Abstract
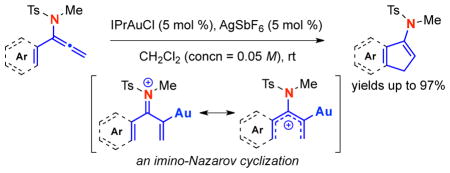
An imino-Nazarov cyclization using α–aryl-substituted allenamides is described. This gold(I)-catalyzed cascade is efficient and regioselective in constructing a diverse array of synthetically useful aromatic-ring fused cyclopentenamides. The success in this transformation represents a solution to the challenge in establishing an imino-Nazarov cyclization process.
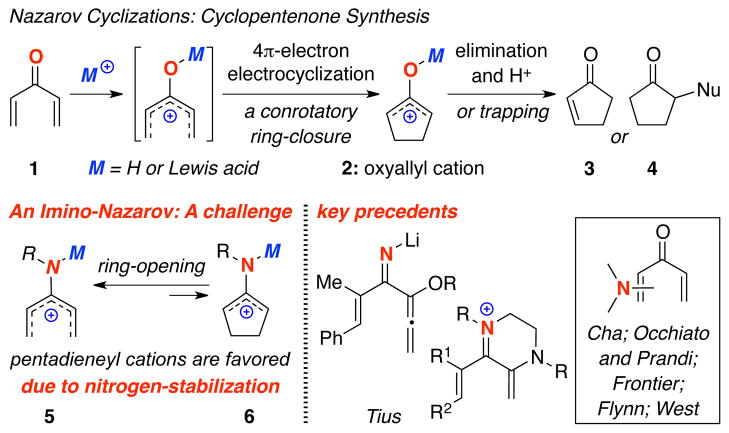
The Nazarov cyclization1 has captured an immense amount of interest from the synthetic community in the last three decades.2,3 This classical pericyclic process proceeds through conrotatory 4π–electron electrocyclic ring-closure of a pentadienyl cation via activation of divinyl ketone 1 or its equivalent, leading to a key oxyallyl cation 2 that can afford cyclopentenone 3 substituted cyclopentanones 4 through trapping [Scheme 1]. Despite a diverse array of innovative structural designs that has been developed,4–6 one aspect that has received very little attention is an imino version of this cascade with the sole exceptions of Tius’ seminal work on lithiated imino-Nazarov,7a and recently, with enamine-iminium ions.7b,c There are examples in which stabilization of the pentadienyl cation is assisted by the presence of an amino or amide substituent, albeit not a formal imino-Nazarov cyclization. Cha’s8 earlier work on cephalotaxine synthesis, along with reports by Occhiato/Prandi,9 Frontier,10 Flynn11 employing ynamides, and recently, by West12 in a clever usage of allenamides represent such elegant cases. The challenge in developing a successful imino-Nazarov cyclization is to overcome the propensity of 2-amino-allyl cation 6 to ring-open in favor of amino-pentadienyl cation 5, which receives a greater stabilization from the amino nitrogen atom.13 We wish to communicate here our solution to this challenge through gold(I)-activation of allenamides.
Scheme 1.
Nazarov Cyclizations: Challenge in an Imino-Version.
