Abstract
Purpose
To evaluate effects of radiotherapy, chemotherapy, cigarette smoking and alcohol consumption on the risk of second primary salivary gland cancer (SGC) in the Childhood Cancer Survivor Study (CCSS).
Methods
Standardized incidence ratios (SIR) and excess absolute risks (EAR) of SGC in the CCSS were calculated using incidence rates from Surveillance, Epidemiology and End Results population-based cancer registries. Radiation dose to the salivary glands was estimated based on medical records. Poisson regression was used to assess risks with respect to radiation dose, chemotherapy, smoking and alcohol consumption.
Results
During the time period of the study, 23 cases of SGC were diagnosed among 14,135 childhood cancer survivors. The mean age at diagnosis of the first primary cancer was 8.3 years, and the mean age at SGC diagnosis was 24.8 years. The incidence of SGC was 39-fold higher in the cohort than in the general population (SIR=39.4; 95% CI: 25.4–7.8). The EAR was 9.8 per 100,000 person years. Risk increased linearly with radiation dose (excess relative risk=0.36 per gray; 95% CI: 0.06 to 2.5) and remained elevated after 20 years. There was no significant trend of increasing risk with increasing dose of chemotherapeutic agents, pack-years of cigarette smoking or alcohol intake.
Conclusion
While the cumulative incidence of SGC was low, childhood cancer survivors treated with radiation experienced significantly increased risk for at least two decades following exposure, and risk was positively associated with radiation dose. Results underscore the importance of long-term follow up of childhood cancer survivors for the development of new malignancies.
Introduction
Exposure to ionizing radiation is a widely accepted risk factor for salivary gland carcinoma (SGC). Supportive evidence has accumulated from cohort studies of patients treated with head and neck irradiation for benign childhood conditions (1, 2), patients irradiated for the treatment of malignancies (3–6), and from studies of atomic bomb survivors (7, 8); however, the magnitude of radiation-related risk for SGC is uncertain. Some studies suggested that young children are more susceptible to radiation-related salivary gland tumors than older individuals (1, 4, 9), but relatively little quantitative information is available concerning risks following radiation exposures early in life. Many childhood cancer patients are treated with substantial doses of radiation to the head and neck region, and even chest irradiation, such as from mantle radiotherapy for Hodgkin lymphoma, can result in appreciable scatter doses to the salivary glands. With respect to possible risk factors other than ionizing radiation, few data are available concerning risk of SGC following chemotherapy, and the evidence concerning cigarette smoking and alcohol consumption is mixed (10–16). We undertook a cohort analysis of second primary SGC using the unique resource of a large cohort of childhood cancer survivors that provides the opportunity to improve knowledge about therapy-related risk factors for SGC and the role of cigarette smoking and alcohol consumption.
Methods
Study Population
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort study of childhood cancer survivors that was established in 1994. Eligibility criteria included: diagnosis before age 21 years with leukemia, central nervous system (CNS) cancer, Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), renal tumor, neuroblastoma, soft-tissue sarcoma or bone sarcoma between January 1, 1970 and December 31, 1986 at one of 26 collaborating institutions in the US and Canada, and survival for at least 5 years after diagnosis. The CCSS study protocol and contact documents were approved by institutional review boards at each participating medical institution. The study design for constructing the cohort and collecting treatment, risk factor and outcome information has been described in detail previously (17, 18). A baseline, self-administered questionnaire sent in 1994 obtained data for demographic characteristics, education, income, employment history, marital status, height, weight, personal health habits, family history of cancer, use of medications, reproductive history, new malignant disorders, and other health outcomes. Among the 20,346 eligible participants, 14,135 (69.5%) were located, agreed to participate, and completed a questionnaire. Information on the cohort was updated in periodic follow-up mail surveys, which elicited further, although less extensive, information. Each survey inquired about the occurrence of new cancers. Copies of questionnaires are available at http://ccss.stjude.org.
Copies of radiotherapy (RT) records for the first cancer diagnosis and treatment were obtained from the treating institution and forwarded to the collaborating radiation dosimetry center. Chemotherapy (CT) data were abstracted from medical records using uniform data-abstraction procedures across institutions. Abstraction of CT information included the beginning and ending dates of all chemotherapy agents and cumulative doses (mg/m2) and routes of administration for 22 specific agents. Abstract forms are available at http://ccss.stjude.org. An alkylating agent score was calculated according to the method of Tucker et al (19).
Pathology reports were obtained and reviewed by the study pathologist to verify self-reported cancers. Questionnaires administered through the year 2004 identified 24 pathologically verified subsequent primary salivary gland carcinomas in the cohort. One case was subsequently excluded because the diagnosis occurred less than 5 years after the diagnosis of the first primary malignancy, leaving 23 cases for analysis.
