Abstract
Background
The cortisol awakening response (CAR) has been shown to predict major depressive episodes (MDEs) over a 1-year period. It is unknown whether this effect: (a) is stable over longer periods of time; (b) is independent of prospective stressful life events; and (c) differentially predicts first onsets or recurrences of MDEs.
Method
A total of 270 older adolescents (mean age 17.06 years at cortisol measurement) from the larger prospective Northwestern-UCLA Youth Emotion Project completed baseline diagnostic and life stress interviews, questionnaires, and a 3-day cortisol sampling protocol measuring the CAR and diurnal rhythm, as well as up to four annual follow-up interviews of diagnoses and life stress.
Results
Non-proportional person-month survival analyses revealed that higher levels of the baseline CAR significantly predict MDEs for 2.5 years following cortisol measurement. However, the strength of prediction of depressive episodes significantly decays over time, with the CAR no longer significantly predicting MDEs after 2.5 years. Elevations in the CAR did not significantly increase vulnerability to prospective major stressful life events. They did, however, predict MDE recurrences more strongly than first onsets.
Conclusions
These results suggest that a high CAR represents a time-limited risk factor for onsets of MDEs, which increases risk for depression independently of future major stressful life events. Possible explanations for the stronger effect of the CAR for predicting MDE recurrences than first onsets are discussed.
Keywords: Cortisol awakening response, diurnal rhythm, late adolescence, major depressive disorder, non-proportional hazards models, stressful life events
Introduction
Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, and particularly the hormone cortisol, has been implicated in the etiology of major depressive disorder by a large body of animal research and cross-sectional research in humans (Chrousos & Gold, 1992; Ehlert et al. 2001). Several prospective studies of HPA functioning and depression have shown that cortisol levels during the morning (rather than average or total cortisol levels throughout the day), around the peak of cortisol’s diurnal rhythm, prospectively predict depressive episodes or symptoms (Harris et al. 2000; Halligan et al. 2007; Goodyer et al. 2010). One aspect of morning cortisol that has received particular attention is the cortisol awakening response (CAR) – the pronounced (on average, 50–60%) increase in cortisol level that peaks approximately 30 to 40 min after waking (Pruessner et al. 1997). The CAR is frequently operationalized as the difference between the level of salivary cortisol sampled immediately after waking and the level sampled 30 to 40 min after waking (Clow et al. 2010). Experimental research indicates that the CAR represents a true response to waking, rather than an underlying diurnal rhythm (Wilhelm et al. 2007). Though the precise function of the CAR has not been fully characterized, current theory suggests that it serves to mobilize resources to meet the perceived demands of the upcoming day (e.g. Adam et al. 2006; Fries et al. 2009).
The CAR is thought to be an important indicator of risk for major depressive episodes (MDE) in part because it captures a large and rapid increase on top of already elevated waking cortisol levels (Adam et al. 2010), potentially reaching physiological levels capable of activating relatively low-affinity glucocorticoid receptors implicated in the etiology of major depressive disorder (e.g. Holsboer, 2000). Recently, we showed that the CAR prospectively predicted the occurrence of MDEs across a 1-year follow-up period (odds ratio 3.0, p = 0.04; Adam et al. 2010). Other basal cortisol indices, namely the diurnal slope and total waking cortisol (area under the curve with respect to ground; AUCg), were not related to MDE onsets during this follow-up period. Participants in this study were a subset of those from the Northwestern-UCLA Youth Emotion Project (YEP), a two-site longitudinal study of risk for emotional disorders (Zinbarg et al. 2010). Since that time, additional follow-up waves have been conducted and the number of participants in the cortisol subsample of the YEP reporting any MDE over the follow-up period has increased from 18 to 42, permitting additional analyses over a longer time period.
Thus, using data from the same study over an extended 4-year follow-up period, the present study examined three questions. First, does the CAR predict MDE onsets beyond 1 year (what we refer to as a question of predictive stability)? Several prospective studies suggest that morning or average daytime cortisol predicts depressive symptoms over 2 to 3 years (Halligan et al. 2007; Shirtcliff & Essex, 2008); to our knowledge, no one has examined the CAR as a predictor of MDE onset over similar time periods, nor has anyone explicitly examined the predictive stability of the CAR. We will use the CAR in relation to MDE onsets over an extended period of time by using a CAR × time interaction variable to detect decay in predictive power. Knowledge regarding the stability of this effect could inform our understanding of the CAR, and has practical significance for the design of prospective research studies. The robustness of the CAR effect and the CAR × time interaction will also be considered. In particular, models will examine whether the CAR and its interaction with time predict MDE above and beyond other known predictors of MDE, including the personality trait neuroticism (e.g. Kendler et al. 2004) and a history of MDE or anxiety disorders at the time of cortisol measurement (e.g. Boland & Keller, 2009). Models will also consider whether the CAR is a significant predictor of MDE above and beyond the effects of possible confounding variables, including fatigue and negative affect at the time of cortisol sampling (e.g. Adam et al. 2006), and chronic and episodic life stress in the year prior to cortisol sampling (Adam & Gunnar, 2001; Gerritsen et al. 2010).
Second, do elevations in the CAR increase vulnerability to prospective major stressful life events? Major stressful life events are known to precipitate MDEs, often within the month after the occurrence of an event (Kendler et al. 1999). Our research has shown that the CAR’s ability to predict depression onset was independent of an index of moderate to severe episodic stressful events occurring in approximately the year prior to cortisol measurement (Adam et al. 2010). What is unknown is whether elevations in the CAR associated with increased risk for MDEs represent enhanced vulnerability to future stressful events, or instead whether such CAR elevations increase risk for MDEs independently of these stressful events. To our knowledge, prior research has demonstrated unique effects of elevated morning cortisol and of stressful life events in the month prior to depressive onset (Goodyer et al. 2000), but research has not tested whether morning cortisol peaks (or an elevated CAR) significantly interact with stressful life events in predicting MDEs.
