This research introduces the concept of a long-term community-based program of individualized exercise as a feasible and effective intervention to improve quality of life for persons with all stages of cancer.
Abstract
Purpose:
To determine the effects of a community-based program of exercise on quality of life (QOL) of persons with cancer over time.
Methods:
Participants were referred by their physician to participate in an individualized program of exercise at one of 14 community centers. The Medical Outcomes Survey, Short Form, version 2.0 (SF-36) was used to assess QOL. Individual participants were monitored for 2 years. Data collection took place at baseline, every 3 months months during year 1, and every 6 months during year 2.
Results:
Enrolled participants (n = 701) had been diagnosed with different cancers and were at all stages; 177 completed data collection for 2 years. One-way analysis of variance (n = 177) supported the positive impact of exercise on QOL over time. Significant subscale scores of the SF-36, including Physical Function (F = 2.13, P ≤ .047), Role Physical (F = 3.78, P ≤ .001), Vitality (F = 5.97, P ≤ .001), Social Function (F = 4.46, P ≤ .001), Role Emotional (F = 2.56, P ≤ .01), Mental Health (F = 2.16, P ≤ .05), and General Health (F = 3.42, P ≤ .01), were sustainable over time.
Conclusion:
This research introduces the concept of a long-term community-based program of individualized exercise as a feasible and effective intervention to improve QOL for persons with all stages of cancer. Improvements, noted at the 3-month time point, appear to be sustainable for extended time (24 months). Attrition is problematic and needs to be addressed. Results from this study have significance for practice recommendations and health policy reimbursement issues.
Introduction
Both conventional wisdom and scientific studies laud the health benefits of exercise. Recommending exercise as the solution to a multitude of health problems has become routine to the point that the extent of its impact on improvement is frequently lost. In addition, perseverance with exercise programs is a recurring problem, suggesting that measurements demonstrating the positive effects are needed to encourage commitment to exercise and health. This study was designed to address this need by specifically measuring the impact of an exercise program on health outcomes of patients with cancer.
Exercise has been associated with decreased cancer recurrence and decreased mortality, as a result of both cancer and other causes, primarily in patients with breast and colon cancer.1–3 Patients with breast cancer who exercised 9 or more hours a week experienced a 43% reduction in relative risk of breast cancer recurrence and a 50% relative improvement in survival.1 Patients with stage III colon cancer who exercised 18 or more hours per week had a 50% reduction in colon cancer recurrence.3 In 2008, the results of a 3-year study of 933 patients with breast cancer showed a 64% reduction in risk of death from all causes in patients who exercised at a moderate intensity for 9 hours per week.2
Exercise also reduces treatment adverse effects and improves quality of life (QOL) in cancer survivors.4–6 The majority of studies have been conducted with breast cancer survivors4,6–7; however, many of the exercise interventions were of limited duration. In a meta-analysis of 78 exercise studies, Ferrer et al5 reported a mean duration of 13.5 weeks. Participants were followed for 52 weeks in only one study, which the authors considered an outlier. Rarely have persons with advanced cancers been included.8 The lack of longitudinal studies that monitor cancer patients' adherence to exercise program for extended periods represent a clear gap in the cancer outcome literature.
The American College of Sports Medicine (ACSM) assembled a panel of experts to establish exercise guidelines for patients with cancer, during and after treatment. In June 2010, ASCO recommended exercise using the ACSM guidelines as an important treatment component for patients during and after chemotherapy. Despite the overwhelming evidence and professional guidelines, exercise is not yet a routine standard of care for most patients with cancer and survivors. Several factors contribute to this lack.