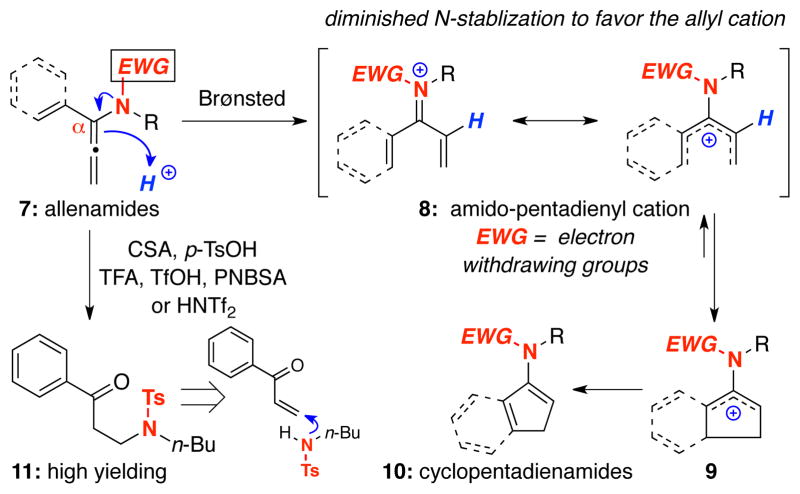
Our long standing interest in allenamides,14–16 and in particular, their electrophilic activations,17,18 led us to explore their potential use in this underdeveloped aspect of the Nazarov cyclization. As shown in Scheme 2, α–vinyl or α–aryl allenamides 7 could be employed in an imino-Nazarov cyclization cascade through Brønsted acid activation.19 This electrophilic activation could give amido-pentadienyl cation 8, which would undergo an electrocyclization to afford 2-amido-allyl cation 9. It is noteworthy that a distinct advantage in achieving a successful imino-Nazarov cyclization is the synthetically useful enamide functionality20 in the end product 10.
Scheme 2.
An Approach to Imino-Nazarov Cyclization.
We reasoned that unlike amino-pentadienyl cation 5, the electrocyclization of 7 could be more favored given the reduced ability of an N-acyl nitrogen atom to stabilize cations relative to an amino group. Such rationalization would find prevalent support in related chemistry of vinyl N-acyl iminium ions21 and aza-Prins cyclizations.22 However, after many attempts, the Brønsted acid activation failed because we were unable to control and overtake the hydrolysis pathway that led to amido ketones 11 through a facile trapping of the initial enone.
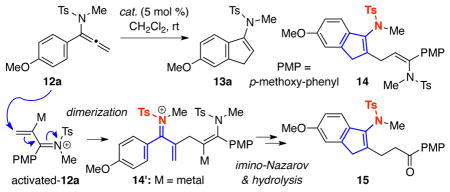
Inspired by recent gold-catalysis23 particularly in the area of Nazarov type process24 with the report by Zhang,19d we turned our attention to gold catalysts. As shown in Table 1, trivalent gold catalyst was first investigated. Reactions of 12a proceeded smoothly in the presence of catalytic amount of AuCl3 or cationic AuCl3/AgSbF6 (entries 1 and 2), but gave an inseparable mixture of dimers 14 and 1525 with no desired Nazarov cyclization product 13a. Attempts to suppress the dimerization by lowering the concentration (entry 3) or addition of 4 Å MS (entry 4) proved to be fruitless.
Table 1.
Screening for a Suitable Metal Catalyst.
| |||||
|---|---|---|---|---|---|
| entrya | catalyst | time (h) | conversion (%)b | 13a (%)c | 14 + 15d (%)c |
| 1 | AuCl3 | 30 | >95 | – | 55 |
| 2 | AuCl3/AgSbF6 | 1 | >95 | – | 45 |
| 3 | AuCl3/AgSbF6e | 22 | 48 | – | 36 |
| 4 | AuCl3/AgSbF6f | 40 | 55g | – | 36 |
| 5 | AuCl/AgSbF6 | 5 | >95 | – | 48 |
| 6 | AuCl(PPh3)/AgSbF6 | 60 | 75g | 50h | 24 |
| 7 | JohnphosAuCl/AgSbF6 | 5 | >95 | 91 | – |
| 8 | IPrAuCl/AgSbF6 | 1 | >95 | 97 | – |
| 9 | IPrAuCl | 21 | <5 | – | – |
| 10 | AgSbF6i | 20 | 64 | – | 24 |
| 11 | PtCl2 | 24 | <5 | – | – |
| 12 | PtCl4i | 12 | >95 | – | 82j |
Unless noted, all reactions were carried out at 0.1 mmol scale in 2 mL of CH2Cl2 (concn = 0.05 M) at room temperature with the addition of 5 mol % catalyst.