Third, does the CAR predict first onsets and recurrences of MDE differentially? While our prior report after a 1-year follow-up presented descriptive data supporting that both first onsets and recurrences of MDE were predicted by elevations in the CAR (Adam et al. 2010), an increased number of MDE cases in the present report permits a formal statistical examination of whether the CAR interacts with MDE history to predict new onsets and recurrences differentially, where one prospective MDE per person is considered. It might be that CARs of similar magnitude are more predictive of MDE risk for first onsets or recurrences. To our knowledge, the present study is the first to examine each of these questions, which are intended to clarify the relationship of the CAR to onsets of depressive episodes.
Method
Participants
Detailed information regarding recruitment and demographics of the larger YEP sample is reported by Zinbarg et al. (2010). In brief, over 1900 high school juniors were screened for neuroticism using the Revised Eysenck Personality Questionnaire Neuroticism Scale (Eysenck et al. 1985). Those scoring high on this measure were oversampled, comprising 59% of the final group of 627 (432 females) who gave consent to participate in a longitudinal study and who completed the baseline interviews and questionnaires described below. Participants were asked to repeat all these measures annually.
Of these participants, 491 individuals were randomly selected for invitation to participate in a cortisol sampling protocol; a total of 344 participants (250 females) completed this protocol, 70.1% of those invited. Participants were excluded from analyses for one or more of the following reasons: corticosteroid medication (n = 13); insufficient cortisol data (n = 29); no follow-up interviews after the baseline interview (n = 18); current major depression at baseline (n = 17); current or past post-traumatic stress disorder at baseline (n = 2), which is associated with lower cortisol levels (for a meta-analysis, see Meewisse et al. 2007); or at any time during the study being diagnosed with dysthymic disorder (n = 8), clinically significant psychotic symptoms (n = 3) or bipolar disorder I or II (n = 7). Individuals with these three diagnoses were excluded a priori from analyses in order to yield a cleaner comparison group. After exclusions, a total of 270 participants remained, yielding 75 males and 195 females in the current analyses. The sample comprises more females than males both because females were more likely than males to agree to participate if invited, and because we oversampled for high levels of neuroticism, a trait on which females score higher on average (Costa et al. 2001). Participants were Caucasian (49%), African American (10%), Hispanic or Latino (18%), Asian (4%), or multiple/other races and ethnicities (19%). Hollingshead’s socio-economic status index (Hollingshead, 1975) was determined from information gathered at the baseline interview.
Procedures
Assessment of psychopathology
The presence or absence of clinically significant MDEs was ascertained at each assessment using the Structured Clinical Interview for DSM-IV, non-patient edition (SCID; First et al. 2001). All diagnoses reported here were clinically significant manifestations. The baseline SCID assessed lifetime diagnoses, and four subsequent annual follow-up SCIDs assessed diagnoses of psychopathology occurring during the interim since the participant’s previous assessment. Interviewers were blind to the results of previous assessments. Inter-rater reliability was assessed for approximately 10% of all SCIDs administered in the larger study. Across the five assessment periods, k for individual interviewers’ diagnoses of MDEs, adjusted due to departure from equiprobability (R. Zinbarg et al. unpublished observations), ranged from 0.82 to 0.94 (mean = 0.89, s.d. = 0.05).
Life stress assessment
Baseline chronic stress, which is used as a covariate in models to rule out the possibility that stress accounts for CAR’s effects on MDE, was measured using the Life Stress Interview (LSI; Hammen et al. 1987). Chronic stress over the past year was measured across 10 life domains: best friend relationship; social circle; romantic relationships; family relationships; academics; work; finances; neighborhood conditions; physical health; and family’s health. Ratings were assigned by the interviewer for each domain on a scale from 1 to 5 in half-point increments, with 1 representing the best possible circumstances, and 5 representing the worst possible circumstances. Scores in each domain were standardized, then averaged to create a chronic stress composite score. Inter-rater reliability (intra-class correlations) for the baseline chronic stress composite score was 0.87 within site and 0.84 across sites.
Episodic life stress was also assessed at annual interviews using the LSI (Hammen, 1991). Episodic events reported by participants in the LSI were presented by the interviewer to a team of independent raters blind to the participant’s diagnoses and response to the event. Event severity ratings were assigned by the consensus of the independent rating team, ranging from 1 (no significant threat or negative implications) to 5 (a very severe event, maximal negative impact or threat) in half-point increments. Inter-rater reliability (intra-class correlations) cross-site for the five interviews examined ranged from 0.69 to 0.76 (mean = 0.72, s.d. = 0.03). Here, events are deemed to be ‘major’ if assigned a severity rating of 2.5 or greater, reflecting moderately to severely stressful events. To covary episodic life stress prior to cortisol, the severity scores of all major events in the year prior were summed to create a single episodic stress score. To examine prospective stressful events following cortisol, the occurrence of a major event in a given month was coded dichotomously (absent = 0, present = 1) in ‘person-month’ datasets, described in more detail in the ‘Analytic plan’ section. If a follow-up interview was missed, the subsequent SCID covered the period since the prior interview, but the LSI covered only the past 12 months due to concerns about reliability of recalling stressful life events occurring over longer durations. As such, when prospective stressful life events are incorporated into statistical models, months for which LSI data were not available were excluded from analyses.
Personality assessment
As previously described (Zinbarg et al. 2010), the personality trait neuroticism was measured using four separate scales measuring neuroticism or one of its facets: the Revised Eysenck Personality Questionnaire Neuroticism Scale (Eysenck et al. 1985), the International Personality Item Pool (IPIP) NEO Revised Personality Inventory (Goldberg, 1999), the Behavioral Inhibition Scale from the Behavioral Inhibition Scale/Behavioral Activation Scale (Carver & White, 1994) and the Big Five Mini-Markers Neuroticism Scale (Saucier, 1994). These were standardized and then averaged to yield a composite neuroticism score (Zinbarg et al. 2010).
