Abstract
Cytomegalovirus (CMV) has been detected in several human cancers but it has not proven to be oncogenic. However, recent studies have suggested mechanisms through which CMV may modulate the tumor environment, encouraging its study as a positive modifier of tumorigenesis. In this study, we investigated the effects of CMV infection in TrP53 heterozygous mice. Animals were infected with murine CMV (MCMV) after birth at 2 days (P2) or 4 weeks of age and then monitored for tumor formation. Mice injected at two days of age developed tumors at a high frequency (43%) by 9 months of age. In contrast, only 3% of mock-infected or mice infected at 4 weeks developed tumors. The majority of tumors from P2 MCMV-infected mice were pleomorphic rhabdomyosarcomas (RMS) harboring MCMV DNA, RNA, and protein. An examination of clinical cases revealed that human RMS (embryonal, alveolar, and pleomorphic) harbored human CMV IE1 and pp65 protein as well as viral RNA. Taken together, our findings offer support for the hypothesis that CMV contributes to the development of pleomorphic RMS in the context of Trp53 mutation, a situation which occurs with high frequency in human RMS.
Keywords: Cytomegalovirus, rhabdomyosarcoma, viral oncology, muscle, p53
Introduction
Rhabdomyosarcoma (RMS) is a lethal tumor believed to originate from immature striated muscle cells that primarily affects children (1). Approximately half of patients with RMS perish and survival has changed little in the last few decades (2). RMS is divided into three subtypes: embryonal, alveolar, and pleomorphic. Both embryonal and alveolar are more common in children, whereas the more lethal pleomorphic subtype occurs primarily in adults with a median survival of 2.25 years (3).
Human CMV (HCMV) is a DNA β-herpesvirus. Like all herpesviridae, acute infection is followed by a lifelong latent infection that is reactivatable. HCMV is present in 70 – 90% of adults and in 40% of one year olds(4). It infects several human cells including RMS (5) and myoblasts (6) in vitro, shown to be a cell of origin for RMS (7). Accumulating evidence suggests a link between persistent HCMV infection and cancer. Several reports have shown HCMV presence in solid tumors including glioma, medulloblastoma, breast, prostate, and colorectal cancer (8-10). This warrants research into how this virus is linked to cancer.
Although some have reported a high frequency of p53 mutations in RMS (11, 12) this is not universally accepted (13). Heterozygous Trp53+/− mice develop a mixed spectrum of tumors occurring at a late age (>9 months) (14). Combining Trp53 heterozygosity with K-Ras oncogene activation in mice, leads to pleomorphic RMS suggesting a cooperative role between the two mutations (15). HCMV has been shown to interact with many cancer pathways, some of which are implicated in RMS. HCMV can interact with p53 (9): IE2 can bind directly to p53, inactivating p53-dependent apoptosis; UL44 inhibits p53 transcriptional activity (16); and IE1 reduces p53 expression in glioma cells.
There is no evidence for a HCMV role in RMS. Utilizing Trp53+/− mice, we report that MCMV infection leads to the development of murine pleomorphic RMS. We demonstrate HCMV gene expression in human RMS, implicating this virus in the pathogenesis of these neoplasms.
Methods and Materials (see supplemental methods)
Mouse handling and virus infection
The Institutional Animal Care and Use Committee at The Ohio State University approved mouse experiments and care. Trp53+/− mice (see supplemental methods) were intraperitoneally (ip) injected on postnatal day 2 (P2) with 103 p.f.u.s. or at four weeks of age with 106 p.f.u.s. with an MCMV strain that possesses an ablated m157 allele to enhance infectivity in B6 mice (17) in 100 μl of PBS or PBS only (Mock). MCMV infection in mice is considered a reasonable surrogate for modeling HCMV infection in humans. An additional cohort received 103 p.f.u.s. of HSV1 at P2 in 100 μl of PBS. To prevent unintentional viral spread, mock-infected mice were housed separately from virus-infected mice, were always handled before MCMV mice, and their cages were changed on different days than MCMV-infected mice. Mice were genotyped on P19 (18) and genotypes were re-confirmed. Mice were sacrificed after tumors became 1.6 cm in diameter or when moribund. Tumors appeared throughout the body, although they favored the abdominal cavity or limb girdles (sTable 1A).