Conversion determined by 1H NMR.
Isolated yields.
14 and 15 could not be separated by column chromatography.
20 mL CH2Cl2 was used (concn = 0.005 M).
35 mg 4 Å MS was added.
Based on recovered 12a.
Inseparable mixture with 12a.
10 mol % of catalyst was used.
An inseparable mixture of 14, 15 and unidentified products.
Nevertheless, we were encouraged by dimers 14 and 15 because they imply that the desired imino-Nazarov reaction had taken place possibly post-dimerization through the intermediacy of 14′ [addition of 12a to activated-12a] Gratifyingly with AuCl(PPh3)/AgSbF6, 13a was obtained for the first time in 50% yield along with 14/15 mixture in 24% yield. Further optimizations revealed that the best yield was reached when using IPrAuCl/AgSbF6, leading to 13a in 97% yield (entry 8) with JohnphosAuCl/AgSbF6 affording 13a in 91% yield. Remarkably, neutral IPrAuCl itself turned out to be completely inactive (entry 9), while AgSbF6 alone gave 14 and 15, albeit sluggishly (entry 10). Lastly, Pt catalysts were also examined and gave very poor results in this process (entries 11 and 12).
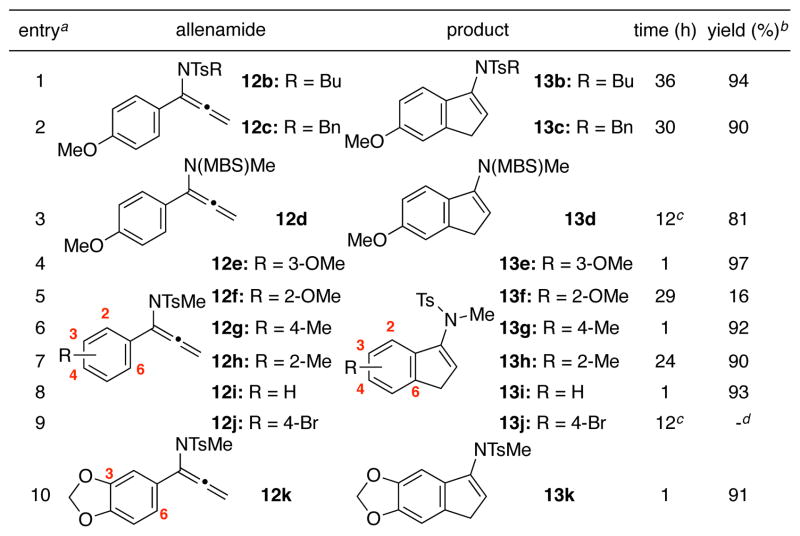
Having established the optimum catalytic system, the scope of the imino-Nazarov process was explored [Table 2]. Notably, for allenamides equipped with more bulky substituents on the nitrogen (n-Bu in 12b and Bn in 12c), reactions were slower, although yields were still excellent (entries 1 and 2). The structure of 13b was unambiguously assigned through its X-ray structure [left in Figure 1]. The cyclization of 12d, bearing a 4-methoxybenzenesulfonyl [MBS] substituent, also worked but at elevated temperature. Lastly, allenamides with different substituents on the aryl ring (entries 4–9) also proved to be quite effective overall with exceptions of 2-MeO and 4-Br substituents (entries 5 and 9). In particular, while the reaction of 12f is very tardy, the cyclization of 12j was completely attenuated. We are not clear of the precise rationale at this point. However, it is noteworthy that the high level of regioselectivity found in cases of 12e and 12k was remarkable. While these two cyclizations appear to favor the less hindered C6-position with no detectable cyclization at C2-position, this preference is likely due to direct donation from the C3-OR group.
Table 2.
An Au(I)-Catalyzed Imino-Nazarov Cyclization.
|
Unless noted, all reactions were carried out at 0.1 mmol scale in 2 mL of CH2Cl2 (concn = 0.05 M) at rt with the addition of 5 mol % catalyst.
