Abstract
Previous research has clearly established associations between low socioeconomic status (SES) and poor youth physical health outcomes. This article provides an overview of the main pathways through which low SES environments come to influence youth health. We focus on two of the most prevalent chronic health problems in youth today, asthma and obesity. We review and propose a model that encompasses (1) multiple levels of influence, including the neighborhood, family and person level, (2) both social and physical domains in the environment, and finally (3) dynamic relationships between these factors. A synthesis of existing research and our proposed model draw attention to the notion of adverse physical and social exposures in youth’s neighborhood environments altering family characteristics and youth psychosocial and behavioral profiles, thereby increasing youth’s risk for health problems. We also note the importance of acknowledging reciprocal influences across levels and domains (e.g., between family and child) that create self-perpetuating patterns of influence that further accentuate the impact of these factors on youth health. Finally, we document that factors across levels can interact (e.g., environmental pollution levels with child stress) to create unique, synergistic effects on youth health. Our model stresses the importance of evaluating influences on youth’s physical health not in isolation but in the context of the broader social and physical environments in which youth live. Understanding the complex relationships between the factors that link low SES to youth’s long-term health trajectories is necessary for the creation and implementation of successful interventions and policies to ultimately reduce health disparities.
Keywords: socioeconomic status, youth, asthma, obesity, family, neighborhood, social environment, physical environment
Socioeconomic status (SES) has long been known to influence physical health, with increases in SES being associated with striking benefits to health (Adler et al., 1994; Adler & Newman, 2002). SES-based disparities have been demonstrated across a range of health outcomes in adults, including cardiovascular disease (Kaplan & Keil, 1993), diabetes (Everson, Maty, Lynch, & Kaplan, 2002), and mortality (Feinglass et al., 2007), and the influence of SES on health begins early in life with lasting influences on youth’s health well into their adult years (Chen, Matthews, & Boyce, 2002; Miller, Chen, & Parker, 2011; Poulton et al., 2002).
These persistent associations between SES and health have challenged researchers to more fully explain why they exist. Various aspects of both the physical and social environment of youth have been postulated to play a role, including neighborhood factors, such as the built environment (Cohen, Inagami, & Finch, 2008) and violence (Wright et al., 2004b), and family factors, such as exposure to indoor pollutants (Diette et al., 2007) and parent psychological characteristics (Wright, Cohen, Carey, Weiss, & Gold, 2002). However, psychologists who have embarked upon SES-health research have primarily focused on the psychosocial factors that explain these relationships. In addition, many mechanistic studies have focused on single categories of influence (e.g., individual-level distress), yet these approaches are limited in their ability to explain SES gradients. For example, accounting for a single category of mechanism, such as health behaviors, does not eliminate the relationship between SES and mortality (Lantz et al., 1998). To ultimately arrive at a more comprehensive understanding of the influences of SES on youth’s health and to be able to properly inform future research and interventions, it is necessary for psychosocial research to be integrated together with an understanding of the physical environment contributors to SES-health gradients, and for the influence of factors at multiple levels to be examined simultaneously.
We note upfront that the detrimental effects of growing up in a low SES environment cannot be explained solely by access to care and genetics. Whereas access to care has some influence on health outcomes and may be reduced among low SES groups, the fact that countries with universal health care, such as Canada (Orpana & Lemyre, 2004) and the UK (Marmot, Shipley, & Rose, 1984; Marmot et al., 1991; Banks, Marmot, Oldfield, & Smith, 2006), nevertheless report gradients in health by SES suggests that access to care may not be at the core of current health inequalities (Pincus, Esther, DeWalt, & Callahan, 1998). With respect to genetics, although genes unquestionably play a role in health, they probably do not serve as a primary confounding factor creating an artificial SES-health gradient (e.g., Adler et al., 1994). Nonetheless, the functional roles that genes play in the body may allow them to act as mediators in other ways, e.g., adverse social environments such as low SES altering patterns of gene expression that have implications for health. Alternatively, genotypes could serve in a moderator fashion, e.g., in particular, moderating the relationships between environmental variables and health (Cole, 2009). In this paper, we do not include a primary focus on either access to care or genetics in this paper, although we do discuss the moderation and mediation possibilities for genes later in the section on biological pathways to asthma.
The focus of the current review is to provide an overview of the literature on mechanisms of the SES-health relationship across multiple levels (i.e., the neighborhood, family, and individual) and across multiple domains (i.e., social and physical) in order to develop a more comprehensive model of how low SES influences physical health. Some of these issues have previously been discussed in reviews, but rarely have they been integrated. Although some reviews have focused only on influences at single levels, be it at the individual (Lehrer, Feldman, Giardino, Song, & Schmaling, 2002; Bloomberg & Chen, 2005), family (Sandel & Wright, 2006), or neighborhood (Pickett & Pearl, 2001; Diez Roux, 2001) level, others have focused on influences in either the social (Yen & Syme, 1999; McNeill, Kreuter, & Subramanian, 2006) or physical domain (Booth, Pinkston, & Poston, 2005). The goal of this review is to provide an overview and highlight the importance for psychologists of accounting for physical exposures (together with the social factors they traditionally study), and of considering factors across multiple levels (neighborhood, family, individual) in efforts to explain SES and health relationships.
To narrow the scope of this review, we chose to focus on childhood health outcomes, given that SES disparities are already documented during this period of life, and given that disparities created in childhood may lay the foundation for life-long health trajectories. More specifically, we will focus on two of the most common health concerns in childhood and adolescence, pediatric asthma and obesity, both of which follow the traditional SES gradient (Simon, Zeng, Wold, Haddock, & Fielding, 2003; Miech et al., 2006; Delva, O’Malley, & Johnston, 2006). As the subsequent section will show, both are widespread among today’s youth and pose significant health risks to youth.
This review is organized into three sections. First, we provide a brief overview of the SES gradient in childhood asthma and obesity. Second, we present an overarching model of the factors ultimately affecting health disparities and discuss possible mechanisms for these effects, broken down into categories by level (neighborhood, family, individual) and domain within level (physical, social). Third, we elaborate on the ways in which factors across multiple levels and domains may operate and interact to affect health disparities.
SES and Childhood Asthma and Obesity
Pediatric Asthma and Obesity
Both asthma and obesity affect millions of children and adolescents, contributing significantly to the overall national disease burden, putting youth at an increased risk for later health problems and resulting in large economic costs (Visscher & Seidell, 2001; Weiss & Sullivan, 2001; Akinbami, Moorman, Garbe, & Sondik, 2009). Previous reviews have documented consistent associations of low SES with poor childhood health outcomes, including asthma and obesity (Starfield, 1982; Starfield, Riley, Witt, & Robertson, 2002; Chen et al., 2002). We refer the reader to these other reviews for additional details about the epidemiological associations of SES with childhood health outcomes (Starfield, 1982; Starfield et al., 2002; Chen et al., 2002). Rates of both obesity and asthma have increased sharply in recent years and hence have become the primary health problems faced by today’s youth (Hedley et al., 2004; Dey & Bloom, 2005).
SES patterns in pediatric asthma
Pediatric asthma has been on the rise in recent years (Akinbami & Schoendorf, 2002). The lifetime prevalence of asthma among American youth under the age of 18 is now estimated at 12.5%, that is about 9 million youth; in addition, about 4 million children and adolescents under the age of 18 experienced an acute asthma attack in the past year (Dey & Bloom, 2005).
Low SES is consistently associated with greater asthma impairment, including more frequent emergency department visits (Simon et al., 2003; Maziak et al., 2004), more frequent hospitalizations (Dales, Choi, Chen, & Tang, 2002; Amre, Infante-Rivard, Gautrin, & Malo, 2002), greater symptoms (Ernst, Demissie, Joseph, Locher, & Becklake, 1995), and more severe asthma (Mielck, Reitmeir, & Wjst, 1996). These findings hold across different types of SES measures (e.g. income, occupation) and across both family and neighborhood SES measures. For example, low SES is associated with higher prevalence rates of asthma, across both family and neighborhood measures (Castro, Schechtman, Halstead, & Bloomberg, 2001; Simon et al., 2003; Cesaroni, Farchi, Davoli, Forastiere, & Perucci, 2003; Claudio, Stingone, & Godbold, 2006). Occasionally studies have failed to find an inverse relationship between pediatric asthma and neighborhood level SES (e.g. Shankardass et al., 2007); however, reduced access to health services in low SES areas may result in underreporting of asthma prevalence in such areas.
SES patterns in pediatric obesity
The last two decades alone have seen significant increases in overweight and obesity, typically defined as a sex- and age-specific body mass index (BMI) ≥ 85th and 95th percentile respectively, among children and adolescents, with current estimates of overweight among US youth between the ages of 2–19 years being ~16% (Ogden, Flegal, Carroll, & Johnson, 2002; Hedley et al., 2004). This pattern has been found to exist among low SES youth in particular (Mei et al., 1998; Vieweg, Johnston, Lanier, Fernandez, & Pandurangi, 2007; Shrewsbury & Wardle, 2008).
Similar to the relationships discussed above, low SES is associated with a greater likelihood of overweight or obesity across a number of both family and neighborhood measures (Lobstein, Baur, Uauy, & TaskForce, 2004; Lamerz et al., 2005; Oliver & Hayes, 2005; Wickrama, Wickrama, & Bryant, 2006; Janssen, Boyce, Simpson, & Pickett, 2006). One review found mixed support for the relationship between low SES in childhood and increased risk of childhood obesity, but reported consistent associations between low childhood SES and obesity in adulthood (Parsons, Power, Logan, & Summerbell, 1999). Overall, however, the relationships between low SES and obesity have been demonstrated by a number of studies to be fairly robust (Lynch, Kaplan, & Salonen, 1997; Everson et al., 2002).
In sum, past research has established associations between living in low SES environments and youth health problems including asthma and obesity. Youth from low SES neighborhoods and families as a group evidence higher rates of, and impairment from, asthma and obesity. In the next section we provide an overview of the types of factors that have been proposed to explain the above SES and health relationships.
Pathways between SES and Child Health
Because SES is such a broad construct reflecting both household and neighborhood characteristics, there are a wide variety of potential mediators that could explain SES effects. Mediators could occur at the neighborhood (e.g., community support), family (e.g., parenting), or individual child level. At the individual level, pathways include not only psychosocial characteristics (e.g., negative mood) but also the various levels of biological processes (e.g., cells, gene expression) that shape disease pathogenesis. Furthermore, influences at the neighborhood and family levels can be broken down into both physical (e.g., pollutants) and social (e.g., interpersonal conflict) exposures. In order to organize the studies included in this review, we structure the next sections first by level – neighborhood, family, and child – and within level by domain, presenting evidence on both physical and social environment characteristics.
With respect to the physical neighborhood and family environments we refer to the presence or absence of physical components or commodities of the environment, such as traffic, grocery stores, parks, and allergens; that is, factors that are descriptive of the structure of a given environment, rather than its people and the interactions between them. Conversely, when we speak of the social environment we refer to factors that have their origin in interactions between people or within people’s psychological perceptions about their environment, for example violence, routines, trust, and safety, or factors that are descriptive of people’s characteristics and traits, such as parenting styles or psychological states. We acknowledge that the physical and social domains do not operate completely independently of each other and in some cases, there will be overlap in factors across the social and physical domains. We discuss this synergistic relationship in more detail in the second part of the paper; however, we keep the distinction between the social and physical environments in the first part because it helps create an organizational structure for reviewing studies, and because many of the studies stem from distinct disciplinary backgrounds (e.g., environmental health for pollution studies, psychology for studies of interpersonal conflict) that are not typically presented together.
The third section of the paper then integrates influences across levels and domains and discusses the bi-directional and synergistic ways in which these factors operate to shape children’s health. For example, factors at the neighborhood level may spill onto the family, altering family characteristics that in turn have implications for health. Or, family dynamics and child psychosocial characteristics may have reciprocal effects on each other, creating feedback loops that accentuate effects on child health. Or, factors at multiple levels may interact to create synergistic, interactive effects on health.
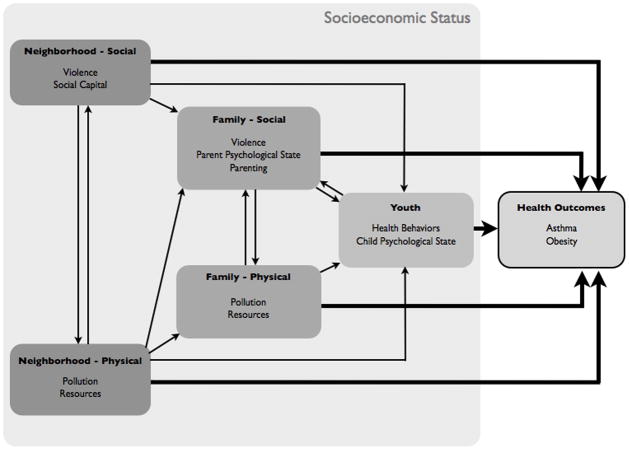
A graphic representation of our model of these influences can be found in Figure 1. This model depicts the primary pathways that lead from various neighborhood, family, and person-level factors to youth physical health. We propose that factors at different levels influence one another in dynamic ways that can alter their effects on child health. That is, factors at different levels and domains should not be conceptualized simply as having independent effects on health (as is implied by studies that examine variables at a single level and their relationship to health), but rather, factors operate in more complex ways that are bi-directional and synergistic to shape children’s health. For example, factors at one level (e.g., the neighborhood) may spill over onto other levels (e.g., the family), affecting how that factor shapes health. Factors at two different levels may also have reciprocal effects on each other (e.g., family dynamics affecting child psychological states and vice versa), creating feedback loops that accentuate effects any one factor can have on child health. Lastly, factors at multiple levels may interact in ways that create synergistic effects on health. We denote these possibilities in Figure 1 by depicting unidirectional arrows (for cross-level, or spillover, effects), and bi-directional arrows for reciprocal effects.
Figure 1.
This type of dynamic model stems from Bronfenbrenner’s ecological systems theory (Bronfenbrenner, 1977) that considered the ways in which children are nested within broader family and neighborhood environments, which both shape them and are shaped by them. Bronfenbrenner’s original model consisted of four nested systems, beginning with a person’s immediate environments, such as the family home and school (microsystem), the interactions between these most immediate settings (mesosystem), formal and informal social structures that influence people and their settings (exosystem) and finally overarching cultural ‘blueprints’ based on which the former three systems develop (macrosystem).
In order to first establish the specific factors that are relevant to SES disparities, in the next sections we provide an overview of the most commonly studied contributors at each level and domain to childhood asthma and obesity. Studies are included below if they discuss factors at any level or within any domain as they are relevant to childhood asthma and obesity, regardless of whether they consider multiple domains or levels simultaneously. In addition, Tables 1–5 summarize relevant studies for each section and are organized by study design (longitudinal followed by cross-sectional studies) and sample size (in decreasing order).
Table 1.
Physical Neighborhood Influences on Youth Health Outcomes
| Citation/Neighborhood level variable | Age range or mean ± SD/Sample size | Type of study/Sample | Outcome/Construct measurement/Finding | Alternatives: Follow-up, covariates, reverse causation |
|---|---|---|---|---|
|
Brauer et al., 2002 Traffic-related air pollution measured at the home over 4 2-week periods across a year |
Followed since birth N = 4146 |
Longitudinal Children part of a Dutch birth cohort study, mothers recruited during the second trimester |
Parent-reported asthma outcomes (physician-diagnosed asthma, asthma symptoms) at 3 months, 1 and 2 years; Robust positive (though not always significant) associations between pollution exposure and asthma outcomes |
Two year follow-up Took into account many confounding variables, including demographics, smoke and other environmental exposures, parental allergies |
|
Gauderman et al., 2007 Traffic exposure; proximity to nearest freeway or major road, estimated pollution at the residence |
10 ± .44 years N = 3677 |
Longitudinal Children recruited from schools in southern California communities |
Pulmonary functioning, tested yearly at the schools using spirometry Children living within 500m of a freeway had worse 8-year growth of forced expiratory volume in one second and maximum midexpiratory flow rate than children living more than 1500m from a freeway |
Eight year follow-up; effect of free-way traffic was independent of regional air quality; model adjusted for demographics, asthma status, exercise, smoking |
|
McConnell et al., 2002 Air pollutant concentration, measured through monitoring stations in communities |
9–16 years N = 3535 |
Longitudinal Children without asthma from school in areas of the communities under study were invited to participate |
Risk of developing asthma (self-reported physician-diagnosed asthma) Greater time spent outside (e.g. because of team sports) was associated with higher incidence of asthma in high (but not low) ozone communities |
Five year follow-up No information on individual exposures, but set up monitoring stations specifically for this study |
|
Chang et al., 2009 Traffic exposure: shortest distance from home to nearest major road, air pollution in 300m buffer zone, total length of major roads in buffer zone, traffic density |
0–18 years N = 3297 |
Longitudinal Youth presenting at one of two hospitals over a 4 year span with asthma as primary or secondary diagnosis |
Repeat ED visits or hospitalization for asthma Living within 300 m of freeways or arterial roads increased risk of repeat hospital visits; associations stronger in females and children without insurance |
Four year follow-up Controlled for gender, age, race, insurance status, residence distance to treating hospital, and census block group median household income |
|
McConnell et al., 2010 Traffic-related air pollution, measured through monitoring stations in communities |
Kindergarten and grade 1 children N = 2,497 |
Longitudinal Healthy children attending kindergarten or grade 1 in California |
Asthma incidence (parent-reported) Greater traffic-related air pollution around the home and youth’s schools was associated with a greater likelihood of developing asthma over the follow-up period |
Three year follow-up Controlled for sociodemographics, pets in the home, second-hand smoke exposure, history of allergy, parental asthma, health insurance, housing characteristics |
|
O’Connor et al., 2008 Daily measurements of outdoor air pollutants from monitoring sites close to participants’ homes |
5–12 years N = 861 |
Longitudinal Youth with persistent asthma from low-income census tracts in urban US areas |
Asthma status; at-home spirometry for two weeks every six months; self-reported asthma symptoms every two months; caretaker-reported asthma symptoms and days of missed school Increases in air pollutant concentrations were associated with lower pulmonary functioning, more missed school and more symptoms |
Two year follow-up Considered 5-day average pollutant concentrations which produced associations as well No influence of use of inhaled corticosteroid, presence of cigarette smoke in the home, more severe asthma, or study group |
|
Zmirou et al., 2004 Retrospectively composed index of lifetime traffic exhaust exposure |
4–14 years N = 390 (195 controls) |
Longitudinal (case-control study) Youth with diagnosed asthma living in one of five French cities under investigation were recruited; healthy controls were matched on city, age, and gender |
Current asthma status Traffic exposure before age 3 (but not lifelong exposure) was positively associated with asthma prevalence, i.e. youth with asthma had been exposed to more traffic exhaust than controls |
Case/control pairs well-matched and investigated on same days Controlled for environmental tobacco smoke, child and parental allergies, and other environmental confounds |
|
Dales et al., 2009 Exposure to roadways; total length of roadways within 200m radius of postal code |
School-age children from grades 1–8 N = 12,693 |
Cross-sectional All grade 1–8 schoolchildren in the Windsor, ON, Canada area were invited to participate (64% did) |
Parent-reported physician-diagnosed asthma and history of wheezing and breathing problems Positive associations between roadway density and wheeze and asthma |
Multiple other family and environmental characteristics accounted for |
|
Nicolai et al., 2003 Traffic exposure assessed through traffic counts and an emission model |
5–7 and 9–11 years N = 7,509 |
Cross-sectional Random samples of schoolchildren from Munich (response rate ~ 84%) |
Self-reported asthma status, symptoms, ETS exposure; among 9–11 year olds also skin-prick tests and sensitization to allergens; spirometry and bronchial challenge task for a subset of 9–11 year olds Greater traffic associated with greater likelihood of current asthma, as were soot and benzene. If also exposed to ETS, greater traffic was also associated with allergic sensitization |
Controlled for age, sex, environmental tobacco smoke exposure, SES, family history of asthma, hay fever, or eczema |
|
Juhn et al., 2005 Census tract facing or not facing major intersection with highways or railroads |
Children < 7 years of age N = 3959 (215 with asthma) |
Cross-sectional Children born in Rochester, Minnesota between 1976 and 1979 and diagnosed with asthma before 1983 |
Incident asthma (including probable and definite cases) Greater relative risk of developing asthma among children from census tracts facing intersections with railroads or highways |
Adjusted for individual-level covariates for asthma and demographics Independent individual-level effects on asthma incident Neighborhood environment influence small to modest |
|
Lin et al., 2004 Exposure to gaseous air pollutants, measured daily using monitoring stations |
6–12 years N = 3754 |
Cross-sectional Data on hospitalization records |
Hospitalization for asthma within a 12-year period For males, exposure to nitrogen dioxide predicted asthma hospitalization among low SES group only; also exposure to sulfur dioxide among low SES females |
Considered cumulative lag exposure; separate analyses for males and females and by SES; took into account weather and humidity |
|
Sahsuvaroglu et al., 2009 Air pollution; distance from roadways, pollution surfaces for air pollutants from fixed-site government pollution monitors, short-term monitors, and land use regression modeling |
Children aged 6– 7 years (grade 1) and 13–14 years (grade 8) N = 1467 |
Cross-sectional Participants from a larger study were selected based on geographic extent of pollution monitoring data available for analysis |
Self-reported asthma and respiratory symptoms Among children (especially girls) without hay fever only, greater exposure to nitrogen dioxide was positively associated with asthma |
Analyses controlled for neighborhood income, dwelling value, state of housing, a deprivation index, and smoking The more refined exposure models produced more robust associations |
|
Edwards et al., 1994 Traffic exposure; residence near roads and traffic flow |
0–5 years N = 1452 (736 hospital controls, number of community controls unspecified) |
Case control study Children hospitalized for asthma or non-respiratory reasons over a 12 months period, and a random sample of community children |
Hospitalization for asthma in Birmingham over a 12 months period Children hospitalized for asthma were exposed to more traffic flow; linear trend for traffic flow for children closer than 500m from main road |
|
|
Gordian et al., 2006 Traffic exposure measured as traffic count in 100 m buffer zones around intersections close to children’s home |
5–7 years N = 1043 |
Cross-sectional Participants recruited from a sample of diverse elementary schools in Anchorage (average return rate by school = 75%) |
Parent-reported asthma prevalence and asthma symptoms Children exposed to more traffic are more likely to have asthma; those with a family history of asthma appear to be more affected by traffic; household smoke exposure also made children more vulnerable to high traffic |
Controlled for gender, parental asthma, household smoking, income Family history also matters |
|
Lin et al., 2002 Traffic exposure; vehicle miles traveled within 200 and 500 m of the home; trucks |
0–14 years N = 888 (461 controls) |
Cross-sectional (case-control study) White children from Erie County, New York, (excluding Buffalo) hospitalized for asthma in a 12 months period and controls |
Hospitalization for asthma Youth hospitalized for asthma lived on roads with greater vehicle miles traveled within 200m and had more trucks and trailers passing within 200m of their home |
Adjusted for age and poverty Controls were children admitted to hospital for non-respiratory reasons Also investigated 500m radius and annual vehicle miles traveled (no association) |
|
Lee et al., 2006 Interquartile range increase in air pollutants, measured at 27 monitoring stations throughout Seoul |
Children < 15 years of age, Average 8.09 patients per day | Cross-sectional Youth hospitalized for aggravated asthma over a 12-month period in Seoul, Korea |
Asthma-related hospitalization and neighborhood SES (average regional health insurance rate) As pollution concentrations increased, more children (especially from low SES areas) were hospitalized |
Considered lag time for pollutants Took into account weather conditions |
|
Ising et al., 2004 Vehicle exhaust and night-time noise (self-reported; also assessed objectively among subsample) |
5–12 years N = 401 |
Cross-sectional Children visiting one of two participating local pediatricians for asthma or bronchitis |
Youth’s physician contacts for asthma and bronchitis for up to the five previous years (from patient files) Children exposed to medium and high levels of traffic exhaust and noise showed dose-dependent increases in asthma compared to those exposed to low levels of traffic emissions |
Controlled for age, sex, presence of smokers and pets in the home, and parent education |
|
Ising & Ising, 2002 Vehicle exhaust and night-time noise level outside the children’s windows |
5–12 years N = 56 |
Cross-sectional From a previous interview study, children with and without bronchitis and from high and low traffic environments were selected |
Bronchitis-related physician visits; salivary cortisol Children living under high traffic noise exposure had higher morning cortisol and more bronchitis-related physician contacts |
Controlled for age, sex, SES |
|
| ||||
|
Sturm & Datar, 2005 Food prices; number of restaurants, grocery and convenience stores in the area |
74.6 ± 4.33 months N = 6,918 |
Longitudinal Children part of a nationally representative study |
BMI changes over 1 and 3 years Lower real prices for fruits and vegetables predicted lower gain in BMI between kindergarten and third grade; bigger effects for poor children and those already at risk for overweight |
4 year follow-up Controlled for baseline BMI, age, family income, and demographics |
|
Bell et al., 2008 Neighborhood vegetation, residential density |
3–16 years N = 3831 |
Longitudinal Identified data of children who had been to routine physician visits in one of seven urban primary care clinics in Marion County, Indiana |
BMI A greener neighborhood was associated with lower BMI 2 years later, regardless of residential density, and with lower odds of increasing BMI scores over 2 years |
2 year follow-up Controlled for demographics, health insurance status, census block group median family income |
|
Powell et al., 2007 Food store availability |
Grade 8 and 10 students N = 73,079 |
Cross-sectional Data from a large, ongoing national study |
BMI (based on self-reported weight and height); overweight: BMI >/= 95th percentile Chain supermarkets negatively associated with BMI; convenience stores positively associated with higher BMI |
Controlled for demographics, parental education, rural/urban area, area SES Food store access slightly more important when mothers working full-time and for African American students |
|
Gordon-Larsen et al., 2006 Physical activity facilities within 8 km buffer zone around residence |
Grade 7–12 students N = 20,745 |
Cross-sectional Recruited from the Add Health Study, a nationally representative sample of US adolescents |
Overweight (BMI > 95th percentile), physical activity Higher SES blocks had more facilities; facilities associated with decreased overweight and increased relative odds of being physically active 5+ times/week |
Controlled for population density within block groups; controlled for census-level population of nonwhite ethnicities for population-level models |
|
Liu et al., 2007 Neighborhood vegetation, proximity to food retail |
3–18 years N = 7334 |
Cross-sectional Identified data of children who had been to routine physician visits in one of seven urban primary care clinics in Marion County, Indiana |
BMI More neighborhood vegetation associated with decreased risk for overweight only in higher population density areas; greater distance to nearest large supermarket associated with greater risk for overweight only in lower density population areas |
Controlled for individual demographics and neighborhood SES |
|
Minaker et al., 2006 School region SES and geographic location |
Grade 9 and 10 students N = 2,621 |
Cross-sectional Students recruited from a stratified random sample of 53 high schools |
Food consumption patterns, nutrient intake, breakfast skipping, cola consumption Higher school region SES was associated with greater fruit and vegetable intake, fiber intake, less sugar, less breakfast skipping |
Controlled for school grade and gender; weighted data by the number of participants per school |
|
Evenson et al., 2007 Physical neighborhood aspects, self-report questionnaire |
Grade 6 girls N = 1554 |
Cross-sectional Recruited from schools in 6 US states |
Physical activity and sedentary behavior (both assessed through accelerometry) and BMI Lower BMI and higher non-school metabolic equivalent weighted moderate to vigorous physical activity were associated with bicycle and walking trails in the neighborhood and access to physical activity facilities |
Controlled for demographics and neighborhood SES |
|
Timperio et al., 2006 Distance to school, busy-road barrier, route along bus route, pedestrian route directness |
5–6 and 10–12 years N = 912 |
Cross-sectional Families recruited from schools in low and high SES areas in Melbourne, Australia |
BMI; active (walking or cycling) school commuting Children with a busy-road barrier, no lights or crossings, or younger children with a steep incline en route were less likely to commute to school actively |
Personal level factors (child doesn’t enjoy physical activity, overweight) and family factors (family structure, car ownership, etc.) were not important with respect to active commuting |
| Norman et al., 2006 Community design, land use mix, access to facilities within 1-mile buffer zone around residence |
11–15 years N = 799 |
Cross-sectional Recruited through primary care providers in San Diego, CA area |
BMI, daily minutes of physical activity, assessed by accelerometer More recreation facilities and parks, lower intersection density predicted greater girls’ activity; greater retail floor area ratio predicted boys’; community design and access to facilities were unrelated to BMI-percentile |
|
|
De Vries et al., 2007 Built environment factors assessed by observational coding |
6–11 years N = 422 |
Cross-sectional Recruited from elementary schools in the 10 Dutch neighborhoods under investigation |
Hours spent performing physical activity, 7-day activity diary Proportion of green space, residential density, perceived activity-friendliness of neighborhood, sports field, water, dog waste, heavy traffic, safe walking and cycling were associated with physical activity |
Controlled for demographics, maternal education, and BMI |
|
Romero, 2005 Self-reported availability and quality of facilities in neighborhood |
10–16 years N = 74 |
Cross-sectional Recruited from local middle schools and community centers |
Self-reported vigorous exercise frequency More physical activity associated with perception of higher quality of local facilities |
Accounted for gender, age, SES |
|
Epstein et al., 2006 Food prices |
10–12 years N = 10 mother-child pairs |
Experimental Families recruited through schools |
Purchase of healthy or snack foods Low SES children bought more unhealthy foods; as the price of (either healthy or unhealthy) foods increased (i.e. were manipulated in the lab environment), parents and children were less likely to buy those foods |
Child age, sex, BMI, average liking for foods in the study, SES, and hunger did not alter the findings |
Table 5.