Radiation Dosimetry
Dose to the salivary glands (average of parotid, submandibular, and submaxillary regions) was estimated for each patient who received radiotherapy for their initial cancer. Dose calculations were based on 26 points located in the three salivary gland regions. If the salivary glands were outside the beam, doses were estimated using measurements in a water phantom (20). If the salivary glands were in the beam, dose was derived using standard radiotherapy techniques (20). Each patient’s dosimetry was assigned a quality score on the basis of the completeness of the records received and the proximity of the salivary glands to the treatment beam. Ninety-one percent of patients who received RT had records adequate for dosimetry.
Statistical Analysis
We calculated standardized incidence ratios (SIR) as the ratio of observed (O) to expected (E) numbers of SGC (O/E). Expected numbers of cases were based on incidence rates from the Surveillance, Epidemiology and End Results (SEER) cancer registries (21). Person-years (PY) of follow-up began five years after diagnosis of the first cancer and continued to the earliest of the following: date of SGC diagnosis, date of death, date of last known follow-up or close of study date (January 1, 2005). Cancer incidence rates in SEER were calculated according to gender, race, 5-year age groups and 5-year calendar intervals, and multiplied by the accumulated number of PY at risk in each category to determine expected numbers of cases. The excess absolute risk (EAR) was calculated as the difference between observed and expected numbers of SGC cases, expressed per 100,000 PY.
Cumulative incidence of SGC as second primary cancer, adjusted for competing risks, was calculated according to the method of Gooley et al (22), beginning five years after diagnosis of the first cancer.
Poisson regression analysis was used to estimate relative risks (RR) and 95% confidence intervals (CI) for SGC according to the variables shown in Tables 2 and 3. The expected number (E) of SGC (defined above) was used as an offset to provide indirect adjustment for sex, race, attained age and attained calendar period (23). Analyses were also adjusted for the identified decline in the SIR with attained age by including attained age as a continuous variable. Analyses evaluating RR for chemotherapy variables were additionally adjusted for radiation dose by including it as a linear variable. Analyses evaluating treatment variables were restricted to patients whose radiation doses could be estimated. The model that was used in analyses with adjustment for both attained age and radiation dose is as follows:
Table 2.
Risk of subsequent salivary gland carcinoma among 14,135 five-year survivors of childhood cancer diagnosed between 1970–1986 and followed through 2004, with respect to demographic, clinical and lifestyle characteristics.
| Characteristics | O | SIR | 95% CI | RR* | 95% CI | P† |
|---|---|---|---|---|---|---|
| Total | 23 | 39.4 | 25.4–57.8 | ~ | ||
| Gender | ||||||
| Male | 13 | 48.7 | 26.8–80.2 | 1.0 | ||
| Female | 10 | 31.6 | 15.8–55.4 | 0.7 | 0.3–1.5 | 0.34 |
| Age at diagnosis of the first cancer (years) | ||||||
| ≤ 4 | 9 | 54.3 | 26.1–97.8 | 1.0 | ||
| 5–9 | 3 | 24.2 | 6.0–62.8 | 0.6 | 0.1–2.0 | |
| 10–14 | 3 | 21.5 | 5.3–55.8 | 0.8 | 0.2–3.0 | 0.20‡ |
| 15–20 | 8 | 51.9 | 23.7–96.6 | 2.8 | 0.8–10.6 | |
| Original diagnosis | ||||||
| Leukemia@ | 10 | 59.3 | 29.7–104.0 | 1.0 | ||
