Abstract
Introduction
Apoptosis and autophagy impact cell death in multiple systems of the body. Development of new therapeutic strategies that target these processes must address their complex role during developmental cell growth as well as during the modulation of toxic cellular environments.
Areas covered
Novel signaling pathways involving Wnt1-inducible signaling pathway protein 1 (WISP1), phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), β-catenin and mammalian target of rapamycin (mTOR) govern apoptotic and autophagic pathways during oxidant stress that affect the course of a broad spectrum of disease entities including Alzheimer’s disease, Parkinson’s disease, myocardial injury, skeletal system trauma, immune system dysfunction and cancer progression. Implications of potential biological and clinical outcome for these signaling pathways are presented.
Expert opinion
The CCN family member WISP1 and its intimate relationship with canonical and non-canonical wingless signaling pathways of PI3K, Akt1, β-catenin and mTOR offer an exciting approach for governing the pathways of apoptosis and autophagy especially in clinical disorders that are currently without effective treatments. Future studies that can elucidate the intricate role of these cytoprotective pathways during apoptosis and autophagy can further the successful translation and development of these cellular targets into robust and safe clinical therapeutic strategies.
Keywords: β-catenin, Akt, Alzheimer’s disease, apoptosis, autophagy, Beclin 1, cancer, cardiovascular disease, caspase, CCN family, diabetes mellitus, erythropoietin, forkhead transcription factors, FoxO, glycogen synthase kinase-3β, mammalian target of rapamycin, neurodegenerative disease, oxidative stress, Parkinson’s disease, phosphoinositide 3-kinase, programmed cell death, Wnt1-inducible signaling pathway protein 1
1. Introduction
The demise of a cell can occur through a number of pathways that involve apoptosis (type I cell death), autophagy (type II cell death) and necrosis (type III cell death). Apoptosis is considered to be one variant of programmed cell death (PCD) that is a directed process that involves reduction in cell size, chromatin condensation and nuclear DNA fragmentation [1]. Autophagy represents another form of PCD that is highly regulated to reduce and recycle cell components into smaller units through lysosomes that leads to the degradation of cellular organelles [2]. Other forms of PCD can include anoikis that describes apoptotic death to cells that lose contact with the extracellular matrix and cornification that is used to maintain the integrity of the epidermis through the replacement of cellular components with keratin. Although Carl Vogt in 1842 initially described the process of apoptosis, it was not until several years later, such as in 1965, that investigators began to take a greater interest in distinguishing apoptotic cell death from necrotic cell death with the work of John Kerr [3,4]. Early descriptions of autophagy surfaced approximately during the same time period with the identification of several of the essential components of autophagy that includes [5] autophagic vacuoles [6]. In contrast to the processes of PCD that may be beneficial such as during the development of an organism, necrosis is an unexpected and premature death of cells as a result of exposure to environmental toxins or trauma that is usually not considered to be beneficial [7].
In disorders involving multiple disease entities and several systems of the body such as the nervous system, immune system, cardiac system, skeletal system and vascular system, PCD that is associated with apoptosis or autophagy has recently been shown to lead to cell demise. As a result, many proposed treatments for the development of new therapeutic strategies seek to modulate apoptosis or autophagy to either prevent cell injury or foster cellular repair and regeneration. However, a fine balance within the processes of apoptosis and autophagy exists since activation of these pathways is sometimes necessary for developmental cell growth or protection against toxic environments. As a result, development of new avenues of treatment must address these challenges to enhance clinical efficacy and limit potential disability or death. Here, the authors discuss an integrated series of novel signaling pathways that involve Wnt1-inducible signaling pathway protein 1 (WISP1), phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), β-catenin and mammalian target of rapamycin (mTOR) signaling for future clinical development that are increasingly being identified as primary targets against apoptotic or autophagic cell death.
2. Cell injury through oxidative stress, apoptosis and autophagy
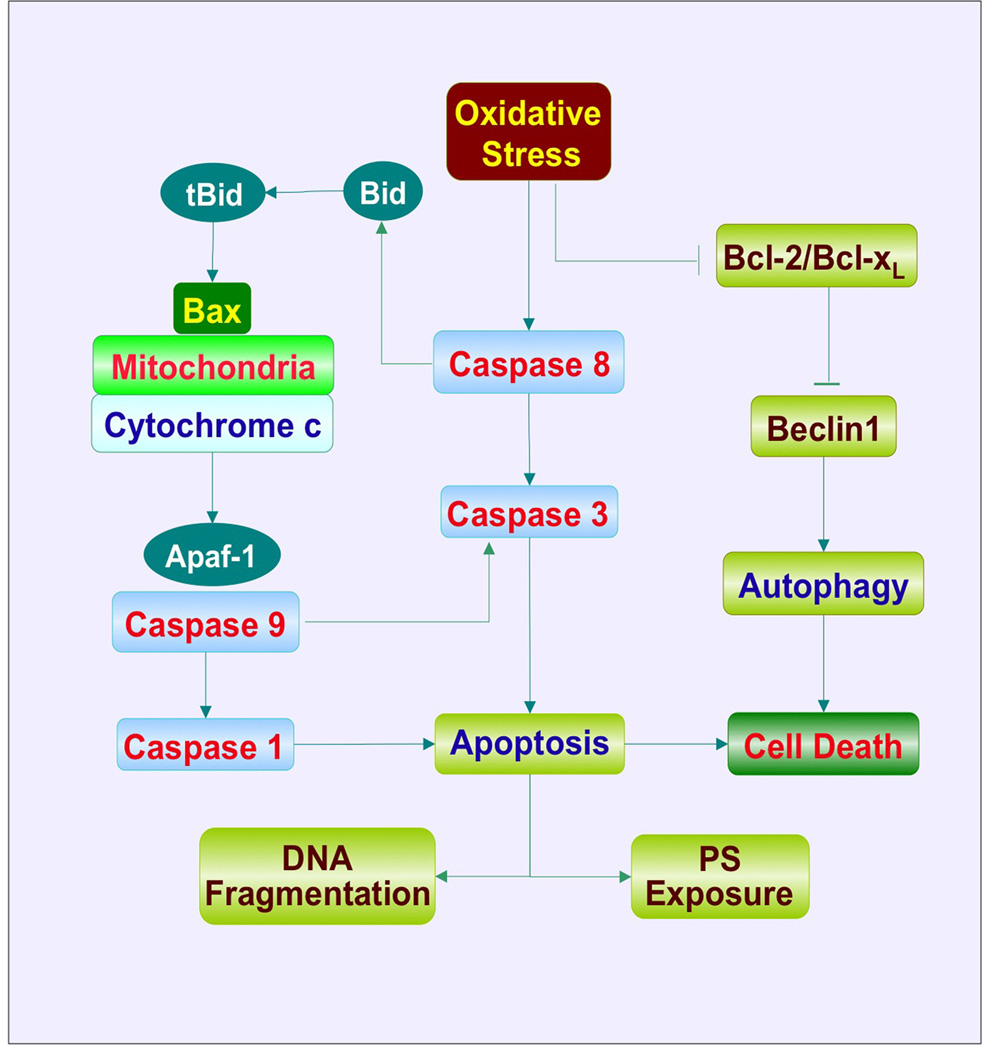
Reactive oxygen species (ROS) are formed through agents such as superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO) and peroxynitrite and lead to oxidative stress [7,8]. Under normal physiological conditions, ROS exist at non-detrimental levels and are kept in check by endogenous antioxidant systems that include superoxide dismutase, catalase, glutathione peroxidase and vitamins C, D, E and K [9–11]. Yet, early studies suggested that exposure to elevated oxygen, a high metabolic rate and ROS could reduce lifespan expectations [12]. Once produced, ROS affect mitochondrial function, DNA integrity and protein folding leading to cellular injury. More current studies have now linked oxygen free radical production to DNA damage in diabetic patients [13], mitochondrial injury and aging mechanisms [14] and nutritional impairment [15]. Oxidative stress can affect multiple systems of the body and result in cardiac disease [16], vasculature dysfunction [17,18], immune-mediated disorders [19–21], aging [22], cerebral ischemia [23,24], diabetic complications [25,26] and cognitive loss [27]. Ultimately, oxidative stress that has pathological consequences can lead to cell death through apoptosis or autophagy (Figure 1).