Cortisol assessment
The cortisol collection protocol used here has been described in detail elsewhere (Adam et al. 2010; Doane et al. in press). In brief, cortisol was collected by passive drool on three consecutive weekdays, six times per day: immediately upon waking, 40 min after waking, three semi-random times throughout the day spaced to avoid mealtimes and signaled by a programmed watch beep, and, finally, at bedtime. Prior to each sample, participants completed a diary report including self-reported mood, which yields the measures of negative affect and fatigue used here. Participants were instructed not to eat, drink or brush their teeth in the 30 min prior to the expected morning and bedtime sample collection times. Several health variables associated with HPA axis functioning were measured by questionnaire or in daily diaries: asthma, hormonal contraceptives, time of waking and bedtime on cortisol measurement days, and daily nicotine use.
Once returned to the laboratory, samples were stored at −20 °C and later shipped to Trier, Germany, where they were assayed in duplicate using time-resolved fluorescent-detection immunoassay (Dressendörfer et al. 1992). Intra-assay variation ranged from 4.0% to 6.7%, while inter-assay variation ranged from 7.1% to 9.0%. Raw cortisol data were logarithmically transformed due to positive skew prior to analyses; raw salivary cortisol values are presented in graphs for ease of interpretation. In addition to cortisol levels at waking, 40 min after waking, and bedtime, three indices of diurnal cortisol were calculated for use in analyses: the CAR (value 40 min after waking minus value at waking), the diurnal rhythm slope (a regression line fit to all measures between waking and bedtime except the measure taken at 40 min after waking), and the average cortisol level (the area under the curve of all six cortisol measures with respect to ground, AUCg, divided by total time awake). Indices represent the mean from the 3 days of cortisol collection.
Analytic plan
Cox regression models (continuous time survival analyses; Cox, 1972) examining the primary research questions utilized a person-month dataset, permitting unique values for stressful life events and MDE onsets for each month. In order to examine the predictive stability of the CAR, non-proportional hazards models were employed. Unlike proportional hazards models, such models do not assume that the hazard associated with a particular predictor is constant across time, permitting the examination of interactions with time (Singer & Willett, 2003). Interactions of the CAR × time were calculated by multiplying median-centered time in months by the CAR value. All Cox models yield hazard ratios (HRs) and their 95% confidence intervals for each predictor. Continuous predictors are standardized to facilitate interpretation of HRs: a one-unit increase in a predictor corresponds to one standard deviation.
Stressful life events and MDE onsets were dated to the nearest month after cortisol measurement based on dates collected during the SCID and LSI interviews. Whether life events indicated as occurring in the same month as MDE onsets in fact occurred before the MDE onsets (i.e. temporal precedence) was carefully scrutinized. When the order of the event and the MDE onset was indeterminate, or when the event clearly occurred after the MDE onset, that event was excluded from consideration. A maximum of one MDE onset per participant was included in the model, whether that MDE represented a first onset or a recurrence of MDE; that is, once a participant experienced an MDE onset after cortisol measurement, their remaining months were not included. Participants without such diagnoses remained in the model until the month corresponding to their last follow-up SCID assessment or a maximum of 54 months.
In models for the prediction of MDEs, unless otherwise specified below, the following sets of variables are entered as four blocks into Cox regression models: demographic variables (i.e. gender, SES, African American race, Hispanic ethnicity), health variables (i.e. hours of sleep, time of waking, nicotine use, asthma, hormonal contraceptives), cortisol variables (CAR, slope, and AUCg), and finally the interaction of CAR × time. For all analyses, p values ≤0.05 are considered statistically significant.
Results
Descriptive statistics for all variables are presented in Table 1. Zero-order correlations for all variables are presented in Table 2. Participants who were included in analyses did not differ significantly from those who were excluded in: gender [χ2(1) = 0.79, n.s.]; minority race/ethnicity [χ2(1) = 3.29, n.s.]; or baseline Hollingshead socio-economic status level [F(1, 334) = 0.66, n.s.]. A total of 42 MDEs were observed (35 in females, seven in males; 27 first onsets, 15 recurrences) after participation in the cortisol protocol, with time to onset ranging from less than 1 month to 53 months. A total of 10 723 person-months were available for analysis of MDE onsets for the primary model.
Table 1.
Descriptive statistics of raw data for cortisol levels and covariates
| Variable | % | Mean (s.d.) | Minimum | Maximum |
|---|---|---|---|---|
| Male gender | 28 | |||
| African American race | 10 | |||
| Hispanic ethnicity | 18 | |||
| Has asthma | 9 | |||
| Uses hormonal contraceptives | 9 | |||
| History of MDE at baseline | 18 | |||
| Current anxiety disorder at baseline | 14 | |||
| Baseline risk category | ||||
| Low | 17 | |||
| Medium | 24 | |||
| High | 59 | |||
| Hollingshead SES index | 47.50 (12.53) | 10.50 | 66.00 | |
| Age at cortisol testing, years | 17.06 (0.39) | 16.07 | 18.14 | |
| Tobacco use, cigarettes/day | 0.22 (1.29) | 0.00 | 13.00 | |
| Mean hours of sleep | 7.14 (0.93) | 4.00 | 10.00 | |
| Mean time of waking | 6.76 (0.80) | 1.75 | 8.59 | |
| Annual follow-ups completed | 3.25 (0.95) | 1.00 | 4.00 | |
| Neuroticism composite | −0.07 (0.78) | −2.11 | 1.97 | |
| Baseline prior year episodic stress | 2.05 (3.19) | 0.00 | 16.80 | |
| Baseline prior year chronic stress | 2.25 (0.34) | 1.45 | 3.35 | |
| Diary reported negative affect | −0.04 (0.62) | −1.00 | 2.03 | |
| Diary reported fatigue | 0.01 (0.52) | −1.52 | 1.36 | |
| S1 wake-upa | 0.44 (0.24) | 0.07 | 2.00 | |
| S2 wake-up + 40 mina | 0.60 (0.28) | 0.03 | 1.38 | |
| S3 mid-morninga | 0.24 (0.16) | 0.02 | 1.13 | |
| S4 mid-afternoona | 0.17 (0.14) | 0.02 | 1.54 | |
| S5 early eveninga | 0.13 (0.13) | 0.01 | 1.14 | |
| S6 bedtimea | 0.10 (0.14) | 0.00 | 1.10 | |
| Average cortisol levels across waking day, AUCg | 3.47 (1.81) | 0.52 | 13.65 | |
| Size of CAR | 0.16 (0.32) | −0.71 | 1.10 | |
| Slope | −0.02 (0.01) | −0.11 | 0.03 |
s.d., Standard deviation; MDE, major depressive episode; SES, Hollingshead socio-economic status index; AUCg, area under the curve with respect to ground, or total waking cortisol; CAR, cortisol awakening response; slope, rate of cortisol decline from wake-up to bedtime.