In 2010, the National Center for Health Statistics reported only 35% of Americans engaged in leisure-time physical activity.9 Persons with cancer are even less likely to exercise, as a result of adverse effects of the disease and treatments.4,10 Fatigue, pain, anemia, depression, and nausea contribute to make exercise more difficult for cancer survivors than for the general population. Survivors are not likely to embark on an exercise program independently.4,10–11
A second challenge for the patient committed to exercising is finding a safe program that is supportive of persons with cancer. The prescription of exercise is limited by the availability of programs designed to handle the special needs of persons receiving treatment. Examples of the special exercise-related needs include awareness and consideration of (1) the high risk for fractures related to bone cancer (either primary or metastatic); (2) risks associated with falls and bleeding associated with thrombocytopenia (low platelet count) as a result of cancer or treatment; (3) decrease in WBC counts (neutropenia) and risk of infection in persons with particular cancers or those receiving chemotherapy; (4) decrease in RBC counts (anemia) related to cancer, cancer treatment, and their impact on oxygenation; and (5) increased risk of falls related to decreased sensation (peripheral neuropathy) in the hands and feet as a result of treatment. Ferrer et al5 reported that only 56% of 78 exercise studies included any type of supervised exercise.
The cost of an exercise program to the patient is yet another challenge. Insurance does not cover long-term exercise rehabilitation programs. Without reimbursement, there is little incentive for physicians to discuss and recommend exercise to their patients and for cancer care centers to provide these services. Most patients with cancer, especially the uninsured/underinsured and single heads of households, are devastated financially by medical expenses and do not have the resources to join a gym or exercise class. Limited reimbursement also directly affects the availability and the sustainability of exercise programs.
The not-for-profit Cancer Foundation for Life (CFFL), headquartered in Tyler, TX, was established in 2001 to meet these challenges. Through an individualized community-based program, titled FitSTEPS for Life (FSFL), all persons with cancer, regardless of type or stage of cancer, complexity of comorbid diseases, or magnitude of disability (including wheelchair dependency), have access to a cost-free, long-term program of tailored and supervised exercise.12 Exercise sites are located in churches, community centers, city-owned facilities, hospital-provided health centers, and free-standing cancer clinics. Personnel are trained to work with the special needs of cancer survivors.
Prior research has focused on a single cancer type or short-term interventions. Thus the purpose of this study was to determine the effects of a community-based program of exercise on the QOL of persons with any type or stage of cancer and magnitude of debilitation (wheelchair and oxygen dependent) over an extended period.
Methods
Bandura's social cognitive theory was used to guide this research.13 Bandura posits a triadic reciprocation among the theoretical concepts of person, environment, and behavior. In the current study, personal factors are represented by demographic data, including health data. The targeted behavior is exercise, and the CFFL centers represent the environment. It was proposed that the interaction among the individuals attending the center, the CFFL staff, and other CFFL participants would contribute to a behavior change (ie, increased exercise), resulting in improved QOL.
A repeated measures pretest/post-test design was used to determine the effect of the individualized FSFL exercise program on QOL of persons with cancer. From April 2006 through March 2009, a convenience sample of patients with cancer was recruited through referrals from 14 oncology practices in the East Texas region and the Dallas metropolitan area. Persons with any type of cancer, at any stage, including those with comorbidities or persons requiring assistance to walk, were eligible to participate on the basis of a physician referral. Persons who chose to participate signed a liability waiver to exercise along with an institutional review board–approved informed consent to have data collected for a period of 24 months. Data collection completed March 31, 2011.
Demographic and health data were collected from each patient at baseline. Standard demographic information such as age, gender, race/ethnicity, and education was obtained; health data included the type and stage of cancer, treatments received or in process, comorbid conditions, and the need for assistive devices to ambulate or breathe. In addition, height, weight, oxygen saturation, and vital signs (heart rate, blood pressure) were measured along with temperature for patients currently receiving chemotherapy. χ2 and t test analyses were used to compare those who elected to participate with those who declined participation to assess for differences on key demographic and health variables such as age, sex, and type or stage of cancer. χ2 and t test were also used to compare those who completed 2 years of follow-up with those who discontinued participation during the course of the 2-year follow-up. Correlation analyses were conducted to explore the relationships among demographic variables and QOL.