Isolated yields.
The reaction was run at 50 °C in DCE.
No reaction.
Figure 1.
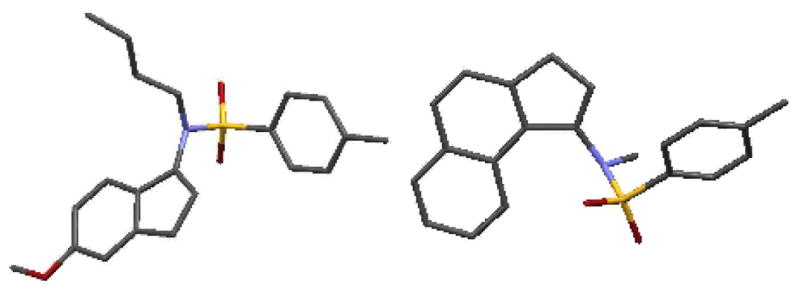
X-Ray Structures of 13b and 20.
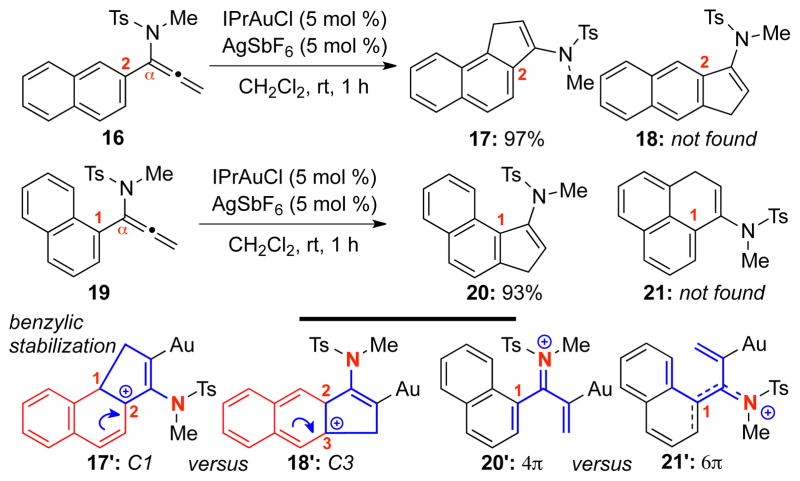
To further investigate the regioselectivity issue, allenamides 16 and 19 were subjected to the cyclization condition [Scheme 3]. For allenamide 16 with an α–(2-naphthyl) substituent, cyclization took place selectively at C1 [see 17′], affording 17 exclusively in 97% yield. This regioselectivity is also likely an electronic preference given that cyclization at C1 would involve the cyclohexadiene potion of the naphthyl ring, thereby leading to a greater cation stabilization: Benzylic stabilization in 17′ versus pseudo-benzylic stabilization in 18′ through cyclization from C3. On the other hand, for allenamide 19 with an α–(1-naphthyl) group, a competing 6π-electron cyclization of the reaction intermediate [see 21′] could deliver 1H-phenalene 21. However, the reaction exclusively proceeded through the imino-Nazarov cyclization pathway via 20′, leading to 20 in excellent yield. The structure of 20 was also confirmed by its single crystal X-ray structure [Figure 1]. It is noteworthy that 17 and 20 are structurally almost identical with the difference residing in the position for the respective enamide motif.
Scheme 3.
Regioselective Imino-Nazarov Cyclizations.