| HL | 6 | 54.0 | 21.5–109.4 | 1.5 | 0.5 – 4.3 | 0.007 |
| NHL | 4 | 85.2 | 26.4–197.8 | 1.8 | 0.5 – 5.6 | |
| Other | 3 | 11.7 | 2.9 – 30.3 | 0.2 | 0.05 – 0.72 | |
| Attained age (years) | ||||||
| 5–14 | 6 | 138.7 | 55.1–281 | 1.0 | ||
| 15–19 | 3 | 29.4 | 7.3–76.3 | 0.2 | 0.04–0.8 | |
| 20–29 | 7 | 31.5 | 13.6–60.9 | 0.2 | 0.1–0.7 | 0.061‡ |
| 30–39 | 6 | 36.3 | 14.4–73.6 | 0.3 | 0.1–0.8 | |
| ≥ 40 | 1 | 19.6 | 1.1–86.4 | 0.1 | 0.01–0.8 | |
| Latency (years)§ | ||||||
| 5–9 | 3 | 31.8 | 7.9–82.5 | 1.0 | ||
| 10–14 | 10 | 73.1 | 36.6–128.2 | 2.7 | 0.8–12.3 | |
| 15–20 | 5 | 32.3 | 11.6–69.4 | 1.5 | 0.3–8.2 | >0.50‡ |
| > 20 | 5 | 25.3 | 9.1–54.4 | 1.6 | 0.3–9.9 | |
| Cigarette smoking, pack-years | ||||||
| Never | 17 | 41.4 | 24.7–64.3 | 1.0 | ||
| 0.05–1.25 | 0 | 0.0 | 0.0–14.1 | 0.0 | 0,0–1.6 | 0.26 |
| 1.3–4 | 0 | 0.0 | 0.0–18.5 | 0.0 | 0.0–1.7 | |
| 4.2–9.6 | 2 | 60.6 | 10.1–187.2 | 1.7 | 0.3–6.0 | 0.35‡ |
| >9.6 | 2 | 47.2 | 7.8–145.7 | 1.6 | 0.2–5.7 | |
| Unknown | 2 | 88.1 | 14.7–271.9 | 2.2 | 0.3–7.5 | |
| Alcohol consumption, grams/day‖ | ||||||
| 0 | 5 | 32.5 | 11.7–69.8 | 1.0 | ||
| <27.4 | 5 | 53.6 | 19.2–115.1 | 2.1 | 0.6–7.7 | 0.10 |
| 41.1 | 4 | 56.4 | 17.5–131.0 | 2.2 | 0.5–8.6 | |
| 54.8–109.6 | 5 | 61.2 | 22.0–131.6 | 2.4 | 0.7–9.0 | >0.5‡ |
| > 109.6 | 1 | 13.5 | 0.8–59.4 | 0.5 | 0.03 – 3.4 | |
| Missing | 3 | 27.5 | 6.8–71.2 | 0.9 | 0.2–3.8 | |
Abbreviations: O: Observed number of cases; SIR: standardized incidence ratio; CI: confidence interval; RR: Relative risk; HL: Hodgkin lymphoma; CNS: central nervous system; NHL: non-Hodgkin lymphoma; ~: not applicable.
All relative risks (RR) were adjusted for attained age and radiation dose.
PHET: P for heterogeneity (refer to text for details).
PTrend: P for trend (refer to text for details).
Leukemia was chosen as the reference category because it had the largest number of cases.
Patients were included in the study if they survived at least 5 years after diagnosis of their first cancer.
A standard drink in the U.S. is equal to 13.7 grams (0.6 ounces) of pure alcohol or 12-ounces of beer, 8-ounces of malt liquor, 5-ounces of wine, 1.5-ounces or a “shot” of 80-proof distilled spirits or liquor (e.g., gin, rum, vodka, or whiskey). 12-ounce beer has about the same amount of alcohol as one 5-ounce glass of wine, or 1.5-ounce shot of liquor. It is the amount of ethanol consumed that affects a person most, not the type of alcoholic drink.
Table 3.
Risk of subsequent salivary gland carcinoma among 14,135 five-year survivors of childhood cancer diagnosed between 1970–1986 and followed through 2004 with respect to treatment received for the primary cancer.
| Characteristics | O | SIR | 95% CI | RR* | 95% CI | P† |
|---|---|---|---|---|---|---|
| Total | 23 | 39.4 | 25.4–57.8 | |||
| Unknown radiation dose excluded‡ | 20 | 39.4 | 25.4–61.0 | |||
| Treatment modalities (CT and RT) | ||||||
| No RT, No CT | 0 | 0.0 | 0.0–47.0 | 1.0 | 0.0–1.0 | |
| CT only | 2 | 18.0 | 3.0–55.5 | 0.3 | 0.053–1.3 | |
| RT only | 4 | 51.3 | 15.9–119.1 | 1.1 | 0.3–3.2 | >0.5 |
| RT and CT combined | 14 | 51.7 | 29.1–83.6 | 1.0 | referent | |
| Radiation dose, Gy (mean)§ | ||||||
| 0 | 2 | 13.1 | 2.2–40.6 | 1.0 | ||
| >0–2.9 (1.4) | 5 | 27.9 | 10.0–60.0 | 2.1 | 0.4–14.5 | |
| 3.0 –11.4 (6.6) | 5 | 73.6 | 26.4–158.1 | 5.7 | 1.2–39.6 | |
| 11.5–80.4 (21.6) | 8 | 73.5 | 33.6–136.7 | 7.2 | 1.7–48.3 | 0.005 |
| ERR/Gy=0.36 (.06 to 2.5) | ||||||
| Chemotherapy (yes/no) | ||||||