Figure 1. Signaling pathways of apoptosis and autophagy during oxidative stress.
Oxidative stress can lead to the activation of initiator caspases, such as caspase 8, which can directly activate caspase 3 and cleave BH3 domainonly protein Bid. The resultant truncated Bid (tBid) promotes the release of cytochrome c from mitochondria through Bax. Cytochrome c interacts with apoptotic protease-activating factor-1 (Apaf-1) resulting in the oligomerizaton of Apaf-1 and the subsequent activation of caspase 9. Activated caspase 9 can activate caspase 3 as well as caspase 1 leading to apoptotic DNA fragmentation and phosphatidylserine (PS) exposure. In response to oxidative stress, expression of the anti-apoptotic protein Bcl-2/Bcl-xL is down-regulated. This process releases the autophagy-related protein Beclin 1 from inhibitory binding by Bcl-2/Bcl-xL and initiates autophagic cell death. In addition, Bcl-2/Bcl-xL also antagonizes Bax-mediated mitochondrial release of cytochrome c to prevent the induction of caspase activation and apoptosis.
Two primary components of apoptosis consist of an early phase that involves the exposure of membrane phosphatidyl-serine (PS) residues and a late phase that involves the destruction of genomic DNA (Figure 1) [7]. The early phase can tag cells with the membrane externalization of PS residues and essentially function as a ‘homing device’ for inflammatory cells to engulf and remove injured cells [28,29]. For this to occur such as during periods of oxidative stress, inflammatory cells increase their expression of the membrane phosphatidylserine receptor (PSR) with a concurrent increase in the proliferation and activation of the inflammatory cells. It is important to note that as a vital therapeutic component, modulation of inflammatory cell activation may be required since removal of temporarily injured cells expressing membrane PS residues may sometimes result in the loss of otherwise functional cells [30,31]. As a potential therapeutic strategy, treatment agents can target the reversal of membrane PS externalization on temporarily injured cells with a simultaneous blockade of the PSR receptor on inflammatory cells to limit their activation and proliferation during apoptosis. Externalization of membrane PS residues can result from a broad array of toxic injuries that result in oxidative stress, such as during oxygen radical exposure, infection, ischemia, vascular clot formation and β-amyloid deposition [32–34].
The second or later phase of apoptosis consists of the cleavage of genomic DNA (Figure 1) [35–37]. Once initiated, genomic DNA degradation usually is not a reversible step. Enzymes responsible for DNA degradation include the acidic cation-independent endonuclease (DNase II), cyclophilins and the 97 kDa magnesium-dependent endonuclease. Three separate endonuclease activities also exist in the nervous system that include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease and an inducible magnesium-dependent endonuclease [1].
During apoptotic cell injury, caspases are activated [38]. Caspases are usually synthesized as inactive zymogens that are proteolytically cleaved into subunits at the onset of apoptosis and function as active caspases after reconstitution to molecular heterodimers. Caspases are composed of three domains including an N-terminal pro-domain, a large subunit and a small subunit. As a result of their activation sequence, caspases are classified as either initiator caspases (also known as apical caspases) or effector caspases. An initiator caspase cleaves and subsequently activates an effector caspase. The apoptotic-associated caspases include initiator caspases, such as caspase 2, 8, 9 and 10, that activate downstream effector caspases leading to an amplification of cascade activity. The initiator caspases consist of long N-terminal pro-domains that contain caspase recruitment domains (CARDs) in caspase 2 and caspase 9 or death effector domains (DEDs) in caspase 8 and caspase 10. The effector caspases consist of caspase 1, 3, 6 and 7 that function to directly cleave crucial cellular protein substrates that result in cell death. The effector caspases contain either short pro-domains or are absent of pro-domains [39].
Activation of caspases can occur through two distinct pathways, namely the extrinsic and intrinsic pathways [1,39]. The extrinsic pathway is initiated by death receptor activation at the cell surface resulting in the recruitment and activation of the initiator caspase 8 or caspase 10 on apoptotic stimuli. The intracellular death domain of death receptors, such as the TNF superfamily Fas/CD95/Apo-1, can bind to extracellular ligands and lead to an intracellular death-inducing signaling complex following recruitment of adaptor molecules, such as the Fas-associated death domain (FADD). FADD recruits caspase 8 and 10 through its DED domain to result in the activation of caspase 8 and 10. Activation of caspase 8 can subsequently lead to caspase 3 activation. In addition, active caspase 8 can cleave BH3-only protein Bid, a pro-apoptotic member of the Bcl-2 family and result in truncated Bid (tBid) that promotes cytochrome c release through Bax resulting in the subsequent activation of executioner caspases. In regards to activity in the intrinsic caspase pathway, this involves mitochondrial membrane depolarization that is associated with the release of cytochrome c and the subsequent activation of caspase 9 followed by activation of caspase 3. The process is regulated by the Bcl-2 subfamily BH3-only proteins including Bid, Bad, Bim, Bmf, Puma and Noxa, which are normally located in cellular compartments other than mitochondria. The translocation of these proteins to mitochondria delivers an apoptotic signal through the interaction with Bax, a multiple Bcl-2 homology domain containing protein, to promote permeabilization of the outer mitochondrial membrane and the release of cytochrome c that then binds to apoptotic protease-activating factor-1 (Apaf-1). Apaf-1 consists of three different domains that include CARD, repeats of tryptophan and aspartate residues (WD-40 repeats) and a nucleotide-binding domain CED-4. Binding of cytochrome c to Apaf-1 results in the removal of the WD-40 domain, masking the CED-4 and CARDs, and leads to the oligomerization of Apaf-1 with the requirement of dATP/ATP. The oligomerization of Apaf-1 promotes the allosteric activation of caspase 9 by forming the Apaf-1 apoptosome. Caspase 9 can subsequently activate caspase 3 as well as caspase 1 through the intermediary caspase 8. Activation of caspase 1 and caspase 3 not only leads to DNA fragmentation, but also results in membrane PS exposure [40,41].
Apoptotic injury is believed to be a significant contributor to the pathogenesis of a variety of disorders. For example, in the brains of patients with Alzheimer’s disease, apoptotic DNA fragmentation [42] and caspase activation has been observed [43]. Experimental models of Alzheimer’s disease also have identified apoptotic proteins in the brain [44]. Apoptotic neuronal nuclei and caspase 3 has been identified in the postmortem nigra of Parkinson’s disease patients, suggesting that neuronal loss during Parkinson’s disease is a result of apoptosis [45]. During cardiac transplant rejection, both early apoptotic membrane PS exposure and later caspase 3 activation has been reported [46]. Furthermore, injury to cells of the immune system, such as microglia, is believed to be mediated through apoptotic mechanisms [47,48].