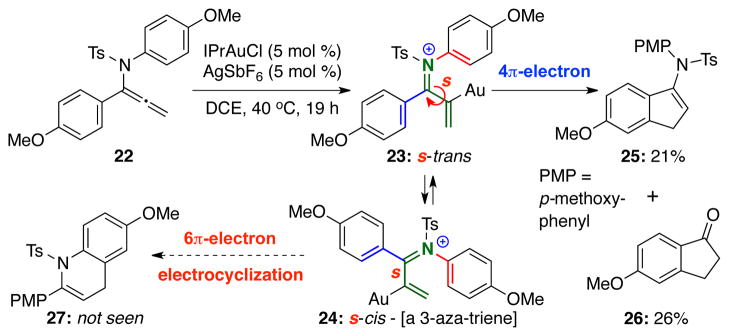
Intrigued by this competition, another reaction with potential competing imino-Nazarov’s 4π-electron and 6π-electron electrocyclization was examined using allenamide 22 [Scheme 4]. The reaction of 22 led to 25 and 26 as the only discernable products with latter likely coming from hydrolysis of the initial Nazarov cyclization product 25. This study suggests that while the cationic imino-Nazarov intermediate could exist in two equilibrating conformations 23 and 24 differing only at the ‘s’-marked C–C bond [or being trans- and cis-1-aza-dienes, respectively, shown in green], 6π-electron electrocyclization of 3-aza-triene 2426,27 was also not competitive in this case.
Scheme 4.
4π– Versus 6π–Electron Electrocyclization.
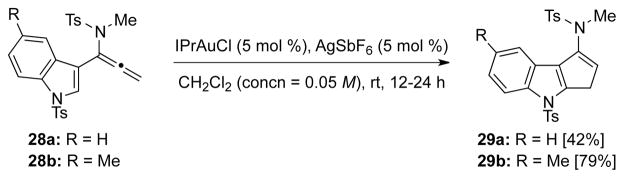
Lastly, while cyclopentenamides are synthetically useful, we recognized that our imino-Nazarov cyclization could provide a concise approach to indole-fused cyclopentenamides [or cyclopenta[b]indoles],24a thereby representing an opportunity to develop new strategy for natural product synthesis.28 Consequently, as shown in Scheme 5, under the optimized conditions, cyclizations of α-indolyl-substituted allenamides 28a and 28b proceeded smoothly to afford indole-fused cyclopentenamides 29a and 29b, respectively, in good yields.
Scheme 5.
Synthesis of Indole-Fused Cyclopentenamides.
We have showcased here an imino-Nazarov cyclization using α-aryl-substituted allenamides. This Au(I)-catalyzed cascade represents a successful solution to overcome the challenge in designing an imino-Nazarov cyclization cascade. It is a highly efficient and regioselective transformation in constructing a diverse array of aromatic-ring fused cyclopentenamides. Further mechanistic studies and applications of this cyclization process in total synthesis are currently underway.
Supplementary Material
Acknowledgments
We thank NIH [GM066055] for funding. We thank Dr. Vic Young of University of Minnesota for providing X-ray structural analysis.
Footnotes
Supporting Information Available: Experimental procedures as well as NMR spectra, X-ray structural files, and characterizations are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.Nazarov IN, Torgov IB, Terekhova LN. Izv Akad Nauk SSSR, Otd Khim Nauk. 1942:200. [Google Scholar]
- 2.For earlier reviews, see: Habermas KL, Denmark SE, Jones TK. In: Organic Reactions. Paquette LA, editor. Vol. 45. John Wiley & Sons; New York: 1994. pp. 1–158.Denmark SE. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, editors. Vol. 5. Pergamon; Oxford, U.K: 1991. pp. 751–784.Santelli-Rouvier C, Santelli M. Synthesis. 1983:429.
- 3.For recent reviews, see: Vaidya T, Eisenberg R, Frontier AJ. ChemCatChem. 2011;3:1531.Shimada N, Stewart C, Tius MA. Tetrahedron. 2011;67:5851. doi: 10.1016/j.tet.2011.05.062.Grant TN, Rieder CJ, West FG. Chem Commun. 2009;45:5676. doi: 10.1039/b908515g.Nakanishi W, West F. Curr Opin Drug Discovery Dev. 2009;12:732.Frontier A, Collison C. Tetrahedron. 2005;61:7577.Tius MA. Eur J Org Chem. 2005:2193.Pellissier H. Tetrahedron. 2005;61:6479.Tius MA. Acc Chem Res. 2003;36:284. doi: 10.1021/ar0200394.Harmata M. Chemtracts. 2004;17:416.