Cortisol levels at times 1–6 are given in µg/dl and represent average cortisol levels over the 3 days of sample collection at the following times: sample immediately upon waking (S1); sample 40 min after waking (S2); sample approximately 3 h post-waking (S3); sample approximately 8 h post-waking (S4); sample approximately 12 h post-waking (S5); and sample immediately before bedtime (S6). All analyses were conducted using natural log-transformed values.
Table 2.
Zero-order Pearson correlations
| Variablea | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | 1.00 | ||||||||||||||||||||||||||
| 2. SES | 0.07 | 1.00 | |||||||||||||||||||||||||
| 3. Age | −0.04 | 0.14* | 1.00 | ||||||||||||||||||||||||
| 4. African American | −0.06 | −0.10 | 0.08 | 1.00 | |||||||||||||||||||||||
| 5. Hispanic | −0.09 | −0.54* | −0.06 | −0.15* | 1.00 | ||||||||||||||||||||||
| 6. Birth control | −0.19* | 0.05 | −0.02 | −0.06 | −0.15* | 1.00 | |||||||||||||||||||||
| 7. Tobacco | 0.07 | 0.05 | 0.09 | −0.05 | −0.07 | 0.14* | 1.00 | ||||||||||||||||||||
| 8. Asthma | −0.08 | 0.05 | 0.00 | 0.03 | −0.05 | 0.04 | 0.02 | 1.00 | |||||||||||||||||||
| 9. Hours of sleep | 0.04 | −0.04 | 0.02 | −0.02 | 0.137 | 0.00 | 0.01 | 0.03 | 1.00 | ||||||||||||||||||
| 10. Wake time | 0.06 | 0.15* | −0.01 | −0.07 | −0.10 | 0.01 | 0.02 | −0.05 | −0.06 | 1.00 | |||||||||||||||||
| 11. Neuroticism | −0.13* | 0.13* | 0.02 | −0.06 | −0.04 | 0.12* | 0.01 | 0.09 | −0.21* | 0.04 | 1.00 | ||||||||||||||||
| 12. MDE historyb | −0.05 | 0.05 | 0.09 | −0.02 | −0.01 | 0.09 | 0.07 | 0.02 | −0.12 | 0.02 | 0.19* | 1.00 | |||||||||||||||
| 13. Anxiety disorder | 0.08 | 0.12* | −0.02 | 0.04 | −0.14* | 0.17* | 0.00 | 0.05 | −0.11 | −0.03 | 0.32* | 0.11 | 1.00 | ||||||||||||||
| 14. Baseline episodic stress | 0.00 | −0.08 | 0.15* | −0.01 | 0.03 | 0.18* | 0.17* | 0.04 | −0.11 | 0.01 | 0.10 | 0.22* | 0.09 | 1.00 | |||||||||||||
| 15. Baseline chronic stress | 0.05 | −0.26* | −0.04 | 0.14* | 0.18* | 0.04 | 0.16* | 0.08 | −0.08 | −0.12* | 0.17* | 0.23* | 0.11 | 0.31* | 1.00 | ||||||||||||
| 16. Negative affect | −0.20* | 0.17* | 0.02 | −0.08 | −0.10 | 0.27* | −0.01 | 0.07 | −0.11 | 0.08 | 0.45* | 0.06 | 0.18* | 0.01 | 0.02 | 1.00 | |||||||||||
| 17. Fatigue | −0.13* | 0.19* | 0.05 | 0.03 | −0.13* | 0.15* | −0.08 | 0.00 | −0.11 | 0.12* | 0.20* | 0.04 | 0.06 | 0.02 | −0.15* | 0.27* | 1.00 | ||||||||||
| 18. S1 | −0.18* | −0.03 | −0.14* | −0.11 | 0.06 | 0.06 | −0.07 | −0.01 | 0.03 | 0.03 | −0.01 | 0.07 | −0.02 | 0.12 | 0.00 | 0.04 | 0.01 | 1.00 | |||||||||
| 19. S2 | −0.32* | 0.00 | 0.01 | −0.07 | −0.02 | 0.16* | −0.07 | −0.02 | 0.03 | 0.00 | 0.01 | 0.03 | 0.02 | 0.04 | −0.03 | 0.08 | 0.07 | 0.28* | 1.00 | ||||||||
| 20. S3 | −0.14* | 0.07 | 0.02 | 0.03 | −0.11 | 0.26* | −0.06 | 0.05 | 0.01 | −0.05 | 0.05 | 0.06 | 0.11 | 0.10 | 0.07 | 0.25* | 0.15* | 0.33* | 0.37* | 1.00 | |||||||
| 21. S4 | −0.14* | 0.00 | −0.03 | −0.03 | 0.01 | 0.15* | 0.01 | 0.04 | 0.07 | −0.04 | 0.02 | 0.07 | 0.01 | 0.05 | 0.07 | 0.08 | 0.05 | 0.19* | 0.30* | 0.42* | 1.00 | ||||||
