Canadian oncologists'/hematologists' knowledge regarding risk factors for hepatitis B carriage seems to be low, potentially undermining the success of a selective screening strategy.
Abstract
Purpose:
Hepatitis B virus (HBV) reactivation is a potentially fatal complication of chemotherapy that can be largely prevented with medication, provided that asymptomatic HBV carriers are identified. We explored the knowledge, beliefs, and practices of Canadian oncologists/hematologists regarding HBV screening before chemotherapy.
Methods:
A novel questionnaire was mailed to all practicing hematologists/oncologists, where publicly accessible online physician registries facilitated identification of these specialists (71% of the Canadian physician population).
Results:
Of 504 potentially eligible practitioners, 311 (62%) responded, of whom 246 indicated that they administered chemotherapy and were thus included in final analyses. Respondents tended to underestimate the risk of HBV reactivation, and recognition of the major risk factor for HBV carriage (ie, birth in an endemic area) was low. Forty percent of respondents reported rarely or never testing for HBV before chemotherapy, and 36% reported screening only those patients with HBV risk factors. In multivariate analysis, having a predominantly hematologic practice, practitioner experience with HBV reactivation, ability to correctly estimate the risk of HBV reactivation, fewer years in practice, and female sex were independently associated with an increased likelihood of screening for HBV.
Conclusion:
Canadian oncologists and hematologists tend to underestimate the risk of HBV reactivation and report relatively low HBV screening rates. Among those practitioners who do screen, the favored strategy is selective screening of patients with HBV risk factors. However, oncologists'/hematologists' knowledge regarding risk factors for HBV carriage seems to be low, potentially undermining the success of a selective screening strategy.
Introduction
Hepatitis B (HBV) is a global health problem, with approximately 300 to 450 million people affected worldwide.1 In the United States, estimates of the number of people with chronic HBV infection range from 800,000 to 1.4 million (approximately 0.3% to 0.5% of the population), of whom 47% to 70% are immigrants.2 In Canada, approximately 2% of the population is affected by HBV.3 Patients with HBV are at risk of reactivation hepatitis if they are administered systemic cancer treatments. Reactivation hepatitis is associated with substantial morbidity and mortality.4 Estimates of the risk of reactivation in asymptomatic HBV carriers range from 20% to more than 70% depending on tumor type and chemotherapy administered.5–16 Risk factors for HBV reactivation include high HBV viral load before treatment, HBeAg positivity, young age, treatment of hematologic malignancies, use of glucocorticoids, use of rituximab, and bone marrow/hemapoeitic stem-cell transplantation.17 Fortunately, provided that HBV carriers are recognized, HBV reactivation can be largely prevented through the administration of oral antinucleoside analogs.9,12–16,18
Current guidelines recommend HBV screening before chemotherapy, although there are some discrepancies among published recommendations. Infectious disease and hepatology bodies recommend universal screening,2,19 whereas the lead oncology society recommends consideration of targeted testing of high-risk individuals.20 Targeted testing is predicated on the assumption that physicians are aware of the risks of HBV reactivation and able to identify those at highest risk of HBV carriage. We undertook a national survey to evaluate the knowledge, attitudes, and testing practices of Canadian hematologists and oncologists with regard to HBV.
Methods
We developed a questionnaire evaluating hematologists'/oncologists' knowledge, attitudes, and practices regarding HBV (Data Supplement). In September 2009, after pilot testing, the questionnaire was mailed to all practicing hematologists/oncologists in provinces or territories where publicly accessible online physician registries facilitated identification of these specialists (British Columbia, Alberta, Manitoba, Ontario, Nova Scotia, Newfoundland, and Prince Edward Island). These provinces represent 71% of the physician population in Canada.21 The St Michael's Hospital Research Ethics Board approved the study.
Statistical Analysis
Multivariate logistic regression was used to determine the independent association between screening for HBV and physician- and practice-level predictor variables. Candidate predictor variables were years in practice, university- versus non–university-based practice, hematologic versus solid tumor–based practice, physician sex, physician experience with HBV reactivation, and physician awareness of the risk of HBV reactivation. Candidate variables were selected for inclusion in the multivariate model using univariate screening with χ2, Fisher's exact, or Mantel-Haenszel test as appropriate, using a threshold for inclusion of P < .25.22,23 Estimates of association in the final model were considered significant with a two-tailed P value of less than .05. Missing data were handled by list-wise deletion. All analyses were completed using SAS 9.2 software (SAS Institute, Cary, NC).