MCMV PCR and RT-PCR
After anesthesia with ip injection of ketamine (100 mg/kg) and xylazine (20 mg/kg), 0.5 mls. of blood were obtained via the facial vein. Mice were perfused via the intracardiac route with ice-cold PBS followed by organ harvest. Genomic DNA was obtained using a DNeasy kit and RNA via RNeasy kit (Qiagen) with on column DNase treatment. MCMV-GB gene was amplified by PCR using the following primers (5′-3′): MCMV-GB forward: TGGGTGAGAACAACGAGAT and MCMV-G B r e v e r s e : CGCAGTCTCCCTTCGAGTA. β-actin was used ascontrol (mouse β-actin forward: AGCCTCGTCCCGTAGACAAAT; mouse β-actin reverse: GAAGACACCAGTAGACTCCACGACAT). For RT-PCR experiments, 1 μg of total RNA was transcribed using a SuperScript First-Strand cDNA Synthesis Kit (Invitrogen). Primers (5′-3′) for MCMV-IE1 were forward: TAGCCAATGATATCTTCGAGCG and reverse: TTAGCTGATCTGAGGAGCACCAGAT.
Mouse Histology
Initially visible tumors reached a large size within one week. After anesthesia, mice were examined for visible pathology. Tumor tissue or other tissues of interest were snap frozen at −80 degrees or placed in 4 % paraformaldehyde, processed for paraffin embedding, and cut into 4-μm sections. A board-certified pathologist (O.H.I) specializing in sarcomas was blinded to infection status and evaluated hematoxylin and eosin stained sectiond. Additionally, IHC was performed. Antibodies were against MyoD1 (Santa Cruz Biotechnology Inc.), Desmin (Sigma), smooth muscle actin (Millipore) and Croma101 (from Stipan Jonjic). MOM kit (Vector) was used for antibodies raised in mouse. Microscopic images were captured with an Olympus BX51 microscope (Olympus America Inc.) equipped with DP72 camera.
HCMV Immunohistochemistry
Human RMS and normal muscle biopsy samples were obtained as per an approved IRB protocol. Immunohistochemistry for human HCMV in tumors has been previously described with minor modifications (8). HCMV-IE1 (Millipore), HCMV-pp65 (Leica Microsystems), or smooth muscle actin (Millipore) primary antibodies were incubated on sections overnight at four degrees Celsius. Secondary antibody was applied for 30 minutes followed by biotin link for 30 minutes (Biogenex). Positive staining was visualized with a DAB substrate.
Results and Discussion
MCMV-infected Trp53+/− mice developed tumors at a high rate by 9 months
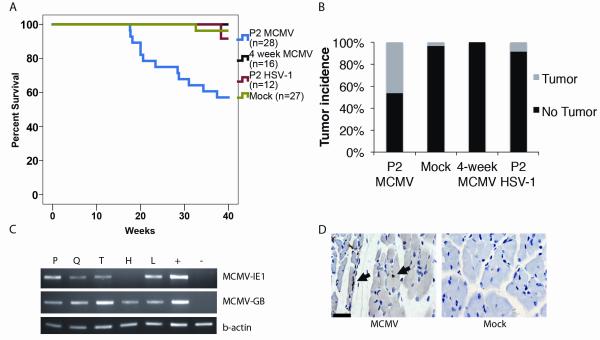
Mice were infected with MCMV (103 p.f.u.s) two days after birth (P2) or at four weeks of age (106 p.f.u.s). Separate Trp53+/− control cohorts were inoculated with PBS vehicle (Mock infection) or with a non-lethal dose of another herpesvirus, herpes simplex virus 1 (HSV-1), at the same time points, in order to test for non-specific viral effects on tumor formation. Virus infection caused no distinguishing behavioral or clinical effects in mice. Since Trp53+/− mice start developing tumors at a late age (>9 months), we followed mice for nine months to detect MCMV-specific effects on tumor development. P2 MCMV-infection was associated with a statistically significant decrease in survival (the point of veterinary need for euthanasia) compared to Mock-infected mice (p<0.05, Log Rank Test) (Fig 1A). 42.8% (12/28) of P2 MCMV-infected Trp53+/− mice developed large, visible tumors over nine months (Fig 1B). Instead, only 1/27 Mock-infected and 1/12 HSV1-infected mice developed a visible tumor during this time period. In addition, MCMV infection at 4 weeks of age did not result in visible tumors. Therefore, MCMV (but not HSV1 or Mock) infection of Trp53+/− mice at P2, but not at 4 weeks, led to a significantly higher incidence of visible tumors over the first nine months of animal life.