| Alkylating agents | 13 | 60.4 | 33.2–99.4 | 2.5 | 0.9–7.9 | 0.065 |
| Anthracyclines | 7 | 42.9 | 18.4–82.9 | 1.6 | 0.6–4.2 | 0.34 |
| Alkylating agents score‖ | ||||||
| 0 | 5 | 20.2 | 7.2–43.4 | 1.0 | ||
| 1 | 8 | 85.3 | 39.0–158.7 | 4.1 | 1.4–13.7 | |
| 2 | 3 | 43.6 | 10.9–113.1 | 2.0 | 0.4–8.1 | >0.5 |
| 3 | 2 | 38.0 | 6.3–117.3 | 1.1 | 0.2–5.3 | |
| Unknown | 2 | 44.4 | 7.4–136.9 | 1.6 | 0.2–7.5 | |
| Anthracyclines, mg/m2 (mean)§ | ||||||
| None | 11 | 34.0 | 17.7–58.2 | 1.0 | ||
| 0.12–174 (109.7) | 2 | 57.9 | 9.6–178.9 | 1.8 | 0.3–6.7 | |
| 175–290 (227.9) | 2 | 49.9 | 8.3–154.0 | 1.8 | 0.3–6.8 | |
| 291–390 (339.1) | 1 | 24.4 | 1.4–107.2 | 0.9 | 0.05–4.8 | 0.42 |
| 391–8370 (498.8) | 2 | 42.1 | 7.0–129.8 | 2.0 | 0.3–7.7 | |
| Unknown | 2 | 94.1 | 15.6–290.4 | 3.1 | 0.5–11.6 | |
Abbreviations: O: Observed number of cases; SIR: standardized incidence ratio; CI: confidence interval; RR: Relative risk; ~: not applicable.
All relative risks were adjusted for attained age. Relative risks for chemotherapy variables were also adjusted for radiation dose.
P for linear trend except for treatment modalities, for which a test of heterogeneity was done.
Radiation dose was missing for 3 salivary gland carcinomas.
Mean value of the range.
Dose scores were assigned to individual alkylating agents on the basis of doses (mg/m2) for each agent, and these scores were then summed across agents.
If Oi and Ei indicate the number of observed and expected cancers, respectively, in a specified group (such as a chemotherapy category), then the statistical expectation of Oi is given by Ei exp(αi + β a)[1 + γ d], where a is attained age in years, d is radiation dose in gray (Gy), α, β and γ are parameters to be estimated, and exp(αi) is the RR for group i.
Two-sided P-values and 95% confidence intervals (CI) were based on the likelihood ratio statistic. Analyses were implemented with the AMFIT module of the software package Epicure (24).
Results
General characteristics of the cohort are shown in Table 1. Of the 14,135 5-year survivors diagnosed between 1970 and 1986, 53.7% were males, and the mean age at diagnosis of the first cancer was 8.3 years. The most common cancer diagnoses were leukemia (33.6%), HL (13.4%), and cancer of the CNS (13.1%). Of the total cohort, 13,279, 12,152 and 8,173 were followed 10+, 15+, and 20+ years after diagnosis of the initial cancer, respectively. At the time of this analysis, 87% of patients were still alive. Sixty-eight percent of the cohort received radiotherapy (excluding those with unknown treatment), and, among them, the average dose to the salivary glands was 7.5 Gy (median, 2.9 Gy; maximum, 80.4 Gy). More than half of the patients received combined RT and CT. Tobacco use was reported in 23% of patients. During the time period of follow-up (228,439 PY), 23 subsequent SGC were diagnosed, among which 10 cases occurred in patients initially treated for leukemia, 6 among HL patients and 4 among NHL patients. The mean interval between diagnosis of the initial cancer and SGC was 14.9 years (median, 12.6 years), and the mean age at diagnosis of SGC was 24.8 years. Most SGC occurred in the parotid gland (O=22), with 1 case observed in the submandibular gland. Fourteen SGC cases were mucoepidermoid carcinoma, 3 were adenocarcinoma, 3 were acinar cell carcinoma and 3 were miscellaneous other types.
Table 1.
Demographic, clinical and lifestyle characteristics of 14,135 five-year survivors of childhood cancer diagnosed between 1970 and 1986.