Individual Influences on Youth Health Outcomes
| Citation/Person level variable | Age range or mean ± SD/Sample size | Type of study/Sampling strategy | Outcome/Construct measurement/Finding | Alternatives: Follow-up, covariates, reverse causation |
|---|---|---|---|---|
|
Weil et al., 1999 Child mental health (Child Behavior Checklist) |
4–9 years N = 1528 |
Longitudinal Recruited from emergency rooms and clinics in 7 inner-city areas in the US |
Asthma morbidity (health care utilization and child functioning) Children with clinically significant behavior problems had more days of wheeze and poorer functional status over the follow-up period |
9-month follow-up controlled for baseline morbidity, demographics, research center site, and other psychosocial variables |
|
McQuaid et al., 2003 Medication adherence (electronically monitored) |
8–16 years N = 106 |
Longitudinal Recruited through emergency department records, flyers, physician referrals, asthma education classes, asthma summer camp attendance |
Asthma morbidity (self-reported frequency of episodes, frequency of symptoms between episodes, intensity of impairment during and in between episodes) Adherence was negatively related to asthma morbidity |
One-month monitoring period of medication adherence |
|
Silverglade et al., 1994 Irrational beliefs, emotionality, anxiety, depression |
12–18 years N = 203 (74 controls) |
Cross-sectional/case-control study Recruited from a pediatric allergy specialist in private practice, controls from a pediatric dentist |
Prevalence of irrational beliefs, emotionality, anxiety and depression according to asthma status Irrational beliefs in the importance of approval, lack of control of emotions, self-reported anxiety, and depression were all more common among youth with moderate to severe asthma as compared to mild or no asthma |
|
| Vila et al., 1999 Anxiety disorders (semistructured diagnostic interview, STAI-C) |
8–17 years N = 186 (94 controls) |
Cross-sectional/case-control study Consecutive outpatients, controls recruited through a nearby school |
Prevalence of anxiety disorders according to asthma status Children scoring higher on the STAI and showing a greater prevalence of anxiety disorders were more likely to have asthma |
Controls matched for demographics, SES |
|
Bussing et al., 1996 Anxiety disorders (semistructured diagnostic interview) |
7–17 years N = 68 (31 controls) |
Cross-sectional/case-control study Enrolled through a pediatric outpatient clinic, controls through the public school system |
Prevalence of anxiety disorders according to asthma status Youth with asthma were more than twice as likely as their healthy counterparts to have an anxiety disorder |
Controls matched for demographics/SES |
|
| ||||
|
Goodman & Whitaker, 2002 Depressed mood (self-reported) |
Grade 7–12 students N = 9374 |
Longitudinal Nationally representative sample (National Longitudinal Study of Adolescent Health) |
Overweight and obesity (BMI > 85th and 95th age- and sex-specific percentile respectively; self-reported height and weight) Depressed mood at baseline predicted obesity at follow-up |
1 year follow-up Controlled for smoking, self-esteem, delinquent behavior, physical activity Baseline obesity did not predict depression at follow-up |
|
Gable et al., 2007 Television watching (parent-reported) |
68.4 ± 4.1 months N = 8000 |
Longitudinal Nationally representative sample (Early Childhood Longitudinal Study – Kindergarten Cohort) |
Overweight (BMI > 95th percentile) Children who watched more television were more likely to be overweight for the first time in third grade and to be persistently overweight |
Followed for 3 years Controlled for demographics, SES |
|
Gortmaker et al., 1996 Television watching (self-reported) |
6–11 years N = 746 |
Longitudinal National probability sample (The National Longitudinal Survey of Labor Market Experience, Youth Cohort) |
Overweight (age- and gender-specific BMI > 85th percentile) Youth who watched more than 5 hours of TV per day were 4.6 as likely to be overweight as those watching 0–2 hours per day; television viewing was also associated with an increased incidence and decreased remission of overweight |
4 year follow-up Controlled for previous weight, baseline maternal overweight, socioeconomic status, household structure, ethnicity, and maternal and child aptitude scores |
| Proctor et al., 2003 Television watching (parent-reported) |
3–5 years N = 106 |
Longitudinal Recruited third and fourth generation offspring of the original Framingham Heart Study cohort |
BMI, triceps skinfolds, sum of five skinfolds (assessed yearly) At age 7, youth who watched 3+ hours of television per day had a greater sum of skinfolds; effects of TV viewing worse for those who were also sedentary and had a higher-fat diet |
Followed for 7 years Controlled for baseline body fat, accelerometer measured physical activity, percent of calories from fat, total calorie intake, parents’ BMI and education |
|
Hamer & Stamatakis, 2008 Psychosocial stress (composite of lower social class, being divorced, psychological distress, and poor education) |
16+ N = 7540 |
Cross-sectional Nationally representative sample (Scottish Health Survey) |
BMI and waist circumference Risk of obesity rose in a dose-response fashion with an increasing number of reported psychosocial stressors |
Adjusted for demographics, health behaviors, inflammatory markers Inflammatory markers mediated the association between psychological stress and obesity |
|
Lohman et al., 2009 Psychological stress (composite of academic problems, maternal lack of future orientation, drug and alcohol use, internalizing, and externalizing problems) |
10–15 years N = 1011 |
Cross-sectional Household-based stratified random sample from 3 US cities (Welfare, Children, and Families: A Three-City Study) |
Overweight and obesity (BMI > 85th and 95th percentile respectively) Greater individual (but not maternal or family) stressors was associated with an increased likelihood of being overweight or obese |
Controlled for demographics, television viewing, low birth weight, limitation because of a disability |
|
Roemmich et al., 2007 Stress reactivity (manipulated through a speech stressor) |
8–12 years N = 63 |
Cross-sectional laboratory study Community samples from two independent studies |
Adiposity (estimated from skin folds and abdominal girth) Change in perceived stress and heart rate reactivity predicted body fat; heart rate reactivity independently predicted abdominal girth |
Controlled for demographics and baseline perceived stress and heart rate Children tested on two days, stress and control condition randomized |
|
Delva et al., 2007 Breakfast skipping, diet, regular exercise, television watching (self-reported) |
Grade 8 and 10 students N = 39,011 |
Cross-sectional Nationally representative samples from the Monitoring the Future Study |
Overweight (age- and sex-specific BMI > 85th percentile; self-reported height and weight) Youth who ate more breakfast, fruits and vegetables, exercised regularly and watched less television were less likely to be overweight |
All analyses adjusted for grade and year of survey Youth lifestyle behaviors were more important than parenting variables |
| Janssen et al., 2004 Leisure time activities (self-reported) |
11–16 years N = 5890 |
Cross-sectional Nationally representative Canadian sample |
Overweight (BMI; weight and height self-reported) Adolescents who watched more television and had lower physical activity levels were more likely to be overweight or obese |
Analyses stratified by age and gender |
|
Crespo et al., 2001 Weekly participation in physical activity, television watching (self-report) |
8–16 years N = 4069 |
Cross-sectional Nationally representative US sample (National Health and Nutrition Examination Survey III) |
BMI Children watching less than one hour television a day were least likely to be obese, those who watched 4+ hours had the highest prevalence of obesity. Television watching was associated with obesity among girls in particular |
Controlled for demographics, family income, weekly physical activity, energy intake |
|
Andersen et al., 1998 Vigorous activity and television watching (self-reported) |
8–16 years N = 4063 |
Cross-sectional Nationally representative US sample (National Health and Nutrition Examination Survey III) |
Body fat, BMI Youth who watched more than 4 hours of television per day had greater body fat and a greater BMI compared to youth who watched less than two hours of television |
Television was more closely associated to body fat than vigorous physical activity |
|
Dowda et al., 2001 Physical activity and television watching (self-reported) |
8–16 years N = 2791 |
Cross-sectional Data from the Third National Health and Nutrition Examination Survey |
Overweight (age- and sex-specific BMI > 85th percentile) More TV watching increased overweight risk; participation in sport teams and exercise programs reduced likelihood of overweight |
Controlled for demographics, physical activity behaviors, and environmental variables |
|
Patrick et al., 2004 Physical activity (accelerometer-assessed) and sedentary behaviors (self-reported) |
11–15 years N = 878 |
Cross-sectional Recruited through 45 primary care provides from six clinic sites in San Diego County |
Overweight (age- and sex-specific BMI > 85th percentile) Less vigorous physical activity associated with a greater likelihood of being overweight; among boys, less moderate physical activity and more television watching were also associated |
Controlled for demographics, highest household education level, and total energy intake |
|
Gable & Lutz, 2000 Television watching, diet quality (parent-reported) |
6–10 years N = 65 |
Cross-sectional Community sample recruited during a community health fair |
BMI Children who watched more hours of television were much more likely to be overweight; those who watched more television also consumed significantly more junk food |
|
Note: Throughout this table we first list papers investigating influences of youth psychological states, followed by papers investigating influences of youth health behaviors.
Neighborhood Factors that Contribute to SES-Health Relationships
By neighborhood factors we refer to the influences outside of the family homes, for example, factors related to the physical structure of a neighborhood or to the social climate among its residents. For a recent review of methodological issues associated with studies focused specifically on neighborhoods and health see Diez Roux and Mair (2010).
Asthma
Below we review how characteristics of the neighborhood environment, both in terms of physical characteristics, such as neighborhood pollution, as well as social characteristics, such as neighborhood violence exposure and perceptions of neighborhood safety, have important consequences for asthma outcomes among youth.
Physical neighborhood environment
One of the primary ways in which the physical neighborhood environment affects asthma is via exposures to outdoor pollutants (see Table 1 for a summary of relevant studies). Generally speaking, low SES neighborhoods contain a greater number of sources of pollution, such as industrial facilities (Chakraborty & Zandbergen, 2007), and have higher levels of air pollutants, including nitrogen dioxide (NO2), ozone (O3), and fine particulate matter (PM; Grineski, Bolin, & Boone, 2007). In turn, there is strong evidence supporting the notion that these pollutants negatively affect youth asthma, as a number of large-scale longitudinal studies, following youth from between two to eight years, uniformly suggest a detrimental impact of air pollution on youth asthma outcomes (see Table 1).
Some of the stronger studies repeatedly assessed clinical asthma outcomes over time. For example, O’Connor et al. (2008) followed five to 12 year olds for two years, asking youth to complete two weeks of at-home spirometry every six months. They found that short-term increases in NO2, sulfur dioxide (SO2), and PM were related to subsequent poorer pulmonary function in youth, including lower peak expiratory flow rate (PEFR) and lower forced expiratory volume in one second (FEV1). Similarly, data from a large sample of children from California, followed for up to eight years with yearly spirometry assessments, suggested that there were independent adverse effects of both local traffic and regional air pollution on subsequent youth lung development, as measured by FEV1 (Gauderman et al., 2007). In terms of directionality, one other study also found that youth living within 300m of arterial roads or freeways were at a greater risk of repeat hospitalizations for asthma over a four-year follow-up period (Chang et al., 2009).
Although less objective, longitudinal studies using self-reported asthma outcomes support the above findings that used objective indicators (McConnell et al., 2002; Brauer et al., 2002; Zmirou et al., 2004; McConnell et al., 2010). Finally, findings from the above discussed longitudinal studies are additionally supported by cross-sectional studies using large sample sizes (Lee, Son, Kim, & Kim, 2006; Sahsuvaroglu et al., 2009).
Increasingly, traffic proximity itself is being linked to asthma outcomes (Salam, Islam, & Gilliland, 2008). Low SES neighborhoods have been found to have more than twice the traffic density of high SES neighborhoods (Houston, Wu, Ong, & Winer, 2004). In turn, increased traffic leads to increased pollution, given that one of the main emission sources contributing to outdoor pollutants is diesel exhaust (Buonocore, Lee, & Levy, 2009). Cross-sectional studies have linked living in a census area marked by higher roadway density or facing major intersections to increased asthma incidence among youth (Juhn et al., 2005; Dales, Wheeler, Mahmud, Frescura, & Liu, 2009) and traffic in general has been linked to asthma outcomes in Germany (Nicolai et al., 2003), the United States (Lin, Munsie, Hwang, Fitzgerald, & Cayo, 2002; Gordian, Haneuse, & Wakefield, 2006; Chang et al., 2009), Great Britain (Edwards, Walters, & Griffiths, 1994) and Canada (Lin et al., 2004). A California-based study furthermore estimated that 6%–9% of pediatric asthma cases could be attributed to automobile and truck traffic as well as to pollution resulting from nearby ports (Perez et al., 2009).
Finally, traffic may also be detrimental to asthma because it creates noise pollution. As shown above, low SES areas are marked by higher levels of traffic and also greater traffic-related noise. Among 5–12 year old children in Germany, for example, there was a dose-dependent increase in youth’s asthma with an increasing pollution index (objectively derived) consisting of both noise and air pollution (Ising, Lange-Asschenfeldt, Moriske, Born, & Eilts, 2004). This may be because noise disturbs sleep; for example, among the younger children in this study, reports of sleep disturbances resulting from road noise were associated with increased asthma-related physician contacts. In part, this may be because noise also alters stress hormone regulation (Ising & Ising, 2002).
Social neighborhood environment
Neighborhood social stressors such as violence exposures have been linked to asthma outcomes in youth (Wright, 2006). See Table 2 for a summary of relevant studies. Low SES neighborhoods are marked by higher levels of crime (Raudenbush, 1997). Case studies of youth with asthma suggest that severe asthma symptoms can come on after exposure to violence (Wright & Steinbach, 2001). Strong support for the negative impact of community violence on youth asthma outcomes comes from two longitudinal studies (Suglia, Ryan, Laden, Dockery, & Wright, 2008; Sternthal, Jun, Earls, & Wright, 2010). Sternthal and colleagues (2010) followed over 2000 children between the ages of 0–9 years for three years on average, linking greater exposure to community violence to a greater likelihood of parent-reported physician-diagnosed asthma at follow-up after controlling for a host of other person-, family-, and neighborhood-level variables. Similarly, lifetime community violence exposure among 6–7 year olds has been linked to worse lung functioning, specifically lower FVC and FEV1, among boys but not girls (Suglia et al., 2008).
Table 2.
Social Neighborhood Influences on Youth Health Outcomes
| Citation/Neighborhood level variable | Age range or mean ± SD/Sample size | Type of study/Sample | Outcome/Construct measurement/Finding | Alternatives: Follow-up, covariates, reverse causation |
|---|---|---|---|---|
|
Sternthal et al., 2010 Exposure to community violence (caregiver reported) |
0–9 years N = 2071 |
Longitudinal Children part of a longitudinal Chicago-based study drawn from a stratified probability sample of 80 Chicago neighborhoods |
Physician-diagnosed asthma and use of prescription asthma medication (both parent-reported) Exposure to community violence was associated with elevated asthma risk; youth with medium or high exposure to violence had a greater likelihood of asthma at follow-up, compared to those exposed to low violence |
~ 3-year follow-up Controlled for sociodemographics, child age, maternal asthma, family violence in the home, neighborhood disadvantage, neighborhood social disorder and neighborhood collective efficacy |
|
Suglia et al., 2008 Exposure to community violence (caregiver reported) |
6–7 years N = 330 |
Longitudinal Children part of a prospective birth cohort from East Boston |
Youth lung functioning, forced vital capacity (FVC) and forced expiratory volume during one second (FEV1) Boys exposed to the most violence had lower FVC and FEV1 compared to those exposed to less violence. No relationship for girls |
Followed since birth Controlled for maternal education, child age, race/ethnicity, birth weight, and pre- and postnatal smoking exposure |
|
Gupta et al., 2009 Community vitality; combination of social capital, economic potential, community amenities |
Kindergarten through grade 8 children N = 45,177 |
Cross-sectional Stratified sample of children attending Chicago public/Catholic schools |
Caregiver-reported asthma prevalence Greater civic engagement, community diversity, economic vigor, community amenities at the neighborhood level associated with lower asthma rates; however, greater neighborhood stability and potential for interaction (more families, more adults not in work force) associated with greater asthma rates |
When considering socio-demographic and individual characteristics, social capital and overall community vitality still contribute significantly to asthma variation |
|
Cagney & Browning, 2004 Collective efficacy; combination of social cohesion and informal social control |
18+ years N = 8,782 |
Cross-sectional Representative sample of Chicago residents, selected by neighborhood clusters and interviewed over the phone |
Self-reported physician-diagnosed asthma Collective efficacy is protective against asthma |
Controlled for demographics, education, income, smoking, family status, insurance, source of care |
|
Wright et al., 2004b Neighborhood violence |
5–12 years N = 937 (851 caretakers) |
Cross-sectional Families with youth with at least 1 hospitalization or 2 emergency department visits for asthma during the 6 months before screening from 7 US cities. |
Caretaker-reported wheezing, sleep disruption, or interference with play activities caused by asthma during the preceding 2 weeks Greater exposure to violence was associated with greater asthma morbidity |
Results remain significant after controlling for income, employment status, care-taker education, housing problems, and other adverse life events. Psychological stress and caretaker behaviors partially explained the findings. |
|
Chen et al., 2007 Neighborhood problems, e.g. crime, drug use |
9–18 years N = 78 |
Cross-sectional Youth with asthma recruited from the general population |
Self-reported asthma symptoms and pulmonary functioning in the laboratory and at home More neighborhood problems associated with greater symptoms |
Controlled for demographics, asthma severity. Youth smoking behaviors mediated the relationship between greater neighborhood problems and greater symptoms. |
|
Wright et al., 2001 Neighborhood violence |
Youth aged 3, 9, 12, and 15 years N = 4 |
Case studies | Exposure to violence led to asthma exacerbations and hospitalizations | |
|
Duke et al., 2010 Neighborhood social capital and safety (parent-reported) |
Parents of 6–17 year olds N = 64,076 |
Cross-sectional Participants of the 2007 National Survey of Children’s Health |
Youth BMI (parent-reported height and weight) and youth aerobic exercise (parent-reported) Youth from neighborhoods characterized as safe and having greater social capital were more likely to have a healthy weight and engage in aerobic exercise |
Controlled for sociodemographics, family structure, household at or below poverty level, parent education |
|
Cecil-Karb & Grogan-Kaylor, 2009 Neighborhood safety (parent-reported) |
5–20 years N = 5,886 |
Cross-sectional Youth part of the National Longitudinal Survey of Youth |
Age- and gender-specific BMI Youth from neighborhoods deemed unsafe by their parents had higher BMIs and were more likely to be overweight, possibly because they spend more time indoors in sedentary activities |
Controlled for sociodemographics, maternal education, and income |
|
Duncan et al., 2009 Neighborhood safety (youth-reported) |
Grade 9–12 students N = 1140 |
Cross-sectional Youth part of the 2006 Boston Youth Survey, selected through a two-stage, stratified sampling procedure |
BMI (self-reported weight and height) Youth belonging to the ‘other race’ category coming from an unsafe environment were more likely to be overweight |
Controlled for sociodemographics, grade, clustering and observations by school |
|
Cohen et al., 2006 Community-level collective efficacy (social cohesion and informal social control; community-reported) |
12–17 years N = 807 |
Cross-sectional In each household with children part of the LA FANS study, a randomly selected child was interviewed |
BMI (self-reported weight and height) Controlling for neighborhood disadvantage, greater collective efficacy is associated with lower BMI and lower risk of overweight |
Adjusted for predictors of BMI at the neighborhood level, and characteristics of primary care giver and adolescents |
|
Franzini et al., 2009 Neighborhood collective efficacy, consisting of social cohesion and informal social control; collective socialization, social ties, favors exchanged in neighborhood, safety |
Grade 5 students N = 650 |
Cross-sectional Recruited as part of a larger study in 3 US cities/metropolitan areas |
BMI, self-reported physical activity All indicators of the social neighborhood environment were positively associated with self-reported physical activity and physical activity was negatively associated with BMI |
Controlled for children’s sociodemographic characteristics |
|
Cradock et al., 2009 Neighborhood social cohesion (assessed through community survey) |
11–15 years N = 680 |
Longitudinal Youth part of the Project of Human Development in Chicago Neighborhoods |
Self-reported general physical activity and parent-reported participation in school- or community-based recreational activities Youth from neighborhoods with greater social cohesion were less likely to be inactive (not participating in school- or community-based activities) at baseline and more likely to be physically active 2 years later |
2 year follow-up Controlled for sociodemographics, youth weight status, household education |
|
Gordon-Larsen et al., 2000 Neighborhood crime (various sources, including national crime statistics) |
Grade 7–12 N = 17,766 |
Cross-sectional Nationally representative sample from the 1996 National Longitudinal Study of Adolescent Health |
Self-reported weekly hours of inactivity and moderate to vigorous physical activity Serious neighborhood crime decreased the likelihood of youth being in the highest physical activity category |
Controlled for sociodemographics, gender, age, household income, maternal education, ethnicity, pregnancy status, mother/father in household, whether respondent was in school at the time of the interview, recreation center use, weekly PE classes |
|
Kimbro et al., 2011 Neighborhood collective efficacy (parent-reported) |
5 years N = 1822 |
Cross-sectional Youth part of the U.S. Fragile Families and Child Wellbeing Study |
Youth (in)activity: parent-reported outdoor play and television viewing Youth living in neighborhoods with higher levels of collective efficacy spent more time on outdoor play and less on television viewing |
Controlled for sociodemographics, parent-reported child health, maternal education, maternal employment, child enrolment in daycare or similar program, presence of siblings, season, family structure, maternal depression, maternal BMI |
|
Merom et al., 2006 Parent-perceived safety of school commute |
5–12 years N = 812 |
Cross-sectional Randomly selected households in New South Wales, Australia |
Parent-reported active commuting, i.e. walking or cycling, to school If perceived safety was greater, youth were more likely to walk or cycle to school |
|
|
Weir et al., 2006 Parent-perceived neighborhood safety (parent-reported) |
5–10 years N = 305 |
Cross-sectional Recruited parents and children with a scheduled appointment at an inner city health center or suburban private practice |
Youth physical activity (parent-reported) Among inner city children only, greater parental anxiety about neighborhood safety was associated with lower youth physical activity |
Controlled for child age, sex, parent education, and ethnicity |
Note: For the second part of this table concerning youth obesity we first list papers investigating neighborhood influences on BMI, followed by papers investigating neighborhood influences on physical activity behaviors.
Additional cross-sectional research yields similar conclusions. Wright et al. (2004b) found that among 5–12 year olds from inner city regions of seven US cities, those exposed to greater levels of violence reported more asthma symptom days after controlling for SES, physical housing conditions, and other negative life events. Finally, among a small sample of 9–18 year olds, youth-reported neighborhood problems, such as crime and drug use, have been linked to increased asthma day-time symptoms, though not to worse pulmonary functioning (Chen, Chim, Strunk, & Miller, 2007).
Conversely, positive social environments marked by greater social capital, for example, can be beneficial to asthma. Broadly speaking, social capital can be defined as a combination of collective efficacy (i.e. cohesion among residents and willingness to contribute to the common good) and community trust (i.e., trust among community members of one another). However, studies assessing social capital at the neighborhood level often rely on different measures and focus on some but not all facets of social capital, making it difficult to evaluate the effects of social capital across different studies. The issues surrounding the definition and measurement of social capital have been discussed in detail elsewhere (Lochner, Kawachi, & Kennedy, 1999). More deprived neighborhoods typically have lower levels of social capital (Lochner, Kawachi, Brennan, & Buka, 2003). In turn, low neighborhood collective efficacy, as defined by residents’ self-reports of perceived social cohesion (e.g., how close-knit their neighborhood is) and informal social control (e.g., whether they could count on adults in the area to watch out for children’s safety), is associated with increased rates of asthma diagnosis in adults (Cagney & Browning, 2004). More research is needed in this area but initial findings suggest that similar relationships may exist among youth. Gupta, Zhang, Sharp, Shannon, and Weiss (2009) assessed neighborhood vitality, defined in this case as a census tract level measure taking into account social capital (a composite of civic engagement, community diversity, interaction potential, and residential stability), economic potential (reflecting, among other things, a community’s commercial vitality and workforce potential, separate from the community’s SES), and community amenities throughout various Chicago neighborhoods. Among over 45,000 kindergarten through grade 8 children, those from communities marked by greater civic engagement and community diversity were less likely to have asthma.
Obesity
We next turn to the relevant neighborhood factors for youth obesity. The physical neighborhood contributes to youth obesity outcomes through available foods and the built environment. The social neighborhood contributes to youth obesity through factors such as neighborhood safety or violence and neighborhood collective efficacy that can influence levels of perceived stress among residents in the area and alter health-related behavior patterns.
Physical neighborhood environment
Regarding obesity, the types of physical characteristics of neighborhoods that form important influences are available resources, such as grocery stores, and the quality of the built environment. Relevant studies are summarized in Table 1. Overall, there are quite a number of studies linking physical neighborhood characteristics to youth’s risk for obesity and overweight via body mass index (BMI) and/or physical activity. However, we also note that most of this research is cross-sectional and relies on self-reports of weight and height.