Figure 1. Kaplan-Meier survival curve for Trp53+/− mice, after infection with MCMV vs. control.
Panel A: Mice were followed for nine months. 12/28 mice infected with MCMV at P2 developed tumors, compared to 1/27 Mock-infected mice, 0/16 infected with MCMV at 4 weeks of age, or 1/12 mice infected with HSV1. The difference in survival was significant (p<0.05 for P2 MCMV-infected vs. Mock, HSV1 or 4 week infection, Logrank test). Panel B: Bar graph displaying tumor incidence for Trp53+/− mice (**P =0.003, Fisher exact test). Panel C: RT-PCR for MCMV-IE1 and MCMV-GB from mouse organs five days post MCMCV infection of P2 mice. The gels are representative of three different experiments. P: peritoneum, Q: quadriceps muscle, T: trapezius muscle, H: heart, L: liver, +: MCMV-infected fibroblasts, and -: Mock-infected fibroblasts. Panel D: Immunohistochemistry for MCMV-IE1 (black arrow) in muscle from P2 MCMV-infected mice or mock-infected (5 days p.i). Arrows indicate positive immunohistochemistry. Scale bar = 50 μm.
MCMV infected muscle and connective tissue
Next, we examined mice for MCMV distribution. Tissues from mice, infected with MCMV at P2 and analyzed five days post-infection (p.i), were positive for MCMV immediate early (MCMV-IE1) and late, MCMV-GB, transcripts (Fig 1C). Additionally, quadriceps exhibited expression of MCMV-IE1 protein (Fig 1D). MCMV RNA expression in muscle was not detected two weeks after infection (data not shown). In addition, tissues from mice infected at four weeks of age were positive for MCMV-specific glycoprotein B DNA (MCMV-GB) (sFig.1A) and for MCMV-IE1 and GB transcripts (sFig. 1B). Mock-infected mice were negative for MCMV-GB (data not shown). These findings confirm that MCMV infects the tissues from which pleomorphic RMS originate.
MCMV-infected Trp53+/− mice developed pleomorphic RMS at an early age
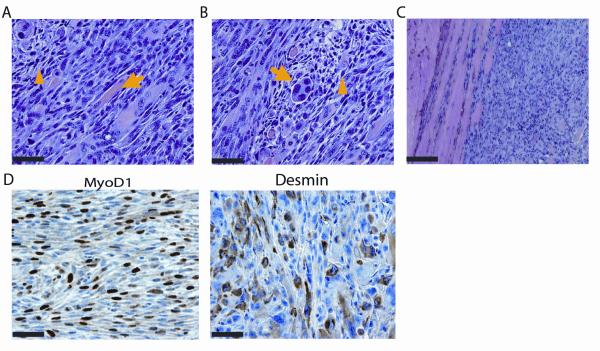
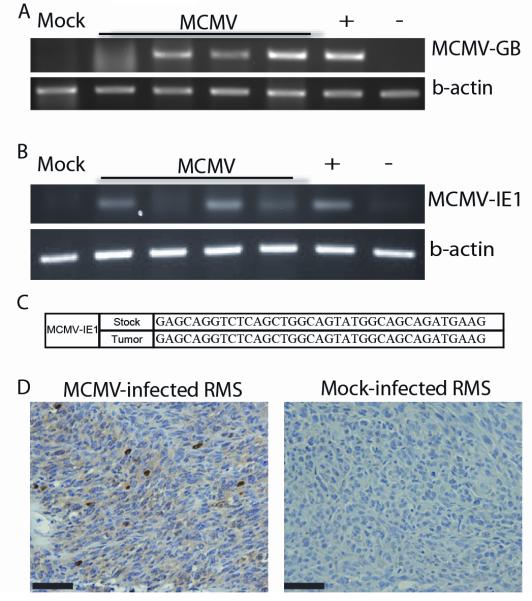
Tumors that developed in MCMV-infected Trp53+/− mice revealed diagnostic features of pleomorphic RMS including: pleomorphic features (Fig 2A), rhabdomyoblasts (Fig 2B) and infiltration into surrounding muscle (Fig 2C). The immunophenotypic expression of MyoD1 and desmin confirmed RMS (Fig 2D). Analysis of a subset of MCMV-infected tumors (n=4) revealed MCMV DNA (Fig 3A), which was absent in the single tumor that had developed in one of the Mock-infected Trp53+/− mice. MCMV-IE1 transcripts were also present in the 4 tested tumors (Fig 3B). Fidelity of PCR reactions was confirmed by DNA sequencing of PCR products (Fig. 3C). Furthermore, MCMV-infected tumors expressed IE1 protein, while this was not seen in the Mock-infected tumor (Fig 3D). Isotype control and no primary antibody confirmed IHC specificity (sFig 2A).Taken together, these data show transcriptionally active MCMV within tested RMS.