Autophagy has three different categories termed microautophagy, macroautophagy and chaperone-mediated autophagy [49]. In general, autophagy allows cells to recycle cytoplasmic components, remove defective organelles andmaintain important cytoskeletal structures during development, cell differentiation and tissue remodeling [50]. Macroautophagy includes the bulk degradation of cytoplasmic material and the sequestration of the cytoplasmic protein and organelles into autophagosomes. The autophagosomes fuse with lysosomes for degradation and reuse by essential cellular processes [51]. In most descriptions of autophagy, macroautophagy is usually depicted. Microautophagy is the sequestration of cytoplasmic components by invagination of the lysosomal membrane. Vesicles subsequently formed are transferred to the lumen of the lysosomes for digestion. In chaperone-mediated autophagy, the cytoplasmic component is delivered by cytosolic chaperones to the receptors on the lysosomal membranes and the cellular organelle is translocated across lysosomal membranes into the lumen.
Induction of autophagy can occur through many environmental stimuli but is frequently described during oxidative stress and nutrient depletion (Figure 1). In some circumstances, activation of autophagy has been reported to be potentially cytoprotective such as during neurodegenerative disorders [52,53] and in the setting of prion protein-mediated neurotoxicity [54]. For example, in Parkinson’s disease, autophagy has been associated with the processing of the protein α-synuclein. Mutation of α-synuclein and accumulation of wild-type α-synuclein in dopaminergic neurons have been associated with progression of Parkinson’s disease [55]. Of the three types of autophagy, both chaperone-mediated autophagy and macroautophagy are involved in the degradation of α-synuclein. Chaperone-mediated autophagy appears to be more critical for the clearance of aberrant α-synuclein in neurons since inhibition of this autophagic pathway leads to accumulation of high molecular weight and detergent insoluble α-synuclein. This leads to neurotoxicity and further inhibition of chaperone-mediated autophagy [55]. Mutant α-synuclein, which is poorly internalized into lysosomes, is degraded by macroautophagy. As a result, activation of autophagic pathway protects against neurodegeneration and α-synuclein in Parkinson’s disease [52].
However in a number of scenarios, autophagy may be detrimental to cell survival. During cellular ischemia, autophagy results in cell death in cerebral astrocytes [56], spinal cord motor neurons [57] and cortical neurons [58]. Autophagic cell death also occurs during growth factor deprivation in Purkinje neurons [59] and in sympathetic neurons [60]. Other toxins such as glutamate, potassium deprivation and staurosporine lead to autophagy [59]. Interestingly, the pathways of autophagy and apoptosis may be closely aligned. For example, methamphatamine leads to cell death not only through apoptosis, but also through autophagy by inhibiting the disassociation of the Bcl-2/Beclin 1 complex [61]. Bcl-2/Bcl-xL is an antiapoptotic protein and a protein that blocks autophagy through its inhibitory interaction with Beclin 1 (Figure 1) [62]. In other circumstances, autophagy and apoptosis may have opposing roles. Some studies report that progression of apoptosis may conversely require the inhibition of autophagy [63–65]. Other work also suggests that some pathways may function as a switch between autophagy that is associated with cell survival and autophagy that is associated with cell death. For example, Draper, the Drosophila melanogaster ortholog of the Caenorhabditis elegans engulfment receptor CED-1, is required for autophagy during cell death in Drosophila salivary glands. Knockdown of Draper has been shown to prevent autophagy in dying salivary glands [66]. However, in the fat body in which autophagy is associated with cell survival, Draper knockdown does not prevent starvation-induced autophagy, suggesting that Draper can have dual roles during autophagy and may be one factor that can distinguish between autophagy-associated cell death and autophagy-associated cell survival [66].
3. The CCN family member WISP1
Initially identified as a downstream target of the wingless Wnt1 signaling pathway and in the mouse mammary epithelial cell line C57MG transformed by Wnt1, WISP1 was later associated with neoplastic growth in the gastrointenstinal tract [67]. WISP1 is expressed in several tissues including the epithelium, heart, kidney, lung, pancreas, placenta, ovaries, small intestine, spleen and brain. WISP1 is a member of the CCN family of proteins and is also known as CCN4. The CCN family of proteins is defined by the first three members of the family that include cysteine-rich protein 61, connective tissue growth factor and nephroblastoma overexpressed gene and consists of six secreted extracellular matrix-associated proteins. Each family member contains four cysteine-rich modular domains that include insulin-like growth factor-binding domain, thrombospondin domain, von Willebrand factor type C module and C-terminal cysteine knot-like domain. This connective tissue growth factor protein family has multiple cellular functions that include skeletal system development, vascular repair, cellular survival and extracellular matrix growth. In regards to the extracellular matrix, WISP1 can bind to leucine-rich proteoglycans that may affect the ability of other cells to anchor to the extracellular matrix [68].
WISP1 has been shown to have elevated expression during events such as cardiac ischemia [69], neuronal exposure to oxidative stress [70,71], lung epithelial damage [72] and cellular repair of fractured bone [73,74]. The increased expression of WISP1 may be suggestive of a reparative and regenerative process that is controlled by this CCN family member. Furthermore, WISP1 is a target of the wingless pathway Wnt1, a cysteine-rich glycosylated protein that controls neuronal development, angiogenesis, tumorigenesis and stem cell proliferation (Figure 2) [75,76].Wnt1 is upregulated during cortical injury [23], on endothelial cell [35,77] and pancreatic cell [78] exposure to elevated glucose [35,77], during spinal cord injury [79], in reactive central nervous system astrocytes [80], in settings of intestinal inflammation [81], during vascular cell aging [82] and during neurodegenerative disease [83]. Wnt1 prevents apoptotic cell injury during stroke injury to the brain [23], protects against ethanol toxicity in osteoblasts [84], blocks cell loss in dopaminergic neurons in models of Parkinson’s disease [85], limits vascular injury during experimental diabetes [35,77] and maintains microglial cell survival during Aβ exposure [21,34,86]. By contrast, inhibition or loss of Wnt1 signaling can lead to apoptosis [76,86–88] as well as the progression of autophagy [89].
