Abstract
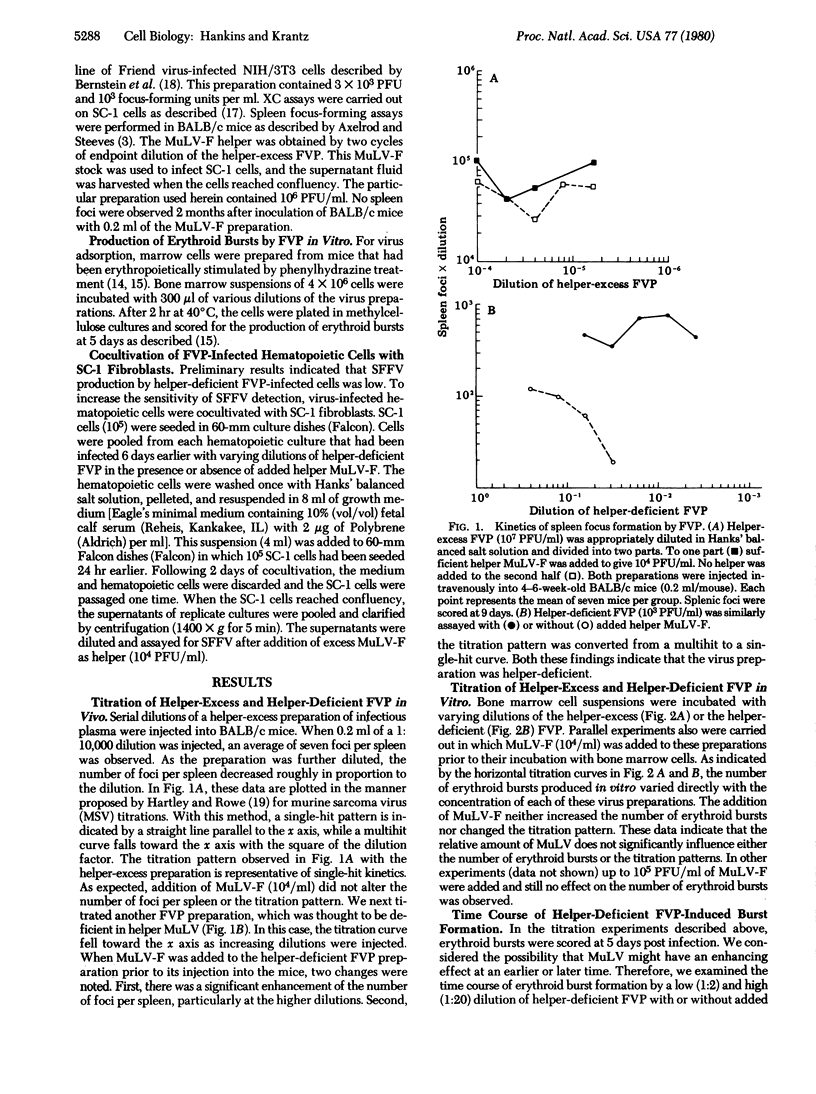
The Friend polycythemia virus complex (FVP), consisting of the replication-defective spleen focus-forming virus (SFFV) and a helper Friend murine leukemia virus (MuLV-F), produces erythroleukemia within 2-3 weeks in vivo. We have recently reported in vitro transformation of bone marrow cells by FVP, producing clusters of erythroid colonies (erythroid bursts) 4-6 days after infection. In contrast to uninfected bone marrow cells, FVP-treated cells proliferated and differentiated (synthesized hemoglobin) in the absence of added erythropoietin, the physiologic regulator of erythropoiesis. The relative roles of helper murine leukemia virus (MuLV) and SFFV in the in vitro erythroid transformation have now been examined. Pseudotype studies and the finding that cloned MuLV-F (free of SFFV) did not induce burst formation indicated that SFFV was essential for this in vitro effect of FVP. Because SFFV could not be obtained free of helper MuLV, we assessed the requirement of MuLV in the transformation by kinetic analyses of helper-deficient and helper-excess FVP preparations. Whereas helper-excess FVP gave single-hit kinetics both in vivo and in vitro, the helper-deficient FVP followed multiple-hit kinetics when titrated for spleen focus formation in vivo. Addition of MuLV-F to helper-deficient FVP prior to injection resulted in a marked enhancement of spleen focus formation and a conversion from multiple-hit to single-hit kinetics. In contrast, titration of this same preparation for erythroid burst transformation in vitro yielded single-hit kinetics, and the addition of helper MuLV-F had no effect. The time course of burst development was similar with or without added MuLV-F. Unlike burst transformation, SFFV production by these infected cultures followed multiple-hit kinetics. Addition of MuLV-F at the time of infection led to an enhancement of SFFV production and conversion of the titration curve from multiple-hit to single-hit. These data are consistent with the idea that SFFV is competent for erythroid transformation in vitro, but requires helper MuLV for its replication.