Results
Five hundred thirty-three eligible practitioners were identified, of whom 29 were excluded because of duplicate listings, exclusively pediatric practice, laboratory-based practice, or recent change to nonactive status. Of 311 respondents (62% response rate), we included the 246 who indicated that they administer chemotherapy (Table 1).
Table 1.
Respondent Demographic Characteristics
| Characteristic | No. | % |
|---|---|---|
| Median age, years | 40-49* | |
| Male sex | 61 | |
| Median time in practice, years | 11-15* | |
| Practice in a university setting | 187 | 76 |
| Tumor type treated | ||
| Solid | 115 | 47 |
| Hematologic | 94 | 38 |
| Both | 33 | 13 |
Respondents were requested to indicate which of a series of intervals represented their age and years in practice. Thus, we report the median interval, not the true median.
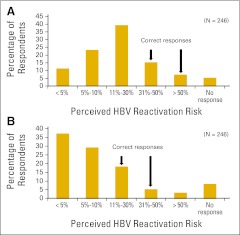
More than half of respondents (51%) were able to correctly identify the prevalence of HBV in Canada as between 1% and 5%; 18% of respondents underestimated the prevalence, 27% overestimated the prevalence, and 4% of respondents did not answer this question (Data Supplement). Respondents underestimated the risk of HBV reactivation during chemotherapy; 40% underestimated the risk in patients with hematologic malignancies, and 32% underestimated the risk in patients with solid tumors (Fig 1). Only 33% of respondents listed birth in an endemic region as a risk factor for HBV carriage, and only 2% were able to correctly identify all continents containing HBV-endemic regions (Appendix Table A1, online only).24
Fig 1.
Respondents' estimation of the risk of hepatitis B virus (HBV) reactivation in patients with (A) hematologic and (B) solid malignancies.
Slightly more than half of respondents (58%) reported screening for HBV before chemotherapy (36% employed selective screening; 22% employed universal screening). Forty percent of respondents never or rarely screened their patients. Respondents were not asked to specify which HBV screening tests they employed. The most commonly cited reasons for not testing were a perception that HBV incidence was low in the respondent's practice, and a perceived absence of guidelines or evidence recommending HBV testing. Only 27% of respondents indicated that they were aware of existing guidelines regarding HBV screening before chemotherapy.
In multivariate analysis, having a predominantly hematologic practice, practitioner experience with HBV reactivation, ability to correctly identify the risk of HBV reactivation with chemotherapy, fewer years in practice, and female sex were independently associated with an increased likelihood of screening for HBV (Table 2).
Table 2.
Practitioner Variables Associated With Increased Likelihood of Testing for HBV Before Chemotherapy
| Predictor Variable | OR | 95% CI |
|---|---|---|
| Predominantly hematologic v solid tumor practice | 8.2 | 4.1 to 16.5 |
| Previous experience with HBV reactivation | 3.8 | 1.9 to 7.6 |
| Able to correctly identify risk of HBV reactivation after treatment of solid tumor or hematologic malignancy | 3.3 | 1.6 to 6.9 |
| Years in practice (≤ 10 v > 10) | 2.2 | 1.1 to 4.3 |
| Female sex | 2.1 | 1.0 to 4.1 |
Abbreviations: HBV, hepatitis B virus; OR, odds ratio.
Discussion
Our survey detected a relatively low rate of HBV screening among Canadian medical oncologists and hematologists. Forty percent of respondents reported rarely or never screening for HBV. Among those respondents who did report screening for HBV, a majority reported employing targeted HBV screening. Of concern, however, most respondents demonstrated relatively little knowledge of the major risk factor for HBV carriage, making it unlikely that they would be able to employ targeted HBV screening successfully.
To our knowledge, this is the only study examining the knowledge, attitudes, and practices of Canadian hematologists/oncologists regarding HBV reactivation. A similar study was conducted in the United States involving oncologists registered with the American Medical Association. Although limited by a low response rate (5%), it showed similar findings: 20% of respondents reported never screening patients for HBV before chemotherapy, 38% reported only screening in the presence of abnormal liver biochemistry results, and 30% reported screening in the presence of risk factors or a known history of hepatitis.25
We identified several factors that may contribute to a low level of HBV screening among hematologists and oncologists. First, in our survey, physicians tended to underestimate the risk of HBV reactivation in both solid and hematologic tumors. For instance, most respondents estimated the risk of HBV reactivation in patients receiving chemotherapy for a hematologic malignancy as being less than 30%, whereas the literature suggests that the actual risk is greater than 50% in this population.10,11 Second, knowledge regarding the major risk factor for HBV carriage (ie, birth in an endemic area) seemed to be low. Only one third of respondents listed country of birth as a risk factor, which is the most important risk factor for chronic HBV infection.26 Third, few respondents were aware of existing guidelines regarding HBV screening in patients receiving chemotherapy.