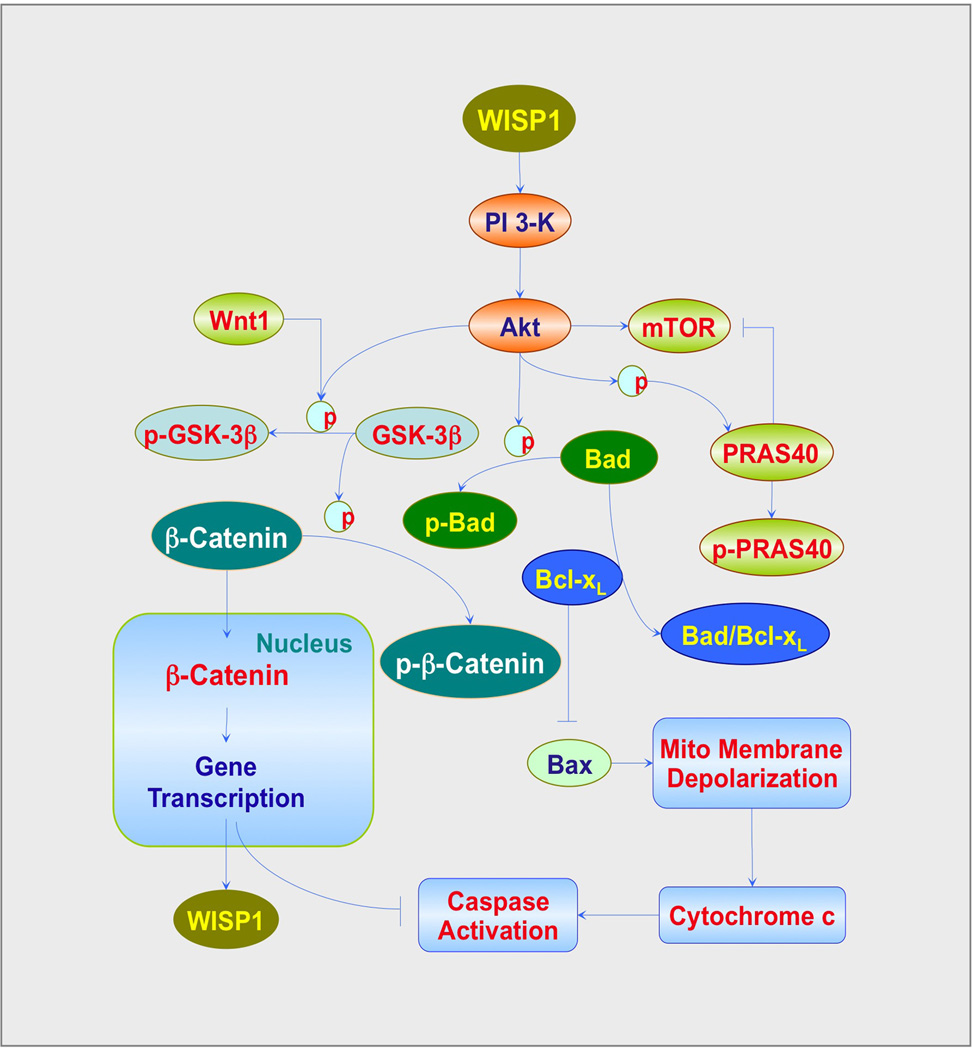
Figure 2. Pathways of WISP1, PI3K, Akt, β-catenin and mTOR that control cell fate.
WISP1 results in the activation of phosphoinositide 3-kinase (PI3K) and Akt (protein kinase B). Following activation, Akt can phosphorylate glycogen synthase kinase-3β (GSK-3β). The phosphorylated (p) GSK-3β loses its ability to phosphorylate β-catenin, allowing β-catenin to enter into the nucleus and promoting gene transcription that fosters WISP1 expression and leads to ‘anti-apoptotic’ protein production. Activation of Akt also phosphorylates the pro-apoptotic protein Bad. This process releases Bcl-xL from the binding to Bad to prevent Baxmediated mitochondrial (Mito) membrane depolarization, cytochrome c release and subsequent caspase activation. In addition, activation of the mammalian target of rapamycin (mTOR) is intimately involved in cell apoptosis and autophagy. Proline-rich Akt substrate 40 kDa (PRAS40) is one target of Akt phosphorylation. PRAS40 is an inhibitory protein of mTOR and its phosphorylation by Akt results in the loss of its ability to prevent mTOR activation.
In regards to WISP1, early work highlighted the ability of WISP1 to prevent p53-mediated DNA damage and apoptosis in kidney fibroblasts [90]. Recent studies have demonstrated both a proliferative and protective role against apoptotic cell injury forWISP1.WISP1may promote cardiac remodeling after myocardial infarction [69], stimulate lung tissue repair [72], lead to cardiomyocyte proliferation [91] and assist with vascular smooth muscle growth [92].WISP1 may be necessary to prevent cell death during bone fractures [73,74], to limit doxorubicin-induced cardiomyocyte death [93] and block oxygen--glucose deprivation injury in primary neuronal cells [70,71].
4. Cytoprotective canonical and non-canonical pathways of PI3K, Akt and β-catenin
Although WISP1 is a target of Wnt1, WISP1 utilizes cytoprotective pathways that are sometimes exclusive of the traditional wingless canonical and non-canonical signaling of Wnt1 (Figure 2). For example, WISP1 phosphorylates and activates Akt under conditions of cardiac tissue injury, oxidative stress, neuronal degeneration and vascular smooth muscle proliferation [70,90,92,93]. WISP1 protects against apoptotic injury through PI3K and Akt pathways. WISP1 has been shown to rely on PI3K and Akt to provide cytoprotection in renal fibroblasts [90], neurons [70,71] and cardiomyocytes [69,92,93]. WISP1 ultimately modulates apoptotic pathways of Bad, glycogen synthase kinase-3β (GSK-3β), Bim, Bcl-xL, mitochondrial membrane permeability, cytochrome c release and caspase activation to prevent cell injury [70]. PI3K and Akt are critical pathways to foster cellular proliferation and block apoptotic injury. During apoptotic injury, the PI3K/Akt pathway can enhance glial cell survival, maintain the integrity of endothelial cells, prevent neurodegeneration, promote cardioprotection and provide tolerance against oxidative stress [94]. Given the proliferative effects of the PI3K/Akt pathway, inhibition of this pathway may be desirable under some circumstances, such as to control tumor growth and promote apoptosis. Experimental strategies targeted to block activation of the PI3K/Akt pathway can suppress medulloblastoma growth [95], reduce colorectal cancer growth [96], increase radiosensitivity in tumors [97] and be beneficial to patients with gynecological malignancies [98]. In addition, inhibition of the PI3K/Akt pathway also can target tumor growth through the induction of autophagy. In oral squamous carcinoma cell lines, application of the agent erufosine that blocks Akt activity leads to cell death through autophagy [99]. Similar results of Akt inhibition that lead to autophagic cell death have been reported in ovarian cancer with other treatments [100].
In regards to the canonical pathways of Wnt1 [75,76], WISP1 controls the post-translational phosphorylation of β-catenin as well as the cellular trafficking of this protein (Figure 2). WISP1 can block phosphorylation of β-catenin in neurons that may be mediated through the inhibition of GSK-3β [71]. GSK-3β inhibition is known to prevent β-catenin phosphorylation [75]. WISP1 also can block GSK-3β activity in other cell systems such as cardiac cells [70,93]. During the inhibition of GSK-3β, β-catenin is not phosphorylated, ubiquinated or degraded and therefore can translocate to the nucleus to initiate ‘antiapoptotic’ pathways and prevent cellular apoptosis [88,101]. In addition to modulating the post-translational phosphorylation of β-catenin [82],WISP1 also controls the subcellular trafficking of β-catenin. In neurons [71], osteoclasts [102], vascular cells [82] and cardiomyocytes [93],WISP1 can increase nuclear expression of β β-catenin. In neurons, WISP1 through a PI3K-mediated pathway promotes the translocation of β-catenin from the cytoplasm of neurons to the nucleus that can allow for the transcription and eventual translation of pathways that can limit apoptotic cell death [71]. WISP1 requires β-catenin to limit neuronal cell injury during oxidative stress, since inhibition of β-catenin activity blocks neuroprotection against apoptosis by WISP1 [71]. The ability of WISP1 to control the phosphorylation and cellular trafficking of β-catenin also appears to be necessary for WISP1 to autoregulate its own expression. WISP1 expression is governed by β-catenin activity and WISP1 regulates its own expression through the ability of WISP1 to control β-catenin phosphorylation and nuclear translocation [71]. Although β-catenin also may function to limit cell injury through the blockade of autophagy [103,104], the ability of WISP1 to control β-catenin activity does not appear to alter autophagy progression, at least in primary neurons during oxidative stress [71].