Several studies have shown that low SES neighborhoods are characterized by fewer supermarkets and more fast food and convenience stores (Moore & Diez Roux, 2006; Powell, Slater, Mirtcheva, Bao, & Chaloupka, 2007; Ford & Dzewaltowski, 2008; Larson, Story, & Nelson, 2009). These factors in turn are known to be associated with a greater likelihood of overweight among adolescents (Powell, Auld, Chaloupka, O’Malley, & Johnston, 2007). Research also suggests that the neighborhood SES of grocery stores may influence BMI because groceries in poorer areas may be of worse quality and because healthy groceries may be less available (Inagami, Cohen, Finch, & Asch, 2006). In addition, if food prices of unhealthy foods are lower than those of healthy foods in low SES neighborhoods, this can drive behavior and lead low SES families to be more likely to purchase unhealthy food options (French, Story, & Jeffery, 2001; Sturm & Datar, 2005; Epstein, Dearing, Handley, Roemmich, & Paluch, 2006; Sturm & Datar, 2008).
The neighborhood built environment can further influence childhood obesity through shaping youth’s physical activity options in a number of ways (Evenson, Scott, Cohen, & Voorhees, 2007; de Vries, Bakker, van Mechelen, & Hopman-Rock, 2007). First, access to physical activity facilities in general is limited in low SES neighborhoods (Powell, Slater, Chaloupka, & Harper, 2006) and fewer available facilities and reduced facility access in turn are related to reduced exercise rates and greater rates of obesity (Gordon-Larsen, Nelson, Page, & Popkin, 2006). Second, whether or not the neighborhood outdoor environment lends itself to physical activity is important. Low SES residents have less access to parks and generally ‘green’ neighborhoods (Estabrooks, Lee, & Gyurcsik, 2003; Martin, Warren, & Kinzig, 2004; Mennis, 2006). One large longitudinal study followed youth for two years to investigate the influence of neighborhood vegetation and residential density on BMI (Bell, Wilson, & Liu, 2008). Regardless of residential density, coming from greener neighborhoods was associated with a lower BMI two years later. Parks too have been linked to lower BMI among youth (Liu, Wilson, Qi, & Ying, 2007; Bell et al., 2008), presumably because coming from greener neighborhoods facilitates outdoor activities among youth. Third, neighborhoods differ greatly in terms of walkability. Low SES youth are especially likely to be attending schools in less walkable neighborhoods (Zhu & Lee, 2008) and characteristics of less walkable neighborhoods such as fewer street lights and safe road crossings are associated with a lower likelihood of youth walking to school (Timperio et al., 2006).
Social neighborhood environment
Psychosocially, perceptions of both positive and negative aspects of one’s neighborhood respectively aid and reduce physical activity behaviors, indirectly affecting youth’s obesity status (Carver, Timperio, & Crawford, 2008). Generally speaking, neighborhoods with greater income inequality are less likely to be marked by social trust and social cohesion (Kawachi, Kennedy, Lochner, & Prothrow-Stith, 1997) and more likely to have crime (Sampson, Raudenbush, & Earls, 1997). Although much of this research is cross-sectional and relies on parent (and occasionally youth) reports for both outcomes and neighborhood level characteristics, one exception is a longitudinal study by Cradock et al. (2009) who followed youth from Chicago neighborhoods for two years and assessed levels of neighborhood social cohesion through a community survey. Not only were youth from more cohesive neighborhoods, meaning from neighborhoods with fewer latent social conflicts and with stronger social bonds, less likely to be inactive at baseline, they were also more likely to be physically active at the two year follow-up, that is more likely to be involved in school- or community-based recreational activities, when compared to youth from less cohesive neighborhoods.
Similarly, cross-sectional studies have linked social neighborhood factors to BMI and youth physical activity levels. Regarding BMI, several large-scale studies, some taking advantage of nationally representative samples of adolescents, found that positive neighborhood characteristics, such as high collective efficacy, safety, and social capital, were negatively associated with youth BMI (Cohen, Finch, Bower, & Sastry, 2006; Cecil-Karb & Grogan-Kaylor, 2009; Duke, Borowsky, & Pettingell, 2010), and positively associated with physical activity (Franzini et al., 2009; Kimbro, Brooks-Gunn, & McLanahan, 2011).
Conversely, negative social characteristics of neighborhoods can have adverse effects on youth’s physical activity behaviors. A large sample of nationally representative grade 7–12 students was shown to engage in less weekly moderate to vigorous physical activity if they came from neighborhoods marked serious crime (Gordon-Larsen, McMurray, & Popkin, 2000). Youth’s perception of a safe environment at physical activity facilities is thought to positively influence physical activity rates (Romero, 2005). Concerns about violence and safety are associated with children commuting to school less often (Merom, Tudor-Locke, Bauman, & Rissel, 2006) and engaging in exercise less frequently (Weir, Etelson, & Brand, 2006), resulting in higher levels of overweight among youth (Duncan, Johnson, Molnar, & Azrael, 2009). Another side effect of dangerous neighborhoods is that since they may prompt parents to keep their children indoors, youth may potentially be exposed to increased levels of household pollutants and social stressors found inside their homes.
Interim conclusion
The neighborhood environments that youth grow up in provide more than a passive backdrop to their everyday lives. Instead they actively influence their health in several important ways. The strongest evidence supports the detrimental influence of physical neighborhood characteristics, such as air pollution, on childhood asthma. This evidence is particularly convincing as it has been demonstrated across a large number of longitudinal studies, some of which were able to take advantage of objective lung functioning assessments, such as spirometry. In contrast, although studies investigating the impact of both positive and negative social neighborhood influences, such as social capital and neighborhood violence, on youth asthma and obesity come to largely congruent findings, such studies are comparatively sparse and almost exclusively cross-sectional in nature. Similarly, studies on the influence of the physical neighborhood environment on obesity-related outcomes among youth provide support for factors such as grocery store accessibility and the presence of physical activity facilities in neighborhoods but would benefit from additional longitudinal research and utilization of objective assessments of overweight and obesity.
Family Factors that Contribute to SES-Health Relationships
The negative effects of low SES are not limited to youth’s neighborhoods but extend into their homes. The family environment consists of influences that are concentrated within the family home, meaning, in the physical realm, aspects related to housing quality, and in the social realm, aspects related to the quality and type of interactions between individuals living in the home.
Asthma
Physical family environment
The homes that poor children grow up in are of lower physical quality (Evans & Kantrowitz, 2002). For example, low SES youth are disproportionally exposed to indoor allergens in the home. Several allergens, such as mouse (Crain et al., 2002; Chew et al., 2003; Levy et al., 2004) and cockroach (Sarpong, Hamilton, Eggleston, & Adkinson, 1996; Matsui et al., 2003) allergen, are commonly found in the homes of low SES youth and results from the Inner City Asthma Study revealed that the majority of inner-city youth were sensitized to three or more indoor allergens and frequently exposed to carpeting, pets, rats and mice (Crain et al., 2002). Low SES youth are also exposed to greater indoor NO2 and PM levels which increase with greater cooking time, gas stove and space heater use in low SES urban households (Baxter, Clougherty, Laden, & Levy, 2007; Hansel et al., 2008). Rauh, Chew, and Garfinkel (2002) furthermore found that among low income families, dilapidated housing and housing instability were related to increased levels of allergens in the kitchens and bedrooms of participating families, respectively.
In turn, indoor allergen exposure has been clearly indicated in worsening asthma. See Table 3. Intensive intervention studies in public housing aimed at reducing indoor allergens and managing pest problems have shown to improve clinical outcomes among youth with asthma (Levy, Brugge, Peters, Clougherty, & Saddler, 2006). Two longitudinal studies have furthermore measured in-home particle and nitrogen dioxide (NO2) concentrations among inner-city families with children between the ages of two to six years with asthma and followed up with youth three and six months later (Hansel et al., 2008; McCormack et al., 2009). Increased indoor NO2 exposure was associated with increased (parent-reported) coughing and nocturnal symptoms over time (Hansel et al., 2008) and greater levels of indoor particulate matter to increased (parent-reported) symptoms and rescue inhaler use over time (McCormack et al., 2009). Cross-sectionally, youth who are exposed to more indoor allergens are more likely to have been diagnosed with asthma (Lanphear et al., 2001). What is more, another study of 4–9 year olds showed that inner-city children who were allergic to (as determined by skin prick) and also exposed to cockroach allergens (as shown by dust samples from their bedrooms), had greater asthma symptoms, more frequent unplanned physician visits and hospitalizations than other children (Rosenstreich et al., 1997).
Table 3.
Physical Family Influences on Youth Health Outcomes
| Citation/Family level variable | Age range or mean ± SD/Sample size | Type of study/Sample | Outcome/Construct measurement/Finding | Alternatives: Follow-up, covariates, reverse causation |
|---|---|---|---|---|
|
Levy et al., 2006 Environmental intervention (allergen reduction) and education in the home |
4–17 years N = 50 |
Intervention Recruited children with asthma living in public housing developments in Boston |
Self-reported asthma symptoms, activity limitations, night-time waking, collected monthly; skin prick testing Following the intervention respiratory symptoms were significantly reduced |
Followed-up for at least three months post-intervention Controlled for demographic, medical, social factors and allergen levels Not clear what factor exactly is responsible for improvement |
|
Martinez et al., 1992 Parental smoking (parent-reported) |
<5 years N = 786 |
Longitudinal Families part of a random, stratified, cluster sample of white households in Tucson, Arizona |
Development of physician-diagnosed asthma during follow-up; spirometry for children aged 6+ Children of mothers who smoked 10+ cigarettes a day and had <12 years of education were more likely to develop asthma and had worse lung functioning |
Followed up again before age 12, an average of 7–8 years later Results independent of self-reported respiratory symptoms in parents |
| Hansel et al., 2009 Indoor nitrogen dioxide concentrations, assessed through indoor air sampling |
2–6 years N = 150 |
Longitudinal Recruited from a random sample of inner-city children with health care encounter for asthma in the previous 12 months |
Caregiver-reported asthma symptoms and health care utilization Greater NO2 exposure associated with more days with limited speech, cough, and nocturnal symptoms |
3- and 6-month follow-ups Controlled for demographics, caregiver education, season, secondhand smoke exposure, distance from curb, type of street in front of the house |
|
McCormack et al., 2009 In-home particle concentration, assessed through indoor air sampling |
2–6 years N = 150 |
Longitudinal Recruited from a random sample of inner-city children with health care encounter for asthma in the previous 12 months |
Caregiver-reported asthma symptoms, medication use Greater indoor particulate and fine particulate matter associated with worse respiratory and exercise-related symptoms and greater rescue medication use |
3- and 6-month follow-ups Controlled for demographics, parent education, season, ambient particulate matter |
|
Lanphear et al., 2001 Presence of allergens in the home, allergen sensitivity (as per skin prick test) |
6–16 years N = 5384 |
Cross-sectional Data from NHANES III, a nationally representative US sample |
Parent-reported physician-diagnosed asthma History of pet allergy, presence of pet, hypersensitivity to dust mite and cockroach allergens increased likelihood of physician-diagnosed asthma |
Adjusted for demographics, urban status, region, parent education, and poverty, parental history of atopy, and history of allergic rhinitis or hay fever |
|
Berz et al., 2007 Smoking in the home (parent-reported) |
2–3 years N = 1158 |
Cross-sectional Random subsample of an initial age- and sex-stratified sample of families from 15 towns in the Northeast of the US |
Parent-reported asthma and wheezing prevalence Smoking in the home associated with greater likelihood of asthma and wheezing, especially for children with short gestational ages and mothers with asthma during pregnancy |
Controlled for child age, sex, ethnicity, birth order, maternal age and education, family type, number of people in the home, receipt of governmental services, and before-tax household income |
|
Rosenstreich et al., 1997 Presence of allergens in the child’s bedroom, assessed through dust sample collection |
4–9 years N = 476 |
Cross-sectional Youth with diagnosed asthma, recruited through the Inner City Asthma Study |
Asthma morbidity (clinical symptoms, use of health care services, activities of daily life, effect on care giver); skin prick testing Children allergic and exposed to cockroach allergen were hospitalized more often, had more unscheduled medical visits, wheezing, missed school days, nights with lost sleep |
Controlled for sex, score on Child Behavior Checklist, family history of asthma |
|
Wang et al., 2007 Tobacco smoke exposure |
.3–15 years N = 355 |
Cross-sectional Recruited children presenting at a US emergency department |
1 or 1+ ED visits over 4-year study period Exposure to tobacco smoke at home was more common among youth with 1+ emergency department visit, as was history of asthma-related hospitalization |
Neighborhood characteristics between groups were similar |
|
| ||||
|
Feunekes et al., 1997 Foods available at home and/or offered to child as indicated by fat intake of other family members, assessed through two-day dietary records |
1–30 years N = 1077 households |
Cross-sectional Data from the Dutch National Food Consumption Surveys, a national stratified probability sample |
Children’s fat intake, assessed through two-day dietary records Parents and children living together were very similar with respect to fat intake, particularly for foods eaten at home, similar relationships for 3–21 year olds |
|
|
Skinner et al., 1998 Foods available at home and/or offered to child as indicated by food preferences of other family members (self-reported) |
28–36 months N = 118 |
Cross-sectional Convenience sample |
Toddler food preferences (parent-reported) Toddler’s food preferences were strongly correlated with likes and dislikes of foods of other family members; foods disliked by parents are only rarely offered to children |
Child gender was unrelated to parents’ influence on food preferences. |
|
Oliveria et al., 1992 Foods available at home and/or offered to child as indicated by 9 day dietary records of parents (over a year) |
3–5 years N = 106 |
Cross-sectional Data from the Framingham Children’s Study |
Children’s 9 day dietary records (over a year) Nutrient intake of parents and children was modestly correlated, more so for mothers and children and parents who eat more meals at home |
|
|
Gibson et al., 1998 Foods available at home and/or offered to child as indicated by maternal diet and nutritional knowledge (self-reported) |
9–11 years N = 92 |
Cross-sectional Recruited through urban primary health-care practices in London, England |
Children’s diet (assessed through diaries) Children’s consumption of fruits, vegetables, and confectionary were predicted by how frequently their mothers ate such items and mothers’ beliefs in how good/bad such foods are |
|
Exposure to smoking (ETS) is also more common in low SES homes, and can exacerbate asthma. In a Chicago-based study of inner-city families about 30% of caregivers and 50% of households reported smoking, respectively (Kumar et al., 2008). However, examination of youth’s salivary cotinine levels, a more objective measure of ETS, suggested that closer to 70% of inner-city youth were indeed exposed to ETS. These estimates are all considerably higher than what is considered the national average rate of youth ETS exposure at home, 7.6% (Singh, Siahpush, & Kogan, 2010). Using a longitudinal study design Martinez et al. (1992) found that young children of mothers who smoked more than ten cigarettes per day and had less than 12 years of education were more likely to develop asthma during this time and had worse lung functioning at follow-up. Hence, ETS may put children of low SES mothers at a particularly high risk for developing asthma. However, this study relied on self-reported smoking habits of parents, suggesting that estimates of smoking rates may be conservative. Two other cross-sectional studies also find that ETS exposure in youth’s homes is associated with greater asthma and wheezing prevalence (Berz et al., 2007) as well as repeat emergency department visits (Wang, McGeady, & Yousef, 2007).
Social family environment
Numerous aspects of the social family environment, including parent psychological states and family dynamics, have been linked to pediatric asthma outcomes, with both longitudinal and cross-sectional research suggesting the considerable importance of the social family environment (see Table 4). For example, the parents of low SES families are more likely to experience greater levels of stress (Baum, Garofalo, & YALI, 1999; Steptoe & Feldman, 2001; Lantz, House, Mero, & Williams, 2005). In turn, higher levels of stress in parents have been prospectively linked to an increased likelihood of asthma onset by age four (Klinnert, Kaugars, Strand, & Silveira, 2008). Some prospective studies of children with a family history of asthma have also linked greater caregiver stress, measured every two months for two years and then yearly thereafter, to a greater likelihood of atopic profiles during youth’s first two years of life (Wright et al., 2004a) and caregiver stress at 2–3 months to an increased risk of wheeze during the following twelve months (Wright et al., 2002). It should also be noted that although greater caregiver stress was associated with undesirable asthma outcomes, asthma outcomes among youth did not predict subsequent caregiver stress, suggesting that associations between parental stress and children’s asthma are not due to the burden of the disease increasing stress levels among parents. Evidence also suggests that parent-perceived stress and parent depression result in undesirable outcomes among youth with asthma by leading to increased amounts of inflammatory markers linked to asthma (Wolf, Miller, & Chen, 2008), indicating youth biological pathways as a link between parent mental health and youth asthma outcomes.
Table 4.
Social Family Influences on Youth Health Outcomes
| Citation/Family level variable | Age range or mean ± SD/Sample size | Type of study/Sample | Outcome/Construct measurement/Finding | Alternatives: Follow-up, covariates, reverse causation |
|---|---|---|---|---|
|
Suglia et al., 2009 Maternal intimate partner violence, supportive caregiving |
Followed since birth N = 3116 |
Longitudinal Nationally representative sample of children from 20 large US cities |
Maternal report of physician-diagnosed asthma at age 3 Children of mothers chronically exposed to intimate partner violence were more likely to be diagnosed with asthma |
Followed for three years Relationship was moderated by level of mother-child activities Adjusted for child age, sex, ethnicity, low birth weight, maternal education, economic hardship, tobacco exposure |
|
Wright et al., 2002 Caregiver stress (caregiver report every 2 months) |
Followed since birth N = 499 |
Longitudinal Parents with a history of asthma/allergy were recruited within 48 hours of delivery |
Bi-monthly parent-report of child wheezing Greater caregiver-reported stress at 2–3 months was associated with a greater risk of repeated child wheezing up to 14 months; dose-response relationship |
Followed for 14 months Controlled for demographics, household income, birth weight, marital status, and maternal active asthma Same association also found cross-sectionally |
|
Wright et al., 2004 Caregiver stress (caregiver report every 2 months for 2 years, then yearly) |
Followed since birth N = 197 |
Longitudinal Parents with a history of asthma/allergy were recruited within 48 hours of delivery |
Immune response outcomes in children – IgE expression, mitogen-induced and allergen-specific proliferative response, cytokine expression Higher caregiver stress in the first 6 months related to greater dust sensitivity among children; greater caregiver stress was associated with high total IgE levels and increased production of TNF-alpha |
Followed for 1.5–2.5 years Controlled for demographics, household income, and maternal active asthma |
|
Bartlett et al., 2001 Maternal depressive symptoms (parent report) |
Kindergarten – grade 5 students N = 158 |
Longitudinal Children with physician-diagnosed asthma, and recent parent-reported asthma symptoms and emergency department visits, recruited through schools |
Parent-reported emergency department use for asthma over the 6-month follow-up period Mothers with high levels of depressive symptoms were more likely to have taken their children to the emergency department over the follow-up period than mothers with low levels of depressive symptoms |
6-month follow-up; prevalence ratios between groups were adjusted for age, asthma morbidity, depression, and family income |
|
Klinnert et al., 2008 Caregiver mental health and family stress (parent report), home observation |
9–24 months N = 98 |
Longitudinal Children from low SES families recruited from pediatric departments of local hospitals and clinics |
Presence or absence of pediatric asthma at age 4 – caregiver-reported symptoms, evidence from medical records Children from families with greater family stress were more likely to be diagnosed with asthma at age 4 |
2–3 year follow-up (to age 4) Included maternal asthma, single parent status, prenatal smoke exposure, and ethnicity in model |
|
Wolf et al., 2008 Parent stress and depression (parent report) |
9–18 years n = 50 (asthma) n = 33 (control) |
Longitudinal Youth with asthma and healthy youth recruited from the general population |
Asthma inflammatory markers, stimulated interleukin-4 and eosinophil cationic protein concentrations Greater parental stress and depressive mood was associated with increases over time in ECP and IL-4 in children |
Six months follow-up Controlled for asthma severity, medication use Relationship between parent psychological state and asthma outcomes not mediated by youth psychological state |
|
Schreier & Chen, 2010 Family routines (parent-reported) |
12.68 ± 2.55 N = 59 |
Longitudinal Community sample of youth with asthma |
Mitogen-stimulated production of Interleukins 4, 5, and 13 Youth from more families with more routines decreased in IL-13 over the 2 year study period; when IL-13 was high, asthma symptoms were also high |
Followed for two years Controlled for demographics and asthma severity Inclusion of medication use eliminated the relationship between routines and IL-13 |
|
Subramanian et al., 2007 Domestic violence (self-report) |
All ages N = 443249 |
Cross-sectional Nationally representative Indian sample |
Self-reported asthma Among younger (5–15 and 15–24 years) groups of this sample, those from a household where women reported domestic violence were more likely to have asthma |
Controlled for a variety of sociodemographics, environmental exposures, health behaviors |
|
Cohen et al., 2008 Physical and sexual abuse (parent and child reports) |
5–13 years N = 1213 |
Cross-sectional Population-based probability sample from Puerto Rico |
Parent-reported physician-diagnosed asthma, Physical or sexual abuse in the previous year was associated with a greater likelihood of current asthma, health care use and medication use for asthma |
Controlled for demographics, SES, parental history of asthma, and caregiver-perceived stress |
|
Barreto do Carmo et al., 2009 Minor maternal psychiatric disorder (self report) |
5–12 years N = 1087 |
Cross-sectional Randomized sampling from 24 regions in Salvador, Brazil |
Presence of asthma, parent report; atopy according to skin prick test Mothers with minor psychiatric disorders were more likely to have children with asthma |
Controlled for demographics, maternal education, history of maternal asthma, mold in the home, dog/cat contact Results independent of child atopy status |
|
Shalowitz, 2001 Maternal depression (parent-report) |
1.5–12 years N = 123 |
Cross-sectional Mothers of children with asthma recruited at subspecialty care |
Asthma morbidity, combined index of health care utilization and recent symptoms Caregivers with more depressive symptoms were more likely to have children with high asthma morbidity |
Controlled for demographics and SES |
|
Chen et al., 2007 Family support (youth report) |
9–18 years N = 78 |
Cross-sectional Youth with asthma recruited from the general population |
Self-reported asthma symptoms and pulmonary functioning in the laboratory and at home; levels of IgE, IL-4, and eosinophils Less family support associated with greater symptoms and poorer pulmonary function |
Controlled for demographics, asthma severity. Biological pathways (more IgE, IL-4, eosinophils) mediated the relationship between less family support and greater symptoms |
|
Meijer et al., 1995 Parental child-rearing attitudes, parental relationship, family functioning (self-report) |
9–15 years N = 70 |
Cross-sectional Youth with asthma who had been pediatric outpatients for at least 2 years |
Controlled versus uncontrolled asthma, as determined by treating pediatrician Children with controlled asthma had parents who were better problem solvers, more rigid mothers, more cohesive families; more structured families better at correctly using medication |
Controlled and uncontrolled cases matched for asthma severity |
|
Brook & Tepper, 1997 Family interaction (youth report) |
12.16 ± 2.56 n = 51 (asthma) 13.28 ± 1.4 n = 32 (control) |
Cross-sectional Randomly chosen from youth treated for asthma; healthy children from nearby school |
Asthma versus controls Youth with asthma were more likely to come from families who showed evidence of lower family interaction |
Controls matched on demographics |
|
Wamboldt et al., 1995 Parental criticism, based on five minute parental speech sample |
11–18 years N = 19 |
Cross-sectional Adolescents admitted to the pediatric inpatient service for severe asthma |
Treatment outcome measures: medication, history, current pulmonary function, physical exam, laboratory data; prednisone equivalents, treatment compliance, length of hospital stay Greater parental criticism associated with lower medication compliance at admission but also greater improvement in asthma severity, reduction in steroid medication dose, and short hospital stays |
Findings independent of vocal cord dysfunction or youth allergy status |
|
| ||||
|
Taveras et al., 2005 Frequency of family dinner (youth-report) |
9–14 years N = 14,431 |
Longitudinal National convenience sample; children of the Nurses’ Health Study II |
Adolescent overweight (age- and sex-specific BMI > 85th percentile; self-reported height and weight) Cross-sectionally at baseline, eating family dinner on most days or every day was associated with lower odds of being overweight; no association with becoming overweight in longitudinal analyses |
Controlled for sociodemographics, physical factors, and hours of physical activity/inactivity |
|
Gable et al., 2007 Frequency of family meals (parent-reported) |
Kindergarten children N = 8000 |
Longitudinal Nationally representative sample of children who entered kindergarten in 1998 |
Overweight (BMI > 95% percentile) Children who ate fewer family meals were more likely to be overweight for the first time at spring semester of third grade and to be persistently overweight |
Followed for three years Controlled for demographics and family SES |
|
Koch et al., 2008 Family stress (serious life events, parenting stress, lack of social support, parental worries) |
Followed since birth N = 7443 (families) |
Longitudinal General population cohort from an area in Sweden |
Child obesity status (international age-adjusted standards) Children from families with stress in at least 2 of 4 areas were more likely to be obese, cross-sectionally and longitudinally |
Five-year follow-up Adjusted for child sex, parental origin, parents’ age at birth, parents’ weight status, parents’ educational level, and marital status |
|
Larson et al., 2007 Frequency of family meals (child-reported) |
Grade 9–12 students N = 1710 |
Longitudinal Recruited from 31 public middle and high schools in the Minneapolis/St. Paul, Minn. area |
Diet quality, meal frequency, social eating and meal structure during young adolescence More family meals during adolescence was associated with greater consumption of fruit, vegetables, and key nutrients and lower consumption of soft drinks during adulthood; also predicted more breakfast meals and higher priority for meal structure and social eating |
Five-year follow-up Adjusted for demographics, energy consumption |
|
Olvera & Power, 2010 Parenting style (parent-reported) |
4–8 years N = 69 |
Longitudinal Community sample of Mexican Americans |
Children’s overweight status (BMI > 85th percentile considered overweight, collected annually) Children of indulgent mothers were more likely to be overweight at follow-up than children of authoritarian or authoritative mothers |
Three-year follow-up Controlled for initial weight status |
|
Videon & Manning, 2003 Parental presence at dinner (adolescent report) |
Grade 8–12 students N = 18177 |
Cross-sectional National stratified, systematic sample of high school students |
Food consumption, fruit and vegetable consumption, breakfast skipping (adolescent report) If parents present for 3+ family meals/week, adolescents were less likely to skip breakfast and report poor consumption of fruits, vegetables, dairy; dose-response relationship |
Controlled for sociodemographics and adolescent body weight perception |
|
Gillman et al., 2000 Frequency of family meals (child-reported) |
9–14 years N = 16,202 |
Cross-sectional National convenience sample; children of the Nurses’ Health Study II |
Food and nutrient intake, self-reported Youth whose families ate more meals together consumed more fruits, vegetables, fiber, and micronutrients, less fried food, soda, and trans fat, and had a lower glycemic load |
Adjusted for sex, BMI, physical activity, hours of television watched, smoking intention, smoking in the home, family structure, household income, frequency of child making his or her own dinner |
|
Neumark-Sztainer et al., 2003 Frequency of family meals (child-reported) |
11–18 years N = 4746 |
Cross-sectional Recruited from 31 public middle and high schools in the Minneapolis/St. Paul, Minn. area |
Food and nutrient intake, self-reported Youth whose families ate more meals together consumed more fruits, vegetables, grains, calcium-rich foods, and micronutrients and fewer soft drinks |
Adjusted for demographics, school level, mother’s employment status, SES, energy consumption and school |
|
Toschke et al., 2005 Daily meal frequency (parent-reported) |
5–6 years N = 4370 |
Cross-sectional German community sample |
Child obesity (sex- and age-specific BMI cut-off points) Children who ate more daily meals were less likely to be obese |
Controlled for a variety of sociodemographic, constitutional and lifestyle factors |
|
Mamun et al., 2005 Maternal attitude towards family meals and frequency of family meals (parent-reported) |
14 years N = 3795 |
Cross-sectional Data from a population-based Australian prospective birth cohort |
Adolescent overweight (BMI > 22.62 for boys and > 23.34 for girls) Children of mothers who did not think eating together was important were more likely to be overweight; frequency of family meals did not impact overweight |
Adjusted for child sex and age, maternal parity, family income, maternal education at birth, and race, child TV watching, child activity, child’s frequency of consumption of unhealthy foods |
|
Garasky et al., 2009 Family stressors (disruption and conflict, mental and physical health problems, housing issues, health care struggles, financial strain, lack of cognitive stimulation/emotional support |
5–11 years n = 1136 12–17 years n = 1001 |
Cross-sectional Second wave of a nationally representative US panel study of income dynamics and children |
Weight status (healthy weight, overweight at BMI > 85th percentile, obese at BMI > 95th percentile) In younger children, lack of emotional support and cognitive stimulation was associated with greater likelihood of overweight and obesity In older children, financial stressors and mental and physical health stressors were associated with greater likelihood of overweight and obesity |
Controlled for age of primary caregiver, whether primary caregiver was child’s mother, number of people in the household and family income |
|
Hoerr et al., 2009 Parenting style (parent-reported) |
Preschool children N = 715 |
Cross-sectional Children and parents selected from a study investigating Head Start families |
Food intake (3 days of dietary recall with children) Compared to children authoritarian parents, those of indulgent or uninvolved parents consumed fewer fruits, vegetables, and juice |
|
|
Moens et al., 2009 Parenting stress (parent-reported) |
6–14 years N = 197 (families, 97 controls) |
Cross-sectional case-control study Overweight children recruited from inpatient/outpatient/school intervention waiting list; control group from the community |
Overweight status Children from families with greater parenting stress were more likely to be overweight |
Controlled for parental education, employment status, family structure, and number of siblings |
|
Coon et al., 2001 Presence of television during meals (parent-reported) |
Grade 4–6 students N = 91 |
Cross-sectional Community sample |
Food and nutrient intake (3 24-hour dietary recall interviews with children) Children from families with more television use during meals derived more of their total energy intake from meats, pizza, salty snacks, soda, and less from fruits, vegetables, and juices; they also consumed more caffeine |
Controlled for demographics, SES, maternal education, maternal weekly working hours, 2-parent household, number of nights per week parents prepared quick suppers and parent’s knowledge and attitudes about nutrition |
|
Johnson & Birch, 1994 Mothers’ degree of control over children’s food intake |
3–5 years N = 77 |
Cross-sectional laboratory study Community sample from one university preschool |
Manipulated caloric density of children’s meals; measured height, weight, skinfolds, ability to regulate energy intake Children who had more controlling mothers were less likely to regulate food intake and had greater body fat |
|
|
Moens et al., 2007 Family Mealtime Interaction (coded videotapes of typical family mealtimes) |
7–13 years N = 56 (families; 28 control) |
Cross-sectional case-control study Overweight children recruited from inpatient/outpatient/school intervention waiting list; control group from the community |
Overweight status Parents who were observed to display less support and use more maladaptive control strategies were more likely to have overweight children. Parents who self-reported being more controlling also were more likely to have overweight children. |
Controlled for maternal BMI and family SES |
| Trost et al., 2003 Parent physical activity beliefs and support behaviors for children’s activity (parent-reported) |
14.0 ± 1.6 years N = 380 |
Cross-sectional Participants recruited from junior and senior high schools in Amherst, MA |
Youth physical activity (child-reported) Greater parental support resulted in greater youth physical activity, directly and through youth’s self-efficacy |
Controlled for child’s age and gender |
|
Davison et al., 2003 Parents’ activity-related parenting (parent-reported) |
9 years N = 180 |
Cross-sectional Convenience sample from an ongoing longitudinal study |
Physical activity (child-report) More modeling of physical activity behaviors and greater provision of logistic support of youth’s activity were associated with greater physical activity among children |
Family income, parent education, parents’ work hours were unrelated to physical activity |
|
Moore et al., 1991 Parental activity levels (assessed through accelerometer) |
4–7 years N = 100 |
Cross-sectional Convenience sample from an ongoing longitudinal study |
Child physical activity levels (accelerometer assessed) Children of active mothers and fathers were more active than those of inactive parents; having two active parents was related to much greater physical activity levels than having two inactive parents |
|
Note: For the second part of this table concerning youth obesity we first list papers investigating family influences on BMI, followed by papers investigating family influences on physical activity behaviors.
