANG II formation in type II diabetes. Am J Physiol Renal Physiol. 2010;298:F37–F48. doi: 10.1152/ajprenal.00519.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sofue T, Kiyomoto H, Kobori H, Urushihara M, Nishijima Y, Kaifu K, et al. Early treatment with olmesartan prevents juxtamedullary glomerular podocyte injury and the onset of microalbuminuria in type 2 diabetic rats. Am J Hypertens. 2012;25:604–611. doi: 10.1038/ajh.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, et al. Young Scholars Award Lecture: intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–550. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sachetelli S, Liu Q, Zhang SL, Liu F, Hsieh TJ, Brezniceanu ML, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–1023. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]