Our findings suggest that practitioners with a predominantly hematologic practice were more likely to screen for HBV. This finding may result from the fact that there is substantially more literature on HBV reactivation in hematologic malignancies compared with solid tumors, and the risk is reported to be greater in this population.17,27,28 Practitioners who had previous experience with HBV reactivation, along with those who provided accurate estimates of the true risks of HBV reactivation, were also more likely to test for HBV. In addition, fewer years in practice and female sex were both associated with increased tendency to test for HBV before chemotherapy. These results are consistent with previous reports that physicians who are more distant from their training are less likely to follow guidelines,29 and female physicians may be more focused on preventive measures.30
Our study is limited by its reliance on physicians' self-reporting and recall. It is possible that actual screening rates are different from reported rates. We believe that our data likely reflect conservative estimates of actual practice, because recall bias may overestimate the rate of HBV screening, and the true rate may be lower than reported. We have previously reported that actual HBV screening rates in an inner-city Canadian oncology clinic were only 14%.31
Our study suggests that HBV screening before chemotherapy is not widely practiced among Canadian oncologists and hematologists. Targeted testing of patients at high risk of HBV was the screening strategy favored by respondents. Yet, risk factors for HBV carriage were not widely recognized, potentially undermining the success of this strategy. These findings are important because HBV reactivation is potentially fatal and is largely preventable. Two recent studies also suggest that in the setting of adjuvant chemotherapy32 and chemotherapy for lymphoma,33 HBV screening may also be cost effective. If our findings are accurate, there may be an unrealized potential to prevent HBV reactivation among Canadian patients with cancer. This potential might be realized through education of oncologists/hematologists regarding risk factors for HBV carriage, or through wider adoption of universal screening. Further research investigating actual patient outcomes with different HBV screening strategies is needed.
Supplementary Material
Acknowledgment
Supported by St Michael's Hospital. C.M.B. is supported by a Canadian Institutes of Health Research and Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care.
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010. We thank Ahmed Bayoumi, MD, MSc, and Kelvin Chan, MD, MSc for critically reviewing the manuscript.
Appendix
Table A1.
Geographic Regions Endemic for HBV
| Geographic Region* |
|---|
| Africa |
| Asia |
| Eastern Europe |
| South America |
NOTE. Data adapted (Centers for Disease Control and Prevention; http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/hepatitis-b.htm).
Abbreviation: HBV, hepatitis B virus.
≥ 2% of population affected.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Ronita S.M. Lee, Lisa K. Hicks
Collection and assembly of data: Ronita S.M. Lee
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.World Health Organization 2008. Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en. [Google Scholar]
- 2.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recommend Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 3.Minuk G, Uhanova J. Chronic hepatitis B infection in Canada. Can J Infect Dis. 2001;12:351–356. doi: 10.1155/2001/650313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang R, Lau GK, Kwong YL. Chemotherapy and bone marrow transplantation for cancer patients who are also chronic hepatitis B carriers: A review of the problem. J Clin Oncol. 1999;17:394–398. doi: 10.1200/JCO.1999.17.1.394. [DOI] [PubMed] [Google Scholar]
- 5.Yeo W, Ho WM, Hui P, et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2004;88:209–215. doi: 10.1007/s10549-004-0725-1. [DOI] [PubMed] [Google Scholar]
- 6.Dai MS, Wu PF, Shyu RF, et al. Hepatitis B virus reactivation in breast cancer patients undergoing cytotoxic chemotherapy and the role of preemptive lamivudine administration. Liver Int. 2004;24:540–546. doi: 10.1111/j.1478-3231.2004.0964.x. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulos CG, Vaslamatzis M, Hatzidimitriou G. Prevalence of hepatitis B virus marker positivity and evolution of hepatitis B virus profile, during chemotherapy, in patients with solid tumors. Br J Cancer. 1999;81:69–74. doi: 10.1038/sj.bjc.6690652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: A prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299–307. doi: 10.1002/1096-9071(200011)62:3<299::aid-jmv1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Idilman R, Arat M, Soydan E, et al. Lamivudine prophylaxis for prevention of chemotherapy-induced hepatitis B virus reactivation in hepatitis B virus carriers with malignancies. J Viral Hepat. 2004;11:141–147. doi: 10.1046/j.1365-2893.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 10.Lok AS, Liang RH, Chiu EK, et al. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy: Report of a prospective study. Gastroenterology. 1999;100:182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Hsiung CA, Su IJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320–1328. doi: 10.1053/jhep.2003.50220. [DOI] [PubMed] [Google Scholar]