- 4.Given the large volume, for some reports in 2012 on Nazarov cyclizations, see: Shimada N, Stewart C, Bow WF, Jolit A, Wong K, Zhou Z, Tius MA. Angew Chem Int Ed. 2012;51:5727. doi: 10.1002/anie.201201724.Lebœuf D, Gandon V, Ciesielski J, Frontier AJ. J Am Chem Soc. 2012;134:6296. doi: 10.1021/ja211970p.Kerr DJ, Flynn BL. Org Lett. 2012;14:1740. doi: 10.1021/ol300332b.Guo F, Wang L, Mao S, Zhang C, Yu J, Han J. Tetrahedron. 2012;68:8367.Álvarez E, Miguel D, García-García P, Fernández-Rodríguez MA, Rodríguez F, Sanz R. Synthesis. 2012:1874.Raja S, Ieawsuwan W, Korotkov V, Rueping M. Chem Asian J. 2012;7:2361. doi: 10.1002/asia.201200391.Dateer RB, Pati K, Liu RS. Chem Commun. 2012;48:7200. doi: 10.1039/c2cc33030j.Gan Z, Wu Y, Gao L, Sun X, Lei J, Song Z, Li L. Tetrahedron. 2012;68:6928. (i) Also see References 11 and 12.
- 5.For leading references on arrested-Nazarov, see: Scadeng O, Ferguson MJ, West FG. Org Lett. 2011;13:114. doi: 10.1021/ol102651k.Rostami A, Wang Y, Arif AM, McDonald R, West FG. Org Lett. 2007;9:703. doi: 10.1021/ol070053m.Song D, Rostami A, West FGJ. Am Chem Soc. 2007;129:12019. doi: 10.1021/ja071041z.White TD, West FG. Tetrahedron Lett. 2005;46:5629.Giese S, Kastrup L, Stiens D, West FG. Angew Chem Int Ed. 2000;39:1970. doi: 10.1002/1521-3773(20000602)39:11<1970::aid-anie1970>3.0.co;2-b.Bender JA, Arif AM, West FG. J Am Chem Soc. 1999;121:7443.
- 6.Also see: Dhoro F, Tius MA. J Am Chem Soc. 2005;127:12472. doi: 10.1021/ja053393g.Dhoro F, Kristensen TE, Stockmann V, Yap GPA, Tius MA. J Am Chem Soc. 2007;129:7256. doi: 10.1021/ja0718873.Nair V, Bindu S, Sreekumar V, Chiaroni A. Org Lett. 2002;4:2821. doi: 10.1021/ol026010h.
- 7.Tius MA, Chu CC, Nieves-Colberg R. Tetrahedron Lett. 2001;42:2419.Bow WF, Basak AK, Jolit A, Vicic DA, Tius MA. Org lett. 2010;12:440. doi: 10.1021/ol9025765.Shimada N, Ashburn BO, Basak AK, Bow WF, Vicic DA, Tius MA. Chem Commun. 2010;46:3774. doi: 10.1039/b927564a.For an example postulating an imino-Nazarov reaction pathway, see: Suarez-Pantiga S, Rubio E, Alvarez-Rua C, González JM. Org Lett. 2009;11:13. doi: 10.1021/ol8025523.