Keywords: Friend polycythemia virus, spleen focus-forming virus, murine leukemia virus, kinetics, helper-involvement
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Jainchill J. L., Todaro G. J. Murine sarcoma virus transformation of BALB-3T3 cells: lack of dependence on murine leukemia virus. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1236–1243. doi: 10.1073/pnas.66.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Mak T. W., Stephenson J. R. The Friend virus genome: evidence for the stable association of MuLV sequences and sequences involved in erythroleukemic transformation. Cell. 1977 Sep;12(1):287–294. doi: 10.1016/0092-8674(77)90206-9. [DOI] [PubMed] [Google Scholar]
- Dawson P. J., Tacke R. B., Fieldsteel A. H. Relationship between Friend virus and an associated lymphatic leukaemia virus. Br J Cancer. 1968 Sep;22(3):569–576. doi: 10.1038/bjc.1968.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R. J., Hettrick K. L. Defective Friend spleen focus-forming virus: interfering properties and isolation free from standard leukemia-inducing helper virus. J Virol. 1977 Oct;24(1):383–396. doi: 10.1128/jvi.24.1.383-396.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Kost T. A., Koury M. J., Krantz S. B. Erythroid bursts produced by Friend leukaemia virus in vitro. Nature. 1978 Nov 30;276(5687):506–508. doi: 10.1038/276506a0. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Krantz S. B. In vitro expression of erythroid differentiation induced by Friend polycythaemia virus. Nature. 1975 Feb 27;253(5494):731–732. doi: 10.1038/253731a0. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Krantz S. B. Rapid in vivo assay for Friend polycythemia virus. J Natl Cancer Inst. 1974 Apr;52(4):1223–1229. doi: 10.1093/jnci/52.4.1223. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Rosenblatt P., Krantz S. B. Splenic erythroid response to friend polycythemia virus: time course in vitro after infection in vivo. J Natl Cancer Inst. 1977 Jul;59(1):107–111. doi: 10.1093/jnci/59.1.107. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Carter W. A. Friend leukemia: rapid development of erythropoietin-independent hematopoietic precursors. J Natl Cancer Inst. 1975 Jan;54(1):265–267. doi: 10.1093/jnci/54.1.265. [DOI] [PubMed] [Google Scholar]
- Liao S. K., Axelrad A. A. Erythropoietin-independent erythroid colony formation in vitro by hemopoietic cells of mice infected with friend virus. Int J Cancer. 1975 Mar 15;15(3):467–482. doi: 10.1002/ijc.2910150313. [DOI] [PubMed] [Google Scholar]
- Mirand E. A. Murine viral-induced polycyhemia. Ann N Y Acad Sci. 1968 Mar 29;149(1):486–496. doi: 10.1111/j.1749-6632.1968.tb15187.x. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Melderis H., Steinheider G., Kluge N., Dube S. Synthesis of mouse haemoglobin and globin mRNA in leukaemic cell cultures. Nat New Biol. 1972 Oct 25;239(95):231–234. doi: 10.1038/newbio239231a0. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., BRODSKY I. A graded-response assay for the Friend leukemia virus. J Natl Cancer Inst. 1959 Dec;23:1239–1248. [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J. Host-induced changes in infectivity of Friend spleen focus-forming virus. J Natl Cancer Inst. 1970 Mar;44(3):587–594. [PubMed] [Google Scholar]
- Steeves R. A. Editorial: Spleen focus-forming virus in Friend and Rauscher leukemia virus preparations. J Natl Cancer Inst. 1975 Feb;54(2):289–297. doi: 10.1093/jnci/54.2.289. [DOI] [PubMed] [Google Scholar]
- Tambourin P., Wendling F. Malignant transformation and erythroid differentiation by polycythaemia-inducing Friend virus. Nat New Biol. 1971 Dec 22;234(51):230–233. doi: 10.1038/newbio234230a0. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Parks W. P., Vass W. C., Scolnick E. M. Isolation of a fibroblast nonproducer cell line containing the Friend strain of the spleen focus-forming virus. Virology. 1977 Feb;76(2):602–615. doi: 10.1016/0042-6822(77)90242-2. [DOI] [PubMed] [Google Scholar]
- Wendling F., Tambourin P. E. Oncogenicity of Friend-virus-infected cells: determination of origin of spleen colonies by the H-2 antigens as genetic markers. Int J Cancer. 1978 Oct 15;22(4):479–486. doi: 10.1002/ijc.2910220418. [DOI] [PubMed] [Google Scholar]