| Characteristic | Number of Patients |
% | Person-years of follow-up |
Salivary gland cancers |
|---|---|---|---|---|
| All patients | 14,135 | 100 | 228,439 | 23 |
| Gender | ||||
| Male | 7,585 | 53.7 | 120,303 | 13 |
| Female | 6,550 | 46.3 | 108,136 | 10 |
| Race | ||||
| White | 13,166 | 93.1 | 214,394 | 22 |
| Black | 713 | 5.1 | 10,402 | 0 |
| Other | 256 | 1.8 | 3,643 | 1 |
| Age at diagnosis of the first cancer (years) | ||||
| ≤ 4 | 5,658 | 32.3 | 91,524 | 9 |
| 5–9 | 3,138 | 30.1 | 50,668 | 3 |
| 10–14 | 2,869 | 20.3 | 46,460 | 3 |
| 15–20 | 2,470 | 17.3 | 39,787 | 8 |
| Original diagnosis | ||||
| Leukemia | 4,741 | 33.6 | 74,003 | 10 |
| HL | 1,902 | 13.4 | 32,414 | 6 |
| CNS cancer | 1,853 | 13.1 | 28,227 | 1 |
| Kidney cancer | 1,240 | 8.8 | 20,794 | 0 |
| Soft tissue cancer | 1,226 | 8.7 | 20,763 | 1 |
| Bone cancer | 1,175 | 8.3 | 18,961 | 0 |
| NHL | 1,059 | 7.5 | 17,316 | 4 |
| Neuroblastoma | 939 | 6.6 | 15,961 | 1 |
| Calendar year of initial cancer diagnosis | ||||
| 1970–1974 | 2,516 | 17.7 | 55,447 | 4 |
| 1975–1979 | 4,016 | 28.3 | 73,918 | 7 |
| 1980–1986 | 7,603 | 54.0 | 99,074 | 12 |
| No of patients entering follow-up interval (years) | ||||
| 5–9 | 14,135 | 100 | 68,458 | 3 |
| 10–14 | 13,279 | 93.9 | 63,811 | 10 |
| 15–19 | 12,152 | 86.0 | 52,677 | 5 |
| ≥ 20 | 8,173 | 57.8 | 29,683 | 5 |
| Treatment modalities (CT and RT)* | ||||
| No RT, No CT | 911 | 6.4 | 15,438 | 0 |
| CT only | 3,022 | 21.4 | 46,863 | 2 |
| RT only | 1,501 | 10.6 | 26,915 | 4 |
| RT and CT combined | 6,934 | 49.1 | 112,239 | 16 |
| Missing all treatment data | 1,767 | 12.5 | 26,984 | 1 |
| Cigarette smoking (pack-years) | ||||
| Never | 10,264 | 72.6 | 165,568 | 17 |
| 0.05–1.25 | 678 | 4.8 | 12,175 | 0 |
| 1.3–4 | 643 | 4.5 | 11,426 | 0 |
| 4.2–9.6 | 611 | 4.3 | 11,414 | 2 |
| >9.6 | 631 | 4.5 | 12,668 | 2 |
| Unknown | 1,308 | 9.3 | 15,188 | 2 |
| Alcohol consumption (grams/day)† | ||||
| 0 | 4,915 | 34.8 | 71,215 | 5 |
| <27.4 | 1,696 | 12.0 | 31,822 | 5 |
| 41.1 | 1,313 | 9.3 | 24,204 | 4 |
| 54.8–109.6 | 1,532 | 10.8 | 28,576 | 5 |
| > 109.6 | 1,431 | 10.1 | 26,815 | 1 |
| Missing | 3,248 | 23.0 | 45,807 | 3 |
Abbreviations: SGC: Salivary gland carcinoma; HL: Hodgkin lymphoma; CNS: central nervous system; NHL: non-Hodgkin lymphoma; CT: Chemotherapy; RT: Radiotherapy.
Information on chemotherapy was not available for 1786 patients; Information on radiotherapy was not available for 1801 patients; Information on surgery was not available for 1804 patients.
A standard drink in the U.S. is equal to 13.7 grams (0.6 ounces) of pure alcohol or 12-ounces of beer, 8-ounces of malt liquor, 5-ounces of wine, 1.5-ounces or a “shot” of 80-proof distilled spirits or liquor (e.g., gin, rum, vodka, or whiskey). 12-ounce beer has about the same amount of alcohol as one 5-ounce glass of wine, or 1.5-ounce shot of liquor. It is the amount of ethanol consumed that affects a person most, not the type of alcoholic drink.
The occurrence of SGC in the cohort was 39-fold higher than that predicted for the general population (SIR=39.4, 95% CI: 25.4 to 57.8), and the SIR remained significantly elevated more than 20 years after diagnosis of the first cancer (Table 2). The EAR was 9.8 per 100,000 PY and was similar for males (EAR=10.8/100,000) and females (EAR=9.2/100,000). The SIR was highest among those whose first cancer was diagnosed before age 5 or after age 14. The cumulative incidence between 5 and 25 years after the initial cancer diagnosis was 0.24%. Elevated risks were seen for adenocarcinoma (SIR=171.1, 95% CI: 55.2 to 530.7), mucoepidermoid carcinoma (SIR=57.7, 95% CI: 34.2–97.5), and acinar cell carcinoma (SIR=16.9, 95% CI: 5.5–2.5), based on small numbers. The highest SIRs were observed for persons whose first cancer was NHL, leukemia or HL.