- 8.Kim SH, Cha JK. Synthesis. 2000:2113. [Google Scholar]
- 9.(a) Larini P, Guarna A, Occhiato EG. Org lett. 2006;8:781. doi: 10.1021/ol053071h. [DOI] [PubMed] [Google Scholar]; (b) Occhiato EG, Prandi C, Ferrali A, Guarna A. J Org Chem. 2005;70:4542. doi: 10.1021/jo0504058. [DOI] [PubMed] [Google Scholar]
- 10.(a) Bitar AY, Frontier AJ. Org Lett. 2009;11:49. doi: 10.1021/ol802329y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Malona JA, Colbourne JM, Frontier AJ. Org Lett. 2006;8:5661. doi: 10.1021/ol062403v. [DOI] [PubMed] [Google Scholar]
- 11.Kerr DJ, Miletic M, Chaplin JH, White JM, Flynn BL. Org Lett. 2012;14:1732. doi: 10.1021/ol300316a. [DOI] [PubMed] [Google Scholar]
- 12.Wu YK, Niuz T, West FG. Chem Commun. 2012;48:9186. doi: 10.1039/c2cc34644c. [DOI] [PubMed] [Google Scholar]
- 13.Smith DA, Ulmer CW., II J Org Chem. 1997;62:5110. [Google Scholar]
- 14.For a leading review on allenamide chemistry, see: Hsung RP, Wei LL, Xiong H. Acc Chem Res. 2003;36:773. doi: 10.1021/ar030029i.
- 15.Also see: Standen PE, Kimber MC. Curr Opin Drug Discovery Dev. 2010;13:645.Deagostino A, Prandi C, Tabasso S, Venturello P. Molecules. 2010;15:2667. doi: 10.3390/molecules15042667.For general reviews on allenes, see: Krause N, Hashmi ASK. Modern Allene Chemistry. 1 and 2 Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004.
- 16.Given the large volume, for reports on allenamide chemistry just in 2012, see: (a) Reference 12. Du Y, Krenske EH, Antoline JE, Lohse AG, Houk KN, Hsung RP. J Org Chem. 2012 doi: 10.1021/jo3011792. ASAP Article.Singh S, Elsegood MRJ, Kimber MC. Synlett. 2012;23:565.Heffernan SJ, Carbery DR. Tetrahedron Lett. 2012;53:5180.Hayashi R, Ma ZX, Hsung RP. Org Lett. 2012;14:252. doi: 10.1021/ol203030a.X-X, Zhu L-L, Zhou W, Chen Z. Org Lett. 2012;14:436. doi: 10.1021/ol202703a.Husinec S, Petkovic M, Savic V, Simic M. Synthesis. 2012:399.Suárez-Pantiga S, Hernández-Díaz C, Piedrafita M, Rubio E, Gonzáleza JM. Adv Syn Cat. 2012;354:1651.Faustino H, Paloma Bernal P, Luis Castedo L, Fernando López F, Mascareñas JL. Adv Synth Catal. 2012;354:1658.Francos J, Grande-Carmona F, Faustino H, Iglesias-Sigüenza J, Díez E, Alonso I, Fernández R, Lassaletta JM, López F, Mascareñas JL. J Am Chem Soc. 2012;134:14322. doi: 10.1021/ja3065446.Broggini G, Borsini E, Fasana A, Poli G, Liron F. Eur J Org Chem. 2012:3617.
- 17.For an account, see: Lohse AG, Hsung RP. Chem-Eur J. 2011;17:3812. doi: 10.1002/chem.201100260.
- 18.Via oxidation: Xiong H, Hsung RP, Berry CR, Rameshkumar C. J Am Chem Soc. 2001;123:7174. doi: 10.1021/ja0108638.Via Brønsted acid: Berry CR, Hsung RP, Antoline JE, Petersen ME, Rameshkumar C, Nielson JA. J Org Chem. 2005;70:4038. doi: 10.1021/jo0503411.Via halogen: Hayashi R, Walton MC, Hsung RP, Schwab J, Yu X. Org Lett. 2010;12:5768. doi: 10.1021/ol102693e.
- 19.For a Brønsted acid activation of allenol ethers, see: Wu YK, West FG. J Org Chem. 2010;75:5410. doi: 10.1021/jo101112t.For an oxidative activation, see: Spencer WT, III, Levin MD, Frontier AJ. Org Lett. 2011;13:414. doi: 10.1021/ol1027255.Malona JA, Cariou K, Frontier AJ. J Am Chem Soc. 2009;131:7560. doi: 10.1021/ja9029736.For a gold-activation, see: Zhang L, Wang S. J Am Chem Soc. 2006;128:1442. doi: 10.1021/ja057327q.