| 22. S5 | −0.01 | 0.02 | −0.01 | 0.06 | −0.04 | 0.09 | −0.02 | 0.06 | −0.08 | 0.02 | 0.08 | 0.08 | 0.07 | 0.02 | 0.12* | 0.09 | 0.05 | 0.22* | 0.17* | 0.38* | 0.43* | 1.00 | |||||
| 23. S6 | −0.12* | 0.01 | 0.05 | 0.01 | 0.05 | 0.15* | −0.01 | 0.17* | −0.06 | 0.05 | 0.07 | 0.10 | −0.02 | 0.06 | 0.10 | 0.14* | 0.03 | 0.28* | 0.17* | 0.45* | 0.38* | 0.54* | 1.00 | ||||
| 24. AUCg | −0.20* | 0.01 | −0.03 | −0.01 | −0.03 | 0.24* | −0.05 | 0.06 | −0.04 | −0.07 | 0.06 | 0.09 | 0.07 | 0.08 | 0.09 | 0.19* | 0.11 | 0.43* | 0.55* | 0.74* | 0.75* | 0.64* | 0.63* | 1.00 | |||
| 25. CAR | 0.11 | 0.04 | 0.17* | 0.12* | −0.03 | 0.02 | 0.07 | 0.09 | −0.10 | −0.01 | 0.06 | −0.01 | 0.01 | −0.08 | 0.07 | 0.06 | 0.02 | −0.81* | −0.17* | −0.05 | 0.04 | 0.10 | 0.31* | −0.04 | 1.00 | ||
| 26. Slope | −0.16* | 0.03 | 0.11 | 0.02 | −0.05 | 0.11 | −0.01 | −0.02 | 0.00 | −0.02 | 0.03 | −0.05 | 0.04 | −0.05 | −0.03 | 0.05 | 0.08 | −0.46* | 0.70* | 0.10 | 0.14* | −0.01 | −0.04 | 0.21* | 0.44* | 1.00 | |
| 27. Prospective major life events | 0.00 | −0.13* | 0.01 | 0.07 | 0.01 | 0.18* | 0.10 | 0.19* | 0.01 | −0.06 | 0.03 | 0.17* | 0.10 | 0.21* | 0.33* | 0.06 | −0.02 | −0.04 | 0.01 | 0.07 | 0.08 | 0.08 | 0.13* | 0.11 | 0.11 | 0.04 | 1.00 |
SES, Hollingshead socio-economic status index; MDE, major depressive episode; AUCg, area under the curve with respect to ground, or total waking cortisol; CAR, cortisol awakening response.
Variable names are condensed to conserve space; refer to Table 1 for full variables names.
‘MDE history ’ (variable 12) refers to whether or not (yes = 1, no = 0) a participant was diagnosed with having experienced at least one clinically significant MDE prior to cortisol measurement.
Statistically significant correlation (p < 0.05).
The CAR predicting MDEs
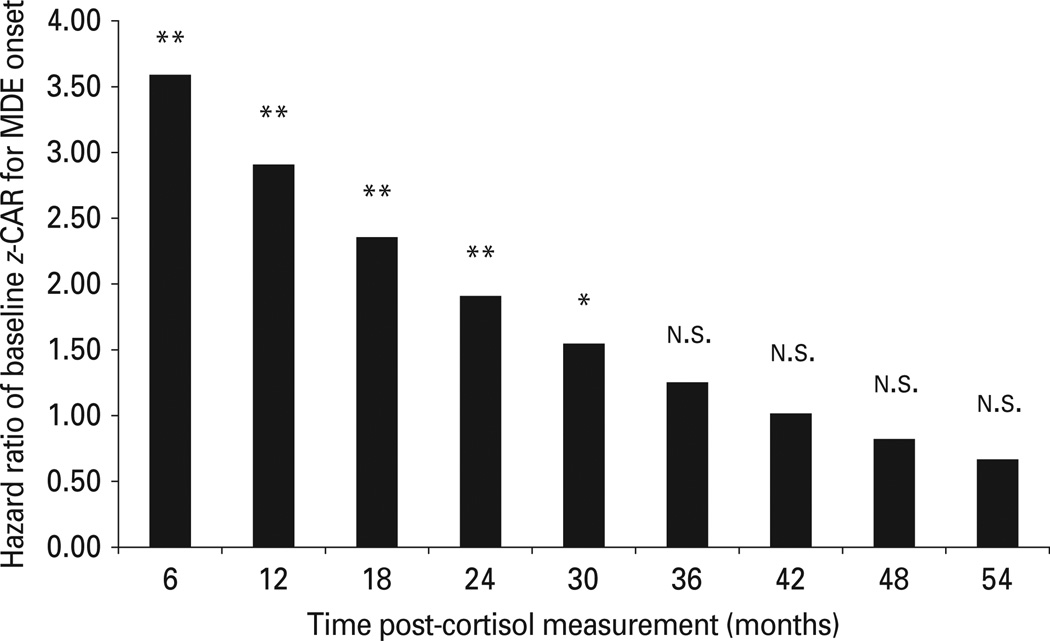
In the final primary model, the CAR was a significant prospective predictor of MDEs; at the median of time (21 months), any one standard deviation increase in the CAR is associated with approximately double the risk for MDE onset. The CAR × time interaction was also significant; the predictive power of the CAR degraded (about 3% per month) with successive months (Table 3, model 1). There was no evidence for a decline in the underlying risk of depressive onsets per person-month (hazard function) over time. To estimate how long the CAR predicts MDEs at a statistically significant level, the time intercept was re-centered to reflect each 6-month time point since the cortisol assessment, providing estimates of effect size at each time point. As implied by the linear decline in prediction over time, maximal effects were seen shortly after cortisol measurement, with a one standard deviation increase in the CAR corresponding to a roughly 3.5-fold increase in risk for MDE onset (Fig. 1). Results also indicate that the CAR significantly predicts MDEs in our sample up to 30 months, or 2.5 years, but not significantly so at 36 months and beyond.
Table 3.