Whereas the SIRs in Table 2 are adjusted only for gender, race, attained age and attained calendar year and are based on external comparisons, the RRs obtained from the internal Poisson regression analysis are further adjusted for radiation dose and for a trend in the SIRs with attained age. Internal analyses yielded RRs that were considerably smaller than the SIRs (Table 2). The risk of SGC was higher for persons followed for more than ten years than for those followed for less than ten years, but the trend over time was not significant. There was no significant association of risk with age at diagnosis of first cancer. Elevated risk for NHL, HL and leukemia as the initial cancer, relative to other cancers (of which there were only three), persisted after adjustment for radiation dose.
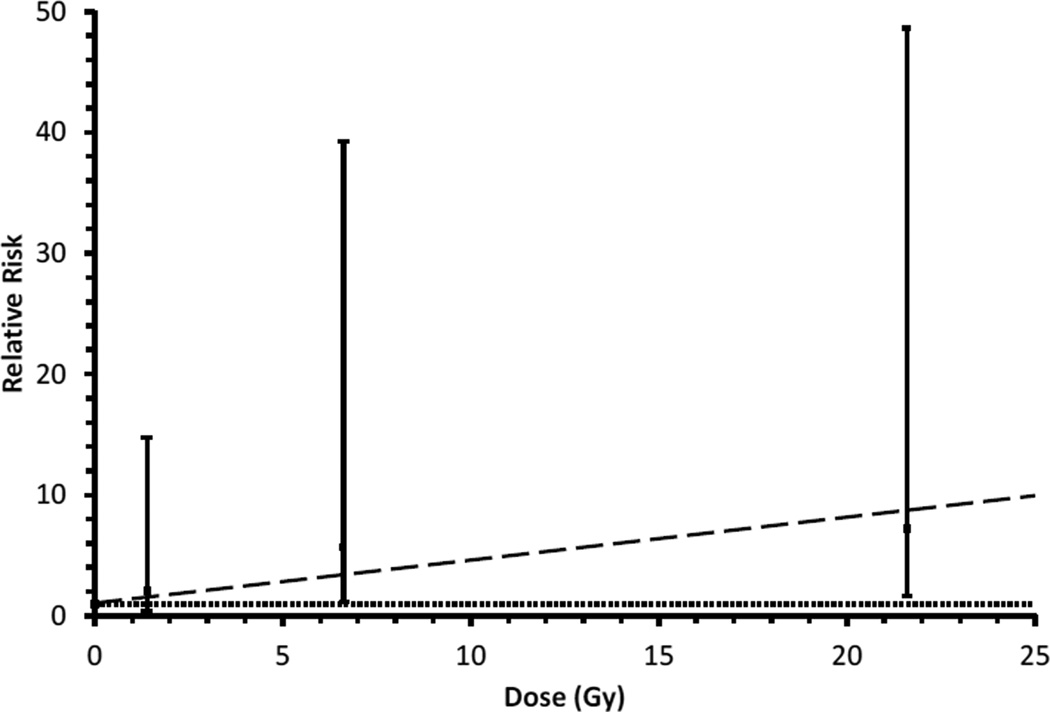
Overall, patients treated with RT had higher risk of SGC compared with patients who did not receive RT (Figure 1, Table 3); however, only two cases occurred among patients who did not receive RT. There was an indication of a radiation dose-response consistent with linearity (P=0.005) (Table 3, Figure 2). The estimated excess relative risk (ERR) was 0.36 per Gy (95% CI: 0.06 to 2.5). Patients treated with alkylating agents and those treated with anthracyclines exhibited non-significantly elevated incidence of SGC. Although risk was not significantly associated with dose, a significantly elevated RR was seen among those with an alkylating agent score of 1 (RR=4.1; 95% CI: 1.4–3.7) (Table 3).
Figure 1.
Cumulative incidence of salivary gland cancers among childhood cancer survivors by time (years) since first cancer diagnosis, overall and separately for those who did and did not receive radiotherapy.
Figure 2.
Risk of subsequent primary salivary gland cancer among five year survivors of childhood cancer with respect to radiation dose to the salivary glands. The sloped dashed line is the fitted dose-response relationship. The horizontal dotted line is a reference line corresponding to relative risk=1.
Patients classified as ever cigarette smokers did not have an increased risk relative to non-smokers (RR=0.9; 95% CI: 0.2–2.4), and SGC risk did not show a significant association with pack-years of cigarette smoking (Table 2). Similarly, SGC risks did not vary significantly for patients who reported drinking alcohol compared with those who had no history of alcohol consumption (RR=1.4; 95% CI: 0.6–4.4). Among those patients who reported alcohol consumption, there was no significant trend of increasing risk of SGC with the amount of alcohol consumed per day.