5. Downstream mTOR signaling during apoptosis and autophagy
Given that PI3K and Akt are central pathways in WISP1 signaling and can determine the onset and progression of apoptosis and autophagy, consideration of intimately linked downstream pathways, such as mTOR, may offer new directions for the modulation of apoptosis and autophagy in multiple disorders (Figure 2). mTOR signaling is controlled through two protein complexes [105–107].mTOR Complex 1 (mTORC1) uses the regulatory-associated protein of mTOR (Raptor) protein to allow mTORC1 to bind to its substrates. mTORC1 consists of the proline-rich Akt substrate 40 kDa (PRAS40), the DEP domain-containing mTOR interacting protein (Deptor) and the mammalian lethal with Sec13 protein 8 (mLST8). mTORC1 controls the serine/threonine kinase ribosomal protein p70S6K and the eukaryotic initiation factor 4E-binding protein 1 (4EBP1). If 4EBP1 is not phosphorylated, it can block protein translation by binding to eukaryotic translation initiation factor 4 epsilon (eIF4E) through the eukaryotic translation initiation factor 4 gamma (eIF4G). Phosphorylation of 4EBP1 by mTORC1 leads to the dissociation of 4EBP1 from eIF4E to allow eIF4G to begin mRNA translation. mTORC1 phosphorylation also increases the kinase activity of p70S6K. Phosphorylation of p70S6K by mTORC1 leads to mRNA biogenesis, translation of ribosomal proteins and cellular proliferation. PRAS40 is an inhibitory protein and can block the binding of the mTORC1 substrates p70S6K and 4EBP1 to Raptor. mTORC2 is similar to mTORC1 in that it also is composed of mTOR, mLST8 and Deptor. mTORC2 is also different from mTORC1 since it contains Rictor as a component rather than Raptor. mTORC2 also associates with the mammalian stress-activated protein kinase interacting protein (mSIN1) and protein observed with Rictor-1 (Protor-1). Rictor is not sensitive to rapamycin and increases the activity of mTORC2. mTORC2 controls actin cytoskeleton organization, cell growth, endothelial cell survival and migration and cell cycle progression. Interestingly, mTORC2 can control the activity of Akt. mTORC2 phosphorylates Akt to lead to its activation. mTORC2 also controls protein kinase C (PKC), P-Rex1, P-Rex2, Rho GTPases and Rho signaling pathways that control cell-to-cell contact [108,109].
Both apoptosis and autophagy can be modulated by mTOR. Activation of mTOR signaling is usually protective during apoptosis. Oxidative stress has been shown to prevent mTOR kinase activity and results in apoptotic cell death in neuronal cells [110]. Inflammatory cells also can sustain apoptotic injury during oxidative stress if deprived of mTOR activation [86,111]. In addition, apoptotic cell death such as in dopaminergic neurons can be blocked during application of agents that increase mTOR activity [112]. During periods of serum deprivation that prevent mTOR activation, insulin has been shown to be unable to rescue cells from apoptotic injury unless mTOR activity is restored [113]. Other growth factors similar to insulin, such as erythropoietin (EPO), also have been shown to be dependent on mTOR activation for cytoprotection against apoptosis [47,86,114]. However, under other circumstances, inhibition of mTOR may increase cell survival and block apoptosis. For example, during Alzheimer’s disease, post-mitotic neurons that attempt to enter the cell cycle do not replicate, but ultimately succumb to apoptotic cell death [115,116]. In work that examines amyloid oligomer exposure, neurons can be prevented from entering the cell cycle during the inhibition of mTOR [117].
mTOR controls apoptotic cell death through 4EBP1 and p70S6K. Activation of p70S6K by mTOR blocks apoptosis through pathways that can increase ‘anti-apoptotic’ Bcl-2/Bcl-xL expression and inactivate the ‘pro-apoptotic’ protein BAD [118]. If mTOR is not active, 4EBP1 binds to eIF4E to result in the translation of pro-apoptotic proteins and cell death [119]. Prevention of apoptotic cell death by mTOR is dependent on Akt. mTOR requires Akt activation to protect endothelial cells against apoptosis [120] and mediate protection through the inactivation of forkhead transcription factors, such as FoxO3a [35,120]. Akt also functions to modulate apoptosis with mTOR through the inhibition of PRAS40. If PRAS40 activity is not prevented, activation of apoptotic pathways can ensue [121]. Phosphorylation of PRAS40 by Akt can block the activity of this substrate and lead to its dissociation from mTORC1 and binding to cytoplasmic 14-3-3 proteins [122].
Similar to studies with mTOR and apoptosis, activation of mTOR is not consistently cytoprotective during autophagy. It is possible that the degree of mTOR activation may be a significant variable. For example, during the early phases of autophagy, mTOR activity can be inhibited [123]. Reactivation of mTOR appears necessary to continue with autophagy, but increased mTOR activity can ultimately block autophagy [123]. In regards to cytoprotection, mTOR activation can prevent neurodegeneration during oxidative stress-mediated autophagy in dopamine neurons [112] and loss of mTOR activity results in autophagic cell death [100]. mTOR activation also appears vital to block autophagy in the cardiac system, limit forkhead transcription FoxO3a activity and prevent cardiac atrophy and dysfunction [124]. During normal physiology, mTOR activity may be required to regulate autophagy, since loss of mTOR activity leads to cardiomegaly and decreased cardiac contractility [125]. However, some chronic disease processes in the nervous or vascular systems may benefit from inhibition of mTOR to allow the progression of autophagy, as suggested in some models of Alzheimer’s disease [53]. Furthermore, the benefits of exercise may require a brief inactivation of mTOR for autophagic pathways to proceed [126].
6. Conclusion
Apoptosis (type I cell death) and autophagy (type II cell death) play a significant role in cell death for multiple disease entities that can include the nervous system, cardiovascular systems, skeletal system and immune system. As a result, development of novel therapeutic strategies that can target these processes are viewed with great excitement. The CCN family member WISP1 and its intimate relationship with canonical and noncanonical wingless signaling pathways offer a novel approach for modulating the pathways of apoptosis and autophagy. WISP1 can have increased expression in a variety of cells during injury that can involve cardiomyocytes, neurons, bone cells and lung cells, suggesting that WISP1 pathways are necessary for tissue repair and re-growth. WISP1 oversees critical cell survival and proliferation cell mechanisms that involve PI3K, Akt1 and β-catenin. Ultimately, these pathways can converge on mTOR signaling that can either repress or promote the induction of apoptosis or autophagy during normal physiology, acute illness or chronic degenerative disorders. Given the intimate relationship WISP1 holds with apoptotic and autophagic pathways that can affect multiple systems throughout the body, WISP1 and its signaling pathways offer promising and novel avenues for the future development of therapeutic strategies, especially for disorders that are currently without effective treatments.
7. Expert opinion
Central to the onset and progression of multiple disease entities are the pathways leading to cell demise through apoptosis or autophagy. Under conditions of toxic insults such as exposure to oxidative stress, activation of apoptosis and autophagy may be instrumental in leading to neurodegeneration, immune system dysfunction or cardiac disease. Yet, apoptotic and autophagic pathways may sometimes be necessary for normal physiological function and balance. Furthermore, prevention of cell death in some disease processes may require the initiation and progression of apoptosis or autophagy. For example, proper early neural development of the nervous system in a variety of organisms requires active apoptotic pathways that require Wnt signaling as well as other pathways [127,128]. Activation of autophagy also may be required during oxidant stress injury to the brain to prevent neuronal cell loss [22].