Results of several Cox regression models for MDE onsetsa
| Variable | β (s.e.) | Hazard ratio: exp(β) |
(95% CI) | p |
|---|---|---|---|---|
| Model 1: Primary model | ||||
| CAR | 0.706 (0.241) | 2.030 | (1.260–3.250) | 0.003 |
| Slope | −0.234 (0.193) | 0.791 | (0.542–1.156) | 0.226 |
| AUCg | 0.030 (0.217) | 1.030 | (0.674–1.575) | 0.891 |
| CAR × time | −0.031 (0.013) | 0.969 | (0.946–0.993) | 0.012 |
| Model 2: Robustness test for the addition of baseline neuroticism | ||||
| CAR | 0.749 (0.262) | 2.114 | (1.264–3.535) | 0.004 |
| CAR × time | −0.032 (0.014) | 0.969 | (0.942–0.996) | 0.025 |
| Baseline neuroticism | 1.212 (0.238) | 3.360 | (2.106–5.359) | <0.001 |
| Model 3: Interaction with prospective major stressful life events | ||||
| CAR | 0.762 (0.269) | 2.143 | (1.265–3.631) | 0.005 |
| CAR × time | −0.034 (0.014) | 0.967 | (0.941–0.993) | 0.014 |
| Prospective events | 2.183 (0.376) | 8.876 | (4.250–18.539) | <0.001 |
| CAR × events | 0.164 (0.350) | 1.179 | (0.594–2.340) | 0.639 |
| Model 4: Interaction with MDE history | ||||
| CAR | 0.736 (0.281) | 2.088 | (1.205–3.619) | 0.009 |
| CAR × time | −0.034 (0.014) | 0.967 | (0.941–0.993) | 0.014 |
| MDE history | 1.291 (0.385) | 3.636 | (1.709–7.735) | 0.001 |
| CAR × MDE history | 1.162 (0.526) | 3.195 | (1.140–8.952) | 0.027 |
MDE, Major depressive episode; s.e., standard error; CI, confidence interval; CAR, cortisol awakening response; AUCg, area under the curve with respect to ground, or total waking cortisol.
All models include CAR, slope, AUCg and the CAR × time interaction as well as additional covariates for gender, Hollingshead socio-economic status index, African American race, Hispanic ethnicity, tobacco use, asthma, time of waking, hours of sleep, and hormonal contraceptive use. Continuous variables were entered in the model as z scores to aid interpretation; dichotomous variables (i.e. gender) were entered untransformed.
Fig. 1.
Model-estimated hazard ratios illustrate the observed cortisol awakening response (CAR) × time interaction. Values are hazard ratios for z-scored baseline ln-transformed CAR in 6-month intervals after baseline. MDE, Major depressive episode. * p < 0.05, ** p < 0.01, n.s., non-significant (p > 0.05).
To examine the robustness of the CAR main effect and CAR × time interaction following the addition of other known predictors of depression and potential confounding variables, four separate models were tested with the respective addition of: (a) baseline neuroticism (Table 3, model 2); (b) anxiety disorder at baseline and history of an MDE; (c) baseline episodic and chronic life stress; and (d) negative affect and fatigue at the time of cortisol participation. Although in each case the additional block made a significant contribution to predicting onsets of MDE [all block changes in χ2 (df≤2) ≥13.60, all p’s <0.05], the CAR and its interaction with time remained significant predictors of MDEs with very similar HRs as in the primary model. Including time interactions for the additional covariates did not alter the findings.
Does the CAR interact with prospective major stressful life events?
When person-months without LSI data were excluded, a total of 9645 person-months were available, including 39 MDE onsets. While a main effect of major stressful life events occurring in the past month robustly predicted MDE onsets, the CAR × major stressful life events interaction was not significant (Table 3, model 3). The CAR and its interaction with time remained significant predictors of MDEs independently of major stressful life events.
Does the CAR predict first onsets and recurrences of MDE differentially?
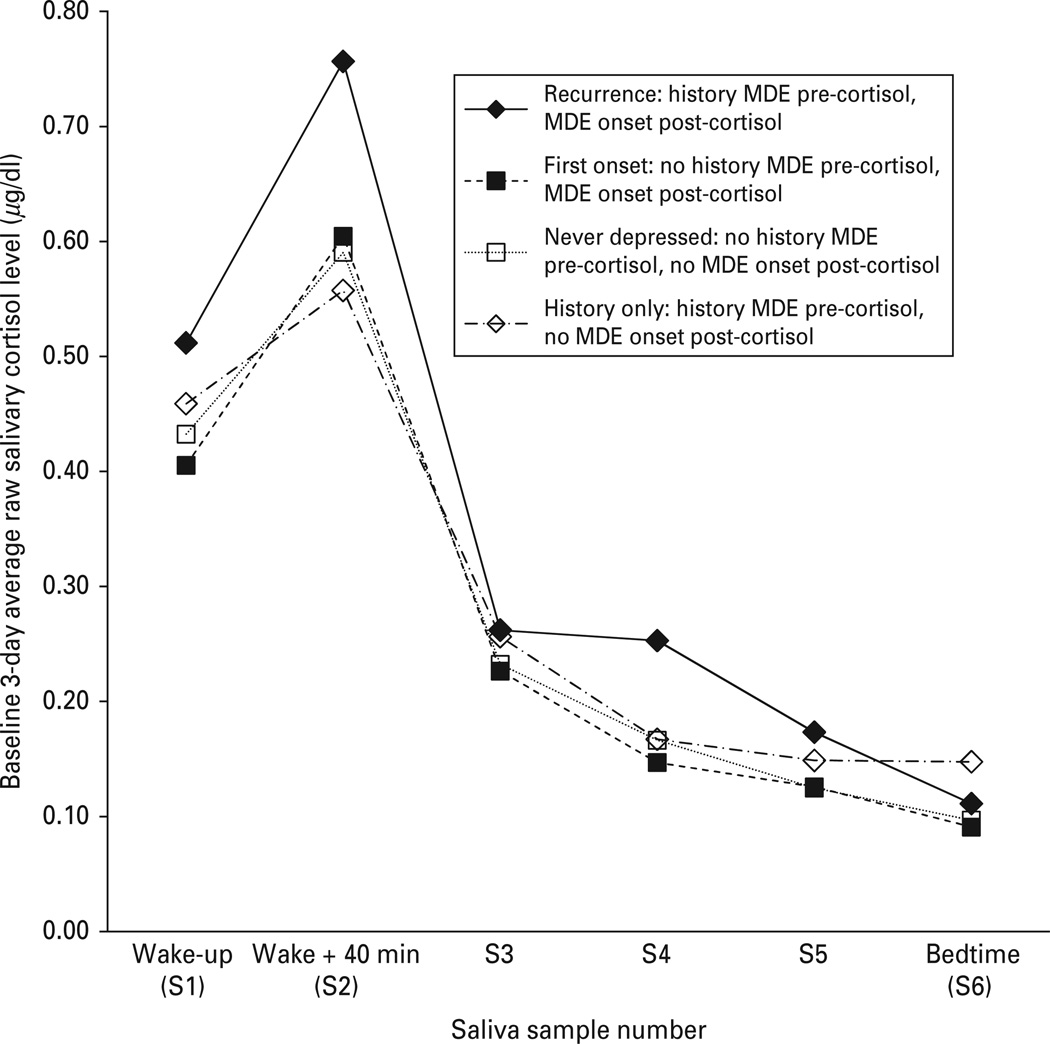
To evaluate whether the CAR similarly predicted both first onsets and recurrences of an MDE when one prospective MDE per person was considered, an interaction term was created between MDE history at the time of the cortisol sampling (no = 0, yes = 1) and the CAR. When this MDE history × CAR interaction was added, the simple main effect of CAR, indicating its relationship to first onsets of MDE, remained significant, as did the CAR × time interaction. The MDE history × CAR interaction effect was also significant (Table 3, model 4). Whereas elevated CARs significantly predicted first onsets, they predicted recurrences with greater strength. Diurnal rhythms of raw salivary cortisol levels are presented by diagnostic group (never depressed, first-onset MDE, recurrent MDE, or history of MDE only) in Fig. 2. This interaction effect was not a matter of differing CAR magnitudes in the first-onset and recurrence groups, as CAR magnitudes did not significantly differ as a function of MDE history [F(1, 40) = 0.22, n.s.]. Further, the number of months between cortisol measurement and MDE onset did not differ significantly between first onsets (mean = 22.19, s.d. = 12.64) and recurrences (mean = 21.13, s.d. = 16.59) [F(1, 40) = 0.053, p = 0.82].
Fig. 2.
Baseline 3-day averages of raw salivary cortisol level (µg/dl) across the day for never-depressed participants, participants who experienced a first onset of major depressive episode (MDE) within the 4-year follow-up, participants who experienced a recurrence of MDE within the 4-year follow-up, and participants with a history of MDE but no recurrence during the 4-year follow-up. Analyses were conducted with natural logarithmically transformed values.