Discussion
To our knowledge, this is the largest study to date of SGC after treatment for childhood cancer and the first to estimate risks by dose of radiation and chemotherapy agents received for the first cancer. We found that risk of SGC after childhood cancer was higher among patients who received RT as part of their initial treatment compared with those who received CT without RT and that risk was positively associated with radiation dose. There was no evidence of a consistent trend in risk with age at diagnosis of the first cancer nor with time since diagnosis of the first cancer. Risk was not significantly associated with dose of alkylating agents or anthracyclines, but RR estimates were elevated, and possible effects cannot be excluded in light of the limited number of cases. Risk was similar among females and males. Risk was significantly higher for patients treated for NHL, HL or leukemia than for other types of cancer. We did not observe an increased risk associated with cigarette smoking or alcohol consumption.
The present findings add to the evidence that exposure to high doses of radiation is a strong risk factor for SGC and, more importantly, provide quantitative evidence of the magnitude of the effect following exposure at young ages. The estimated radiation-related risk observed in the present study (ERR=0.36/Gy) is somewhat lower than values reported in previous studies that included radiation dosimetry. In a study of 2,945 persons irradiated as children for benign conditions of the head and neck, Schneider et al. (1) reported an ERR/Gy for salivary gland tumors of 0.82; however, the 95% confidence interval overlapped broadly with that from the present study, so risk estimates from the two studies are consistent. However, in the Schneider study, the positive association was determined primarily by benign tumors, and a dose-response was not seen for malignant tumors. A contrary finding was reported for atomic bomb survivors, with a steeper radiation dose-response for malignant tumors (7). The estimated relative risk at 1 sievert (Sv) for all salivary gland cancers and all ages at exposure combined was 4.5, implying an average ERR of 3.5 per Sv. The atomic bomb survivors experienced instantaneous rather than fractionated exposures, and risk might vary according to the nature of the radiation exposure.
In our investigation, most of the cases of SGC occurred in the parotid gland, and this finding was to be expected based on results from other series (25). Our analyses showing mucoepidermoid carcinomas to be strongly associated with radiotherapy for childhood cancer are consistent with the especially strong radiation dose-response found for mucoepidermoid carcinomas seen in studies of atomic bomb survivors (7). Patients treated with external radiation to the head, neck or upper chest, also have shown a higher frequency of mucoepidermoid carcinoma compared to other SGC cell types (1, 25).
Our study provides new data indicating that risk of SGC remains elevated among long-term survivors of childhood cancer, indicating the need for continued surveillance. Another finding is the similarity of risk of SGC in women and men (Table 2). Consistent with this is the finding of Dores et al. (26) who reported no significant difference in risk of SGC between male and female HL survivors, and the absence of a significant gender difference among atomic bomb survivors (7, 8) and among children irradiated with low doses to the head or neck for benign medical conditions (1). In contrast, Boukheris et al. (25) reported an increased risk for SGC among women treated for HL compared with men. Given the inconsistent results, additional data are needed before any conclusions about gender differences can be drawn.
In the present study, there was no significant trend of decreasing relative risk with increasing age at initial cancer diagnosis after adjustment for radiation dose. The high SIRs for the youngest and oldest age at first cancer groups can be explained by the predominance of leukemia and lymphoma, respectively, in these groups. An increased risk of SGC among patients exposed to radiation at younger ages was seen in the Life Span Study of atomic bomb survivors (8), bone marrow transplant patients (4) and patients receiving RT for a brain tumor (6); however, within a narrow age range of childhood exposure, there was little or no evidence of an age at exposure trend among persons irradiated for benign conditions of the head or neck (1). That was the case in the present study as well.
Our investigation showed no clear evidence of association between cigarette smoking or alcohol consumption and risk of SGC in survivors of childhood and young adult cancers. Some studies have suggested an association of SGC with alcohol and tobacco use, whereas others have found little or no evidence of a relationship (10–16). Given the limited number of SGC cases in the present investigation and the relatively young average age of the cohort, further studies are needed to clarify the role of cigarette smoking and alcohol consumption.
Strengths of our study include a large, well defined and characterized cohort of childhood cancer survivors with nearly complete information on treatment, detailed radiation dosimetry, and the availability of information about other possible risk factors, such as tobacco smoking and alcohol intake. Limitations include the small number of subsequent SGC, which is a rare cancer, and the potential for under-ascertainment, since we relied on self-report for initial ascertainment, followed by pathologic confirmation. The small number of subsequent SGC precluded detailed evaluation of the shape of the radiation dose-response relationship and of the combined effects of therapy, cigarette smoking and alcohol intake. In addition, some of the SGC excess relative to the general population could be related to increased screening of childhood cancer survivors, resulting in the early detection of asymptomatic or indolent cancers. Thirty percent of potentially eligible survivors either could not be traced (15%) or declined to participate (15%), and the study cohort may not be a representative sample; however, comparisons of known cancer risk factors for which information was available showed no significant difference between participants and nonparticipants (18).