Low income and inner-city mothers also show evidence of significantly higher rates of depression than those found in the general population, up to 50% in some studies (Bartlett et al., 2001). In turn, children growing up with depressed mothers visit the emergency department more frequently for asthma over a 6-month period (Bartlett et al., 2001) and experience greater asthma morbidity in cross-sectional studies (Shalowitz, Berry, Quinn, & Wolf, 2001). Similarly, children of mothers with minor psychiatric disorders, including not only depression but also anxiety and somatic complaints, are more likely to have asthma (Barreto do Carmo et al., 2009).
Research also points to the importance of the structure of day-to-day family life in childhood asthma. The home environments of poor youth have been described as more chaotic (Evans, 2004). In turn, a study from our own research laboratory showed that youth with asthma from family environments with lower levels of family routines prospectively showed increasing levels of asthma inflammatory markers across an 18-month period (Schreier & Chen, 2010). This relationship between youth’s levels of inflammatory markers and family routines disappeared when controlling for medication, suggesting that more structured family environments are beneficial to youth’s daily asthma management. This corroborates findings by Meijer, Griffioen, van Nierop, and Oppenheimer (1995) who investigated differences between youth with controlled versus uncontrolled asthma and found that greater interdependence among family members and rigid behaviors on part of the parents were associated with controlled asthma. This may be because more cohesive families may be better at staying adherent to youth’s medication regimens.
Other family relationship characteristics, such as conflict and support, have been linked to youth asthma outcomes in a number of small-scale, cross-sectional studies. Greater family economic stress also is associated with heightened family conflict (Conger, Ge, Elder, & Lorenz, 1994), and poor families have been found to provide less stimulating family environments, with less affection from mothers (Bradley, Corwyn, McAdoo, & Coll, 2001) and greater turmoil in the family (Evans, 2004). In turn, increased parental criticism and less parental tolerance were found among families of youth with asthma compared to healthy youth (Brook & Tepper, 1997b). Parental criticism towards adolescents with asthma, as assessed through a short speech sample, has also been associated with more severe asthma among youth admitted to a tertiary care setting for asthma (Wamboldt, Wamboldt, Gavin, Roesler, & Brugman, 1995). Similarly, children and adolescents with asthma who reported less family social support experienced more daytime, nighttime, and exertional symptoms, as well as reduced pulmonary functioning (Chen et al., 2007). These relationships were further assumed to be a result of biological pathways, that is, increased levels of inflammatory markers of asthma, rather than behavioral ones, such as smoking. Together these studies suggest that the nature of interactions between youth and their parents are linked to youth’s asthma outcomes.
Finally, family violence is more prominent among low SES families (Emery & Laumann-Billings, 1998). Results from a prospective nationally representative US study suggest that 2–3 year olds of mothers who were exposed to chronic intimate partner violence had twice the risk of asthma onset, although this relationship was moderated by maternal caregiving, such that youth of mothers who were able to maintain positive caregiving were protected from these negative effects (Suglia, Enlow, Kullowatz, & Wright, 2009). Cross-sectional data from other countries further support this notion. Negative effects on asthma-related outcomes have been observed among Indian youth whose mothers experienced domestic violence (Subramanian, Ackerson, Subramanyam, & Wright, 2007) as well as among Puerto Rican youth who reported personal experiences with physical or sexual abuse (Cohen, Canino, Bird, & Celedon, 2008).
Obesity
With respect to youth obesity, physical family influences include factors such as available foods in the household, whereas social family environment influences include factors such as the quality of family relationships, mealtime routines, and parents’ psychological states.
Physical family environment
The physical family environment influences youth’s eating patterns by shaping the types of food that are available and presented to children. Table 3 lists relevant studies. What children eat is shaped by the types of foods that are present in the home, such as the quantity of fruits and vegetables (Hearn et al., 1998; Patrick & Nicklas, 2005). Because parents are ultimately in control of purchasing the foods their children are exposed to at home, the role of parents’ own food preferences is also discussed here.
Several studies have shown that youth from low SES backgrounds are less likely to have a healthy diet. They consume fewer daily micronutrients and get more energy from snacks and fat (Ruxton, Kirk, Belton, & Holmes, 1996) while at the same time consuming fewer fruits and vegetables (Giskes, Turrell, Patterson, & Newman, 2002). This is in part because low SES parents buy foods that yield a higher calorie/price ratio, and which are less healthy, thus exposing low SES youth to more unhealthy foods (Campbell et al., 2002).
Furthermore, parents’ food preferences shape both what foods their children are exposed to and what foods are available in the family home, which in turn contribute to youth’s dietary patterns and ultimately pediatric obesity (Campbell & Crawford, 2001; Haire-Joshu & Nanney, 2002). This is problematic in that low SES adults are more likely to have an unhealthy diet (Darmon & Drewnowski, 2008) and foods that are preferred by parents are more likely to be presented to children in the household (Skinner et al., 1998). Hence, fruit and vegetable consumption, as well as fat and confectionary consumption are similar between children and their mothers (Oliveria et al., 1992; Feunekes, Stafleu, de Graaf, & van Staveren, 1997; Gibson, Wardle, & Watts, 1998). However, more research is needed to investigate the influences on food availability longitudinally as all of the above studies are cross-sectional, and to examine these effects in older youth.
Social family environment
Various family social factors, such as family routines around meals, parenting and parental stress, as well as role modeling from parents have been linked to pediatric obesity. For a summary of studies investigating social family influences on youth obesity, see Table 4.
Research on SES and family mealtimes is mixed, with some studies reporting that high SES families eat more meals together (Neumark-Sztainer, Hannan, Story, Croll, & Perry, 2003) and others finding higher rates among low SES families (Campbell et al., 2002). However, among low SES families common mealtimes are often marked by TV watching, which presumably limits communication and active engagement with other family members during mealtimes and has been shown to be related to less healthy dietary patterns among youth (Coon, Goldberg, Rogers, & Tucker, 2001).
Nonetheless, common family mealtimes have been shown to be an important pathway related to obesity. For a relatively recent discussion of the importance and benefits of common family mealtimes, as well as of the aspects of family mealtimes that result in these benefits, see Fiese and Schwartz (2008). Among a nationally representative sample of students followed from kindergarten to grade three, those whose parents reported fewer family meals were at an increased rate of becoming and being persistently overweight (Gable, Chang, & Krull, 2007). Another study investigating a national convenience sample of 9–14 year olds found a positive association between family dinners and overweight at baseline, but not in longitudinal analyses (Taveras et al., 2005). Research following adolescents from age 15 to 20 indicates that having more family meals during adolescence predicts consuming more fruits and vegetables as young adults (Larson, Neumark-Sztainer, Hannan, & Story, 2007). This is supported by various cross-sectional studies that show associations between family meals and improved overall food consumption patterns (Videon & Manning, 2003) and, more specifically, increased rates of fruit, vegetable, and micronutrient intake and decreased soda and fat consumption (Gillman et al., 2000; Neumark-Sztainer et al., 2003). Conversely, in a large-scale Australian study investigating over 3,000 youth from a population-based sample, youth whose mothers did not think eating together as a family was important were more likely to be overweight (Mamun, Lawlor, O’Callaghan, Williams, & Najman, 2005). This may in part be because youth from families implementing more family meals are more likely to consume regular meals, which has been linked to a decreased risk for obesity (Toschke, Kuchenhoff, Koletzko, & von Kries, 2005).
In addition to routines around mealtimes, parenting behaviors are also linked to obesity-related outcomes. Parents from low SES families are more likely to engage in harsh and punitive parenting (Grant et al., 2003). One study investigated preschoolers’ abilities to self-regulate energy intake (Johnson & Birch, 1994) and found that preschoolers who had more controlling mothers were less able to regulate their energy intake during ad lib consumption trials and had greater fat stores. Similarly, overweight 7–13 year old children were more likely to have controlling mothers; indeed, coding of videos taken during families’ mealtimes suggested that these parents were twice as likely as the parents of non-overweight children to use maladaptive control strategies during mealtimes (Moens, Braet, & Soetens, 2007), although directionality could not be established in this cross-sectional study. On the other hand, indulgent or permissive parenting has also been linked to a greater likelihood of obesity at follow-up in a 3 year longitudinal study of 4–8 year old children (Olvera & Power, 2010) and to the consumption of fewer nutrient rich foods among children in low SES families (Hoerr et al., 2009), suggesting that either parenting extreme may be detrimental to subsequent youth obesity.
Both the psychological state of parents and the support they provide to their children can contribute to child obesity outcomes. As mentioned previously, rates of both parent stress and parent depression are higher among low SES parents (Baum et al., 1999; Bartlett et al., 2001). In turn, greater overall parent stress (Koch, Sepa, & Ludvigsson, 2008; Moens, Braet, Bosnians, & Rosseel, 2009) and lack of provision of emotional support (Garasky, Stewart, Gundersen, Lohman, & Eisenmann, 2009) have been linked to increased rates of obesity among youth. Some convincing evidence in this area comes from a study based on a large Swedish general population cohort followed for five years (Koch et al., 2008). In this study, children from families with stress in at least 2 of 4 areas were more likely to be obese, both cross-sectionally and longitudinally.
Finally, with respect to physical activity, role modeling of physical activity behaviors from parents can also influence youth’s physical activity. Low SES adults are themselves less likely to be physically active (Palmer & Jaworski, 2004), suggesting that low SES youth benefit from such physical activity role modeling less frequently. In turn, parental role modeling of physical activity behaviors (mostly by fathers) relates to more physical activity among youth (Davison, Cutting, & Birch, 2003). Similarities in the physical activity behaviors of parents and their children have also been noted using objective measures such as accelerometry (Moore et al., 1991).
Interim conclusion
The family home environment of children and adolescents is an important contributor to their health. Again, evidence supporting the impact of the physical home environment (allergens, ETS) on asthma is particularly strong and builds on numerous longitudinal studies and findings from an intervention study. On the other hand, although the influence of the physical home environment on youth obesity, i.e. available foods, may seem intuitive, this is only supported by (largely small scale) cross-sectional studies. In the social family domain, many good quality cross-sectional and longitudinal studies underscore the importance in particular of family relationships, family routines and parent mental health (more strongly than parenting styles or role modeling), on youth asthma and obesity outcomes. In addition, research also suggests that negative environmental home influences tend to ‘cluster’ and that low SES youth are significantly more likely than higher SES youth to be exposed to multiple environmental risk factors in their homes, such as crowding, noise, and housing problems (Evans & Marcynyszyn, 2004), thereby putting them at an especially high risk for asthma and obesity problems.
Person Factors that Contribute to SES-Health Relationships
In this section of individual level influences we include youth’s traits and behaviors that can affect their health, as well as biological processes within children that explain how low SES comes to affect disease outcomes. Hence in this section we review evidence linking individual psychological states, such as child anxiety and depression, to asthma and obesity, the biological processes connecting psychological states to asthma and obesity, and the types of health behaviors that children engage in that may help explain health disparities in asthma and obesity. Refer to Table 5 for studies regarding person level influences on asthma and obesity, respectively.
Asthma
Low SES adolescents have higher rates of anxiety and depression than their high SES counterparts (Miech, Caspi, Moffitt, Wright, & Silva, 1999) as well as more socioemotional problems (McLoyd, 1998). In turn, a number of psychological factors, such as youth’s emotions and psychological states (Lehrer, 1998) are associated with pediatric asthma outcomes (Lehrer et al., 2002). Unfortunately, almost all studies assessing the influence of emotional states on asthma have small sample sizes and should be interpreted with caution until replicated in larger samples.
One longitudinal study followed over 1,500 4–9 year olds over 9 months to investigate the link between mental health and asthma morbidity (Weil et al., 1999). They observed increased rates of significant behavior problems among these inner city children with asthma which in turn were linked to more days of wheeze and a lower functional asthma status across the 9-month follow-up. Several small case-control studies further show that compared to healthy controls, youth with asthma have more anxiety disorders and greater rates of family histories of emotional problems (e.g., Bussing, Burket, & Kelleher, 1996). Finally, adolescents with moderate/severe, as opposed to no or mild asthma, were less able to control their emotions and reported higher levels of depression, anxiety, and hostility (Silverglade, Tosi, Wise, & D’Costa, 1994). We note, however, that an alternative possibility is that having a chronic illness such as asthma is psychologically distressing, and many of the above studies could be picking up on that association. Hence, more research needs to be done to establish whether psychological distress is primarily a cause or consequence of asthma exacerbations.
Health behaviors also play an important role in asthma, particularly in terms of adherence to asthma medications. Although adherence to daily asthma medications is an integral part of successful pediatric asthma management, adherence is notoriously low (Bender, Milgrom, Rand, & Ackerson, 1998), especially among low SES youth (Cope, Ungar, & Glazier, 2008). McQuaid, Kopel, Klein, and Fritz (2003) have furthermore shown youth’s adherence to asthma medication to be negatively correlated with asthma activity limitations and symptoms. Other more general health behaviors, such as cigarette smoking, are also relevant to asthma. Smoking is more common among low SES compared to high SES youth (Harrell, Bangdiwala, Deng, Webb, & Bradley, 1998) and in spite of its well-known negative consequences on asthma (Boulet et al., 2006; Thomson, 2007), several studies report higher rates of cigarette smoking among youth with asthma than among healthy youth (Forero, Bauman, Young, Booth, & Nutbeam, 1996; Precht, Keiding, Nielsen, & Madsen, 2006).
Biological Pathways
Also at the individual level, experiences of stress or distress can directly alter physiological systems that have implications for disease. For example, both negative emotions and psychological stress, can alter the sympathetic-adrenal-medullary (SAM) and hypothalamic-pituitary-adrenocortical (HPA) axis, and various immunological processes. The SAM and HPA axes react to acute stress exposure by releasing increased amounts of hormones, such as cortisol and epinephrine (Segerstrom & Miller, 2004; Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009), which in turn influence metabolic, cardiovascular and inflammatory processes. For more in-depth reviews on this topic, see Kiecolt-Glaser, McGuire, Robles and Glaser (2002) and Gallo and Matthews (1999).
More specific to asthma are immunological pathways involved in the airway inflammation and hyperresponsiveness. For example, allergen exposure induces T helper (Th) cells to produce certain cytokines, such as interleukins-4, 5, and 13 (IL-4, IL-5, IL-13). IL-4 and IL-13 bind to B cells, leading to the release of IgE antibodies. These antibodies then bind to mast cells in the airways. When mast cells degranulate they release allergic mediators, such as histamines and leukotrienes, which cause asthma symptoms including edema, smooth muscle constriction, and increased mucus production in the airways. Second, airway inflammation and obstruction is also promoted through the cytokine IL-5, which has been shown to increase eosinophil production and hence, the production of eosinophil cationic protein (ECP) and leukotrienes.
Children with asthma who come from lower SES homes display greater stimulated production of these asthma-relevant cytokines, including IL-5 and IL-13, as well as higher eosinophil counts (Chen, Fisher, Bacharier, & Strunk, 2003; Chen et al., 2006). Proceeding a level deeper biologically, there is also evidence suggesting that the ways in which genes function may be altered by low SES. Chen et al. (2009) documented that low SES is linked to differential patterns of gene expression in children with asthma – that is, low SES children showed bioinformatic indications of heightened activation of gene transcription control pathways related to inflammation and reduced activation of pathways related to catecholamine signaling compared to high SES children with asthma.
In addition, research on gene-environment interactions suggests that genes may moderate the relationships between environmental influences such as SES and various psychological and health outcomes. For example, the influence of genetics varies across the SES spectrum. In general, twin studies suggest that the influence of genes may be weaker among low SES individuals and increase among people with increasing income (Johnson & Krueger, 2005; Johnson et al., 2010). This is thought to be because studies focusing on a variety of outcomes suggest that there is less variation in environmental factors in high SES environments, leading to genes being more influential in the absence of these environmental risk factors (Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003; Tuvblad, Grann, & Lichtenstein, 2006; Harden, Turkheimer, & Loehlin, 2007).
Furthermore, psychological stress is also linked to inflammatory processes. For example, students with asthma exhibit greater immune responses when experimentally presented with an allergen-sensitization task during stressful times, i.e. during final exam time, compared to less stressful times outside of their exam period (Liu et al., 2002). Specifically, when participants were instructed to inhale increasing dosages of allergens, they exhibited greater levels of eosinophils in their blood during exam time. Stress resulting from academic exams has furthermore been linked to the alteration of other immunological processes, including lower natural killer cell proliferation and greater lymphocyte proliferation and neutrophil release (Kang, Coe, & McCarthy, 1996; Kang, Coe, McCarthy, & Ershler, 1997). Research by Marin, Chen, Munch and Miller (2009) has shown that youth with asthma who experience acute stressors in the context of high chronic stress are particularly vulnerable to increased asthma symptoms as well as greater stimulated production of asthma-relevant cytokines such as IL-4, IL-5, and IFN-γ, again suggesting that stressors in youth’s environments come to influence their physical health through inflammatory pathways commonly associated with asthma morbidity. Finally, tests of autonomic nervous system pathways relevant to asthma revealed that in laboratory studies exposing youth with asthma to emotional movies, when watching sad, but not happy, movie scenes, children evidenced increased parasympathetic activity, suggesting that distress increases airway instability (Miller & Wood, 1997).
Obesity
At the individual level, both health behaviors and child psychological distress are also associated with childhood obesity. With respect to health behaviors, youth engaging in less physical activity are not surprisingly at greater risk for overweight and obesity (Sallis, Prochaska, & Taylor, 2000; Dowda, Ainsworth, Addy, Saunders, & Riner, 2001; Patrick et al., 2004). This is particularly relevant since low SES children engage in less physical activity than their high SES counterparts (Gordon-Larsen et al., 2000; Janssen et al., 2006). Related health behaviors, such as less healthy dietary and exercise behaviors, lower breakfast and fruit and vegetable consumption and increased television viewing have also been shown to be related to increased risk for overweight and obesity among youth from minority and low income populations (Delva et al., 2006; Delva, Johnston, & O’Malley, 2007).
Several longitudinal studies provide clear support for the negative impact of television watching on youth weight status, although objective measures of hours of TV watched are generally not available. Low SES youth spend more time watching TV compared to high SES youth (Hancox, Milne, & Poulton, 2005; Lioret, Maire, Volatier, & Charles, 2007). In turn, two studies, one of which involved a large, nationally representative sample of children (Gable et al., 2007) followed youth for between three and seven years and found that increased television watching is associated with youth being, becoming, and staying overweight (Gortmaker et al., 1996; Gable et al., 2007). This is further strengthened by numerous cross-sectional studies that find time spent watching TV to increase youth’s risk of obesity and overweight (e.g., Andersen, Crespo, Bartlett, Cheskin, & Pratt, 1998; Crespo et al., 2001).
Aside from being an entirely sedentary activity, television viewing also has indirect influences on diet. For example, children between the ages of 3–10 years who watch more TV are also more likely to eat junk food, thereby further increasing the negative health consequences of TV watching (Gable & Lutz, 2000). Research also suggests that youth’s TV watching influences the family diet (Taras, Sallis, Patterson, Nader, & Nelson, 1989) as children are more likely to ask for foods they saw as part of TV advertisements, thereby influencing their own caloric intake through their parents’ purchasing behaviors.
Apart from health behaviors, youth’s psychological state also influences obesity. As described earlier, low SES children experience more emotional problems (McLoyd, 1998; Miech et al., 2006), and in turn, emotional problems can affect obesity. Data from a large nationally representative sample of adolescents showed that baseline depression was associated with obesity one year later (Goodman & Whitaker, 2002). Grade 7–12 students were followed for a year, and depression and obesity assessed repeatedly. There was no relationship between baseline depression and obesity; however, youth who reported greater depressed mood at baseline were more likely to become or have remained obese at one year follow-up, independent of a number of demographic and child psychological and exercise covariates. In contrast, there was no support for the opposite relationship of obesity at baseline predicting depressed mood at follow-up. Goossens, Braet, Van Vlierberghe, and Mels (2009) also found that, among overweight youth, psychological problems including depression and anxiety were associated with emotional eating, which mediated the relationship between psychological problems and a loss of control over eating.
More generally, cross-sectional research has also linked greater stress as well as stress reactivity to an increased likelihood of adiposity and obesity (Roemmich, Smith, Epstein, & Lambiase, 2007; Hamer & Stamatakis, 2008; Lohman, Stewart, Gundersen, Garasky, & Eisenmann, 2009). One way in which stress may influence obesity is by shaping people’s health behaviors, for example by facilitating unhealthy eating behaviors both in response to, and as a coping mechanism, for stress (Jenkins, Rew, & Sternglanz, 2005). Finally, stress and psychological difficulties in adolescents have also been linked to health behaviors such as a greater likelihood of intake of fatty foods and less weekend exercise (Simon, Wardle, Jarvis, Steggles, & Cartwright, 2003), that have implications for obesity (Maffeis, 2000).
Biological Pathways
As described in the above section on biological pathways and asthma, individual experiences of stress influence physiological systems, most notably the HPA axis. This is relevant to obesity, given that glucocorticoids are increasingly implicated in the metabolic syndrome, a clustering of risk factors for cardiovascular disease, including insulin resistance, visceral obesity, hypertension, high levels of triglycerides and low levels of high-density lipoprotein (Björntorp & Rosmond, 2000b; Rosmond, 2005).
An upregulation of the HPA axis is thought to lead to higher cortisol levels among people experiencing greater stress. This is turn has ultimately been linked to greater insulin resistance and visceral adiposity (Björntorp & Rosmond, 2000a) and provides a potential explanation for studies finding both greater rates of obesity and cortisol among adults working in low as opposed to high SES jobs (Rosmond & Björntorp, 2000). In addition, glucocorticoids may inhibit the stimulation of certain growth and sex hormones that would normally assist in keeping cortisol levels low, thereby further increasing the risk for visceral adiposity and metabolic syndrome in general (Björntorp & Rosmond, 2000c; Chrousos, 2000). Other studies have shown that glucocorticoids stimulate caloric intake and together with insulin may steer people’s food preferences (Dallman et al., 2004). For example, random assignment of healthy adults to either a placebo or glucocorticoid (methylprednisolone) treatment has shown that the link between glucocorticoids and obesity risk may occur via increased energy intake in response to glucocorticoids (Tataranni et al., 1996).
Leptin, a hormone that helps regulate appetite, possibly represents another physiological pathway to obesity. Not only are leptin and cortisol related to each other, a small study which assigned people to shorter or longer sleep durations found that sleep deprivation (4 hour nights) resulted in an altered leptin-cortisol relationship (Spiegel et al., 2004). Furthermore, increased evening cortisol and lower leptin levels resulted in increased appetite among participants who had been assigned to shorter sleep durations. Hence, health behaviors such as sleep, which are themselves influenced by perceived stress, alter biological pathways to obesity.
Finally, obesity is associated with systemic low-grade inflammation which may contribute to an ongoing stimulation of the stress system. This may potentially lead to a self-reinforcing cycle through which stress leads to obesity which itself helps maintain greater activation of stress systems (Hotamisligil, 2006; Kyrou & Tsigos, 2009).
