Abstract
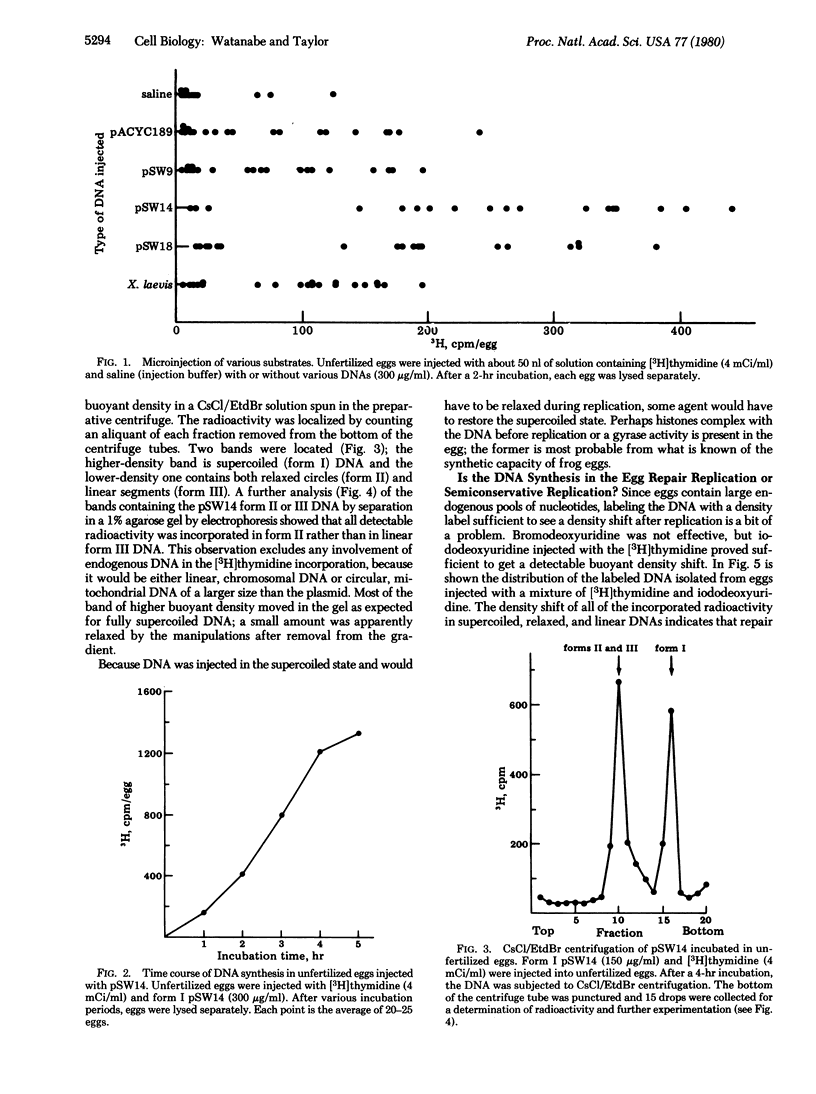
DNA fragments of Xenopus laevis, the African frog, were cloned in the EcoRI site of the Escherichia coli plasmid pACYC189 and tested for ability to initiate and complete replication of the recombinant plasmid when injected into unfertilized eggs of X. laevis. After measurement of the [3H]-thymidine incorporation per egg for a number of recombinant plasmids, pSW14 and pSW9, which respectively contain a small segment (550 base pairs) and several kilobases of frog DNA, were selected for more extensive analysis. In spite of the small size of the segment in pSW14, it incorporates in 2 hr at least 3 times as much labeled thymidine as either pSW9 or the vector alone. The DNA synthesis in pSW14 was shown to be replication rather than repair synthesis, based on a buoyant density shift of the product when iododeoxyuridine was used for labeling. To determine the number of replications of pSW14, a novel method was employed. Because pSW14 is a head-to-head dimer of the vector with the Xenopus fragment inserted at an EcoRI site, the plasmid has three methylatable sites--two bracketing the Xenopus fragment and one opposite the fragment. By cotransformation of E. coli with pSW14 and pBR322 containing the EcoRI methylase gene, supercoiled pSW14 was methylated and injected into eggs with [3H]thymidine. Disappearance of modified EcoRI sites by semiconservative replication was followed by measuring the sensitivity to EcoRI endonuclease over time. The results showed that about 50% of the labeled, supercoiled DNA recovered from eggs after 4 hr was sensitive to EcoRI digestion, which indicates that most of the DNA that incorporated [3H]thymidine had replicated twice during the 4 hr in the unfertilized eggs of X. laevis. We conclude that pSW14 has a functional origin in the Xenopus DNA segment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Crews S. T., Nishiguchi J., Ojala D. K., Posakony J. W. Nucleotide sequence of a fragment of HeLa-cell mitochondrial DNA containing the precisely localized origin of replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):179–192. doi: 10.1101/sqb.1979.043.01.024. [DOI] [PubMed] [Google Scholar]
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Gurdon J. B. Cloned single repeating units of 5S DNA direct accurate transcription of 5S RNA when injected into Xenopus oocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2849–2853. doi: 10.1073/pnas.75.6.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Gurdon J. B. High-fidelity transcription of 5S DNA injected into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 May;74(5):2064–2068. doi: 10.1073/pnas.74.5.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan H. G. Replication of DNA in eukaryotic chromosomes. Br Med Bull. 1973 Sep;29(3):192–195. doi: 10.1093/oxfordjournals.bmb.a071006. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Dawid I. B. Biogenesis of mitochondria during Xenopus laevis development. Dev Biol. 1972 Apr;27(4):504–518. doi: 10.1016/0012-1606(72)90189-3. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Mertz J. E. Coupled transcription-translation of DNA injected into Xenopus oocytes. Cell. 1977 Sep;12(1):175–182. doi: 10.1016/0092-8674(77)90195-7. [DOI] [PubMed] [Google Scholar]