- 20.For a leading review on enamides, see: Carbery DR. Org Biomol Chem. 2008;9:3455. doi: 10.1039/b809319a.Also see: Rappoport Z. In The Chemistry of Functional Groups. John Wiley and Sons; New York: 1994. Tracey MR, Hsung RP, Antoline J, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: In Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb SM, editor. Chapter 21.4. Georg Thieme Verlag KG; 2005.
- 21.For reviews, see: Maryanoff BE, Zhang HC, Cohen JH, Turchi IJ, Maryanoff C. Chem Rev. 2004;104:1431. doi: 10.1021/cr0306182.Royer J, Bonin M, Micouin L. Chem Rev. 2004;104:2311. doi: 10.1021/cr020083x.
- 22.For reviews, see: Hiemstra H, Speckamp WN. In: Comprehensive Organic Synthesis. Brossi A, editor. Vol. 2. Academic Press; San Diego: 1988. pp. 271–339.Hanessian S, Tremblay M, Marzi M, Del Valle JR. J Org Chem. 2005;70:5070. doi: 10.1021/jo050326w.
- 23.For reviews, see: Boorman TC, Larrosa I. Chem Soc Rev. 2011;40:1910. doi: 10.1039/c0cs00098a.Bandini M. Chem Soc Rev. 2011;40:1358. doi: 10.1039/c0cs00041h.Krause N, Winter C. Chem Rev. 2011;111:1994. doi: 10.1021/cr1004088.Fürstner A. Chem Soc Rev. 2009;38:3208. doi: 10.1039/b816696j.Gorin DJ, Sherry BD, Toste FD. Chem Rev. 2008;108:3351. doi: 10.1021/cr068430g.Fürstner A, Davies PW. Angew Chem Int Ed. 2007;46:3410. doi: 10.1002/anie.200604335.Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x.
- 24.For examples of Nazarov-like processes through activations of vinyl allenes, see: Chen B, Fan W, Chai G, Ma S. Org Lett. 2012;14:3616. doi: 10.1021/ol301408g.Lemière G, Gandon V, Cariou K, Hour A. J Am Chem Soc. 2009;131:2993. doi: 10.1021/ja808872u.Oh CH, Karmakar S. J Org Chem. 2009;74:370. doi: 10.1021/jo802103g.Bhunia S, Liu RS. J Am Chem Soc. 2008;130:16488. doi: 10.1021/ja807384a.Shi FQ, Li X, Xia Y, Zhang L, Yu ZX. J Am Chem Soc. 2007;129:15503. doi: 10.1021/ja071070+.Lee JH, Toste FD. Angew Chem Int Ed. 2007;46:912. doi: 10.1002/anie.200604006.Funami H, Kusama H, Iwasawa N. Angew Chem Int Ed. 2007;46:909. doi: 10.1002/anie.200603986.Marion N, Díez-González S, de Frémont P, Noble AR, Nolan SP. Angew Chem Int Ed. 2006;45:3647. doi: 10.1002/anie.200600571.Shi X, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5802. doi: 10.1021/ja051689g.
- 25.See Supporting Information.
- 26.Okamura WH, de Lera AR. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Paquette LA, editors. Vol. 5. Pergamon Press; New York: 1991. pp. 699–750. [Google Scholar]
- 27.For leading references, see: Tanaka K, Katsumura S. J Am Chem Soc. 2002;124:9660. doi: 10.1021/ja026464+.Sklenicka HM, Hsung RP, McLaughlin MJ, Wei LL, Gerasyuto AI, Brennessel WW. J Am Chem Soc. 2002;124:10435. doi: 10.1021/ja020698b.
- 28.For some examples, see: Feng T, Li Y, Wang YY, Cai XH, Liu YP, Luo XD. J Nat Prod. 2010;73:1075. doi: 10.1021/np100086x.He YL, Chen WM, Feng XZ. Phytochemistry. 1994;37:1055.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.