Figure 2. Histological and molecular analyses of tumors from MCMV infected Trp53+/− mice.
Hematoxylin and eosin staining of MCMV-infected ectopic tumor showing pleomorphism (yellow arrowhead) and immature muscle (yellow arrow) (panel A), rhabdomyoblasts (yellow arrowhead) and multi-nucleated giant cells (yellow arrow) (panel B), and invasive tumor margins (panel C). Panel D: Immunohistochemistry for myogenic markers, MyoD1 and desmin.
Figure 3. MCMV analysis of mouse tumors.
Panel A: PCR for MCMV DNA in tumors from MCMV (n=4)- and Mock-infected (n=1) mice. Panel B: RT-PCR for MCMV-IE1 in tumors from MCMV (n=4)- and mock-infected (n=1) mice. Panel C: RT-PCR product fidelity was validated by sequencing the amplified MCM-IE1. Panel D: MCMV-IE1 immunohistochemistry in MCMV- and Mock-infected tumor. Scale bars = 50 μm.
Published data for uninfected Trp53+/− mice show variable tumor types occurring after the age of nine months. In one study, 28% (28/100) of Trp53+/− mice develop tumors by seventeen months. These tumors were primarily lymphomas, osteosarcomas, and fibrosarcomas; only one was a RMS (14). Pathologic analyses of MCMV-infected tumors in this study revealed that 84.6% were RMS (10/12) while 15.4% (2/12) were lymphomas. Nine out of ten RMS tumors were pleomorphic (sTable 1A). Therefore, our data suggests that MCMV accelerates tumor formation in mice with heterozygous Trp53 mutation, leading preferentially to pleomorphic RMS.
Human cytomegalovirus antigen presence in human rhabdomyosarcomas, but not in normal muscle
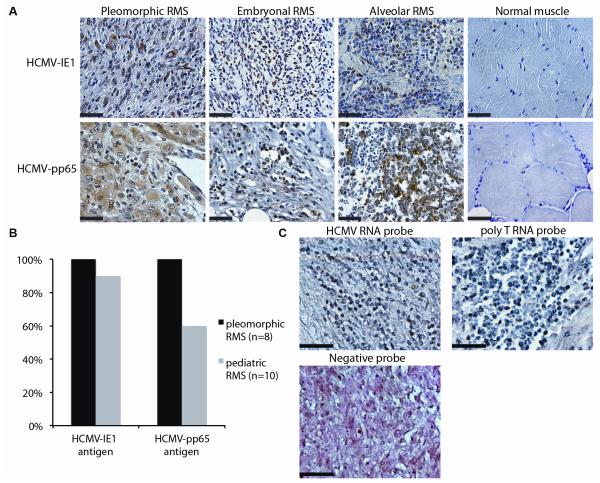
Since the majority of tumors from MCMV-infected mice were pleomorphic RMS, we tested whether this finding was clinically relevant by examining human tumors for the presence of HCMV (sTable 1B). Pleomorphic RMS cases were first examined for HCMV-IE1 via immunohistochemistry. All human pleomorphic RMS (8/8) stained strongly and diffusely for HCMV-IE1 (Fig 4A), with expected isotype and negative control IHC (sFig 2B). As a control, HCMV-IE1 immunoreactivity was not observed in 5/5 normal human adult muscle (Fig 4A). Staining was expanded to include embryonal and alveolar (collectively referred to as pediatric) RMS tumors (Fig 4A). Ninety percent (9/10) of these tumors stained positive for HCMV-IE1 (Fig 4B). Because HCMV-IE1 transcription can frequently be detected even after the virus becomes latent, we also stained for the presence of HCMV-pp65, a late protein expressed during active viral replication. All adult pleomorphic RMS cases and most (6/10) pediatric RMS tumors stained strongly for pp65 (Fig 4B) and pp65 positive cells were abundant throughout the positive tumors (Fig 4A). Altogether, 100% (8/8) of adult and 90% (9/10) of pediatric RMS cases expressed HCMV antigen (Fig 4B). Only one pediatric case did not stain positive for HCMV-IE1 or HCMV-pp65 (sFig2C and not shown). Rhabdomyoblasts stained strongly for HCMV antigen (sFig 2D, arrow), suggesting that the RMS-associated primitive cell type was indeed infected with the virus. In several cases, cells in muscle beyond the tumor margin were also positive for HCMV antigen (sFig 2E). One limitation of the presented immunopositive findings in