- Ford C. C., Woodland H. R. DNA synthesis in ocytes and eggs of Xenopus laevis injected with DNA. Dev Biol. 1975 Mar;43(1):189–199. doi: 10.1016/0012-1606(75)90140-2. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Birnstiel M. L., Speight V. A. The replication of purified DNA introduced into living egg cytoplasm. Biochim Biophys Acta. 1969 Feb 18;174(2):614–628. doi: 10.1016/0005-2787(69)90291-3. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Yasuda S., Yamada M., Nishimura A., Sugimoto K., Sugisaki H., Oka A., Takanami M. Structural and functional properties of the Escherichia coli origin of DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):129–138. doi: 10.1101/sqb.1979.043.01.019. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Scangos G. A., Ruddle F. H. DNA-mediated gene transfer of a circular plasmid into murine cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5820–5824. doi: 10.1073/pnas.76.11.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Sekiya T. Cloning and characterization of the replication origin from rat mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):193–201. doi: 10.1101/sqb.1979.043.01.025. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Gurdon J. B. Induction of polyoma DNA synthesis by injection into frog-egg cytoplasm. Eur J Biochem. 1973 Sep 3;37(3):467–471. doi: 10.1111/j.1432-1033.1973.tb03007.x. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockey C. H., Saffhill R. The comparative effects of short-term DNA Inhibition on replicon synthesis in mammalian cells. Exp Cell Res. 1976 Dec;103(2):361–373. doi: 10.1016/0014-4827(76)90272-x. [DOI] [PubMed] [Google Scholar]
- Rungger D., Türler H. DNAs of simian virus 40 and polyoma direct the synthesis of viral tumor antigens and capsid proteins in Xenopus oocytes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6073–6077. doi: 10.1073/pnas.75.12.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. H., Hozier J. C. Evidence for a four micron replication unit in CHO cells. Chromosoma. 1976 Sep 24;57(4):341–350. doi: 10.1007/BF00332159. [DOI] [PubMed] [Google Scholar]
- de Vries F. A., Collins C. J., Jackson D. A. Joining of simian virus 40 DNA molecules at endonuclease R Eco Ri sites by polynucleotide ligase and analysis of the products by agarose gel electrophoresis. Biochim Biophys Acta. 1976 Jul 2;435(3):213–227. doi: 10.1016/0005-2787(76)90103-9. [DOI] [PubMed] [Google Scholar]