On the converse side, it is clear that in other disorders, inhibition of apoptosis and autophagy is necessary to prevent disease progression. The presence of apoptotic proteins in the brain may be a significant contributor to the progression of Alzheimer’s disease as well as alter immune system response [8,44]. During acute cerebral ischemia, the onset and progression of autophagy may ultimately increase brain infarction size [58]. Furthermore, cell death becomes more elaborate with evidence that apoptosis and autophagy may synergistically lead to cell death under some circumstances [61], but under other scenarios, apoptosis may oppose the progression of autophagy to result in cell death. For example, apoptosis can block autophagy by increasing caspase-mediated cleavage of Beclin 1 [64].
Given the complexities of cell death that are controlled by apoptosis and autophagy and the variable relationship between these processes of PCD, new therapeutic strategies must address a number of critical concerns. For example, why does it appear that processes such as autophagy appear to be protective during chronic disorders, such as neurodegeneration [52,53], but detrimental during acute injury such as cellular ischemia [56–58]? Are there cellular pathways that differ between acute and chronic disorders that can alter the modulation of apoptosis or autophagy to affect cellular survival? Are there also specific genetic pathways that can be altered by different toxic environments that determine the role of PCD pathways during disease? New work suggests that in some organisms this may be the case. Draper is required for autophagy during cell death in Drosophila salivary glands, but Draper does not appear to have a role with autophagy during starvation [66].
Future strategies for new treatments also must address the role of novel new targets, such as WISP1, PI3K, Akt, β-catenin and mTOR, during apoptotic and autophagic cell death. Although each of these pathways in combination or independently can be highly effective in controlling cell death during apoptosis or autophagy, several unanswered questions remain. For example, WISP1 can effectively limit injury in bone cells [73,74], cardiac cells [69], lung tissue [72] and neurons [70,71] during oxidative stress through blockade of apoptotic pathways. However, autophagy appears to have little or no role with WISP1 cytoprotection or cell death [71] even though pathways that are responsible for WISP1 cytoprotection such as PI3K, Akt and β-catenin are closely tied to modulating autophagic cell death [71,99]. Can cytoprotective pathways such as WISP1 alter endogenous cellular PCD pathways to have one PCD pathway become dominant over another? In addition, are specific cell types, such as neurons, more susceptible to one type of PCD over another dependent on age, toxin exposure or other variables? The specific pathways that are activated during cell injury also may influence biological outcome. In some disorders, mTOR activation is considered beneficial. mTOR signaling may prevent insulin resistance during diabetes mellitus [129] and lessen the toxicity of β-amyloid in the brain [86,130]. However, other parameters such as duration of activity with mTOR may influence outcome. Acute activation of mTOR yields cardioprotection [131], but long-term mTOR activity may lead to vasculopathy [132]. As a result, WISP1, PI3K, Akt, β-catenin and mTOR offer significant excitement and potential to target apoptotic and autophagic pathways in multiple disorders throughout the body. Yet, it is vital that well-focused future investigations provide essential knowledge of the intricate role these cellular targets play during complex PCD processes that involve apoptosis and autophagy for the successful translation and development of these pathways into robust and safe clinical treatment strategies.
Article Highlights.
Programmed cell death that is associated with apoptosis or autophagy can lead to pathological consequences in multiple disease entities through oxidative stress that can involve the nervous system, immune system, cardiac system, skeletal system, and vascular system
Apoptosis leads to cell death through an early phase that involves the exposure of membrane phosphatidylserine residues and a late phase that involves the destruction of genomic DNA
Autophagy recycles cytoplasmic components, removes defective organelles, and maintains important cytoskeletal structures, but can also determine whether a cell will survive a toxic insult
Apoptosis and autophagy can work in unison or in an opposing fashion to modulate cell survival in multiple systems of the body
WISP1, a target of the wingless Wnt1 pathway, is cytoprotective in a variety of tissues that include cells of the nervous system, cardiovascular system, renal system, and musculoskeletal system
WISP1 and its integrated pathways of PI 3-K, Akt, and β-catenin as well as the downstream signaling of mTOR govern apoptosis and autophagy during normal physiology as well as during cell injury
Acknowledgments
This research was supported by the following grants to K Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, NIH NIEHS, NIH NIA, NIH NINDS and NIH ARRA (NS059346-04 and NS059346-03S1).
Footnotes
Declaration of interest
The authors state no other conflicts of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Deretic V, Jiang S, Dupont N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012;22(8):397–406. doi: 10.1016/j.tcb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerr JF. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol. 1965;90:419–435. doi: 10.1002/path.1700900210. •• One of the initial studies that served to distinguish apoptotic cell death from necrotic cell death.
- 4.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Novikoff AB, Beaufay H, De Duve C. Electron microscopy of lysosomerich fractions from rat liver. J Biophys Biochem Cytol. 1956;2:179–184. •• Early work that identified lysosomes as a component of autophagy.
- 6. Clark SL., Jr Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957;3:349–362. doi: 10.1083/jcb.3.3.349. •• Early studies that identified the existence of autophagic vacuoles.
- 7.Maiese K, Chong ZZ, Shang YC, et al. Novel avenues of drug discovery and biomarkers for diabetes mellitus. J Clin Pharmacol. 2011;51:128–152. doi: 10.1177/0091270010362904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong ZZ, Shang YC, Wang S, et al. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiese K, Chong ZZ, Hou J, et al. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzen S, Cihaner SS, Coban T. Synthesis and comparison of antioxidant properties of indole-based melatonin analogue indole amino acid derivatives. Chem Biol Drug Des. 2012;79:76–83. doi: 10.1111/j.1747-0285.2011.01216.x. [DOI] [PubMed] [Google Scholar]
- 11.Yuan H, Wan J, Li L, et al. Therapeutic benefits of the group B3 vitamin nicotinamide in mice with lethal endotoxemia and polymicrobial sepsis. Pharmacol Res. 2012;65:328–337. doi: 10.1016/j.phrs.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Pearl R. The rate of living. London: University of London Press; 1928. [Google Scholar]
- 13.Zengi A, Ercan G, Caglayan O, et al. Increased oxidative DNA damage in lean normoglycemic offspring of type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2011;119:467–471. doi: 10.1055/s-0031-1275289. [DOI] [PubMed] [Google Scholar]
- 14.Vendelbo MH, Nair KS. Mitochondrial longevity pathways. Biochim Biophys Acta. 2011;1813:634–644. doi: 10.1016/j.bbamcr.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tupe RS, Tupe SG, Agte VV. Dietary nicotinic acid supplementation improves hepatic zinc uptake and offers hepatoprotection against oxidative damage. Br J Nutr. 2011;1–9 doi: 10.1017/S0007114510005520. [DOI] [PubMed] [Google Scholar]
- 16.Tanno M, Kuno A, Yano T, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J, Chong ZZ, Shang YC, et al. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jourde-Chiche N, Dou L, Cerini C, et al. Vascular incompetence in dialysis patients-protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–337. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 19.Nerurkar PV, Johns LM, Buesa LM, et al. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang YC, Chong ZZ, Hou J, et al. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009;6:223–238. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang YC, Chong ZZ, Hou J, et al. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22:1317–1329. doi: 10.1016/j.cellsig.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulose SM, Bielinski DF, Carrihill-Knoll K, et al. Exposure to (16) O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat Res. 2011;176:761–769. doi: 10.1667/rr2605.1. [DOI] [PubMed] [Google Scholar]
- 23.Chong ZZ, Shang YC, Hou J, et al. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3:153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raza SS, Khan MM, Ashafaq M, et al. Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: a behavioral, biochemical and immunohistological study in Wistar rats. J Neurol Sci. 2011;309:45–54. doi: 10.1016/j.jns.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Hou J, Chong ZZ, Shang YC, et al. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321:194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Stanojevic V, Brindamour LJ, et al. GLP1-derived nonapeptide GLP1 (28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol. 2012;213:143–154. doi: 10.1530/JOE-11-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cechetti F, Worm PV, Elsner VR, et al. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem. 2012;97:90–96. doi: 10.1016/j.nlm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Bailey TJ, Fossum SL, Fimbel SM, et al. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91:601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong ZZ, Kang J, Li F, et al. mGluRI targets microglial activation and selectively prevents neuronal cell engulfment through akt and caspase dependent pathways. Curr Neurovasc Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh PO. Nicotinamide attenuates the decrease of astrocytic phosphoprotein PEA-15 in focal cerebral ischemic injury. J Vet Med Sci. 2012;74:377–380. doi: 10.1292/jvms.11-0392. [DOI] [PubMed] [Google Scholar]