human RMS cases results from our inability to secure serum from these same historical subjects to determine their CMV serology. To confirm the selective presence of HCMV DNA in human RMS, quantitative PCR analysis showed that tumors expressed an average of 81.1 copies/ng of HCMV-UL83 and of 22.2 copies/ng of HCMV-UL146 compared to 0 and 0.49 copies/ng in normal muscle, respectively (sFig 3A, p<0.05, student’s T-test). The amplified products’ nucleotide sequence was greater than 95% identical to that of a clinical strain (sFig. 3B).
Figure 4. HCMV detection in human RMS.
Panel A: Immunohistochemistry for HCMV-IE1 and HCMV-pp65 IHC in pleomorphic, embryonal, and alveolar RMS and normal muscle. Panel B: Bar graph detailing antigen presence in pleomorphic and pediatric RMS. Panel C: In situ hybridization for HCMV in pleomorphic RMS. The same tumor was stained with a probe against poly T RNA, present in all cells, as a positive control. A negative control probe is included. This section was counterstained with eosin to show cell architecture. Scale bar = 50 μm.
To further validate the presence of HCMV in RMS we next tested for the presence of HCMV RNA by using in situ hybridization (ISH) in a subset of tumors. A commercially available RNA probe targeted to an unspecified early gene of HCMV revealed HCMV RNA in RMS (Fig 4C). ISH with a negative control probe or no probe was negative (Fig 4C & data not shown). Altogether 100% (5/5) of RMS cases tested, but none of the normal muscle biopsies, contained HCMV RNA. The one pediatric case that did not express HCMV-IE1 or HCMV-pp65 protein was positive for HCMV via ISH (data not shown). Taken together, 100% (18/18) of tested RMS cases contained HCMV genetic material.
Our data suggests a link between RMS and CMV. During the nine months of our study, wild type mice infected with MCMV did not develop tumors (data not shown). However, when MCMV infection was introduced into a neonatal mouse with a Trp53+/− tumor suppressor deficiency, mice develop tumors at a higher rate and earlier onset than Mock-infected mice. Interestingly, tumor formation primarily occurred in Trp53+/− mice infected at P2. Additionally, mice preferentially develop pleomorphic RMS as opposed to a wide array of tumors. Molecular analyses reveal the presence of MCMV in these mouse tumors. In addition, examination of human RMS samples revealed that 100% of tumors contain HCMV genetic material. This implicates CMV in the pathogenesis of the disease. CMV has never been shown to be a transforming virus. Our data shows for the first time that CMV infection combined with Trp53 heterozygosity promotes pleomorphic RMS. Furthermore, the idea that CMV can promote tumorigenesis in an organism with genetic aberrations may help to explain the difficulty of epidemiologically linking CMV infection with relatively rare cancers.
Supplementary Material
Acknowledgements
The authors thank Ulrich Koszinowski (Max von Pettenkofer-Institute, Munich, Germany) for kindly providing MCMV and Stipan Jonjic (University of Rijeka, Rijeka, Croatia) for providing Croma101 antibody. Denis Guttridge (Ohio State University, Columbus, OH) provided constructive guidance to the project.