Discussion
Using data from 270 participants from the larger Youth Emotion Project over four annual follow-ups, this is the first study to explicitly test the extent to which the CAR predicts MDE onsets consistently over an extended period of time. Results showed that at 6 months after cortisol sampling, any one-unit increase in the baseline standardized CAR was associated with a greater than 3-fold increase in risk per month for MDE onset. This risk then decays, remaining a statistically significant predictor in our sample for approximately 2.5 years (out of 4 years), but not significantly so after such time. These effects persisted despite covariance of other known predictors of MDEs and possible confounding variables. Thus, the CAR appears to serve as a time-limited risk factor for depression. Studies interested in predicting onsets of MDE over a several-year period may benefit from including a baseline CAR measure, but longitudinal studies using the CAR as a predictor of MDE onsets over more extended periods of time may benefit from using repeated measures of the CAR. This is also the first study to examine whether the CAR predicts MDE onsets in interaction with prospectively occurring major stressful life events. The results indicate that both the CAR and major events predict MDEs uniquely and independently of one another.
In addition, the results showed that when a single prospective MDE onset per person is considered, the CAR significantly predicts both first onsets and recurrences of depression, but that the CAR predicts recurrences with greater strength than it predicts first onsets. Both individuals experiencing first onsets and those experiencing recurrences have CARs of similar magnitude (Fig. 2), but the CAR appears particularly important in predicting whether someone with a history of depression will experience a recurrence. Because the CAR prospectively predicts first onsets, these data suggest that an elevated CAR should not be construed solely as a ‘scar’ of depression (Cowen, 2010), i.e. a marker that is only present following an MDE, which may be indicative of damage caused by the episode. The observed effect is consistent with several possible explanations. Individuals with a history of MDE who have recurrences during the follow-up period experienced earlier first onsets than those whose first onsets were observed during the follow-up period. Earlier age of first onset is associated with a worse course of illness (Boland & Keller, 2009) and may account for the observed effect. Alternatively, Post’s (1992) kindling theory has suggested that within-person change occurs as a result of a first MDE, which may erode resilience and increase an individual’s propensity for later episodes. While the present data do not examine within-person change in the relation of the CAR to MDE, a stronger relationship between the CAR and recurrences of MDE compared with first onsets of MDE may be consistent with such within-person change.
Taken together, these findings suggest that the CAR and not other aspects of the diurnal cortisol rhythm predict future MDE onsets. Current theory posits that the CAR functions to prepare the individual to rise to the expected demands of the upcoming day (Adam et al. 2006; Fries et al. 2009); thus, the presence of the CAR can be seen as adaptive. However, frequent large CAR values may indicate excessive perceived demands and/or insufficient perceived resources (e.g. competencies and social support) to meet those demands. In such instances, large CAR increases may be indicative of struggle above and beyond anything captured by measuring mood. By contrast, flattened diurnal cortisol slopes may serve as a marker of the day’s levels of negative emotions such as tension and anger, leading to elevated evening cortisol levels (Adam et al. 2006). Flattened diurnal slopes are also suggested to serve as a ‘scar’ marker for prior depression (Doane et al. in press), suggesting they may indicate allostatic load associated with accommodating recent psychopathology, among other stressors.
Although the present data do not speak to the biological mechanism by which the CAR is related to MDE risk, several possibilities exist. As noted earlier, the CAR reflects both the highest and the most rapidly increasing cortisol levels of the day. Over time, an elevated CAR may contribute to more intense occupation and eventual down-regulation of glucocorticoid receptors, a pattern which has been implicated in depression (Holsboer, 2000). Further, the CAR appears to be regulated by a range of factors, including extra-pituitary efferent pathways from the suprachiasmatic nucleus to the adrenal influencing sensitivity to adrenocorticotropic hormone (ACTH), and a role for hippocampal functioning, perhaps particularly pre-awakening (Clow et al. 2010). Such pathways may uniquely influence the CAR, and not the diurnal slope or evening levels, leading to the differential importance of the CAR for prediction of MDE onsets. Finally, it is possible that the CAR does not play a causal role in precipitating MDE onsets, but rather serves as a proxy marker for risk conferred by unknown but correlated neural processes.