- 31.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: new paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contrib Nephrol. 2011;174:119–128. doi: 10.1159/000329249. [DOI] [PubMed] [Google Scholar]
- 33.Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong ZZ, Hou J, Shang YC, et al. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kook YH, Ka M, Um M. Neuroprotective cytokines repress PUMA induction in the 1-methyl-4-phenylpyridinium (MPP(+)) model of Parkinson’s disease. Biochem Biophys Res Commun. 2011;411:370–374. doi: 10.1016/j.bbrc.2011.06.151. [DOI] [PubMed] [Google Scholar]
- 37.Ullah N, Lee HY, Naseer MI, et al. Nicotinamide inhibits alkylating agent-induced apoptotic neurodegeneration in the developing rat brain. PLoS One. 2011;6:e27093. doi: 10.1371/journal.pone.0027093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Troy CM, Akpan N, Jean YY. Regulation of caspases in the nervous system implications for functions in health and disease. Prog Mol Biol Transl Sci. 2011;99:265–305. doi: 10.1016/B978-0-12-385504-6.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- 40.Chong ZZ, Lin SH, Kang JQ, et al. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71:659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 41.Shang YC, Chong ZZ, Hou J, et al. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broe M, Shepherd CE, Milward EA, et al. Relationship between DNA fragmentation, morphological changes and neuronal loss in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2001;101:616–624. doi: 10.1007/s004010000337. [DOI] [PubMed] [Google Scholar]
- 43.Louneva N, Cohen JW, Han LY, et al. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am J Pathol. 2008;173:1488–1495. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grammas P, Tripathy D, Sanchez A, et al. Brain microvasculature and hypoxia-related proteins in Alzheimer’s disease. Int J Clin Exp Pathol. 2011;4:616–627. [PMC free article] [PubMed] [Google Scholar]
- 45.Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson’s disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- 46.Narula J, Acio ER, Narula N, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7:1347–1352. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]
- 47.Shang YC, Chong ZZ, Wang S, et al. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011;198:270–285. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou X, Wang L, Wang M, et al. Emodin-induced microglial apoptosis is associated with TRB3 induction. Immunopharmacol Immunotoxicol. 2011;33:594–602. doi: 10.3109/08923973.2010.549135. [DOI] [PubMed] [Google Scholar]
- 49.Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61:272–280. doi: 10.2337/db11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Exp Neurol. 2010;223:28–37. doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva DF, Esteves AR, Oliveira CR, et al. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Curr Alzheimer Res. 2011;8:563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- 52.Spencer B, Potkar R, Trejo M, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong JK, Moon MH, Bae BC, et al. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res. 2012;73:99–105. doi: 10.1016/j.neures.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Xilouri M, Vogiatzi T, Vekrellis K, et al. Aberrant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS ONE. 2009;4:e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qin AP, Liu CF, Qin YY, et al. Autophagy. was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6:738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 57.Baba H, Sakurai M, Abe K, et al. Autophagy-mediated stress response in motor neuron after transient ischemia in rabbits. J Vasc Surg. 2009;50:381–387. doi: 10.1016/j.jvs.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Wang JY, Xia Q, Chu KT, et al. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 2011;70:314–322. doi: 10.1097/NEN.0b013e31821352bd. [DOI] [PubMed] [Google Scholar]
- 59.Canu N, Tufi R, Serafino AL, et al. Role of the autophagic-lysosomal system on low potassium-induced apoptosis in cultured cerebellar granule cells. J Neurochem. 2005;92:1228–1242. doi: 10.1111/j.1471-4159.2004.02956.x. [DOI] [PubMed] [Google Scholar]
- 60.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 61.Nopparat C, Porter JE, Ebadi M, et al. The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. J Pineal Res. 2010;49(4):382–389. doi: 10.1111/j.1600-079X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 62.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Carayol N, Vakana E, Sassano A, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci USA. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maiese K. The many facets of cell injury: angiogenesis to autophagy. Curr Neurovasc Res. 2012;9:1–2. doi: 10.2174/156720212800410911. [DOI] [PubMed] [Google Scholar]
- 66. McPhee CK, Logan MA, Freeman MR, et al. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature. 2010;24465:1093–1096. doi: 10.1038/nature09127. •• Early work that identified components of autophagy to involve lysosomes and autophagic vacuoles.
- 67.Davies SR, Davies ML, Sanders A, et al. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010;36:1129–1136. doi: 10.3892/ijo_00000595. [DOI] [PubMed] [Google Scholar]
- 68.Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. J Biol Chem. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 69.Colston JT, de la Rosa SD, Koehler M, et al. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007;293:H1839–H1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Chong ZZ, Shang YC, et al. Wnt1 inducible signaling pathway protein 1 (WISP1) blocks neurodegeneration through phosphoinositide 3 kinase/Akt1 and apoptotic mitochondrial signaling involving Bad, Bax, Bim, and Bcl-xL. Curr Neurovasc Res. 2012;9:20–31. doi: 10.2174/156720212799297137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang S, Chong ZZ, Shang YC, et al. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012;9:89–99. doi: 10.2174/156720212800410858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heise RL, Stober V, Cheluvaraju C, et al. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011;286:17435–17444. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.French DM, Kaul RJ, D’Souza AL, et al. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am J Pathol. 2004;165:855–867. doi: 10.1016/S0002-9440(10)63348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Macsai CE, Georgiou KR, Foster BK, et al. Microarray expression analysis of genes and pathways involved in growth plate cartilage injury responses and bony repair. Bone. 2012;50:1081–1091. doi: 10.1016/j.bone.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Maiese K, Li F, Chong ZZ, et al. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noguti J, CF DEM, Hossaka TA, et al. The role of canonical WNT signaling pathway in oral carcinogenesis: a comprehensive review. Anticancer Res. 2012;32:873–878. [PubMed] [Google Scholar]
- 77.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang YL, Ning Y, Ma XL, et al. Alteration of the proteome profile of the pancreas in diabetic rats induced by streptozotocin. Int J Mol Med. 2011;28:153–160. doi: 10.3892/ijmm.2011.696. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, et al. Differential expression of wnts after spinal cord contusion injury in adult rats. PLoS One. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.L’Episcopo F, Tirolo C, Testa N, et al. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Neurobiol Dis. 2011;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. doi: 10.1053/j.gastro.2010.05.037. 81 e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchand A, Atassi F, Gaaya A, et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011;10:220–232. doi: 10.1111/j.1474-9726.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 83.Wexler EM, Rosen E, Lu D, et al. Genome-wide analysis of a wnt1-regulated transcriptional network implicates neurodegenerative pathways. Sci signal. 2011;4:ra65. doi: 10.1126/scisignal.2002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen JR, Lazarenko OP, Shankar K, et al. A role for ethanol-induced oxidative stress in controlling lineage commitment of mesenchymal stromal cells through inhibition of Wnt/beta-catenin signaling. J Bone Miner Res. 2010;25(5):1117–1127. doi: 10.1002/jbmr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.L’Episcopo F, Tirolo C, Testa N, et al. Plasticity of subventricular zone neuroprogenitors in MPTP (1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/beta-catenin signaling pathways: functional consequences for neuroprotection and repair. J Neurosci. 2012;32:2062–2085. doi: 10.1523/JNEUROSCI.5259-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shang YC, Chong ZZ, Wang S, et al. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY) 2012;4:187–201. doi: 10.18632/aging.100440. •• Study identifies novel protection by the cytokine EPO against amyloid toxicity through combined pathways of Wnt1 and mTOR.