Grant Support RLP was funded by a seed grant from the American Medical Association Foundation. This study was funded by a Viral Oncology Program Grant, the Dardinger Neuro-oncology Fund, the Jeffrey Thomas Hayden Foundation (to E.A.C.), OSU MBCG Program grant (to E.A.C. and C.-H.K.), and OSU CCC Start-up Fund (to C.-H.K.).
Financial support: RLP was funded by a seed grant from the American Medical Association Foundation. This study was funded by a Viral Oncology Program Grant, the Dardinger Neuro-oncology Fund, the Jeffrey Thomas Hayden Foundation (to E.A.C.), OSU MBCG Program grant (to E.A.C. and C.-H.K.), and OSU CCC Start-up Fund (to C.-H.K.).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Paulino AC, Okcu MF. Rhabdomyosarcoma. Curr Probl Cancer. 2008;32:7–34. doi: 10.1016/j.currproblcancer.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–25. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Simon JH, Paulino AC, Ritchie JM, Mayr NA, Buatti JM. Presentation, prognostic factors and patterns of failure in adult rhabdomyosarcoma. Sarcoma. 2003;7:1–7. doi: 10.1080/1357714031000114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onorato IM, Morens DM, Martone WJ, Stansfield SK. Epidemiology of cytomegaloviral infections: recommendations for prevention and control. Rev Infect Dis. 1985;7:479–97. doi: 10.1093/clinids/7.4.479. [DOI] [PubMed] [Google Scholar]
- 5.Cinatl J, Cinatl J, Radsak K, Rabenau H, Weber B, Novak M, et al. Replication of human cytomegalovirus in a rhabdomyosarcoma cell line depends on the state of differentiation of the cells. Arch Virol. 1994;138:391–401. doi: 10.1007/BF01379143. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Yu P, Zhang P, Shi Y, Bu H, Zhang L. The infection of human primary cells and cell lines by human cytomegalovirus: new tropism and new reservoirs for HCMV. Virus Res. 2008;131:160–9. doi: 10.1016/j.virusres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Saab R, Spunt SL, Skapek SX. Myogenesis and rhabdomyosarcoma the Jekyll and Hyde of skeletal muscle. Curr Top Dev Biol. 2011;94:197–234. doi: 10.1016/B978-0-12-380916-2.00007-3. [DOI] [PubMed] [Google Scholar]
- 8.Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–50. [PubMed] [Google Scholar]
- 9.Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? Virus Res. 2011;157:193–203. doi: 10.1016/j.virusres.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baryawno N, Rahbar A, Wolmer-Solberg N, Taher C, Odeberg J, Darabi A, et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest. 2011;121:4043–55. doi: 10.1172/JCI57147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felix CA, Kappel CC, Mitsudomi T, Nau MM, Tsokos M, Crouch GD, et al. Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res. 1992;52:2243–7. [PubMed] [Google Scholar]
- 12.Mulligan LM, Matlashewski GJ, Scrable HJ, Cavenee WK. Mechanisms of p53 loss in human sarcomas. Proc Natl Acad Sci USA. 1990;87:5863–7. doi: 10.1073/pnas.87.15.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ognjanovic S, Martel G, Manivel C, Olivier M, Langer E, Hainaut P. Low Prevalence of TP53 Mutations and MDM2 Amplifications in Pediatric Rhabdomyosarcoma. Sarcoma. 2012;2012:492086. doi: 10.1155/2012/492086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch DG, Dinulescu DM, Miller JB, Grimm J, Santiago PM, Young NP, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–7. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 16.Kwon Y, Kim M-N, Young Choi E, Heon Kim J, Hwang E-S, Cha C-Y. Inhibition of p53 transcriptional activity by human cytomegalovirus UL44. Microbiol Immunol. 2012;56:324–31. doi: 10.1111/j.1348-0421.2012.00446.x. [DOI] [PubMed] [Google Scholar]
- 17.Bubić I, Wagner M, Krmpotić A, Saulig T, Kim S, Yokoyama WM, et al. Gain of virulence caused by loss of a gene in murine cytomegalovirus. Journal of Virology. 2004;78:7536–44. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–30. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.