Limitations
Despite strengths of the present study, including the use of structured diagnostic and life stress interviews, the longitudinal design, the naturalistic measurement of the CAR over a 3-day period, and inclusion of potentially confounding covariates, this study has several limitations. First, due to concerns about feasibility with 17-year-olds and participant burden, only two samples were collected to define the CAR (0 and 40 min after waking), rather than the original approach, which relied on samples taken every 10–15 min for 1 h after waking (Pruessner et al. 1997). Second, although the examination of stressful life events in person-month format represents an advancement over other approaches, we have examined major stressful life events in an aggregate fashion. It may be that CAR increases vulnerability to certain types of major stressful events, but not all major events. Third, we cannot rule out a developmental interpretation whereby the CAR’s interaction with time indicates that the CAR was particularly important during the age range closest to the CAR measurement (i.e. roughly ages 17 to 19 years), rather than a decay in predictive ability. Fourth, we would have liked to examine whether the CAR’s relation to prospective MDE onsets differed between males and females, i.e. a gender × CAR interaction; however, the number of onsets among males remains too low to address this question (seven cases). Finally, given that the present study oversampled for participants with high levels of neuroticism (59%), it may be important to replicate these findings among a representative community sample or a sample selected based on a different risk factor, such as family history of depression.
Conclusions
This is the first study to demonstrate that the CAR predicts depression onsets for 2.5 years of a 4-year follow-up, initially serving as a strong predictor but ‘decaying’ in its predictive ability with the passage of time. That is, the CAR appears to function as a time-limited risk factor for depression. Further, the CAR does not significantly enhance vulnerability to major stressful life events. Third, while the CAR predicts both first onsets and recurrences of depression when a maximum of one prospective MDE onset per person is considered, it predicts recurrences with greater strength than first onsets. Research which examines factors contributing to individual differences in the CAR, and within-person change in the CAR over time may be particularly important for understanding the role of the HPA axis in the etiology and course of major depressive disorder.
Acknowledgements
The present study was supported by a two-site grant from the National Institute of Mental Health to S.M. and R.E.Z. (no. R01-MH065652) and to M.G.C. (no. R01-MH065651), by a William T. Grant Foundation Scholars Award to E.K.A., a Faculty Fellowship (E.K.A.) and Graduate Fellowship (L.D.D.) from the Institute for Policy Research at Northwestern University, and a Postdoctoral NRSA from the National Institute of Mental Health to S.V.-S. (no. F32-MH091955). The content is the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health.
Footnotes
A portion of these results was presented by S.V.-S. at annual meetings of the Society for Research in Psychopathology (2010) and the International Society for Psychoneuroendocrinology (2011).
Declaration of Interest
None.
References
- Adam E, Doane L, Zinbarg R, Mineka S, Craske M, Griffith J. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam E, Gunnar M. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam E, Hawkley L, Kudielka B, Cacioppo J. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland R, Keller M. Course and outcome of depression. In: Gotlib I, Hammen C, editors. Handbook of Depression. New York: Guilford Press; 2009. pp. 23–44. [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Chrousos G, Gold P. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Costa PT, Terracciano A, McCrae RR. Gender differences in personality traits across cultures: robust and surprising findings. Journal of Personality and Social Psychology. 2001;81:322–331. doi: 10.1037/0022-3514.81.2.322. [DOI] [PubMed] [Google Scholar]
- Cowen P. Not fade away: the HPA axis and depression. Psychological Medicine. 2010;40:1–4. doi: 10.1017/S0033291709005558. [DOI] [PubMed] [Google Scholar]
- Cox D. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187–220. [Google Scholar]
- Doane LD, Adam EK, Mineka S, Zinbarg R, Craske M, Griffith J. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology. doi: 10.1017/S0954579413000060. (in press) [DOI] [PubMed] [Google Scholar]
- Dressendörfer R, Kirschbaum C, Rohde W, Stahl F, Strasburger C. Synthesis of a cortisol–biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus–pituitary–adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6:21–29. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Non-Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 2001. [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Geerlings M, Beekman A, Deeg D, Penninx B, Comijs H. Early and late life events and salivary cortisol in older persons. Psychological Medicine. 2010;40:1569–1578. doi: 10.1017/S0033291709991863. [DOI] [PubMed] [Google Scholar]
- Goldberg L. A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. In: Mervielde I, Deary I, De Fruyt F, Ostendorf F, editors. Personality Psychology in Europe. Tilburg, The Netherlands: Tilburg University Press; 1999. pp. 7–28. [Google Scholar]
- Goodyer I, Croudace T, Dudbridge F, Ban M, Herbert J. Polymorphisms in BDNF (Val66Met) and 5-HTTLPR, morning cortisol and subsequent depression in at-risk adolescents. British Journal of Psychiatry. 2010;197:365–371. doi: 10.1192/bjp.bp.110.077750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer I, Tamplin A, Herbert J, Altham P. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British Journal of Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Halligan S, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C, Hiroto D. Children of depressed mothers: maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- Harris T, Borsanyi S, Messari S, Stanford K, Brown G, Cleary S, Shiers H, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. British Journal of Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Kendler K, Karkowski L, Prescott C. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler K, Kuhn J, Prescott C. The interrelationship of neuroticism, sex, stressful life events in the prediction of episodes of major depression. American Journal of Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, De Vries GJ, Gersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Post R. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Saucier G. Mini-markers: a brief version of Goldberg’s unipolar big-five markers. Journal of Personality Assessment. 1994;63:506–516. doi: 10.1207/s15327752jpa6303_8. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J, Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Wilhelm I, Born J, Kudielka B, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Zinbarg R, Mineka S, Craske M, Griffith J, Sutton J, Rose R, Nazarian M, Mor N, Waters A. The Northwestern-UCLA Youth Emotion Project: associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48:347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]