Interim Conclusion
At the individual level, both the psychological state of youth and the health behaviors they engage in affect physical health outcomes. Although the evidence linking youth’s mental health to asthma is largely based on small cross-sectional studies, one larger longitudinal study provides support for the link between child behavior problems and asthma at least. However, youth’s emotional and psychological states and how they relate to pediatric asthma need to be investigated in larger samples. In contrast, with obesity, there are both large-scale longitudinal and cross-sectional studies that support the link between youth depression and obesity. In addition, the negative influence of television watching as well as the positive influence of physical activity on obesity outcomes have been shown very convincingly both cross-sectionally and longitudinally and across both children and adolescents. Finally, both low SES and individual psychosocial profiles shape responses of the autonomic, endocrine, and immune systems, which provides mechanistic explanations at the individual-biological level for how low SES can affect pediatric health outcomes.
Indirect Pathways to Youth’s Physical Health
Although research has investigated the many influences of SES-related factors on youth asthma and obesity outcomes, most studies have limited themselves to the examination of associations between single variables in the social or physical environments and youth health outcomes. Few studies have explored the ways in which factors at the child, family, and neighborhood levels might operate through one another or interact with one another to affect health disparities. The second part of this paper highlights the most important of these pathways through an overview of studies that have addressed factors at multiple levels. We note here that in contrast to the first part of the paper, which sought to provide a comprehensive review of factors at the individual, family, and neighborhood levels, this section is intended to describe, but not exhaustively review, the dynamic ways in which factors at different levels may affect one another. We chose not to conduct a comprehensive review in this section because most of the relevant studies focus on psychosocial outcomes, and hence are only tangentially relevant to the topic of asthma and obesity disparities; however, where possible, we point readers to other reviews that more thoroughly cover one or more of the pathways described below.
Figure 1 includes indirect and synergistic pathways to youth’s physical health. As discussed previously, the original transactional model put forward by Urie Bronfenbrenner, highlights interactions between various levels of influence. Although traditionally focused on psychological well-being, this approach has argued that youth’s well-being is the result of a complex interplay of factors that are transactional, or reciprocal. For example, influences between children and their families are thought to be reciprocal in that not only are children influenced by their families, but children themselves also influence their families, thereby creating a dynamic cycle of influences dependent on the experiences and behavior of all family members.
Most research has focused on how transactional family patterns shape psychosocial outcomes among youth (Dodge, Bates, & Pettit, 1990; Rudolph & Hammen, 1999). Drawing on this literature, we focus below on evidence explaining how factors across levels may operate in conjunction to affect the types of individual-level psychosocial outcomes linked earlier to youth asthma and obesity.
Direct (Cross-Level) Relationships
With some factors, evidence suggests that relationships are primarily unidirectional in nature, meaning that factors at one level influence factors at another level (but not generally vice-versa). As can be seen in Fig. 1 both the social and physical characteristics of neighborhood environments provide a background that shapes how families interact with one another, as well as shapes the psychological states of both parents and youth. We start first with the cross-level effects that neighborhoods can have on families, and then move to the effects that neighborhoods can have on youth themselves.
Neighborhoods ⇒ Families
In the first part of the paper we reviewed the links at the family level from parent mental health, parenting styles, and housing quality to pediatric asthma and obesity outcomes (see, e.g., Bartlett et al., 2001; McCormack et al., 2009; Olvera & Power, 2010), and found that the evidence for the impact of parent-perceived stress and parent mental health was particularly convincing. However, these relationships may be preceded, or influenced, by factors in the neighborhood environment that represent the beginning of a ‘downstream’ cascade of influences which leads through the family environment to youth, ultimately influencing youth asthma and obesity outcomes.
Psychosocially, neighborhood factors may come to influence downstream youth asthma and obesity through shaping parent mental health and parenting styles. Specifically, living in violent neighborhoods has been linked to worse overall parent mental health, more restrictive parenting, and less warmth and closeness from parents towards their children (Burton, 1990; Furstenberg et al., 1993; Klebanov, Brooks-Gunn, & Duncan, 1994; Stockdale et al., 2007). Conversely, living in a positive neighborhood environment marked by trust and cohesion is known to protect parents’ mental health (Kohen, Leventhal, Dahinten, & McIntosh, 2008; Ann Wright & Kloos, 2007; Kim & Ross, 2009; Kim, 2010) and is associated with improved family functioning, such as greater support, acceptance, and warmth within families (Chung & Steinberg, 2006; Law & Barber, 2006; Kohen et al., 2008; Vieno, Nation, Perkins, Pastore, & Santinello, 2010). These connections are highly relevant, given that both parent mental health problems (Bartlett et al., 2001; Klinnert et al., 2008; Koch et al., 2008) and parenting behaviors (Wamboldt et al., 1995; Brook & Tepper, 1997a; Chen et al., 2007; Garasky et al., 2009) have been linked to youth asthma and obesity outcomes.
The physical characteristics of a neighborhood further influence family behavior. Neighborhoods of lower quality, with fewer parks and public facilities result in parents being less likely to encourage physical activity in their children and reporting lower levels of parental efficacy (Shumow & Lomax, 2002; Ceballo & Hurd, 2008; Kotchick & Forehand, 2002) with subsequent negative effects on youth’s overweight status. In addition, neighborhood air pollution can have spillover effects onto home air quality (Jones, Thornton, Mark, & Harrison, 2000; Ilgen et al., 2001; McCormack et al., 2008), particularly when insufficient ventilation (found more frequently in low SES households) traps outdoor pollution indoors.
While beyond the scope of this paper to describe in detail, we remind interested readers to consider other indirect exosystem effects on youth, including parents’ work environments. Parents working in a positive environment tend to be warmer and more responsive when interacting with their children at home (Greenberger, O’Neil, & Nagel, 1994), whereas overwhelming work conditions increase conflict at home (Galambos, Sears, Almeida, & Kolaric, 1995). Conflict in turn has previously been linked to factors such as youth mental health that can affect youth physical health (see, e.g., DeCarlo Santiago & Wadsworth, 2009).
Overall, as indicated in Figure 1, these relationships appear to be unidirectional in nature, meaning that the neighborhood environment predominantly exhibits spillover effects onto the family environment, but that in general, reverse causation (e.g., factors within a family influencing the broader neighborhood environments) does not occur frequently and is not supported empirically in the literature.
Neighborhoods ⇒ Individuals
We previously concluded that the impact of individual-level factors such as youth’s psychological state and health behaviors especially are important with respect to asthma and obesity outcomes. However, psychological states and perceived stress, may have their origins within the neighborhood environments youth live in. Specifically, neighborhood characteristics including exposure to violence (Wright et al., 2004b) and physical properties, such as housing arrangements (Evans, Lercher, & Kofler, 2002) and noise pollution (Evans, Bullinger, & Hygge, 1998) influence youth psychological states.
Children growing up in poor neighborhoods are exposed to particularly high rates of neighborhood violence (Aneshensel & Sucoff, 1996; Sheehan, DiCara, LeBailly, & Christoffel, 1997) which has been linked to greater youth distress (Xue, Leventhal, Brooks-Gunn, & Earls, 2005; Meltzer, Vostanis, Goodman, & Ford, 2007), as well as greater post-traumatic stress syndrome (PTSD), depressive symptoms, and behavioral problems (Fitzpatrick & Boldizar, 1993; Boney-McCoy & Finkelhor, 1995; Aneshensel & Sucoff, 1996; Fitzpatrick, Wright, Piko, & LaGory, 2005). These psychological symptoms, in turn, have been associated with youth asthma and obesity rates (Silverglade et al., 1994; Weil et al., 1999; Goodman & Whitaker, 2002; Wright, Cohen, & Cohen, 2005; Hamer & Stamatakis, 2008). Similarly, physical neighborhood characteristics, such as aircraft and traffic noise, negatively affect youth’s quality of life and perceived stress (Evans, Hygge, & Bullinger, 1995; Evans et al., 1998; Evans, Lercher, Meis, Ising, & Kofler, 2001). In turn, greater stress worsens asthma and increases risk of obesity (Wright et al., 2005; Hamer & Stamatakis, 2008).
The above associations are supported by a small number of studies that explicitly tested spillover effects across multiple levels (e.g., neighborhood → family → child), although all in the context of youth psychological well-being. Two longitudinal studies provide strong evidence of neighborhood environments, either positive or marked by violence, influencing both family environments and youth problem and antisocial behaviors. A 2-year longitudinal study found that exposure to violence at the neighborhood level prospectively predicted decreases in family discipline over time, which in turn increased the likelihood of youth engaging in future problematic behaviors such as acts of violence (Spano, Vazsonyi, & Bolland, 2009). Conversely, a positive social neighborhood environment can increase maternal acceptance and monitoring, consequently lowering youth antisocial behavior over four years (Law & Barber, 2006). Two cross-sectional studies using smaller samples also find that undesirable neighborhood factors, such as violence, negatively influence youth adjustment and mental health by increasing parent psychological stress and family conflict (Overstreet & Braun, 2000; Gutman, McLoyd, & Tokoyawa, 2005).
Spillover effects have also been documented from SES→ family→ child. Similarly to the above discussed research on neighborhoods, findings to date suggest that low SES (economic pressure experienced by parents) increases family conflict and parenting stress, which in turn increase youth internalizing and externalizing behavior problems and depressed mood (Conger et al., 1994; Hammack, Robinson, Crawford, & Li, 2004; DeCarlo Santiago & Wadsworth, 2009). In turn these child psychological states have been linked to asthma and obesity (e.g., Roemmich et al., 2007; Marin et al., 2009); hence, we hypothesize that these types of spillover effects from neighborhood to family to child relationships and from SES to family interaction patterns to child psychological states will also have implications for childhood asthma and obesity.
Overall, the associations connecting the neighborhood environment to individual characteristics are thought to be largely unidirectional (see Fig. 1). We acknowledge that there could be small effects, such as when youth who grow up in more violent neighborhoods develop behavioral problems themselves and consequently later contribute to neighborhood violence; on the whole however, these apply to only certain select neighborhood characteristics, as, for example, youth’s psychological states are unlikely to affect neighborhood traffic or the availability of food stores.
Other microsystem influences
It is beyond the scope of this paper to discuss all microsystem influences on youth. However, we briefly mention some additional factors not covered in detail in this review, including school and work environments, and provide citations to more detailed papers for interested readers. Regarding schools, low SES schools are more likely to have more crowded classrooms, greater student and teacher turnover (creating more unstable school environments), lower teacher quality and fewer resources for physical activity and healthy food options, all of which increase the stressfulness of the school environment and the barriers to healthy behaviors in school, with implications for both obesity and asthma (Alexander, Entwisle, & Dauber, 1996; Maxwell, 1996; Sallis, Zakarian, Hovell, & Hofstetter, 1996; Pianta & Early, 2001; Lankford, Loeb, & Wyckoff, 2002; Hanushek, Kain, & Rivkin, 2004; Minaker et al., 2006; Scafidi, Sjoquist, & Stinebrickner, 2007). In addition, youth’s home and school air pollution, crowding, and noise have been shown to interact to influence youth health (Cohen, Evans, Stokols, & Krantz, 1986; Maxwell, 1996; Maxwell, 2003; McConnell et al., 2010).
Employment outside of school is common among US youth with about 50–70% of high school seniors being employed part-time (Entwisle, Alexander, Olson, & Ross, 1999; Entwisle, Alexander, & Olson, 2000; Hirschman & Voloshin, 2007). Minority and low SES youth are more likely to be in less desirable and high intensity jobs, with poorer working conditions and greater injury rates (Hirschman & Voloshin, 2007; Rauscher & Myers, 2008). In addition, youth who spend more time working outside of school face negative effects on their family, social, and academic lives (Greenberger & Steinberg, 1986; Monahan, Lee, & Steinberg, 2011; Mihalic & Elliott, 1997) and, as shown above, greater stress in any or all of these areas may further predispose youth to negative health outcomes.
Interim conclusion
These studies illustrate potentially cascading effects from one level to another that may ultimately come to influence youth asthma and obesity. The neighborhood environment is an important influence on both parent mental health and parenting behaviors. Neighborhood stressors also directly influence youth’s psychological well-being, and influences of youth’s school and work environments should not be ignored. Overall, evidence suggests that these types of cross-level effects from neighborhoods are largely unidirectional, as characteristics measured at the child and family level have not been found to longitudinally predict changes in neighborhood characteristics.
Reciprocal Relationships
We now turn to influences that create reciprocal, self-perpetuating patterns, both across levels (e.g. family conflict increasing youth distress, which in turn shapes future family interactions), and across domains (e.g. the built environment shaping social interactions among residents which in turn can shape social action to improve the physical layout of their neighborhood). See Figure 1. These reciprocal patterns can serve to further amplify the impact of individually discussed factors on youth asthma and obesity. This includes the amplification of both factors at the individual level (e.g., youth’s psychological state), and at the family level (e.g., family conflict), all of which have already been shown to play important roles in pediatric asthma and obesity outcomes.
Family ⇔ Youth
Parents’ and youth’s mental health and behaviors have reciprocal effects on each other (Belsky, 1984). Poorer parent mental health has been linked to worse mental health among their children (Lindsey et al., 2008; Mellins et al., 2008). Youth of mothers who are depressed are themselves more likely to be depressed (Downey & Coyne, 1990) and to suffer from adjustment, behavior and emotional problems (Downey & Coyne, 1990; Riley et al., 2009). This may in part be due to independent effects of shared environmental influences or common genetic risk factors (Kendler, 2001; Rice, Harold, & Thapar, 2002). However, the literature also clearly suggests negative parenting as a mediator between poverty and youth mental health (Grant et al., 2003).
In turn, youth’s mental health and behavior problems also affect their parents’ psychological state and behaviors (Elgar, McGrath, Waschbusch, Stewart, & Curtis, 2004; Fite, Colder, Lochman, & Wells, 2006) and begin to do so early on. Intriguing longitudinal research that takes advantage of latent growth curve and structural equation modeling has shown that children’s and adolescents’ behavioral problems and noncompliance both precede and are consequences of maternal depression and negative parenting (Elgar, Curtis, McGrath, Waschbusch, & Stewart, 2003; Combs-Ronto, Olson, Lunkenheimer, & Sameroff, 2009; Gross, Shaw, Moilanen, Dishion, & Wilson, 2008; Gross, Shaw & Moilanen, 2008; Shaw, Gross, Moilanen, & Sameroff, 2009). As outlined above, both maternal depression and negative parenting, for example harsh parenting, have been linked to negative outcomes with respect to youth asthma and obesity, including greater asthma morbidity (Shalowitz, Berry, Quinn, & Wolf, 2001) and greater fat stores (Johnson & Birch, 1994).
Family conflict and violence also have reciprocal effects with youth well-being. Low-income youth are more likely to experience family assault (Finkelhor & Dziuba-Leatherman, 1994). In turn, experiencing parental assault or corporal punishment is associated with greater PTSD symptomatology, depression, and hopelessness among youth (Boney-McCoy & Finkelhor, 1995). Similarly, parental conflict has been linked to greater depressed mood and anxiety (Mechanic & Hansell, 1989), internalizing and externalizing problems (Grych, Fincham, Jouriles, & McDonald, 2000; El-Sheikh, Harger, & Whitson, 2001), and child-perceived parent rejection (Shelton & Harold, 2008) among youth. However, youth also actively contribute to this reinforcing cycle of negative interactions, potentially leading to negative or coercive behavior patterns across family members that escalate over time (Patterson, DeBaryshe, & Ramsey, 1989). For example, parents of highly disobedient and violent youth experienced more anger, fear and negative emotions towards their child (Bradshaw, Glaser, Calhoun, & Bates, 2006), suggesting that youth’s adversive behavior patterns may not only increase the emotional burden on parents, but may also increase parents’ likelihood of engaging in negative and violent behaviors themselves. In addition, two studies suggest that adolescent maladjustment and behavioral problems can have significant negative repercussions on parenting pleasure, marital satisfaction, and marital functioning (Cui, Donnellan, & Conger, 2007; Johnston & Mash, 2001). These reinforcing patterns between violence and behavioral problems are particularly relevant as both violent family environments and youth behavioral problems have been linked above to worse health outcomes, for example with respect to asthma (e.g. Suglia, Enlow, Kullowatz, & Wright, 2009). In addition, living in a violent environment leads to greater everyday stress among youth, which in turn can also worsen asthma and obesity outcomes (Marin, Chen, Munch & Miller, 2009; Lohman, Stewart, Gundersen, Garasky, & Eisenmann, 2009).
This section provides evidence that reciprocal relationships exist between parent and youth mental health, and as well between family conflict and youth mental health. We suggest that the effects of any one of these factors can get amplified by their reciprocal relationships with these other factors, in turn amplying the effects that parent mental health, family conflict, and youth mental health all have associations with childhood asthma and obesity (Silverglade et al., 1994; Wamboldt et al., 1995; Goodman & Whitaker, 2002; Moens et al., 2007; Koch et al., 2008; Barreto do Carmo et al., 2009),
Physical ⇔ Social
Reciprocal effects also exist between the physical and social domains. For example, some reviews now discuss the notion that greater psychosocial stress may increase vulnerability to physical environmental stressors (Gee & Payne-Sturges, 2004; Evans, 2004; Morello-Frosch & Shenassa, 2006; Sandel & Wright, 2006).
At the neighborhood level, physical features of the built environment, such as the presence of parks, create a space that determines how residents interact with each other socially, leading to stronger or weaker social connections among residents depending on how conducive physical neighborhood layouts are to social interactions (Leyden, 2003; Cohen et al., 2008). In turn, social characteristics of residents can shape the physical properties of neighborhoods; for example, residents living in a neighborhood higher in social capital are more politically engaged and invest more time and effort into shaping their physical neighborhood (Lake & Huckfeldt, 1998). As both social capital and the physical built environment play a role in pediatric asthma and obesity (see e.g., Cagney & Browning, 2004; Juhn et al., 2005; Gordon-Larsen et al., 2006; Cradock et al., 2009), the reciprocal relationships between them become especially important to understand.
Important reciprocal relationships between social and physical factors also exist at the family level (Sandel & Wright, 2006). For example, families living in less physically desirable homes – e.g., noisier and more crowded homes – experience more conflictual parent-child relationships and provide less stimulating and responsive parenting to their infants (Evans, Lepore, Shejwal, & Palsane, 1998; Corapci & Wachs, 2002). In addition, lower observer-rated physical housing quality has been associated with greater psychological distress among mothers (Evans, Wells, Chan, & Saltzman, 2000). Conversely, psychological problems, such as depressed mood, among caregivers is predictive of worse physical home environments, perhaps because these caregivers have fewer resources in the form of motivation, time, and materials, at their disposal to provide a positive physical home environment (Klebanov et al., 1994). Persistently depressed parents also report more negative physical home environments when compared to parents from a healthy control group (Billings & Moos, 1981).
In sum, social influences have been shown to change physical environments and physical environments to influence social ones in reciprocal ways that suggest the importance of considering the two domains together, particularly given that both physical family environment exposures (such as indoor allergens and food availability), as well as social family environment exposures (such as family routines) have been linked to asthma and obesity (Campbell & Crawford, 2001; Levy et al., 2006; Gable et al., 2007; Schreier & Chen, 2010).
Interim conclusion
Multiple factors at the family level, most notably parent mental health and parenting styles, operate in a reciprocal fashion with factors at the individual child level, including youth mental health and behaviors. Relatedly, the physical and social domains of neighborhood and family environments can reciprocally influence each other. For example, at the neighborhood level, the physical properties of neighborhoods can facilitate or hinder social cohesion, which in turn can shape community action toward changing neighborhood physical characteristics. At the family level, physical characteristics of homes, such as crowding, influence family dynamics, which in turn can shape the ability of families to maintain their home environment physically. These reciprocal relationships then create spiraling sources of exposures that contribute to risk for childhood health problems such as asthma and obesity.
Interactions
In addition to unidirectional and reciprocal effects, factors across different levels may interact to create effects on youth health. Because several recent studies address cross-level interactions in the context of pediatric asthma, we focus on these to provide examples of cross-level interaction research.
Three recent studies have examined how the social and physical environment, specifically, stress and air pollution, interact to affect childhood asthma. Although the direct links between air pollution and asthma are well-established, studies now suggest that certain social risk factors may exacerbate the effects of physical environmental risks on asthma outcomes. Shankardass et al. (2009) prospectively investigated whether parent-reported stress moderated the impact of traffic-related pollution and in utero tobacco smoke exposure in children. They found that traffic-related pollution had worse effects on asthma incidence in youth whose parents reported more stress, suggesting that social stressors such as family stress can make youth more susceptible to the negative effects of risk factors in their physical environment. Similarly, chronic life stress in the form of lifetime exposure to violence among youth in conjunction with elevated air pollution has been linked to increased risk of asthma diagnosis (Clougherty et al., 2007). Finally, among children already diagnosed with asthma, Chen, Schreier, Strunk, and Brauer (2008) found that asthma morbidity was greater among youth who had high levels of chronic family stress even in spite of only modest exposures to traffic-related pollution. That is, children and adolescents who reported greater chronic life stress in their family in the context of modest air pollution exposures showed evidence of greater stimulated cytokine production and eosinophil counts (indicative of greater inflammation), resulting in profiles similar to those of children who lived in high pollution neighborhoods. This furthermore carried over to clinical asthma outcomes longitudinally as these youth also experienced increasing symptoms and decreasing lung functioning over time.
More research needs to investigate the mechanisms underlying these findings, but it is possible that social exposures such as high chronic stress can result in a physiological shift, possibly through sensitizing physiological systems, which then makes youth more vulnerable to physical pollutants (Chen & Miller, 2007). Consequently, youth who are exposed to ongoing social stressors may be more vulnerable to negative effects of physical environment characteristics, possibly because both can operate through similar biological pathways. This points to the need for investigating the effects of physical environment characteristics in the context of youth’s social environment in order to more fully understand the contributors to complex diseases such as childhood asthma.
The Importance of Timing
We acknowledge that the relationships between SES and environmental influences have thus far been conceptualized in a rather static way. However, as emphasized by Bronfenbrenner and Evans (2000), taking into account settings, that is the duration, timing, and regularity of exposures, is also important. Several ways in which timing may have effects on health have been proposed (Pollitt, Rose, & Kaufman, 2005). For example, critical period models suggest that SES is particularly influential during particular life stages, such as early childhood. Others suggest that duration of exposure – for example, extended exposure to a low SES environment across longer periods of time (accumulation models) or inconsistent exposure (consistency models) - is particularly detrimental. All of these are supported by empirical research to some extent (Evans, Gonnella, Marcynyszyn, Gentile, & Salpekar, 2005; Evans & Kim, 2007; Timberlake, 2007; Miller et al., 2009). In addition, simultaneous exposure to more than one variable is not only possible but presumably common. Youth growing up in poverty are more likely to be exposed to multiple risks simultaneously (Adler & Stewart, 2010; Evans & Kim, 2010) and consequently are at an especially high risk for health problems. Hence, beyond considering reciprocal and synergistic effects of variables, future research will need to consider in more detail both timing and co-occurrence issues.
Future Directions
The model proposed above, together with insights from relevant existing research can inform future research in a number of ways. Overall, a relatively large number of previous studies has established connections between the individual variables in our model. However, research to date has not fully addressed the complex ways in which these variables interact and influence each other. Consequently, several types of questions are in need of being evaluated further.
First, reciprocal influences between the physical and social domains at both the family and neighborhood level should be investigated with respect to pediatric asthma and obesity in greater detail, especially as research discussed in this paper – such as the synergistic effects of family stress or neighborhood violence and air pollution on asthma – suggest that such investigations are worthwhile. Some people have begun to advocate the integration of the physical and social domains (e.g. Sandel & Wright, 2006) but most research so far has viewed these two domains separately and different academic disciplines have tended towards investigating either one or the other. Hence, although this paper shows that there is strong support for many of the links between SES and social/physical factors and in turn between these social/physical factors and health outcomes, too few studies include an investigation of dynamic mediating relationships, such as the potential spiraling effects of the social and physical domains on each other, and in turn how these cyclical relationships affect physical health outcomes such as asthma and obesity. Future research projects should involve researchers across disciplines on collaborative projects to make the most effective use of the expertise scientists can bring from their respective disciplines to better understand the collective social and physical contributors to disparities in asthma and obesity outcomes in childhood.
Second, reciprocal influences between the individual, family, and neighborhood environments demand greater attention in studies of pediatric asthma and obesity. In many cases influences at the neighborhood level represent the beginnings of a cascade of factors that move down through the family environment to influence youth’s psychological state and health behaviors, and vice versa, in turn influencing youth physical health. For example, with respect to obesity, perceptions of neighborhood violence may alter parenting styles, which in turn may result in youth staying at home more, adopting a more sedentary lifestyle, and over time becoming more obese. The dynamics of these relationships need to be investigated in a health-context in longitudinal studies, in order to trace pathways over time and to ascertain the temporal order in which influences in one domain or on one level affect other factors and the eventual implications for physical health. For example, it may be possible to test whether physical neighborhood factors (e.g., violence) at time 1 influence family dynamics (e.g., greater psychological stress) at time 2, and whether these family dynamics in turn influence youth characteristics (e.g., poorer mental health) and health outcomes such as asthma or obesity at a further time point. Measurement across multiple levels simultaneously would also allow for a better evaluation of the relative contributions of factors at different levels.
Third, recent findings suggest that interactions across the physical and social domains warrant greater attention in health research. So far these interactions have primarily focused on asthma outcomes and encompassed influences in the social domain of one level, e.g. the social home environment, and influences in the physical domain of another level, e.g. neighborhood air pollution. Aside from investigating these relationships in greater detail it would be of interest to extend these investigations to different variables and to different outcomes, such as obesity, since it is possible that similar interactions across levels and domains are also influential with regards to youth’s physical health outcomes other than asthma. For example, the social home environment of youth, including the nature of the relationships between youth and other family members, may influence how youth take advantage of opportunities for physical activity in their physical neighborhood environment.
With respect to investigating various influences on asthma and obesity specifically, more studies are needed that use a longitudinal study design to allow for stronger conclusions regarding cause and effect, and that evaluate effects across different age groups, as effects may differ between children and adolescents. In addition, measurement issues exist for the assessment of asthma and obesity outcomes alike. The majority of studies rely on self-reported outcomes, including self-reported medication use and symptoms in the case of asthma and self-reported physical activity or height and weight information in the case of obesity. This leads to apparent problems, most notably the questionable accuracy of such reports, which may be further compounded by participants’ young age. Taking advantage of existing technologies such as spirometry and accelerometers would greatly increase the reliability of findings.