- 12.Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: A randomized trial. Hepatology. 2008;47:844–853. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 13.Yeo W, Chan PK, Ho WM, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927–934. doi: 10.1200/JCO.2004.05.161. [DOI] [PubMed] [Google Scholar]
- 14.Li YH, He YF, Jiang WQ, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006;106:1320–1325. doi: 10.1002/cncr.21701. [DOI] [PubMed] [Google Scholar]
- 15.Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziakas PD, Karsaliakos P, Mylonakis E. Effect of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in lymphoma: A meta-analysis of published clinical trials and a decision tree addressing prolonged prophylaxis and maintenance. Haematologica. 2009;94:998–1005. doi: 10.3324/haematol.2009.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubel JS, Testro AG, Angus PW. Hepatitis B virus reactivation following immunosuppressive therapy: Guidelines for prevention and management. Intern Med J. 2007;37:705–712. doi: 10.1111/j.1445-5994.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 18.Katz LH, Fraser A, Gafter-Gvili, et al. Lamivudine prevents reactivation of hepatitis B and reduces mortality in immunosuppressed patients: Systematic review and meta-analysis. J Viral Hepat. 2008;15:89–102. doi: 10.1111/j.1365-2893.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 19.Sherman M, Shafran S, Burak K, et al. Management of chronic hepatitis B: Consensus guidelines. Can J Gastroenterol. 2007;21(suppl):5C–24C. [PMC free article] [PubMed] [Google Scholar]
- 20.Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 21.Canadian Medical Association 2012. Statistical information on Canadian physicians: Physicians by province/territory, subspecialty, Canada. http://www.cma.ca/multimedia/CMA/Content_Images/Inside_cma/Statistics/01Spec&Prov.pdf. [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Logistic Regression. ed 2. New York, NY: Wiley; 2000. [Google Scholar]
- 23.Austin PC, Tu JV. Automated variable selection methods for logistic regression produced unstable models for predicting acute myocardial infarction mortality. J Clin Epidemiol. 2004;57:1138–1146. doi: 10.1016/j.jclinepi.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention 2012. Infectious diseases related to travel. http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/hepatitis-b.htm. [Google Scholar]
- 25.Tran TT, Rakoski MO, Martin P, et al. Screening for hepatitis B in chemotherapy patients: Survey of current oncology practices. Aliment Pharmacol Ther. 2010;31:240–246. doi: 10.1111/j.1365-2036.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- 26.Chang MH. Hepatitis B virus infection. Semin Fetal Neonatal Med. 2007;12:160–167. doi: 10.1016/j.siny.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Lubel JS, Angus PW. Hepatitis B reactivation in patients receiving cytotoxic chemotherapy: Diagnosis and management. J Gastroenterol Hepatol. 2010;25:864–871. doi: 10.1111/j.1440-1746.2010.06243.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu CJ, Chen PJ, Chen DS, et al. Hepatitis B virus reactivation in patients receiving cancer chemotherapy: Natural history, pathogenesis, and management. Hepatol Int. doi: 10.1007/s12072-011-9279-6. [epub ahead of print on June 14, 2011] [DOI] [PubMed] [Google Scholar]
- 29.Bikdeli B, Sharif-Kashani B, Raeissi S, et al. Chest physicians' knowledge of appropriate thromboprophylaxis: Insights from the PROMOTE study. Blood Coagul Fibrinolysis. 2011;22:667–672. doi: 10.1097/MBC.0b013e32834ad76d. [DOI] [PubMed] [Google Scholar]
- 30.Lurie N, Slater J, McGovern P, et al. Preventive care for women: Does the sex of the physician matter? N Engl J Med. 1993;329:478–482. doi: 10.1056/NEJM199308123290707. [DOI] [PubMed] [Google Scholar]
- 31.Lee R, Vu K, Bell CM, et al. Screening for hepatitis B surface antigen before chemotherapy: Current practice and opportunities for improvement. Curr Oncol. 2010;17:32–38. doi: 10.3747/co.v17i6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumours. J Clin Oncol. 2011;29:3270–3277. doi: 10.1200/JCO.2011.35.1635. [DOI] [PubMed] [Google Scholar]
- 33.Zurawska U, Hicks LK, Woo G, et al. Screening for hepatitis B virus (HBV) prior to chemotherapy: A cost-effectiveness analysis. J Clin Oncol. 2011;29(suppl):398S. doi: 10.1200/JCO.2011.40.7510. abstr 6059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.