QOL was measured with the Medical Outcomes Survey Short Form, version 2.0 (SF-36). This 36-item measure of physical and mental health has been used in more than 1,000 studies, including many in patients with cancer, and has well-established reliability and validity.14 Individual items contain choices in verbal descriptors (eg, “all of the time,” “most of the time,” “some of the time,” “a little of the time,” or “none of the time”). The SF-36 comprises eight subscales: Physical Function, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional, and Mental Health. These subscales are then standardized and norm-based according to the specifications of Quality Metric, publisher of the SF-36.14 The standardized, norm-based subscales are next condensed into two summary scores, the Physical Component Summary (PCS) and Mental Component Summary (MCS), again according to specifications of the instrument's publisher. Thus, scoring of the SF-36 results in eight subscales and two summary scores ranging from 0′ to 100′. Higher scores are indicative of better physical and mental health.
Phone calls were made to participants who did not return to the CFFL center within a 2-week time frame. The purpose of the call was to determine participant status and encourage return to the exercise program. During a 13-month period (March 2009 through March 2010), CFFL staff also collected data regarding the reason for participants not returning. Descriptive statistics were used to summarize the reasons for withdrawal.
Physicians who referred patients to the program faxed a referral form to the CFFL office. A phone call was made by the CFFL Clinical Director to explain the program and schedule an initial visit. At that first visit, a health history was taken. Participants completed demographic forms and the SF-36 before beginning the exercise session. The patient was then placed on a treadmill and monitored by an exercise kinesiologist. An elliptical was used for those with weight-bearing joint disease. On completion of the initial session, an individualized exercise prescription was recommended. The prescription included walking, stretching, resistance training, and core muscle strengthening exercises. Participants were encouraged to attend the centers at least three times a week and to exercise at home when possible. Pedometers were provided at cost to help participants track their progress.
During subsequent exercise sessions, trained staff monitored participants. At each session visit, measured metrics were recorded before, during, and after exercise. The SF-36 survey was completed at 3, 6, 9, 12, 18, and 24 months. A complete description of the FSL program is reported elsewhere.12
Results
Over a 3-year period, 1,665 patients were referred to the FSFL program and came for an initial evaluation and consultation. Of those, 701 elected to participate in the program. All cancer types were represented; the majority of participants had breast (50%), prostate (9%), colorectal (9%), lymphoma (7%), or lung (6%). Persons at all stages (I = 20%; II = 20%; III = 13%; IV = 9%; unknown = 38%) participated in the program. Nearly 70% of participants had one or more comorbid conditions (eg, heart disease, diabetes, arthritis), and 10% required an assistive device (walker, cane, or wheelchair) for mobility. Four percent required oxygen. Thirty percent were actively receiving cancer treatment (radiation and/or chemotherapy). Attrition was observed at each data collection point; participant numbers are summarized in Table 1.
Table 1.
FitSTEPS for Life Attrition Over Time
| Data Collection Time Point | No. of Participants | Attrition (lost from baseline No. of participants) |
|
|---|---|---|---|
| No. | % | ||
| Referred to program | 1,665 | N/A | N/A |
| Began program/baseline | 701 | N/A | N/A |
| 3 months | 600 | 101 | 14 |
| 6 months | 420 | 180 | 26 |
| 9 months | 318 | 102 | 15 |
| 12 months | 298 | 20 | 03 |
| 18 months | 223 | 75 | 11 |
| 24 months | 177 | 46 | 06 |
Abbreviation: N/A, not applicable.
χ2 analysis and t tests were conducted to compare those referred to the program who declined participation with those who elected to begin the program. t tests and χ2 were also used to compare those who completed the program with those who did not. There were no significant differences in sex, race/ethnicity, or education level between those who declined participation and those who entered the program. Those who were younger, single parents, and of lower socioeconomic status were more likely to decline program participation. In addition, those who had diabetes (χ2 = 7.95; P = .005) or were actively receiving chemotherapy (χ2 = 22.19; P < .001) were less likely to participate. Those who completed the program were more likely to be older, male, and not working. As expected, those with advanced-stage disease and lung cancer were less likely to complete the 24-month follow-up. Demographic differences are summarized in Table 2.
Table 2.