Finally, in terms of intervention implications, we caution against the idea that just because low SES environments lack certain qualities, providing them would be the solution to alleviating health disparities. Some factors that are beneficial in high SES neighborhoods, e.g. community attachment, may in fact have undesirable consequences in low SES neighborhoods. Caughy, O’Campo, and Muntaner (2003) showed that among families from high SES neighborhoods, mothers’ lack of community attachment was linked to greater internalizing disorders in their children, whereas among families from low SES neighborhoods mothers’ lack of community attachment was associated with lower rates of internalizing disorders in their children. Similarly, neighborhood social participation was associated with positive mental health outcomes in communities with few stressors, whereas living on an ‘isolated’ block was advantageous for mental health if a neighborhood experienced multiple stressors (Dupere & Perkins, 2007). The existence of strong social ties in poorly functioning communities may also have negative effects if it results in increased exposure to deviant peers (Darling & Steinberg, 1997; Andersen, 1999; Pattillo-McCoy, 2000). Lastly, one experimental study investigated the effects of moving to a higher SES neighborhood as part of a neighborhood desegregation program (Fauth, Leventhal, & Brooks-Gunn, 2007). Follow-up data from seven years later revealed that this move to a ‘better’ neighborhood had largely negative influences on youth anxiety and depressive symptoms, a relationship that was mediated by the social climate of the new neighborhood environment. Families who moved ultimately ended up with fewer informal social contacts compared to families who did not have to move, which negatively influenced youth’s psychological health. These findings are consistent with a systematic review (De Silva, McKenzie, Harpham, & Huttly, 2005) that found inconclusive evidence for an overall main effect of neighborhood social capital on residents’ mental health. Instead of simply evaluating what characterizes high SES neighborhoods, research should focus also on how such factors may play out differently in different situations, i.e. in affluent versus disadvantaged neighborhoods. Rather than working toward ‘importing’ characteristics of high SES environments, we need to work toward developing interventions that are context-specific, and that are beneficial for health given the circumstances under which many low SES families live.
Conclusion
In this review, we have documented that the socioeconomic environment that children come from shapes both the physical and social characteristics of neighborhoods, families, and children themselves, with implications for childhood physical health problems, such as obesity and asthma. We have furthermore emphasized the importance of recognizing that the factors shaped by low SES do not exist in isolation, but rather influence one another, creating complex reciprocal relationships that work together to alter physical health outcomes in youth. In some cases, effects at one level spill over and influence other levels (direct, cross-level effects, e.g. the built neighborhood environment shaping parenting behaviors). In other cases, factors at different levels have reciprocal effects on one another (e.g. parenting affecting child behavioral problems, which in turn shape future parenting behaviors). And finally, factors across levels can interact to create unique, synergistic effects (e.g. family stress accentuating the effect of physical environmental exposures on asthma).
To effectively reduce the burden of pediatric asthma and obesity, both acutely and in the long-term, a more integrative approach is necessary for understanding the contributing factors to childhood health disparities and for ultimately designing interventions to reduce these disparities. Research has long investigated how SES influences youth’s risk for asthma and obesity through multiple pathways. However, future research should move beyond studying relationships between single environmental influences and youth physical health outcomes and turn toward viewing the more complex structures at play between factors at multiple levels of influence and across both the social and the physical domains. A more in-depth understanding of the reciprocal relationships connecting these factors represents an important first step on the way to creating maximally effective intervention strategies and ultimately disrupting the pathways through which low SES comes to negatively influence youth’s physical health.
Acknowledgments
This work was supported by NIH grant # HL073975, the Social Sciences and Humanities Research Council of Canada, and the Canadian Institutes of Health Research grant FRN: 97872.
Contributor Information
Hannah M. C. Schreier, Department of Psychology, University of British Columbia.
Edith Chen, Department of Psychology and Cells to Society (C2S): The Center on Social Disparities and Health, Institute for Policy Research, Northwestern University.
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. American Psychologist. 1994;49 (1):15–24. doi: 10.1037/0003-066X.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Annals of the New York Academy of Sciences. 2010;1186(1):5–23. doi: 10.1111/j.1749-6632.2009.05337.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2009.05337.x/full. [DOI] [PubMed] [Google Scholar]
- Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Affairs. 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Supplement 3):S131. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- Alexander KL, Entwisle DR, Dauber SL. Children in motion: School transfers and elementary school performance. The Journal of Educational Research. 1996;90(1):3–12. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/00220671.1996.9944438. [Google Scholar]
- Amre DK, Infante-Rivard C, Gautrin D, Malo J-L. Socioeconomic status and utilization of health care services among asthmatic children. Journal of Asthma. 2002;39(7):625–631. doi: 10.1081/JAS-120014927. [DOI] [PubMed] [Google Scholar]
- Andersen E. Code of the Street: Decency, Violence, and the Moral Life of the Inner City. New York: WW Norton; 1999. [Google Scholar]
- Andersen RE, Crespo CJ, Bartlett SJ, Cheskin LJ, Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(12):938–942. doi: 10.1001/jama.279.12.938. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Sucoff CA. The neighborhood context of adolescent mental health. Journal of Health & Social Behavior. 1996;37(4):293–310. doi: 10.2307/2137258. [DOI] [PubMed] [Google Scholar]
- Ann Wright P, Kloos B. Housing environment and mental health outcomes: A levels of analysis perspective. Journal of Environmental Psychology. 2007;27(1):79–89. doi: 10.1016/j.jenvp.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Barreto do Carmo MB, Neves Santos D, Alves Ferreira Amorim LD, Fiaccone RL, Souza da Cunha S, Cunha Rodrigues L, et al. Minor psychiatric disorders in mothers and asthma in children. Social Psychiatry and Psychiatric Epidemiology. 2009;44(5):416–420. doi: 10.1007/s00127-008-0450-x. [DOI] [PubMed] [Google Scholar]
- Bartlett SJ, Kolodner K, Butz AM, Eggleston P, Malveaux FJ, Rand CS. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Archives of Pediatrics & Adolescent Medicine. 2001;155(3):347–353. doi: 10.1001/archpedi.155.3.347. [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, YALI ANN. Socioeconomic status and chronic stress: Does stress account for SES effects on health? Annals of the New York Academy of Sciences. 1999;896(1):131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.1999.tb08111.x/full. [DOI] [PubMed] [Google Scholar]
- Baxter LK, Clougherty JE, Laden F, Levy JI. Predictors of concentrations of nitrogen dioxide, fine particulate matter, and particle constituents inside of lower socioeconomic status urban homes. Journal of Exposure Science & Environmental Epidemiology. 2007;17(5):433–444. doi: 10.1038/sj.jes.7500532. [DOI] [PubMed] [Google Scholar]
- Bell JF, Wilson JS, Liu GC. Neighborhood greenness and 2-year changes in body mass index of children and youth. American Journal of Preventive Medicine. 2008;35(6):547–553. doi: 10.1016/j.amepre.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. The determinants of parenting: A process model. Child development. 1984;55(1):83–96. doi: 10.2307/1129836. [DOI] [PubMed] [Google Scholar]
- Bender B, Milgrom H, Rand C, Ackerson L. Psychological factors associated with medication nonadherence in asthmatic children. Journal of Asthma. 1998;35(4):347–353. doi: 10.3109/02770909809075667. [DOI] [PubMed] [Google Scholar]
- Berz JB, Carter AS, Wagmiller RL, Horwitz SM, Murdock KK, Briggs-Gowan M. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2- to 3-year olds. Journal of Pediatric Psychology. 2007;32(2):154–166. doi: 10.1093/jpepsy/jsj123. [DOI] [PubMed] [Google Scholar]
- Billings AG, Moos RH. The role of coping responses and social resources in attenuating the stress of life events. Journal of Behavioral Medicine. 1981;4(2):139–157. doi: 10.1007/BF00844267. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. International journal of obesity Supplement. 2000a;24(2):S80–S85. doi: 10.1038/sj.ijo.0801285. Retrieved from http://cat.inist.fr/?aModele=afficheN&cpsidt=1408175. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000b;16(10):924–936. doi: 10.1016/s0899-9007(00)00422-6. Retrieved from http://www.sciencedirect.com/science/article/pii/S0899900700004226. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. The metabolic syndrome—a neuroendocrine disorder? British Journal of Nutrition. 2000c;83(S1):S49–S57. doi: 10.1017/s0007114500000957. Retrieved from http://journals.cambridge.org/abstract_S0007114500000957. [DOI] [PubMed] [Google Scholar]
- Bloomberg GR, Chen E. The relationship of psychologic stress with childhood asthma. Immunology & Allergy Clinics of North America. 2005;25(1):83–105. doi: 10.1016/j.iac.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Boney-McCoy S, Finkelhor D. Psychosocial sequelae of violent victimization in a national youth sample. Journal of Consulting & Clinical Psychology. 1995;63(5):726–736. doi: 10.1037/0022-006X.63.5.726. [DOI] [PubMed] [Google Scholar]
- Booth KM, Pinkston MM, Poston WSC. Obesity and the built environment. Journal of the American Dietetic Association. 2005;105(5)(Suppl 1):S110–7. doi: 10.1016/j.jada.2005.02.045. [DOI] [PubMed] [Google Scholar]
- Boulet LP, Lemière C, Archambault F, Carrier G, Descary MC, Deschesnes F. Smoking and Asthma. Chest. 2006;129(3):661. doi: 10.1378/chest.129.3.661. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, Coll CG. The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child Development. 2001;72(6):1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Bradshaw CP, Glaser BA, Calhoun GB, Bates JM. Beliefs and practices of the parents of violent and oppositional adolescents: An ecological perspective. The Journal of Primary Prevention. 2006;27(3):245–263. doi: 10.1007/s10935-006-0030-3. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. American Journal of Respiratory & Critical Care Medicine. 2002;166(8):1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Evans GW. Developmental science in the 21st century: Emerging questions, theoretical models, research designs and empirical findings. Social development. 2000;9(1):115–125. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/1467-9507.00114/full. [Google Scholar]
- Bronfenbrenner U. Toward an experimental ecology of human development. American Psychologist. 1977;32(7):513–531. doi: 10.1037/0003-066X.32.7.513. [DOI] [Google Scholar]
- Brook U, Tepper I. Self image, coping and familial interaction among asthmatic children in Israel. Patient Education and Counseling. 1997a;30(2):187–192. doi: 10.1016/S0738-3991(96)00945-7. [DOI] [PubMed] [Google Scholar]
- Brook U, Tepper I. Self image, coping and familial interaction among asthmatic children and adolescents in Israel. Patient Education & Counseling. 1997b;30(2):187–192. doi: 10.1016/S0738-3991(96)00945-7. [DOI] [PubMed] [Google Scholar]
- Buonocore JJ, Lee HJ, Levy JI. The Influence of Traffic on Air Quality in an Urban Neighborhood: A Community-University Partnership. American Journal of Public Health. 2009;99(S3):S629. doi: 10.2105/AJPH.2008.149138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton LM. Teenage childbearing as an alternative life-course strategy in multigeneration black families. Human Nature. 1990;1(2):123–143. doi: 10.1007/BF02692149. [DOI] [PubMed] [Google Scholar]
- Bussing R, Burket RC, Kelleher ET. Prevalence of anxiety disorders in a clinic-based sample of pediatric asthma patients. Psychosomatics. 1996;37(2):108–115. doi: 10.1016/S0033-3182(96)71576-1. [DOI] [PubMed] [Google Scholar]
- Cagney KA, Browning CR. Exploring neighborhood-level variation in asthma and other respiratory diseases: the contribution of neighborhood social context. Journal of General Internal Medicine. 2004;19(3):229–236. doi: 10.1111/j.1525-1497.2004.30359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, Crawford D. Family food environments as determinants of preschool-aged children’s eating behaviours: implications for obesity prevention policy. A review. Australian Journal of Nutrition and Dietetics. 2001;58(1):19–25. Retrieved from http://en.scientificcommons.org/38193039. [Google Scholar]
- Campbell K, Crawford D, Jackson M, Cashel K, Worsley A, Gibbons K, et al. Family food environments of 5–6-year-old-children: does socioeconomic status make a difference? Asia Pacific Journal of Clinical Nutrition. 2002;11(Suppl 3):S553–61. doi: 10.1046/j.0964-7058.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- Carver A, Timperio A, Crawford D. Playing it safe: the influence of neighbourhood safety on children’s physical activity. A review. Health & Place. 2008;14(2):217–227. doi: 10.1016/j.healthplace.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Castro M, Schechtman KB, Halstead J, Bloomberg G. Risk factors for asthma morbidity and mortality in a large metropolitan city. Journal of Asthma. 2001;38(8):625–635. doi: 10.1081/JAS-100107540. [DOI] [PubMed] [Google Scholar]
- Caughy MOB, O’Campo PJ, Muntaner C. When being alone might be better: neighborhood poverty, social capital, and child mental health. Social Science & Medicine. 2003;57 (2):227–237. doi: 10.1016/S0277-9536(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Cecil-Karb R, Grogan-Kaylor A. Childhood body mass index in community context: neighborhood safety, television viewing, and growth trajectories of BMI. Health and Social Work. 2009;34(3):169–177. doi: 10.1093/hsw/34.3.169. Retrieved from http://www.ingentaconnect.com/content/nasw/hsw/2009/00000034/00000003/art00002. [DOI] [PubMed] [Google Scholar]
- Cesaroni G, Farchi S, Davoli M, Forastiere F, Perucci CA. Individual and area-based indicators of socioeconomic status and childhood asthma. European Respiratory Journal. 2003;22(4):619–624. doi: 10.1183/09031936.03.00091202. [DOI] [PubMed] [Google Scholar]
- Chakraborty J, Zandbergen PA. Children at risk: measuring racial/ethnic disparities in potential exposure to air pollution at school and home. Journal of Epidemiology & Community Health. 2007;61(12):1074–1079. doi: 10.1136/jech.2006.054130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Delfino RJ, Gillen D, Tjoa T, Nickerson B, Cooper D. Repeated respiratory hospital encounters among children with asthma and residential proximity to traffic. Occupational & Environmental Medicine. 2009;66(2):90–98. doi: 10.1136/oem.2008.039412. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64(1):38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. American Journal of Respiratory & Critical Care Medicine. 2007;176(7):644–649. doi: 10.1164/rccm.200610-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Fisher EB, Bacharier LB, Strunk RC. Socioeconomic status, stress, and immune markers in adolescents with asthma. Psychosomatic Medicine. 2003;65(6):984–992. doi: 10.1097/01.PSY.0000097340.54195.3C. [DOI] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. Journal of Allergy & Clinical Immunology. 2006;117(5):1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: how and why do these relationships change with age? Psychological Bulletin. 2002;128(2):295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain, Behavior, & Immunity. 2007;21(8):993–999. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Schreier HMC, Strunk RC, Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environmental Health Perspectives. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environmental Health Perspectives. 2003;111(10):1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000;24:S50. doi: 10.1038/sj.ijo.0801278. Retrieved from http://ukpmc.ac.uk/abstract/MED/10997609. [DOI] [PubMed] [Google Scholar]
- Chung HL, Steinberg L. Relations between neighborhood factors, parenting behaviors, peer deviance, and delinquency among serious juvenile offenders. Developmental Psychology. 2006;42(2):319–331. doi: 10.1037/0012-1649.42.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio L, Stingone JA, Godbold J. Prevalence of childhood asthma in urban communities: the impact of ethnicity and income. Annals of Epidemiology. 2006;16(5):332–340. doi: 10.1016/j.annepidem.2005.06.046. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environmental Health Perspectives. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Finch BK, Bower A, Sastry N. Collective efficacy and obesity: The potential influence of social factors on health. Social Science & Medicine. 2006;62(3):769–778. doi: 10.1016/j.socscimed.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Cohen DA, Inagami S, Finch B. The built environment and collective efficacy. Health & Place. 2008;14(2):198–208. doi: 10.1016/j.healthplace.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RT, Canino GJ, Bird HR, Celedon JC. Violence, abuse, and asthma in Puerto Rican children. American Journal of Respiratory & Critical Care Medicine. 2008;178(5):453–459. doi: 10.1164/rccm.200711-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Evans GW, Stokols D, Krantz DS. Behavior, health, and environmental stress. Plenum Press; New York, NY: 1986. [Google Scholar]
- Cole SW. Social regulation of human gene expression. Current Directions in Psychological Science. 2009;18(3):132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs-Ronto LA, Olson SL, Lunkenheimer ES, Sameroff AJ. Interactions between maternal parenting and children’s early disruptive behavior: Bidirectional associations across the transition from preschool to school entry. Journal of Abnormal Child Psychology. 2009;37(8):1151–1163. doi: 10.1007/s10802-009-9332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH, Lorenz FO. Economic stress, coercive family process, and developmental problems of adolescents. Child Development. 1994;65(2):541–561. doi: 10.2307/1131401. [DOI] [PubMed] [Google Scholar]
- Coon KA, Goldberg J, Rogers BL, Tucker KL. Relationships between use of television during meals and children’s food consumption patterns. Pediatrics. 2001;107(1):E7. doi: 10.1542/peds.107.1.e7. [DOI] [PubMed] [Google Scholar]
- Cope SF, Ungar WJ, Glazier RH. Socioeconomic factors and asthma control in children. Pediatric Pulmonology. 2008;43(8):745–752. doi: 10.1002/ppul.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corapci F, Wachs TD. Does parental mood or efficacy mediate the influence of environmental chaos upon parenting behavior? Merrill-Palmer Quarterly. 2002;48(2):182–201. doi: 10.1353/mpq.2002.0006. [DOI] [Google Scholar]
- Cradock AL, Kawachi I, Colditz GA, Gortmaker SL, Buka SL. Neighborhood social cohesion and youth participation in physical activity in Chicago. Social Science & Medicine. 2009;68(3):427–435. doi: 10.1016/j.socscimed.2008.10.028. Retrieved from http://www.sciencedirect.com/science/article/pii/S0277953608005601. [DOI] [PubMed] [Google Scholar]
- Crain EF, Walter M, O’Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environmental Health Perspectives. 2002;110(9):939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo CJ, Smit E, Troiano RP, Bartlett SJ, Macera CA, Andersen RE. Television watching, energy intake, and obesity in US children: results from the third National Health and Nutrition Examination Survey, 1988–1994. Archives of Pediatrics & Adolescent Medicine. 2001;155(3):360–365. doi: 10.1001/archpedi.155.3.360. [DOI] [PubMed] [Google Scholar]
- Cui M, Donnellan MB, Conger RD. Reciprocal influences between parents’ marital problems and adolescent internalizing and externalizing behavior. Developmental psychology. 2007;43(6):1544–1552. doi: 10.1037/0012-1649.43.6.1544. [DOI] [PubMed] [Google Scholar]
- Dales RE, Choi B, Chen Y, Tang M. Influence of family income on hospital visits for asthma among Canadian school children. Thorax. 2002;57(6):513–517. doi: 10.1136/thorax.57.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales R, Wheeler AJ, Mahmud M, Frescura A-M, Liu L. The influence of neighborhood roadways on respiratory symptoms among elementary schoolchildren. Journal of Occupational & Environmental Medicine. 2009;51(6):654–660. doi: 10.1097/JOM.0b013e3181a0363c. [DOI] [PubMed] [Google Scholar]
- Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids—food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145(6):2633. doi: 10.1210/en.2004-0037. Retrieved from http://endo.endojournals.org/content/145/6/2633.short. [DOI] [PubMed] [Google Scholar]
- Darling N, Steinberg L. Community influences on adolescent achievement and deviance. Neighborhood poverty: Policy implications in studying neighborhoods. 1997;2:120–131. [Google Scholar]
- Darmon N, Drewnowski A. Does social class predict diet quality? The American journal of clinical nutrition. 2008;87(5):1107. doi: 10.1093/ajcn/87.5.1107. Retrieved from http://www.ajcn.org/content/87/5/1107.short. [DOI] [PubMed] [Google Scholar]
- Davison KK, Cutting TM, Birch LL. Parents’ activity-related parenting practices predict girls’ physical activity. Medicine & Science in Sports & Exercise. 2003;35(9):1589–1595. doi: 10.1249/01.MSS.0000084524.19408.0C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva MJ, McKenzie K, Harpham T, Huttly SRA. Social capital and mental illness: a systematic review. Journal of Epidemiology and Community Health. 2005;59:619–627. doi: 10.1136/jech.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries SI, Bakker I, van Mechelen W, Hopman-Rock M. Determinants of activity-friendly neighborhoods for children: results from the SPACE study. American Journal of Health Promotion. 2007;21(4 Suppl):312–316. doi: 10.4278/0890-1171-21.4s.312. [DOI] [PubMed] [Google Scholar]
- DeCarlo Santiago C, Wadsworth ME. Coping with family conflict: What’s helpful and what’s not for low-income adolescents. Journal of Child and Family Studies. 2009;18(2):192–202. doi: 10.1007/s10826-008-9219-9. [DOI] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47 (3):864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Delva J, Johnston LD, O’Malley PM. The epidemiology of overweight and related lifestyle behaviors: racial/ethnic and socioeconomic status differences among American youth. American Journal of Preventive Medicine. 2007;33(4 Suppl):S178–86. doi: 10.1016/j.amepre.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Delva J, O’Malley PM, Johnston LD. Racial/ethnic and socioeconomic status differences in overweight and health-related behaviors among American students: national trends 1986–2003. Journal of Adolescent Health. 2006;39(4):536–545. doi: 10.1016/j.jadohealth.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dey AN, Bloom B. Summary health statistics for US children: National Health Interview Survey, 2003. Vital and health statistics. Series 10, Data from the National Health Survey. 2005;(223):1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15792295. [PubMed]
- Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, et al. Home indoor pollutant exposures among inner-city children with and without asthma. Environmental Health Perspectives. 2007;115(11):1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV. Investigating neighborhood and area effects on health. American Journal of Public Health. 2001;91(11):1783–1789. doi: 10.2105/AJPH.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186(1):125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250(4988):1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Dowda M, Ainsworth BE, Addy CL, Saunders R, Riner W. Environmental influences, physical activity, and weight status in 8-to 16-year-olds. Archives of Pediatrics and Adolescent Medicine. 2001;155(6):711. doi: 10.1001/archpedi.155.6.711. Retrieved from http://archpedi.ama-assn.org/cgi/content/abstract/155/6/711. [DOI] [PubMed] [Google Scholar]
- Downey G, Coyne JC. Children of depressed parents: an integrative review. Psychological Bulletin. 1990;108(1):50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- Duke NN, Borowsky IW, Pettingell SL. Parent Perceptions of Neighborhood: Relationships with US Youth Physical Activity and Weight Status. Maternal and Child Health Journal. 2010:1–9. doi: 10.1007/s10995-010-0731-3. Retrieved from http://www.springerlink.com/index/TTP4019M01X578X7.pdf. [DOI] [PubMed]
- Duncan D, Johnson R, Molnar B, Azrael D. Association between neighborhood safety and overweight status among urban adolescents. BMC Public Health. 2009;9(1):289. doi: 10.1186/1471-2458-9-289. Retrieved from http://www.biomedcentral.com/1471-2458/9/289/PREPUB/ABSTRACT/abstract/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupere V, Perkins DD. Community types and mental health: a multilevel study of local environmental stress and coping. American journal of community psychology. 2007;39(1):107–119. doi: 10.1007/s10464-007-9099-y. [DOI] [PubMed] [Google Scholar]
- Edwards J, Walters S, Griffiths RK. Hospital admissions for asthma in preschool children: relationship to major roads in Birmingham, United Kingdom. Archives of Environmental Health. 1994;49(4):223–227. doi: 10.1080/00039896.1994.9937471. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Harger J, Whitson SM. Exposure to interparental conflict and children’s adjustment and physical health: the moderating role of vagal tone. Child Development. 2001;72(6):1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- Elgar FJ, Curtis LJ, McGrath PJ, Waschbusch DA, Stewart SH. Antecedent-consequence conditions in maternal mood and child adjustment: A four-year cross-lagged study. Journal of Clinical Child & Adolescent Psychology. 2003;32(3):362–374. doi: 10.1207/S15374424JCCP3203_05. [DOI] [PubMed] [Google Scholar]
- Elgar FJ, McGrath PJ, Waschbusch DA, Stewart SH, Curtis LJ. Mutual influences on maternal depression and child adjustment problems. Clinical Psychology Review. 2004;24(4):441–459. doi: 10.1016/j.cpr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Emery RE, Laumann-Billings L. An overview of the nature, causes, and consequences of abusive family relationships. Toward differentiating maltreatment and violence. American Psychologist. 1998;53(2):121–135. doi: 10.1037/0003-066X.53.2.121. [DOI] [PubMed] [Google Scholar]
- Entwisle DR, Alexander KL, Olson LS. Early work histories of urban youth. American Sociological Review. 2000:279–297. Retrieved from http://www.jstor.org/stable/10.2307/2657441.