In conclusion, our study found that the major salivary glands are highly susceptible to the induction of malignancy after radiation treatment for childhood cancer. Risk increased with radiation dose, and excess risk was apparent at doses below 3 Gy. The elevated risk among long-term survivors highlights the importance of regularly following childhood cancer patients throughout their lifetime and the need for education of childhood cancer survivors on the risk of development of new malignancies. Future studies will be needed to determine if current efforts to diminish radiation exposures for cancer patients, such as restricting radiation to involved fields with lower doses, will reduce the risk of secondary SGC among long-term survivors.
Acknowledgments
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived five or more years after diagnosis of childhood cancer. The CCSS study population is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss.
Sources of research support: This study was funded by National Cancer Institute grant #U24 CA55727 to St. Jude Children’s Research Hospital, the Lance Armstrong Foundation grant 147149, and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: Actual or potential conflicts of interest do not exist.
References
- 1.Schneider AB, Lubin J, Ron E, et al. Salivary gland tumors after childhood radiation treatment for benign conditions of the head and neck: dose-response relationships. Radiat Res. 1998;149:625–630. [PubMed] [Google Scholar]
- 2.Shore-Freedman E, Abrahams C, Recant W, et al. Neurilemomas and salivary gland tumors of the head and neck following childhood irradiation. Cancer. 1983;51:2159–2163. doi: 10.1002/1097-0142(19830615)51:12<2159::aid-cncr2820511202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Ramsay NK, Steinbuch M, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87:3633–3639. [PubMed] [Google Scholar]
- 4.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 5.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 6.Inskip PD. Multiple primary tumors involving cancer of the brain and central nervous system as the first or subsequent cancer. Cancer. 2003;98:562–570. doi: 10.1002/cncr.11554. [DOI] [PubMed] [Google Scholar]
- 7.Land CE, Saku T, Hayashi Y, et al. Incidence of salivary gland tumors among atomic bomb survivors, 1950–1987. Evaluation of radiation-related risk. Radiat Res. 1996;146:28–36. [PubMed] [Google Scholar]
- 8.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 9.Preston-Martin S, Thomas DC, White SC, et al. Prior exposure to medical and dental X-rays related to tumors of the parotid gland. J Natl Cancer Inst. 1988;80:943–949. doi: 10.1093/jnci/80.12.943. [DOI] [PubMed] [Google Scholar]
- 10.Boice JD, Jr, Fraumeni JF., Jr Second cancer following cancer of the respiratory system in Connecticut, 1935–1982. Natl Cancer Inst Monogr. 1985;68:83–98. [PubMed] [Google Scholar]
- 11.Hayes RB, Bravo-Otero E, Kleinman DV, et al. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999;10:27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- 12.Spitz MR, Fueger JJ, Goepfert H, et al. Salivary gland cancer. A case-control investigation of risk factors. Arch Otolaryngol Head Neck Surg. 1990;116:1163–1166. doi: 10.1001/archotol.1990.01870100057012. [DOI] [PubMed] [Google Scholar]
- 13.Zheng W, Shu XO, Ji BT, et al. Diet and other risk factors for cancer of the salivary glands:a population-based case-control study. Int J Cancer. 1996;67:194–198. doi: 10.1002/(SICI)1097-0215(19960717)67:2<194::AID-IJC8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414–419. doi: 10.1097/00001648-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Sadetzki S, Oberman B, Mandelzweig L, et al. Smoking and risk of parotid gland tumors: a nationwide case-control study. Cancer. 2008;112:1974–1982. doi: 10.1002/cncr.23393. [DOI] [PubMed] [Google Scholar]
- 16.Muscat JE, Wynder EL. A case/control study of risk factors for major salivary gland cancer. Otolaryngol Head Neck Surg. 1998;118:195–198. doi: 10.1016/S0194-5998(98)80013-2. [DOI] [PubMed] [Google Scholar]
- 17.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker MA, Meadows AT, Boice JD, Jr, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 20.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 21.Horner MJRL, Krapcho M, Neyman N, et al. Bethesda, MD: National Cancer Institute; 2009. SEER Cancer Statistics Review, 1975–2006. Available at http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 24.Preston DL, Lubin JH, Pierce DA, et al. Epicure Users Guide. Seattle, WA: Hirosoft International, editor; 1993. [Google Scholar]
- 25.Boukheris H, Ron E, Dores GM, et al. Risk of radiation-related salivary gland carcinomas among survivors of Hodgkin lymphoma: a population-based analysis. Cancer. 2008;113:3153–3159. doi: 10.1002/cncr.23918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]