Demographic Comparison of Participants Over Time
| Demographic Variable | Referred; No Participation (n = 964) | Baseline (n = 701) | Completed 24-Month Follow-Up (n = 177) | Significant Differences Referrals/Baseline | Significant Differences Baseline to 24 Months |
|---|---|---|---|---|---|
| Age, years | |||||
| Mean | 61.60 | 65.44 | 68.4 | F = −26.56, P < .001 | F = −26.56, P < .001 |
| SD | 12.6 | 12.4 | 10.4 | ||
| Range | 17-97 | 27-90 | 37-90 | ||
| Sex, % | |||||
| Female | 76 | 72 | 67 | ns | ns |
| Male | 24 | 28 | 33 | ||
| Race/ethnicity, % | |||||
| White | 79.3 | 78.1 | 88.2 | ns | χ2 = 10.66, P < .05 |
| African-American | 14.7 | 13.9 | 9.6 | ||
| Hispanic | 3.3 | 5 | 1.7 | ||
| High school or higher, % | 96 | 96 | 97.7 | ns | ns |
| Financially stable, % | 79 | 85 | 87 | ns | ns |
| Retired, % | 40.8 | 50.7 | 65.9 | χ2 = 15.02, P < .01 | χ2 = 14.96, P < .05 |
| Has one or more co-morbidities (eg, diabetes, heart disease), % | 69.6 | 69.4 | 75.1 | ns | ns |
| Cancer | |||||
| Breast | 49 | 50 | 49 | ns | ns |
| Prostate | 5 | 9 | 12 | ||
| Colorectal | 8 | 9 | 11 | ||
| Lung | 9 | 6 | 5 | ||
| Lymphoma | 6 | 7 | 7 | ||
| Other | 23 | 19 | 16 | ||
| Stage, % | |||||
| I | 18 | 20 | 21 | ns | χ2 = 19.21, P < .001 |
| II | 20 | 20 | 23 | ||
| III | 17 | 13 | 5 | ||
| IV | 13 | 9 | 6 | ||
| Unknown | 32 | 38 | 45 | ||
| Receiving radiation or chemotherapy, % | 69 | 31 | 23 | χ2 = 10.59, P < .001 | χ2 = 4.86, P < .05 |
Abbreviations: ns, nonsignificant; SD, standard deviation.
Demographic and health variables were assessed for possible significant correlations with the eight SF-36 subscales at baseline. Pearson's r was used to assess continuous variables, and Spearman's rho was used for categorical and nominal data. Several significant relationships are reported. Those variables with significant correlation on at least four of the eight subscales are portrayed in Appendix Table A1 (online only). Those struggling financially reported a lower QOL. Obesity, arthritis, and lung disease were also associated with lower QOL scores. Participants who were receiving chemotherapy or who needed assistance to walk also reported lower QOL scores.
Repeated measures ANOVA was used to assess changes in SF-36 subscale scores over time. When the 701 participants who returned for at least one subsequent assessment are included, QOL scores improved significantly in all eight subscales. When limited to the 177 participants wo completed 24-month surveys, all subscales, with the exception of bodily pain, were significant (Table 3).
Table 3.
Change in Quality of Life Over Time As Measured by SF-36 (n = 177)
| SF-36 Subscale | F | Significance |
|---|---|---|
| Physical Functioning | 2.13 | < .05 |
| Role Performance | 3.78 | < .001 |
| Bodily Pain | 1.60 | ns |
| General Health | 3.42 | < .01 |
| Vitality | 5.97 | < .001 |
| Social Functioning | 4.46 | < .001 |
| Role Emotional | 2.56 | < .05 |
| Mental Health | 2.16 | < .05 |
Abbreviations: ns, nonsignificant; SF-36, Short Form–36.
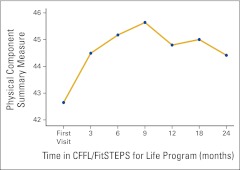
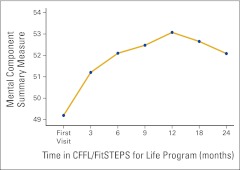
The subscale scores were then condensed into the two SF-36 summary scales, the PCS and MCS measures. Component scores were then standardized to permit comparison with US norms for patients with cancer. When compared with the normed SF-36 scores for patients with cancer among the general US population, baseline scores for FSFL participants were slightly higher for both the PCS and MCS; the differences were nonsignificant. ANOVA revealed significant change for both the PCS (F = 2.33; P = .031) and the MCS (F = 3.36; P = .003) over time. Changes are depicted in Appendix Figures A1 and A2 (online only).