- 87.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu YL, Yang HP, Zhou XD, et al. The hypomethylation agent bisdemethoxycurcumin acts on the WIF-1 promoter, inhibits the canonical Wnt pathway and induces apoptosis in human non-small-cell lung cancer. Curr Cancer Drug Targets. 2011;11(9):1098–1110. doi: 10.2174/156800911798073041. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y, Wang F, Han L, et al. GABARAPL1 negatively regulates Wnt/beta-catenin signaling by mediating Dvl2 degradation through the autophagy pathway. Cell Physiol Biochem. 2011;27:503–512. doi: 10.1159/000329952. [DOI] [PubMed] [Google Scholar]
- 90. Su F, Overholtzer M, Besser D, et al. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16:46–57. doi: 10.1101/gad.942902. • Early work that identified the cytoprotective ability of WISP1 to block p53-mediated apoptotic injury in kidney fibroblasts.
- 91.Shanmugam P, Valente AJ, Prabhu SD, et al. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reddy VS, Valente AJ, Delafontaine P, et al. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol. 2011;226:3303–3315. doi: 10.1002/jcp.22676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Venkatesan B, Prabhu SD, Venkatachalam K, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010;22:809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baryawno N, Sveinbjornsson B, Eksborg S, et al. Small-molecule inhibitors of phosphatidylinositol 3-kinase/Akt signaling inhibit Wnt/beta-catenin pathway cross-talk and suppress medulloblastoma growth. Cancer Res. 2010;70:266–276. doi: 10.1158/0008-5472.CAN-09-0578. [DOI] [PubMed] [Google Scholar]
- 96.Chung CY, Park YL, Song YA, et al. Knockdown of RON Inhibits AP-1 activity and induces apoptosis and cell cycle arrest through the modulation of Akt/FoxO signaling in human colorectal cancer cells. Dig Dis Sci. 2012;57:371–380. doi: 10.1007/s10620-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 97.Fokas E, Yoshimura M, Prevo R, et al. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat Oncol. 2012;7:48. doi: 10.1186/1748-717X-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kapoor V, Zaharieva MM, Das SN, et al. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012;319:39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 100.Le XF, Mao W, Lu Z, et al. Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010;116(21):4980–4990. doi: 10.1002/cncr.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Case N, Ma M, Sen B, et al. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen TM, Subramanian IV, Xiao X, et al. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 beta-catenin levels. J Cell Mol Med. 2009;13:3687–3698. doi: 10.1111/j.1582-4934.2009.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Ding L, Wang X, et al. Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells. Am J Transl Res. 2012;4:44–51. [PMC free article] [PubMed] [Google Scholar]
- 105.Chong ZZ, Maiese K. Mammalian target of rapamycin signaling in diabetic cardiovascular disease. Cardiovasc Diabetol. 2012;11:45. doi: 10.1186/1475-2840-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chong ZZ, Shang YC, Maiese K. Cardiovascular disease and mTOR signaling. Trends Cardiovasc Med. 2011;21:151–155. doi: 10.1016/j.tcm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang SK, Kim HH. The functions of mTOR in ischemic diseases. BMB Rep. 2011;44:506–511. doi: 10.5483/bmbrep.2011.44.8.506. [DOI] [PubMed] [Google Scholar]
- 108.Benjamin D, Colombi M, Moroni C, et al. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 109.Chong ZZ, Shang YC, Zhang L, et al. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen L, Xu B, Liu L, et al. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab Invest. 2010;90:762–773. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- 112.Choi KC, Kim SH, Ha JY, et al. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010;112:366–376. doi: 10.1111/j.1471-4159.2009.06463.x. [DOI] [PubMed] [Google Scholar]
- 113.Wu X, Reiter CE, Antonetti DA, et al. Insulin promotes rat retinal neuronal cell survival in a p70S6K-dependent manner. J Biol Chem. 2004;279:9167–9175. doi: 10.1074/jbc.M312397200. [DOI] [PubMed] [Google Scholar]
- 114.Kim J, Jung Y, Sun H, et al. Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem. 2012;113:220–228. doi: 10.1002/jcb.23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu Y, Ren QG, Zhang ZH, et al. Phospho-Rb mediating cell cycle reentry induces early apoptosis following oxygen-glucose deprivation in rat cortical neurons. Neurochem Res. 2012;37:503–511. doi: 10.1007/s11064-011-0636-6. [DOI] [PubMed] [Google Scholar]
- 117.Bhaskar K, Miller M, Chludzinski A, et al. The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Mol Neurodegener. 2009;4:14. doi: 10.1186/1750-1326-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pastor MD, Garcia-Yebenes I, Fradejas N, et al. mTOR/S6 kinase pathway contributes to astrocyte survival during ischemia. J Biol Chem. 2009;284:22067–22078. doi: 10.1074/jbc.M109.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang D, Contu R, Latronico MV, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282:23679–23686. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thedieck K, Polak P, Kim ML, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS One. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nascimento EB, Snel M, Guigas B, et al. Phosphorylation of PRAS40 on Thr246 by PKB/AKT facilitates efficient phosphorylation of Ser183 by mTORC1. Cell Signal. 2010;22:961–967. doi: 10.1016/j.cellsig.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Yu L, McPhee CK, Zheng L, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schips TG, Wietelmann A, Hohn K, et al. FoxO3 induces reversible cardiac atrophy and autophagy in a transgenic mouse model. Cardiovasc Res. 2011;91:587–597. doi: 10.1093/cvr/cvr144. [DOI] [PubMed] [Google Scholar]
- 125.Jaber N, Dou Z, Chen JS, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ogura Y, Iemitsu M, Naito H, et al. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun. 2011;414:756–760. doi: 10.1016/j.bbrc.2011.09.152. [DOI] [PubMed] [Google Scholar]
- 127.Maiese K, Chong ZZ, Shang YC, et al. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets. 2008;12:905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274:233–244. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 129.Wang RH, Kim HS, Xiao C, et al. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lafay-Chebassier C, Perault-Pochat MC, Page G, et al. The immunosuppressant rapamycin exacerbates neurotoxicity of Abeta peptide. J Neurosci Res. 2006;84:1323–1334. doi: 10.1002/jnr.21039. [DOI] [PubMed] [Google Scholar]
- 131.Hernandez G, Lal H, Fidalgo M, et al. A novel cardioprotective p38-MAPK/mTOR pathway. Exp Cell Res. 2011;317:2938–2949. doi: 10.1016/j.yexcr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sinha SS, Pham MX, Vagelos RH, et al. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. Am Heart J. 2008;155:889. doi: 10.1016/j.ahj.2008.02.004. e1-6. [DOI] [PubMed] [Google Scholar]