- Entwisle DR, Alexander KL, Olson LS, Ross K. Paid Work in Early Adolescence. The Journal of Early Adolescence. 1999;19(3):363–388. Retrieved from http://jea.sagepub.com/content/19/3/363.short. [Google Scholar]
- Epstein LH, Dearing KK, Handley EA, Roemmich JN, Paluch RA. Relationship of mother and child food purchases as a function of price: a pilot study. Appetite. 2006;47(1):115–118. doi: 10.1016/j.appet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ernst P, Demissie K, Joseph L, Locher U, Becklake MR. Socioeconomic status and indicators of asthma in children. American journal of respiratory and critical care medicine. 1995;152(2):570. doi: 10.1164/ajrccm.152.2.7633709. Retrieved from http://ajrccm.atsjournals.org/cgi/content/abstract/152/2/570. [DOI] [PubMed] [Google Scholar]
- Estabrooks PA, Lee RE, Gyurcsik NC. Resources for physical activity participation: does availability and accessibility differ by neighborhood socioeconomic status? Annals of Behavioral Medicine. 2003;25(2):100–104. doi: 10.1207/S15324796ABM2502_05. [DOI] [PubMed] [Google Scholar]
- Evans GW, Lepore SJ, Shejwal BR, Palsane MN. Chronic residential crowding and children’s well-being: an ecological perspective. Child Development. 1998;69(6):1514–1523. [PubMed] [Google Scholar]
- Evans GW, Lercher P, Meis M, Ising H, Kofler WW. Community noise exposure and stress in children. Journal of the Acoustical Society of America. 2001;109(3):1023–1027. doi: 10.1121/1.1340642. [DOI] [PubMed] [Google Scholar]
- Evans GW, Wells NM, Chan HY, Saltzman H. Housing quality and mental health. Journal of Consulting & Clinical Psychology. 2000;68(3):526–530. doi: 10.1037/0022-006X.68.3.526. [DOI] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Marcynyszyn LA, Gentile L, Salpekar N. The role of chaos in poverty and children’s socioemotional adjustment. Psychological Science. 2005;16(7):560. doi: 10.1111/j.0956-7976.2005.01575.x. Retrieved from http://pss.sagepub.com/content/16/7/560.short. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annual Review of Public Health. 2002;23(1):303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. Retrieved from http://www.annualreviews.org/doi/abs/10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health. Psychological Science. 2007;18(11):953. doi: 10.1111/j.1467-9280.2007.02008.x. Retrieved from http://pss.sagepub.com/content/18/11/953.short. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status–health gradient. Annals of the New York Academy of Sciences. 2010;1186(1):174–189. doi: 10.1111/j.1749-6632.2009.05336.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2009.05336.x/full. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Bullinger M, Hygge S. Chronic noise exposure and physiological response: A prospective study of children living under environmental stress. Psychological Science. 1998;9(1):75–77. doi: 10.1111/1467-9280.00014. [DOI] [Google Scholar]
- Evans GW, Hygge S, Bullinger M. Chronic noise and psychological stress. Psychological Science. 1995;6(6):333–338. doi: 10.1111/j.1467-9280.1995.tb00522.x. [DOI] [Google Scholar]
- Evans GW, Lercher P, Kofler WW. Crowding and children’s mental health: The role of house type. Journal of Environmental Psychology. 2002;22(3):221–231. doi: 10.1006/jevp.2002.0256. [DOI] [Google Scholar]
- Evans GW, Marcynyszyn LA. Environmental justice, cumulative environmental risk, and health among low- and middle-income children in upstate New York. American Journal of Public Health. 2004;94(11):1942–1944. doi: 10.2105/AJPH.94.11.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, Scott MM, Cohen DA, Voorhees CC. Girls’ perception of neighborhood factors on physical activity, sedentary behavior, and BMI. Obesity. 2007;15(2):430–445. doi: 10.1038/oby.2007.502. [DOI] [PubMed] [Google Scholar]
- Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. Journal of Psychosomatic Research. 2002;53(4):891–895. doi: 10.1016/S0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Fauth RC, Leventhal T, Brooks-Gunn J. Welcome to the neighborhood? Long-term impacts of moving to low-poverty neighborhoods on poor children’s and adolescents’ outcomes. Journal of Research on Adolescence. 2007;17(2):249–284. doi: 10.1111/j.1532-7795.2007.00522.x. [DOI] [Google Scholar]
- Feinglass J, Lin S, Thompson J, Sudano J, Dunlop D, Song J, et al. Baseline health, socioeconomic status, and 10-year mortality among older middle-aged Americans: findings from the Health and Retirement Study, 1992–2002. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 2007;62(4):S209–17. doi: 10.1093/geronb/62.4.s209. [DOI] [PubMed] [Google Scholar]
- Feunekes GI, Stafleu A, de Graaf C, van Staveren WA. Family resemblance in fat intake in The Netherlands. European Journal of Clinical Nutrition. 1997;51(12):793–799. doi: 10.1038/sj.ejcn.1600494. [DOI] [PubMed] [Google Scholar]
- Fiese BH, Schwartz M. Reclaiming the family table: Mealtimes and child health and wellbeing. Social Policy Report. 2008;22(4):1–18. Retrieved from http://www.yaleruddcenter.org/resources/upload/docs/what/reports/reclaimingfamilytable.pdf. [Google Scholar]
- Finkelhor D, Dziuba-Leatherman J. Children as victims of violence: a national survey. Pediatrics. 1994;94(4 Pt 1):413–420. [PubMed] [Google Scholar]
- Fite PJ, Colder CR, Lochman JE, Wells KC. The mutual influence of parenting and boys’ externalizing behavior problems. Journal of Applied Developmental Psychology. 2006;27(2):151–164. doi: 10.1016/j.appdev.2005.12.011. [DOI] [Google Scholar]
- Fitzpatrick KM, Boldizar JP. The prevalence and consequences of exposure to violence among African-American youth. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32(2):424–430. doi: 10.1097/00004583-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick KM, Wright DR, Piko BF, LaGory M. Depressive symptomatology, exposure to violence, and the role of social capital among African American adolescents. American Journal of Orthopsychiatry. 2005;75(2):262–274. doi: 10.1037/0002-9432.75.2.262. [DOI] [PubMed] [Google Scholar]
- Ford PB, Dzewaltowski DA. Disparities in obesity prevalence due to variation in the retail food environment: three testable hypotheses. Nutrition reviews. 2008;66(4):216–228. doi: 10.1111/j.1753-4887.2008.00026.x. [DOI] [PubMed] [Google Scholar]
- Forero R, Bauman A, Young L, Booth M, Nutbeam D. Asthma, health behaviors, social adjustment, and psychosomatic symptoms in adolescence. Journal of Asthma. 1996;33(3):157–164. doi: 10.3109/02770909609054547. [DOI] [PubMed] [Google Scholar]
- Franzini L, Elliott MN, Cuccaro P, Schuster M, Gilliland MJ, Grunbaum JA, et al. Influences of physical and social neighborhood environments on children Äôs physical activity and obesity. American Journal of Public Health. 2009;99(2):271–278. doi: 10.2105/AJPH.2007.128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annual Review of Public Health. 2001;22:309–335. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- Furstenberg FF, Belzer A, Davis C, Levine JA, Morrow K, Washington M. How families manage risk and opportunity in dangerous neighborhoods. Sociology and the public agenda; 1993. pp. 231–258. [Google Scholar]
- Gable S, Chang Y, Krull JL. Television watching and frequency of family meals are predictive of overweight onset and persistence in a national sample of school-aged children. Journal of the American Dietetic Association. 2007;107(1):53–61. doi: 10.1016/j.jada.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Gable S, Lutz S. Household, parent, and child contributions to childhood obesity. Family Relations. 2000;49(3):293–300. doi: 10.1111/j.1741-3729.2000.00293.x. [DOI] [Google Scholar]
- Galambos NL, Sears HA, Almeida DM, Kolaric GC. Parents’ work overload and problem behavior in young adolescents. Journal of Research on Adolescence. 1995;5(2):201–223. Retrieved from http://www.tandfonline.com/doi/abs/10.1207/s15327795jra0502_3. [Google Scholar]
- Gallo LC, Matthews KA. Do negative emotions mediate the association between socioeconomic status and health? Annals of the New York Academy of Sciences. 1999;896(1):226–245. doi: 10.1111/j.1749-6632.1999.tb08118.x. [DOI] [PubMed] [Google Scholar]
- Garasky S, Stewart SD, Gundersen C, Lohman BJ, Eisenmann JC. Family stressors and child obesity. Social Science Research. 2009;38(4):755–766. doi: 10.1016/j.ssresearch.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369(9561):571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environmental Health Perspectives. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EL, Wardle J, Watts CJ. Fruit and vegetable consumption, nutritional knowledge and beliefs in mothers and children. Appetite. 1998;31(2):205–228. doi: 10.1006/appe.1998.0180. [DOI] [PubMed] [Google Scholar]
- Gillman MW, Rifas-Shiman SL, Frazier AL, Rockett HR, Camargo CA, Jr, Field AE, et al. Family dinner and diet quality among older children and adolescents. Archives of Family Medicine. 2000;9(3):235–240. doi: 10.1001/archfami.9.3.235. [DOI] [PubMed] [Google Scholar]
- Giskes K, Turrell G, Patterson C, Newman B. Socio-economic differences in fruit and vegetable consumption among Australian adolescents and adults. Public Health Nutrition. 2002;5(5):663–669. doi: 10.1079/PHN2002339. [DOI] [PubMed] [Google Scholar]
- Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110(3):497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- Goossens L, Braet C, Van Vlierberghe L, Mels S. Loss of control over eating in overweight youngsters: The role of anxiety, depression and emotional eating. European Eating Disorders Review. 2009;17(1):68–78. doi: 10.1002/erv.892. [DOI] [PubMed] [Google Scholar]
- Gordian ME, Haneuse S, Wakefield J. An investigation of the association between traffic exposure and the diagnosis of asthma in children. Journal of Exposure Science & Environmental Epidemiology. 2006;16(1):49–55. doi: 10.1038/sj.jea.7500436. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, McMurray RG, Popkin BM. Determinants of adolescent physical activity and inactivity patterns. Pediatrics. 2000;105(6):E83. doi: 10.1542/peds.105.6.e83. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117(2):417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- Gortmaker SL, Must A, Sobol AM, Peterson K, Colditz GA, Dietz WH. Television viewing as a cause of increasing obesity among children in the United States, 1986–1990. Archives of Pediatrics & Adolescent Medicine. 1996;150(4):356–362. doi: 10.1001/archpedi.1996.02170290022003. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: moving from markers to mechanisms of risk. Psychological Bulletin. 2003;129(3):447–466. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Greenberger E, O’Neil R, Nagel SK. Linking workplace and homeplace: Relations between the nature of adults’ work and their parenting behaviors. Developmental Psychology. 1994;30(6):990. Retrieved from http://psycnet.apa.org/journals/dev/30/6/990/ [Google Scholar]
- Greenberger E, Steinberg L. When teenagers work: The psychological and social costs of adolescent employment. Basic Books. 1986 Retrieved from http://psycnet.apa.org/psycinfo/1986-98356-000.
- Grineski S, Bolin B, Boone C. Criteria air pollution and marginalized populations: Environmental inequity in metropolitan Phoenix, Arizona. Social Science Quarterly. 2007;88(2):535–554. doi: 10.1111/j.1540-6237.2007.00470.x. [DOI] [Google Scholar]
- Gross HE, Shaw DS, Moilanen KL. Reciprocal associations between boys’ externalizing problems and mothers’ depressive symptoms. Journal of Abnormal Child Psychology: An official publication of the International Society for Research in Child and Adolescent Psychopathology. 2008;36(5):693–709. doi: 10.1007/s10802-008-9224-x. Retrieved from 10.1007/s10802-008-9224-x http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2008-07245-006&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross HE, Shaw DS, Moilanen KL, Dishion TJ, Wilson MN. Reciprocal models of child behavior and depressive symptoms in mothers and fathers in a sample of children at risk for early conduct problems. Journal of Family Psychology. 2008;22(5):742–751. doi: 10.1037/a0013514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grych JH, Fincham FD, Jouriles EN, McDonald R. Interparental conflict and child adjustment: Testing the mediational role of appraisals in the cognitive-contextual framework. Child Development. 2000;71(6):1648–1661. doi: 10.1111/1467-8624.00255. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Zhang X, Sharp LK, Shannon JJ, Weiss KB. The protective effect of community factors on childhood asthma. Journal of Allergy & Clinical Immunology. 2009;123(6):1297–304.e2. doi: 10.1016/j.jaci.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman LM, McLoyd VC, Tokoyawa T. Financial strain, neighborhood stress, parenting behaviors, and adolescent adjustment in urban African American families. Journal of Research on Adolescence. 2005;15(4):425–449. doi: 10.1111/j.1532-7795.2005.00106.x. [DOI] [Google Scholar]
- Haire-Joshu D, Nanney MS. Prevention of overweight and obesity in children: influences on the food environment. Diabetes Educator. 2002;28(3):415–423. doi: 10.1177/014572170202800311. [DOI] [PubMed] [Google Scholar]
- Hamer M, Stamatakis E. Inflammation as an intermediate pathway in the association between psychosocial stress and obesity. Physiology & behavior. 2008;94(4):536–539. doi: 10.1016/j.physbeh.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Hammack PL, Robinson WL, Crawford I, Li ST. Poverty and depressed mood among urban African-American adolescents: A family stress perspective. Journal of Child and Family Studies. 2004;13(3):309–323. doi: 10.1023/B:JCFS.0000022037.59369.90. [DOI] [Google Scholar]
- Hancox RJ, Milne BJ, Poulton R. Association of television viewing during childhood with poor educational achievement. Archives of pediatrics and adolescent medicine. 2005;159(7):614. doi: 10.1001/archpedi.159.7.614. Retrieved from http://archpedi.ama-assn.org/cgi/content/abstract/159/7/614. [DOI] [PubMed] [Google Scholar]
- Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DAL, et al. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environmental Health Perspectives. 2008;116(10):1428–1432. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanushek EA, Kain JF, Rivkin SG. Why public schools lose teachers. Journal of Human Resources. 2004;39(2):326–354. Retrieved from http://jhr.uwpress.org/content/XXXIX/2/326.short. [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype by environment interaction in adolescents’ cognitive aptitude. Behavior Genetics. 2007;37(2):273–283. doi: 10.1007/s10519-006-9113-4. Retrieved from http://www.springerlink.com/index/u840p0h638726207.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell JS, Bangdiwala SI, Deng S, Webb JP, Bradley C. Smoking initiation in youth: the roles of gender, race, socioeconomics, and developmental status. Journal of Adolescent Health. 1998;23(5):271–279. doi: 10.1016/S1054-139X(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Hearn MD, Baranowski T, Baranowski J, Doyle C, Smith M, Lin LS, et al. Environmental influences on dietary behavior among children: Availability and accessibility of fruits and vegetables enable consumption. Journal of Health Education. 1998;29(1):26–32. Retrieved from http://eric.ed.gov/ERICWebPortal/recordDetail?accno=EJ561955. [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Hirschman C, Voloshin I. The structure of teenage employment: Social background and the jobs held by high school seniors. Research in social stratification and mobility. 2007;25(3):189–203. doi: 10.1016/j.rssm.2007.07.001. Retrieved from http://www.sciencedirect.com/science/article/pii/S0276562407000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerr SL, Hughes SO, Fisher JO, Nicklas TA, Liu Y, Shewchuk RM. Associations among parental feeding styles and children’s food intake in families with limited incomes. The International Journal of Behavioral Nutrition and Physical Activity. 2009:6. doi: 10.1186/1479-5868-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Houston D, Wu J, Ong P, Winer A. Structural Disparities of Urban Traffic in Southern California: Implications for Vehicle-Related Air Pollution Exposure in Minority and High-Poverty Neighborhoods. Journal of Urban Affairs. 2004;26(5):565–592. doi: 10.1111/j.0735-2166.2004.00215.x. [DOI] [Google Scholar]
- Inagami S, Cohen DA, Finch BK, Asch SM. You are where you shop: grocery store locations, weight, and neighborhoods. American Journal of Preventive Medicine. 2006;31 (1):10–17. doi: 10.1016/j.amepre.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Ising H, Ising M. Chronic cortisol increases in the first half of the night caused by road traffic noise. Noise and Health. 2002;4(16):13–21. Retrieved from http://www.ingentaconnect.com/content/nrn/nh/2002/00000004/00000016/art00002. [PubMed] [Google Scholar]
- Ising H, Lange-Asschenfeldt H, Moriske HJ, Born J, Eilts M. Low frequency noise and stress: bronchitis and cortisol in children exposed chronically to traffic noise and exhaust fumes. Noise & Health. 2004;6(23):21–28. [PubMed] [Google Scholar]
- Janssen I, Boyce WF, Simpson K, Pickett W. Influence of individual- and area-level measures of socioeconomic status on obesity, unhealthy eating, and physical inactivity in Canadian adolescents. American Journal of Clinical Nutrition. 2006;83(1):139–145. doi: 10.1093/ajcn/83.1.139. [DOI] [PubMed] [Google Scholar]
- Jenkins SK, Rew L, Sternglanz RW. Eating behaviors among school-age children associated with perceptions of stress. Issues in Comprehensive Pediatric Nursing. 2005;28 (3):175–191. doi: 10.1080/01460860500227580. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Birch LL. Parents’ and children’s adiposity and eating style. Pediatrics. 1994;94(5):653–661. [PubMed] [Google Scholar]
- Johnson W, Krueger RF. Genetic effects on physical health: Lower at higher income levels. Behavior genetics. 2005;35(5):579–590. doi: 10.1007/s10519-005-3598-0. Retrieved from http://www.springerlink.com/index/R087V61173114221.pdf. [DOI] [PubMed] [Google Scholar]
- Johnson W, Kyvik KO, Mortensen EL, Skytthe A, Batty GD, Deary IJ. Education reduces the effects of genetic susceptibilities to poor physical health. International journal of epidemiology. 2010;39(2):406. doi: 10.1093/ije/dyp314. Retrieved from http://ije.oxfordjournals.org/content/39/2/406.short. [DOI] [PubMed] [Google Scholar]
- Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clinical Child and Family Psychology Review. 2001;4(3):183–207. doi: 10.1023/A:1017592030434. [DOI] [PubMed] [Google Scholar]
- Juhn YJ, Sauver JS, Katusic S, Vargas D, Weaver A, Yunginger J. The influence of neighborhood environment on the incidence of childhood asthma: a multilevel approach. Social Science & Medicine. 2005;60(11):2453–2464. doi: 10.1016/j.socscimed.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Kang DH, Coe CL, McCarthy DO. Academic examinations significantly impact immune responses, but not lung function, in healthy and well-managed asthmatic adolescents. Brain, Behavior, and Immunity. 1996;10(2):164–181. doi: 10.1006/brbi.1996.0015. [DOI] [PubMed] [Google Scholar]
- Kang DH, Coe CL, McCarthy DO, Ershler WB. Immune responses to final exams in healthy and asthmatic adolescents. Nursing research. 1997;46(1):12. doi: 10.1097/00006199-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 Pt 1):1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Kennedy BP, Lochner K, Prothrow-Stith D. Social capital, income inequality, and mortality. American Journal of Public Health. 1997;87(9):1491–1498. doi: 10.2105/AJPH.87.9.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: An update. Archives of General Psychiatry. 2001;58(11):1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Psychoneuroimmunology: Psychological influences on immune function and health. Journal of Consulting and Clinical Psychology. 2002;70(3):537. doi: 10.1037/0022-006X.70.3.537. [DOI] [PubMed] [Google Scholar]
- Kim J. Neighborhood disadvantage and mental health: The role of neighborhood disorder and social relationships. Social Science Research. 2010 Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0049089X09000878.
- Kim J, Ross CE. Neighborhood-specific and general social support: which buffers the effect of neighborhood disorder on depression? Journal of Community Psychology. 2009;37 (6):725–736. doi: 10.1002/jcop.20327. [DOI] [Google Scholar]
- Kimbro RT, Brooks-Gunn J, McLanahan S. Young Children in Urban Areas: Links Among Neighborhood Characteristics, Weight Status, Outdoor Play, and Television- Watching. Social Science & Medicine. 2011;72(5):668–676. doi: 10.1016/j.socscimed.2010.12.015. Retrieved from http://www.sciencedirect.com/science/article/pii/S0277953611000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanov PK, Brooks-Gunn J, Duncan GJ. Does neighborhood and family poverty affect mothers’ parenting, mental health, and social support? Journal of Marriage & the Family. 1994;56(2):441–455. doi: 10.2307/353111. [DOI] [Google Scholar]
- Klinnert MD, Kaugars AS, Strand M, Silveira L. Family psychological factors in relation to children’s asthma status and behavioral adjustment at age 4. Family Process. 2008;47 (1):41–61. doi: 10.1111/j.1545-5300.2008.00238.x. [DOI] [PubMed] [Google Scholar]
- Koch F-S, Sepa A, Ludvigsson J. Psychological stress and obesity. The Journal of Pediatrics. 2008;153(6):839–844. doi: 10.1016/j.jpeds.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Kohen DE, Leventhal T, Dahinten VS, McIntosh CN. Neighborhood disadvantage: Pathways of effects for young children. Child Development. 2008;79(1):156–169. doi: 10.1111/j.1467-8624.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- Kumar R, Curtis LM, Khiani S, Moy J, Shalowitz MU, Sharp L, et al. A community-based study of tobacco smoke exposure among inner-city children with asthma in Chicago. Journal of Allergy & Clinical Immunology. 2008;122(4):754–759.e1. doi: 10.1016/j.jaci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Current Opinion in Pharmacology. 2009;9(6):787–793. doi: 10.1016/j.coph.2009.08.007. Retrieved from http://www.sciencedirect.com/science/article/pii/S1471489209001313. [DOI] [PubMed] [Google Scholar]
- Lake RLD, Huckfeldt R. Social capital, social networks, and political participation. Political Psychology. 1998;19(3):567–584. doi: 10.1111/0162-895X.00118. [DOI] [Google Scholar]
- Lamerz A, Kuepper-Nybelen J, Wehle C, Bruning N, Trost-Brinkhues G, Brenner H, et al. Social class, parental education, and obesity prevalence in a study of six-year-old children in Germany. International Journal of Obesity. 2005;29(4):373–380. doi: 10.1038/sj.ijo.0802914. [DOI] [PubMed] [Google Scholar]
- Lankford H, Loeb S, Wyckoff J. Teacher sorting and the plight of urban schools: A descriptive analysis. Educational Evaluation and Policy Analysis. 2002;24(1):37–62. Retrieved from http://epa.sagepub.com/content/24/1/37.short. [Google Scholar]
- Lanphear BP, Kahn RS, Berger O, Auinger P, Bortnick SM, Nahhas RW. Contribution of residential exposures to asthma in us children and adolescents. Pediatrics. 2001;107(6):E98. doi: 10.1542/peds.107.6.e98. [DOI] [PubMed] [Google Scholar]
- Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: results from the Americans’ Changing Lives Study. Journal of Health and Social Behavior. 2005;46(3):274. doi: 10.1177/002214650504600305. Retrieved from http://hsb.sagepub.com/content/46/3/274.short. [DOI] [PubMed] [Google Scholar]
- Larson NI, Story MT, Nelson MC. Neighborhood Environments:: Disparities in Access to Healthy Foods in the US. American Journal of Preventive Medicine. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Larson NI, Neumark-Sztainer D, Hannan PJ, Story M. Family meals during adolescence are associated with higher diet quality and healthful meal patterns during young adulthood. Journal of the American Dietetic Association. 2007;107(9):1502–1510. doi: 10.1016/j.jada.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Law JHJ, Barber BK. Neighborhood conditions, parenting, and adolescent functioning. Journal of Human Behavior in the Social Environment. 2006;14(4):91–118. doi: 10.1300/J137v14n04_05. [DOI] [Google Scholar]
- Lee J-T, Son J-Y, Kim H, Kim S-Y. Effect of air pollution on asthma-related hospital admissions for children by socioeconomic status associated with area of residence. Archives of Environmental & Occupational Health. 2006;61(3):123–130. doi: 10.3200/AEOH.61.3.123-130. [DOI] [PubMed] [Google Scholar]
- Lehrer PM. Emotionally triggered asthma: a review of research literature and some hypotheses for self-regulation therapies. Applied Psychophysiology & Biofeedback. 1998;23(1):13–41. doi: 10.1023/A:1022170028979. [DOI] [PubMed] [Google Scholar]
- Lehrer P, Feldman J, Giardino N, Song H-S, Schmaling K. Psychological aspects of asthma. Journal of Consulting & Clinical Psychology. 2002;70(3):691–711. doi: 10.1037/0022-006X.70.3.691. [DOI] [PubMed] [Google Scholar]
- Levy JI, Brugge D, Peters JL, Clougherty JE, Saddler SS. A community-based participatory research study of multifaceted in-home environmental interventions for pediatric asthmatics in public housing. Social Science & Medicine. 2006;63(8):2191–2203. doi: 10.1016/j.socscimed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Levy JI, Welker-Hood LK, Clougherty JE, Dodson RE, Steinbach S, Hynes HP. Lung function, asthma symptoms, and quality of life for children in public housing in Boston: a case-series analysis. Environmental Health: A Global Access Science Source. 2004;3(1):13. doi: 10.1186/1476-069X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden KM. Social capital and the built environment: the importance of walkable neighborhoods. American Journal of Public Health. 2003;93(9):1546–1551. doi: 10.2105/AJPH.93.9.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Villeneuve PJ, Burnett RT, Lemyre L, Hertzman C, et al. Gaseous air pollutants and asthma hospitalization of children with low household income in Vancouver, British Columbia, Canada. American Journal of Epidemiology. 2004;159(3):294–303. doi: 10.1093/aje/kwh043. [DOI] [PubMed] [Google Scholar]
- Lin S, Munsie JP, Hwang S-A, Fitzgerald E, Cayo MR. Childhood asthma hospitalization and residential exposure to state route traffic. Environmental Research. 2002;88 (2):73–81. doi: 10.1006/enrs.2001.4303. [DOI] [PubMed] [Google Scholar]
- Lindsey MA, Browne DC, Thompson R, Hawley KM, Graham JC, Weisbart C, et al. Caregiver mental health, neighborhood, and social network influences on mental health needs among African American children. Social Work Research. 2008;32(2):79–88. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2009-05569-003&site=ehost-live. [Google Scholar]
- Lioret S, Maire B, Volatier JL, Charles MA. Child overweight in France and its relationship with physical activity, sedentary behaviour and socioeconomic status. European Journal of Clinical Nutrition. 2007;61(4):509–516. doi: 10.1038/sj.ejcn.1602538. [DOI] [PubMed] [Google Scholar]
- Liu GC, Wilson JS, Qi R, Ying J. Green neighborhoods, food retail and childhood overweight: differences by population density. American Journal of Health Promotion. 2007;21(4S):317–325. doi: 10.4278/0890-1171-21.4s.317. Retrieved from http://www.goforyourlife.vic.gov.au/hav/admin.nsf/Images/Green_Neighborhoods.pdf/$File/Green_Neighborhoods.pdf. [DOI] [PubMed] [Google Scholar]
- Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. American Journal of Respiratory & Critical Care Medicine. 2002;165(8):1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- Lobstein T, Baur L, Uauy R, TaskForce IIO. Obesity in children and young people: a crisis in public health. Obesity Reviews. 2004;5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- Lochner K, Kawachi I, Kennedy BP. Social capital: a guide to its measurement. Health & Place. 1999;5(4):259–270. doi: 10.1016/S1353-8292(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Lochner KA, Kawachi I, Brennan RT, Buka SL. Social capital and neighborhood mortality rates in Chicago. Social Science & Medicine. 2003;56(8):1797–1805. doi: 10.1016/s0277-9536(02)00177-6. Retrieved from http://www.sciencedirect.com/science/article/pii/s0277953602001776. [DOI] [PubMed] [Google Scholar]
- Lohman BJ, Stewart S, Gundersen C, Garasky S, Eisenmann JC. Adolescent overweight and obesity: Links to food insecurity and individual, maternal, and family stressors. Journal of Adolescent Health. 2009;45(3):230–237. doi: 10.1016/j.jadohealth.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Science & Medicine. 1997;44(6):809–819. doi: 10.1016/S0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- Lynch M, Cicchetti D. Links between community violence and the family system: Evidence from children’s feelings of relatedness and perceptions of parent behavior. Family Process. 2002;41(3):519–532. doi: 10.1111/j.1545-5300.2002.41314.x. [DOI] [PubMed] [Google Scholar]
- Maffeis C. Aetiology of overweight and obesity in children and adolescents. European journal of pediatrics. 2000;159(13):35–44. doi: 10.1007/PL00014361. [DOI] [PubMed] [Google Scholar]
- Mamun AA, Lawlor DA, O’Callaghan MJ, Williams GM, Najman JM. Positive maternal attitude to the family eating together decreases the risk of adolescent overweight. Obesity Research. 2005;13(8):1422–1430. doi: 10.1038/oby.2005.172. [DOI] [PubMed] [Google Scholar]
- Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosomatic Medicine. 2009;71(4):378–384. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG, Shipley MJ, Rose G. Inequalities in death--specific explanations of a general pattern? The Lancet. 1984;323(8384):1003–1006. doi: 10.1016/s0140-6736(84)92337-7. Retrieved from http://www.sciencedirect.com/science/article/pii/S0140673684923377. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Stansfeld S, Patel C, North F, Head J, White I, et al. Health inequalities among British civil servants: the Whitehall II study. The Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-K. [DOI] [PubMed] [Google Scholar]