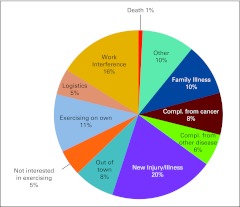
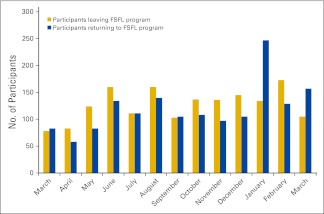
Additional analyses were conducted to ascertain the reasons for attrition over time. Over a 13-month period (March 2009 through March 2010), FSFL participants who withdrew were contacted to determine their reasons for leaving the program. The most common reason for program withdrawal was related to work demands. Of those who stopped attending the FSFL centers, 11% continued to exercise but did so on their own. Reasons for leaving the program are summarized in Appendix Figure A3 (online only). During the same 13-month period, the number of participants who returned to the program was assessed. The number of participants who left the program and the number who re-enrolled are summarized in Appendix Figure A4 (online only).
Discussion
Results from this longitudinal study suggest that QOL for persons with all types and at all stages of cancer can be improved through an individualized program of exercise. The CFFL has increased availability of and access to survivorship programs and services designed to improve QOL to ensure optimum care for all phases of cancer survivorship. On the basis of previous research by Holmes et al,1 Irwin et al,2 and Myerhardt et al,3 it is possible the program will also maximize survival time, though this was not a measured outcome in this study. Results of this study extend the current research supporting exercise for patients with cancer4–6 to include those with more advanced-stage disease and comorbidities. Since its inception in 2001, FSFL has been a safe and effective program that has enhanced QOL in cancer survivors.12 No injuries associated with exercise have occurred among FSFL participants.
The fitness industry reports a consistent 50% attrition rate within the first 6 months of an exercise program.15 FSFL witnesses a similar trend (40% attrition at 6 months), although the reasons for FSFL attrition differ from those for the commercial fitness industry and are more complicated. Seventy percent of FSFL participants have comorbidities (heart disease, diabetes, lung disease). FSFL participants are also at high risk for complications related to cancer and its treatment (infection, anemia, bone fractures), thus making commercial fitness centers inappropriate venues for them to exercise. They are also more likely to experience mental health issues (anxiety, depression). The FSFL program was specifically designed to accommodate the unique circumstances of the cancer population. Thus, there is no limit to the length of time a patient may participate in the program, enabling them to achieve optimal benefits. Because patients have no limit to duration of participation and are always welcomed back, an FSFL participant is rarely considered a permanent drop-out. This is in contrast to exercise programs of limited duration that have more definitive drop-out rates. CFFL has observed participants may need to drop out temporarily as a result of complications or comorbid conditions but frequently return to the program (Appendix Figure A4). The pattern of participants returning after a period of nonattendance suggests that the program may be effective in changing long-term behavior, even though participants may need to stop temporarily to attend to health concerns. The slightly lower attrition rate (40% of FSFL at 6 months compared with 50% for the general public) and the impressive return to the program suggest the program offers support that encourages persons with cancer to exercise.
This study raises several additional research questions. The impact of an exercise-based health promotion program on QOL patients with cancer should be studied to determine time points for optimal engagement, QOL improvement, and stopping points. These target areas may be managed by the addition of other interventions to improve retention in exercise programs. The role of social support and self-efficacy in contributing to improved QOL in patients with cancer who participate in exercise programs should be studied. The fluidity of the participation rate is a concern, so a support mechanism to encourage continuation of exercise at home should be tested by using such interventions as ongoing telephone or Web-based support. Finally, the efficacy of using an exercise-based intervention should be promoted to oncology providers, with funding recommendations to expand the availability of programs such as FSFL to patients with cancer and survivors throughout the nation and the world.
Results of this study also have implications for practice and health policy. Health care providers caring for persons with cancer are advised to refer patients to exercise programs and to encourage patients to be active throughout all phases of cancer treatment and beyond. When possible, referral to a program specializing in exercise for cancer survivors is recommended. Health policy change is needed to require financial support for persons with cancer to exercise in a supervised setting. The evidence suggests that exercise is safe and effective for all cancer survivors, regardless of type, stage, or comorbidities. It is time to incorporate exercise as a standard of care for every cancer survivor.
Acknowledgment
This study was partially funded by a faculty research grant from The University of Texas at Tyler. Presented at the 41st Sigma Theta Tau International Nursing Society Biennial Convention, Grapevine, TX, October 29-November 2, 2011.
Appendix
Table A1.
Significant Correlations Among SF-36 Subscales and Demographic/Health Variables (n = 701)
| Variable | Physical Function | Social Function | Bodily Pain | Role Emotion | Role Physical | General Health | Vitality | Mental Health |
|---|---|---|---|---|---|---|---|---|
| Age | −.147* | 0.174* | 0.086† | 0.151* | ||||
| Finance | −.106* | −.181* | −.128* | −.128* | −.130* | −.189* | −.165* | −.224* |
| Lung disease | −.190* | −.090† | −.138* | −.165* | ||||
| Arthritis | −.229* | −.180* | 0.081† | −.094† | −.108* | −.093† | ||
| Obese | −.184* | −.087† | −.159* | −.110* | ||||
| Assist walk | −.339* | −.178* | −.178* | −.199* | −.261* | −.169* | −.180* | |
| Chemotherapy | −.275* | −.111* | −.126* | −.241* | −.126* | −.139* | −.123* |
Abbreviation: SF-36, Short Form–36.
P = .01.
P = .05.
Figure A1.
Changes in the Medical Outcomes Survey, Short Form, version 2.0 (SF-36) Physical Component Summary scores over time (n = 177). CFFL, Cancer Foundation for Life.
Figure A2.
Changes in the Medical Outcomes Survey, Short Form, version 2.0 (SF-36) Mental Component Summary scores over time (n = 177). CFFL, Cancer Foundation for Life.
Figure A3.
Reasons for leaving the FitSTEPS for Life program (n = 1,735). Compl., complications.
Figure A4.
FitSTEPS for Life (FSFL) participant attrition (n = 1,735) and return (n = 1,675), March 2009 through March 2010.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Barbara K. Haas, Gary Kimmel
Financial support: Barbara K. Haas, Gary Kimmel
Administrative support: Gary Kimmel
Provision of study materials or patients: Gary Kimmel
Collection and assembly of data: Barbara K. Haas
Data analysis and interpretation: Barbara K. Haas, Belinda Deal
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 2.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The Health, Eating, Activity, and Lifestyle study. J Oncol Pract. 2008;24:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CLGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society's SCS-II. J Clin Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer RA, Huedo-Medina TB, Johnson BT, et al. Exercise interventions for cancer survivors: A meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41:32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: A systematic review. Cancer Treat Rev. 2010;36:185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Bicego D, Brown K, Ruddick M, et al. Effects of exercise on quality of life in women living with breast cancer: A systematic review. Breast J. 2009;15:45–51. doi: 10.1111/j.1524-4741.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 8.Beaton R, Pagdin-Friesen W, Robertson C, et al. Effects of exercise intervention on persons with metastatic cancer: A systematic review. Physiotherapy Canada. 2009;61:141–153. doi: 10.3138/physio.61.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey. National Center for Health Statistics: Vital Health Stat. 2009;10:2010. [PubMed] [Google Scholar]
- 10.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43:32–38. doi: 10.1136/bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 11.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: Five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. doi: 10.1136/bmj. 38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas BK, Kimmel G. A community-based exercise program for cancer survivors: Taking patient care to the next level. J Oncol Pract. 2011;7:252–256. doi: 10.1200/JOP.2010.000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs NJ: Prentice-Hall; 1986. [Google Scholar]
- 14.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric; 2000. [Google Scholar]
- 15.Livestrong. Attrition rates for exercise programs, 2011. www.livestrong.com/article/445409-attrition-rates-for-exercise-programs/ [Google Scholar]