- Martin CA, Warren PS, Kinzig AP. Neighborhood socioeconomic status is a useful predictor of perennial landscape vegetation in residential neighborhoods and embedded small parks of Phoenix, AZ. Landscape and Urban Planning. 2004;69(4):355–368. Retrieved from http://www.sciencedirect.com/science/article/pii/S0169204603002512. [Google Scholar]
- Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics. 1992;89(1):21–26. [PubMed] [Google Scholar]
- Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Curtin-Brosnan J, et al. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. Journal of Allergy & Clinical Immunology. 2003;112(1):87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- Maxwell LE. Multiple effects of home and day care crowding. Environment and Behavior. 1996;28(4):494–511. Retrieved from http://eab.sagepub.com/content/28/4/494.short. [Google Scholar]
- Maxwell LE. Home and school density effects on elementary school children. Environment and behavior. 2003;35(4):566–578. Retrieved from http://eab.sagepub.com/content/35/4/566.short. [Google Scholar]
- Maziak W, von Mutius E, Keil U, Hirsch T, Leupold W, Rzehak P, et al. Predictors of health care utilization of children with asthma in the community. Pediatric Allergy & Immunology. 2004;15(2):166–171. doi: 10.1046/j.1399-3038.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. Childhood Incident Asthma and Traffic-Related Air Pollution at Home and School. Environmental health perspectives. 2010;118(7):1021. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359(9304):386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams DA, Curtin-Brosnan J, et al. In-home particle concentrations and childhood asthma morbidity. Environmental Health Perspectives. 2009;117(2):294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037/0003-066X.53.2.185. [DOI] [PubMed] [Google Scholar]
- McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Social Science & Medicine. 2006;63(4):1011–1022. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. Journal of Pediatric Psychology. 2003;28 (5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- Mechanic D, Hansell S. Divorce, family conflict, and adolescents’ well-being. Journal of Health & Social Behavior. 1989;30(1):105–116. doi: 10.2307/2136916. [DOI] [PubMed] [Google Scholar]
- Mei Z, Scanlon KS, Grummer-Strawn LM, Freedman DS, Yip R, Trowbridge FL. Increasing prevalence of overweight among US low-income preschool children: the Centers for Disease Control and Prevention pediatric nutrition surveillance, 1983 to 1995. Pediatrics. 1998;101(1):E12. doi: 10.1542/peds.101.1.e12. [DOI] [PubMed] [Google Scholar]
- Meijer AM, Griffioen RW, van Nierop JC, Oppenheimer L. Intractable or uncontrolled asthma: Psychosocial factors. Journal of Asthma. 1995;32(4):265–274. doi: 10.3109/02770909509044834. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Brackis-Cott E, Dolezal C, Leu CS, Valentin C, Meyer-Bahlburg HFL. Mental health of early adolescents from high-risk neighborhoods: The role of maternal HIV and other contextual, self-regulation, and family factors. Journal of Pediatric Psychology. 2008;33(10):1065–1075. doi: 10.1093/jpepsy/jsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H, Vostanis P, Goodman R, Ford T. Children’s perceptions of neighbourhood trustworthiness and safety and their mental health. Journal of Child Psychology and Psychiatry. 2007;48(12):1208–1213. doi: 10.1111/j.1469-7610.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- Mennis J. Socioeconomic-vegetation relationships in urban, residential land: the case of Denver, Colorado. Photogrammetric engineering and remote sensing. 2006;72(8):933. Retrieved from http://www.asprs.org/a/publications/pers/2006journal/august/2006_aug_911-921.pdf. [Google Scholar]
- Merom D, Tudor-Locke C, Bauman A, Rissel C. Active commuting to school among NSW primary school children: implications for public health. Health & Place. 2006;12 (4):678–687. doi: 10.1016/j.healthplace.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Miech RA, Caspi A, Moffitt TE, Wright BRE, Silva PA. Low socioeconomic status and mental disorders: A longitudinal study of selection and causation during young adulthood. American Journal of Sociology. 1999;104(4):1096–1131. doi: 10.1086/210137. [DOI] [Google Scholar]
- Miech RA, Kumanyika SK, Stettler N, Link BG, Phelan JC, Chang VW. Trends in the association of poverty with overweight among US adolescents, 1971–2004. JAMA. 2006;295(20):2385–2393. doi: 10.1001/jama.295.20.2385. [DOI] [PubMed] [Google Scholar]
- Mielck A, Reitmeir P, Wjst M. Severity of childhood asthma by socioeconomic status. International Journal of Epidemiology. 1996;25(2):388. doi: 10.1093/ije/25.2.388. [DOI] [PubMed] [Google Scholar]
- Mihalic S, Elliott D. Short- and long-term consequences of adolescent work. Youth & Society. 1997;28(4):464–498. [Google Scholar]
- Miller BD, Wood BL. Influence of specific emotional states on autonomic reactivity and pulmonary function in asthmatic children. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(5):669–677. doi: 10.1097/00004583-199705000-00018. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106(34):14716–14721. doi: 10.1073/pnas.0902971106. Retrieved from http://www.pnas.org/content/106/34/14716.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving towards a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaker LM, McCargar L, Lambraki I, Jessup L, Driezen P, Calengor K, et al. School region socio-economic status and geographic locale is associated with food behaviour of Ontario and Alberta adolescents. Canadian Journal of Public Health, Revue Canadienne de Sante Publique. 2006;97(5):357–361. doi: 10.1007/BF03405342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens E, Braet C, Bosnians G, Rosseel Y. Unfavourable family characteristics and their associations with childhood obesity: A cross-sectional study. European Eating Disorders Review. 2009;17(4):315–323. doi: 10.1002/erv.940. [DOI] [PubMed] [Google Scholar]
- Moens E, Braet C, Soetens B. Observation of family functioning at mealtime: a comparison between families of children with and without overweight. Journal of Pediatric Psychology. 2007;32(1):52–63. doi: 10.1093/jpepsy/jsl011. [DOI] [PubMed] [Google Scholar]
- Monahan KC, Lee JM, Steinberg L. Revisiting the Impact of Part-Time Work on Adolescent Adjustment: Distinguishing Between Selection and Socialization Using Propensity Score Matching. Child development. 2011;82(1):96–112. doi: 10.1111/j.1467-8624.2010.01543.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1467-8624.2010.01543.x/full. [DOI] [PubMed] [Google Scholar]
- Moore LL, Lombardi DA, White MJ, Campbell JL, Oliveria SA, Ellison RC. Influence of parents’ physical activity levels on activity levels of young children. Journal of Pediatrics. 1991;118(2):215–219. doi: 10.1016/S0022-3476(05)80485-8. [DOI] [PubMed] [Google Scholar]
- Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. American Journal of Public Health. 2006;96(2):325–331. doi: 10.2105/AJPH.2004.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environmental Health Perspectives. 2006;114(8):1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Hannan PJ, Story M, Croll J, Perry C. Family meal patterns: associations with sociodemographic characteristics and improved dietary intake among adolescents. Journal of the American Dietetic Association. 2003;103(3):317–322. doi: 10.1053/jada.2003.50048. [DOI] [PubMed] [Google Scholar]
- Nicolai T, Carr D, Weiland SK, Duhme H, von Ehrenstein O, Wagner C, et al. Urban traffic and pollutant exposure related to respiratory outcomes and atopy in a large sample of children. European Respiratory Journal. 2003;21(6):956–963. doi: 10.1183/09031936.03.00041103a. [DOI] [PubMed] [Google Scholar]
- O’Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, et al. Acute respiratory health effects of air pollution on children with asthma in US inner cities. Journal of Allergy & Clinical Immunology. 2008;121(5):1133–1139.e1. doi: 10.1016/j.jaci.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Oliver LN, Hayes MV. Neighbourhood socio-economic status and the prevalence of overweight Canadian children and youth. Canadian Journal of Public Health, Revue Canadienne de Sante Publique. 2005;96(6):415–420. doi: 10.1007/BF03405180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SA, Ellison RC, Moore LL, Gillman MW, Garrahie EJ, Singer MR. Parent-child relationships in nutrient intake: the Framingham Children’s Study. American Journal of Clinical Nutrition. 1992;56(3):593–598. doi: 10.1093/ajcn/56.3.593. [DOI] [PubMed] [Google Scholar]
- Olvera N, Power TG. Brief report: parenting styles and obesity in Mexican American children: a longitudinal study. Journal of pediatric psychology. 2010;35(3):243. doi: 10.1093/jpepsy/jsp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orpana HM, Lemyre L. Explaining the social gradient in health in Canada: Using the National Population Health Survey to examine the role of stressors. International journal of behavioral medicine. 2004;11(3):143–151. doi: 10.1207/s15327558ijbm1103_3. Retrieved from http://www.springerlink.com/index/M68X86T8313L5751.pdf. [DOI] [PubMed] [Google Scholar]
- Overstreet S, Braun S. Exposure to community violence and post-traumatic stress symptoms: mediating factors. American Journal of Orthopsychiatry. 2000;70(2):263–271. doi: 10.1037/h0087828. [DOI] [PubMed] [Google Scholar]
- Palmer T, Jaworski CA. Exercise prescription for underprivileged minorities. Current Sports Medicine Reports. 2004;3(6):344. doi: 10.1007/s11932-996-0010-7. Retrieved from http://journals.lww.com/acsm-csmr/Abstract/2004/12000/Exercise_Prescription_for_Underprivileged.10.aspx. [DOI] [PubMed] [Google Scholar]
- Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1999;23(Suppl 8):S1–107. [PubMed] [Google Scholar]
- Patrick H, Nicklas TA. A review of family and social determinants of children’s eating patterns and diet quality. Journal of the American College of Nutrition. 2005;24(2):83–92. doi: 10.1080/07315724.2005.10719448. [DOI] [PubMed] [Google Scholar]
- Patrick K, Norman GJ, Calfas KJ, Sallis JF, Zabinski MF, Rupp J, et al. Diet, physical activity, and sedentary behaviors as risk factors for overweight in adolescence. Archives of Pediatrics & Adolescent Medicine. 2004;158(4):385–390. doi: 10.1001/archpedi.158.4.385. [DOI] [PubMed] [Google Scholar]
- Patterson GR, DeBaryshe BD, Ramsey E. A developmental perspective on antisocial behavior. American psychologist. 1989;44(2):329–335. doi: 10.1037/0003-066X.44.2.329. [DOI] [PubMed] [Google Scholar]
- Pattillo-McCoy M. Black picket fences: Privilege and peril among the Black middle class. University of Chicago Press; 2000. [Google Scholar]
- Perez L, Kunzli N, Avol E, Hricko AM, Lurmann F, Nicholas E, et al. Global Goods Movement and the Local Burden of Childhood Asthma in Southern California. American Journal of Public Health. 2009;99(S3):S622. doi: 10.2105/AJPH.2008.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianta RC, Early D. Turnover in kindergarten classroom membership in a national sample. Early Education and Development. 2001;12(2):239–252. Retrieved from http://www.tandfonline.com/doi/abs/10.1207/s15566935eed1202_5. [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: A critical review. Journal of Epidemiology & Community Health. 2001;55 (2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Annals of Internal Medicine. 1998;129(5):406. doi: 10.7326/0003-4819-129-5-199809010-00011. Retrieved from http://www.annals.org/content/129/5/406.short. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Auld MC, Chaloupka FJ, O’Malley PM, Johnston LD. Associations between access to food stores and adolescent body mass index. American Journal of Preventive Medicine. 2007;33(4):S301–S307. doi: 10.1016/j.amepre.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Powell LM, Slater S, Chaloupka FJ, Harper D. Availability of physical activity-related facilities and neighborhood demographic and socioeconomic characteristics: a national study. American Journal of Public Health. 2006;96(9):1676. doi: 10.2105/AJPH.2005.065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Slater S, Mirtcheva D, Bao Y, Chaloupka FJ. Food store availability and neighborhood characteristics in the United States. Preventive Medicine. 2007;44 (3):189–195. doi: 10.1016/j.ypmed.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Precht DH, Keiding L, Nielsen GA, Madsen M. Smoking among upper secondary pupils with asthma: reasons for their smoking behavior: a population-based study. Journal of Adolescent Health. 2006;39(1):141–143. doi: 10.1016/j.jadohealth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Chew GR, Garfinkel RS. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environmental Health Perspectives. 2002;110(Suppl 2):323–327. doi: 10.1289/ehp.02110s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher KJ, Myers DJ. Socioeconomic disparities in the prevalence of work- related injuries among adolescents in the United States. Journal of Adolescent health. 2008;42(1):50–57. doi: 10.1016/j.jadohealth.2007.08.003. Retrieved from http://www.sciencedirect.com/science/article/pii/S1054139X0700328X. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. Assessing the effects of age, sex and shared environment on the genetic aetiology of depression in childhood and adolescence. Journal of Child Psychology and Psychiatry. 2002;43(8):1039–1051. doi: 10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- Riley AW, Coiro MJ, Broitman M, Colantuoni E, Hurley KM, Bandeen-Roche K, et al. Mental health of children of low-income depressed mothers: Influences of parenting, family environment, and raters. Psychiatric Services. 2009;60(3):329–336. doi: 10.1176/appi.ps.60.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemmich JN, Smith JR, Epstein LH, Lambiase M. Stress reactivity and adiposity of youth. Obesity. 2007;15(9):2303–2310. doi: 10.1038/oby.2007.273. [DOI] [PubMed] [Google Scholar]
- Romero AJ. Low-income neighborhood barriers and resources for adolescents’ physical activity. Journal of Adolescent Health. 2005;36(3):253–259. doi: 10.1016/j.jadohealth.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. New England Journal of Medicine. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30(1):1–10. doi: 10.1016/j.psyneuen.2004.05.007. Retrieved from http://www.sciencedirect.com/science/article/pii/S0306453004000733. [DOI] [PubMed] [Google Scholar]
- Rosmond R, Björntorp P. Occupational status, cortisol secretory pattern, and visceral obesity in middle-aged men. Obesity. 2000;8(6):445–450. doi: 10.1038/oby.2000.55. Retrieved from http://www.nature.com/oby/journal/v8/n6/abs/oby200055a.html. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70 (3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Ruxton CH, Kirk TR, Belton NR, Holmes MA. Relationships between social class, nutrient intake and dietary patterns in Edinburgh schoolchildren. International Journal of Food Sciences & Nutrition. 1996;47(4):341–349. doi: 10.3109/09637489609041034. [DOI] [PubMed] [Google Scholar]
- Sahsuvaroglu T, Jerrett M, Sears MR, McConnell R, Finkelstein N, Arain A, et al. Spatial analysis of air pollution and childhood asthma in Hamilton, Canada: comparing exposure methods in sensitive subgroups. Environmental Health: A Global Access Science Source. 2009;8:14. doi: 10.1186/1476-069X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam MT, Islam T, Gilliland FD. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Current Opinion in Pulmonary Medicine. 2008;14(1):3–8. doi: 10.1097/MCP.0b013e3282f1987a. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Medicine & Science in Sports & Exercise. 2000;32(5):963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Zakarian JM, Hovell MF, Hofstetter CR. Ethnic, socioeconomic, and sex differences in physical activity among adolescents. Journal of Clinical Epidemiology. 1996;49(2):125–134. doi: 10.1016/0895-4356(95)00514-5. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhood and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Sandel M, Wright RJ. When home is where the stress is: expanding the dimensions of housing that influence asthma morbidity. Archives of Disease in Childhood. 2006;91(11):942–948. doi: 10.1136/adc.2006.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpong SB, Hamilton RG, Eggleston PA, Adkinson NF., Jr Socioeconomic status and race as risk factors for cockroach allergen exposure and sensitization in children with asthma. Journal of Allergy & Clinical Immunology. 1996;97(6):1393–1401. doi: 10.1016/S0091-6749(96)70209-9. [DOI] [PubMed] [Google Scholar]
- Scafidi B, Sjoquist DL, Stinebrickner TR. Race, poverty, and teacher mobility. Economics of Education Review. 2007;26(2):145–159. Retrieved from http://www.sciencedirect.com/science/article/pii/S0272775705000993. [Google Scholar]
- Schreier H, Chen E. Longitudinal Relationships Between Family Routines and Biological Profiles Among Youth With Asthma. Health psychology. 2010;29(1):82–90. doi: 10.1037/a0018311. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin. 2004;130(4):601. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalowitz MU, Berry CA, Quinn KA, Wolf RL. The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambulatory Pediatrics. 2001;1(4):185–193. doi: 10.1367/1539-4409(2001)001<0185:TROLSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankardass K, McConnell RS, Milam J, Berhane K, Tatalovich Z, Wilson JP, et al. The association between contextual socioeconomic factors and prevalent asthma in a cohort of Southern California school children. Social Science & Medicine. 2007;65(8):1792–1806. doi: 10.1016/j.socscimed.2007.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Gross HE, Moilanen KL, Sameroff A. The transactional model of development: How children and contexts shape each other. Washington, DC US: American Psychological Association; 2009. Developmental transactions between boys’ conduct problems and mothers’ depressive symptoms; pp. 77–96. [DOI] [Google Scholar]
- Sheehan K, DiCara JA, LeBailly S, Christoffel KK. Children’s exposure to violence in an urban setting. Archives of Pediatrics & Adolescent Medicine. 1997;151(5):502–504. doi: 10.1001/archpedi.1997.02170420072012. [DOI] [PubMed] [Google Scholar]
- Shelton KH, Harold GT. Interparental conflict, negative parenting, and children’s adjustment: Bridging links between parents’ depression and children’s psychological distress. Journal of Family Psychology. 2008;22(5):712–724. doi: 10.1037/a0013515. [DOI] [PubMed] [Google Scholar]
- Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990–2005. Obesity. 2008;16(2):275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- Silverglade L, Tosi DJ, Wise PS, D’Costa A. Irrational beliefs and emotionality in adolescents with and without bronchial asthma. Journal of General Psychology. 1994;121(3):199–207. doi: 10.1080/00221309.1994.9921196. [DOI] [PubMed] [Google Scholar]
- Simon AE, Wardle J, Jarvis MJ, Steggles N, Cartwright M. Examining the relationship between pubertal stage, adolescent health behaviours and stress. Psychological Medicine. 2003;33(08):1369–1379. doi: 10.1017/S0033291703008390. [DOI] [PubMed] [Google Scholar]
- Simon PA, Zeng Z, Wold CM, Haddock W, Fielding JE. Prevalence of childhood asthma and associated morbidity in Los Angeles County: impacts of race/ethnicity and income. Journal of Asthma. 2003;40(5):535–543. doi: 10.1081/JAS-120018788. [DOI] [PubMed] [Google Scholar]
- Singh GK, Siahpush M, Kogan MD. Disparities in children’s exposure to environmental tobacco smoke in the United States, 2007. Pediatrics, peds. 2010:2009–2744v1. doi: 10.1542/peds.2009-2744. Retrieved from http://pediatrics.aappublications.org/cgi/content/abstract/peds.2009-2744v1. [DOI] [PubMed]
- Skinner J, Carruth BR, Moran J, III, Houck K, Schmidhammer J, Reed A, et al. Toddlers’ food preferences: Concordance with family members’ preferences. Journal of Nutrition Education. 1998;30(1):17–22. doi: 10.1016/S0022-3182(98)70270-5. [DOI] [Google Scholar]
- Spano R, Vazsonyi AT, Bolland J. Does parenting mediate the effects of exposure to violence on violent behavior? An ecological, Äìtransactional model of community violence. Journal of Adolescence. 2009;32(5):1321–1341. doi: 10.1016/j.adolescence.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L’Hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology & Metabolism. 2004;89(11):5762. doi: 10.1210/jc.2004-1003. Retrieved from http://jcem.endojournals.org/content/89/11/5762.short. [DOI] [PubMed] [Google Scholar]
- Starfield B, Riley AW, Witt WP, Robertson J. Social class gradients in health during adolescence. Journal of Epidemiology and Community Health. 2002;56(5):354. doi: 10.1136/jech.56.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starfield BH. Child health and socioeconomic status. American Journal of Public Health. 1982;72(6):532. doi: 10.2105/AJPH.72.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Annals of Behavioral Medicine. 2001;23(3):177–185. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- Sternthal MJ, Jun HJ, Earls F, Wright RJ. Community violence and urban childhood asthma: a multilevel analysis. European Respiratory Journal. 2010;36(6):1400. doi: 10.1183/09031936.00003010. Retrieved from http://erj.ersjournals.com/content/36/6/1400.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale SE, Wells KB, Tang L, Belin TR, Zhang L, Sherbourne CD. The importance of social context: Neighborhood stressors, stress-buffering mechanisms, and alcohol, drug, and mental health disorders. Social Science & Medicine. 2007;65(9):1867–1881. doi: 10.1016/j.socscimed.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, Datar A. Body mass index in elementary school children, metropolitan area food prices and food outlet density. Public Health. 2005;119(12):1059–1068. doi: 10.1016/j.puhe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Sturm R, Datar A. Food prices and weight gain during elementary school: 5-year update. Public health. 2008;122(11):1140. doi: 10.1016/j.puhe.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SV, Ackerson LK, Subramanyam MA, Wright RJ. Domestic violence is associated with adult and childhood asthma prevalence in India. International Journal of Epidemiology. 2007;36(3):569–579. doi: 10.1093/ije/dym007. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Ryan L, Laden F, Dockery DW, Wright RJ. Violence exposure, a chronic psychosocial stressor, and childhood lung function. Psychosomatic medicine. 2008;70(2):160–169. doi: 10.1097/PSY.0b013e318160687c. Retrieved from http://www.psychosomaticmedicine.org/content/70/2/160.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Enlow MB, Kullowatz A, Wright RJ. Maternal intimate partner violence and increased asthma incidence in children: buffering effects of supportive caregiving. Archives of Pediatrics & Adolescent Medicine. 2009;163(3):244–250. doi: 10.1001/archpediatrics.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taras HL, Sallis JF, Patterson TL, Nader PR, Nelson JA. Television’s influence on children’s diet and physical activity. Journal of Developmental & Behavioral Pediatrics. 1989;10(4):176–180. doi: 10.1097/00004703-198908000-00003. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. American Journal of Physiology-Endocrinology And Metabolism. 1996;271(2):E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. Retrieved from http://ajpendo.physiology.org/content/271/2/E317.short. [DOI] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Berkey CS, Rockett HRH, Field AE, Frazier AL, et al. Family dinner and adolescent overweight. Obesity Research. 2005;13(5):900–906. doi: 10.1038/oby.2005.104. [DOI] [PubMed] [Google Scholar]
- Thomson NC. The role of environmental tobacco smoke in the origins and progression of asthma. Current allergy and asthma reports. 2007;7(4):303–309. doi: 10.1007/s11882-007-0045-8. [DOI] [PubMed] [Google Scholar]
- Timberlake JM. Racial and ethnic inequality in the duration of children’s exposure to neighborhood poverty and affluence. Social Problems. 2007;54(3):319–342. Retrieved from http://www.jstor.org/stable/10.1525/sp.2007.54.3.319. [Google Scholar]
- Timperio A, Ball K, Salmon J, Roberts R, Giles-Corti B, Simmons D, et al. Personal, family, social, and environmental correlates of active commuting to school. American Journal of Preventive Medicine. 2006;30(1):45–51. doi: 10.1016/j.amepre.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Toschke AM, Kuchenhoff H, Koletzko B, von Kries R. Meal frequency and childhood obesity. Obesity Research. 2005;13(11):1932–1938. doi: 10.1038/oby.2005.238. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14(6):623. doi: 10.1046/j.0956-7976.2003.psci_1475.x. Retrieved from http://pss.sagepub.com/content/14/6/623.short. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Grann M, Lichtenstein P. Heritability for adolescent antisocial behavior differs with socioeconomic status: gene–environment interaction. Journal of Child Psychology and Psychiatry. 2006;47(7):734–743. doi: 10.1111/j.1469-7610.2005.01552.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1469-7610.2005.01552.x/full. [DOI] [PubMed] [Google Scholar]
- Videon TM, Manning CK. Influences on adolescent eating patterns: the importance of family meals. Journal of Adolescent Health. 2003;32(5):365–373. doi: 10.1016/S1054-139X(02)00711-5. [DOI] [PubMed] [Google Scholar]
- Vieno A, Nation M, Perkins DD, Pastore M, Santinello M. Social capital, safety concerns, parenting, and early adolescents’ antisocial behavior. Journal of Community Psychology. 2010;38(3):314–328. Retrieved from 10.1002/jcop.20366 http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2010-05706-004&site=ehost-live. [Google Scholar]
- Vieweg VR, Johnston CH, Lanier JO, Fernandez A, Pandurangi AK. Correlation between high risk obesity groups and low socioeconomic status in school children. Southern Medical Journal. 2007;100(1):8–13. doi: 10.1097/01.smj.0000253479.03665.6f. [DOI] [PubMed] [Google Scholar]
- Visscher TLS, Seidell JC. The public health impact of obesity. Annual review of public health. 2001;22(1):355–375. doi: 10.1146/annurev.publhealth.22.1.355. Retrieved from http://www.annualreviews.org/doi/abs/10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- Wamboldt FS, Wamboldt MZ, Gavin LA, Roesler TA, Brugman SM. Parental criticism and treatment outcome in adolescents hospitalized for severe, chronic asthma. Journal of Psychosomatic Research. 1995;39(8):995–1005. doi: 10.1016/0022-3999(95)00507-2. [DOI] [PubMed] [Google Scholar]
- Wang HC, McGeady SJ, Yousef E. Patient, home residence, and neighborhood characteristics in pediatric emergency department visits for asthma. Journal of Asthma. 2007;44 (2):95–98. doi: 10.1080/02770900601180610. [DOI] [PubMed] [Google Scholar]
- Weil CM, Wade SL, Bauman LJ, Lynn H, Mitchell H, Lavigne J. The relationship between psychosocial factors and asthma morbidity in inner-city children with asthma. Pediatrics. 1999;104(6):1274–1280. doi: 10.1542/peds.104.6.1274. [DOI] [PubMed] [Google Scholar]
- Weir LA, Etelson D, Brand DA. Parents’ perceptions of neighborhood safety and children’s physical activity. Preventive Medicine: An International Journal Devoted to Practice and Theory. 2006;43(3):212–217. doi: 10.1016/j.ypmed.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. Journal of Allergy and Clinical Immunology. 2001;107(1):3–8. doi: 10.1067/mai.2001.112262. Retrieved from http://www.sciencedirect.com/science/article/pii/S0091674901673329. [DOI] [PubMed] [Google Scholar]
- Wickrama KAT, Wickrama KAS, Bryant CM. Community Influence on Adolescent Obesity: Race/Ethnic Differences. Journal of Youth and Adolescence. 2006;35(4):647–657. doi: 10.1007/s10964-006-9050-9. [DOI] [Google Scholar]
- Wolf JM, Miller GE, Chen E. Parent psychological states predict changes in inflammatory markers in children with asthma and healthy children. Brain, Behavior, and Immunity. 2008;22(4):433–441. doi: 10.1016/j.bbi.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Steinbach SF. Violence: an unrecognized environmental exposure that may contribute to greater asthma morbidity in high risk inner-city populations. Environmental Health Perspectives. 2001;109(10):1085–1089. doi: 10.1289/ehp.011091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ. Health effects of socially toxic neighborhoods: the violence and urban asthma paradigm. Clinics in Chest Medicine. 2006;27(3):413–421. doi: 10.1016/j.ccm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Current Opinion in Allergy & Clinical Immunology. 2005;5(1):23–29. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. American Journal of Respiratory & Critical Care Medicine. 2002;165(3):358–365. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. Journal of Allergy & Clinical Immunology. 2004a;113(6):1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. American Journal of Public Health. 2004b;94(4):625–632. doi: 10.2105/AJPH.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Leventhal T, Brooks-Gunn J, Earls FJ. Neighborhood Residence and Mental Health Problems of 5- to 11-Year-Olds. Archives of General Psychiatry. 2005;62(5):554–563. doi: 10.1001/archpsyc.62.5.554. [DOI] [PubMed] [Google Scholar]
- Yen IH, Syme SL. The social environment and health: a discussion of the epidemiologic literature. Annual Review of Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee C. Walkability and safety around elementary schools economic and ethnic disparities. American Journal of Preventive Medicine. 2008;34(4):282–290. doi: 10.1016/j.amepre.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Zmirou D, Gauvin S, Pin I, Momas I, Sahraoui F, Just J, et al. Traffic related air pollution and incidence of childhood asthma: results of the Vesta case-control study. Journal of Epidemiology & Community Health. 2004;58(1):18–23. doi: 10.1136/jech.58.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]