In a time of rapid change in practice and research in cancer, ASCO can play a pivotal role in patient care through major revisions to guideline development, accessibility, and integration with quality metrics.
Abstract
ASCO produces guidelines for oncologists, utilizing a systematic review process. Although this resource-intense process results in authoritative and widely cited guidelines, they can cover only a few specific clinical issues. Hence, the ASCO guidelines presently do not fully address many clinical situations. Expanding the scope of ASCO guidelines will require major revisions to the guidelines development process. Changes likely to improve the process include establishing disease-specific committees composed of content experts, improving methods to resolve conflicts of interest, simplifying steps to engage members to suggest topics for new guidelines, and linking guidelines utilization with quality indices. In a time of rapid change in practice and research in cancer, ASCO can play a pivotal role in patient care through major revisions to guideline development, accessibility, and integration with quality metrics.
Introduction
Utilization of evidence-based guidelines can improve cancer care by helping to bring clinical practice in line with the state of the art in oncology and support quality improvement initiatives within practices.1 The 2011 Institute of Medicine (IOM) report, Clinical Practice Guidelines We Can Trust,2 defined clinical practice guidelines as “statements that include recommendations intended to optimize patient care that are informed by a systematic review of the evidence and an assessment of the benefits and harms of alternative care options.” Scientific advancements are being made rapidly, and evidence-based, clinical practice guidelines are of paramount importance.
Since 1994, ASCO has developed a series of documents to provide guidance to the oncology community. ASCO guidelines are created by multidisciplinary teams; the process adheres to a strict conflict of interest (COI) policy and a rigorous systematic review methodology. The ASCO Clinical Practice Guidelines Committee (CPGC) oversees guideline development and the creation of expert panels for input to that process. The ASCO guidelines, like all such documents, are intended to improve patient care. The ASCO guidelines are utilized by physicians in private health care settings, academic medical centers, and health maintenance organizations, as well as by third-party payers and policy makers. Published guidelines are among the articles most commonly accessed in Journal of Clinical Oncology (JCO) and Journal of Oncology Practice (JOP). In prior surveys of ASCO membership, the ASCO guidelines have ranked as the third most important service provided by ASCO to members, ranking only behind JCO and the ASCO annual meeting. Yet, data gathered from the ASCO membership have also highlighted certain limitations of the guidelines. In particular, ASCO guidelines have been criticized for their limited scope,3 lack of timeliness, and difficulty of access.4
Apart from the ASCO guidelines, oncologists have alternative resources for information on delivering high-quality care to their patients. The guidelines developed by the National Comprehensive Cancer Network (NCCN) are a resource commonly used by oncologists. The NCCN guidelines, like those produced by ASCO, are frequently referenced by insurance companies for reimbursement purposes.5 Some oncology groups develop and follow their own cancer guidelines designed to meet the needs of their patients and practice, or adopt existing guidelines for their setting. Guidelines are generated by a wide range of methods. The algorithm for identifying the data to be reviewed and for the panel to build consensus on that review is unique to each organization. NCCN has formed disease-specific panels that use clinical evidence and consensus opinion to formulate their guideline product. In a recently published analysis of the NCCN guidelines, only 6% of guidelines were classified as level I evidence, whereas 83% were level IIA (lower level of evidence with universal consensus).6 Although there are many clinical situations in oncology for which level I evidence to guide decision making is lacking, clear reporting of how recommendations are reached in these scenarios is crucial to their use. The importance of making a guideline trustworthy is addressed in the recent IOM report on guidelines,2 and thus the means by which guidelines are developed must be transparent.
Assessing Current Perceptions of ASCO Guidelines
To assess the current perceptions and use of ASCO guidelines, we performed the following tasks: (1) researched the guideline process used by organizations that develop guidelines (2) interacted with the ASCO CPGC, and (3) surveyed clinical oncology providers. These coordinated efforts permitted assessment of the current use of ASCO guidelines in oncology practice, evaluated existing barriers to guideline development, and provided insight into how and where changes might be applied to improve the development and implementation of ASCO guidelines. Listed below are the key findings.
1. Evaluation of the methods used by other organizations to generate guidelines revealed wide variations in identification and resolution of COIs for experts that serve on panels. Another area of variability is the means by which the guideline panel generates consensus and the transparency of that method. Appendix Table A1 (online only) summarizes the process used by prominent international organizations with a formal guideline development process.
2. The ASCO CPGC uses a rigorous methodology to compose the guideline panel, taking into account the balance of content expertise and COIs. There are also defined criteria for the quality of evidence needed to generate a standard guideline versus creating other clinically useful documents such as a Provisional Clinical Opinion (PCO). The steps to forming the panel, data collation, data review, and manuscript preparation are defined within the Committees Guideline Procedure Manual, which is accessible online by ASCO members.
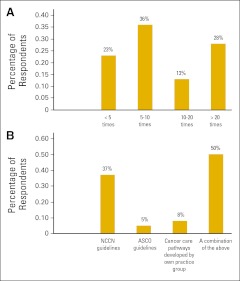
3. Surveys of ASCO membership and focus groups have demonstrated that production of ASCO guidelines is perceived as being a highly important service of ASCO. Yet, satisfaction with the guidelines among members is not high. The results of our recent survey (Appendix Figure A1, online only) suggest that ASCO members value the rigor and transparency of ASCO guidelines but do not find them inclusive and broad enough for routine use. Notably, 77% of respondents reported frequent use of guidelines, with the majority of the respondents using them more than five times a week, but the use of ASCO guidelines was relatively low (Figure 1A). Those surveyed reported use of guidelines from a variety of other sources, with NCCN being the most common (Figure 1B).
Figure 1.
Responses to survey questions (A) How often do you use cancer treatment guidelines in a week? and (B) Which of the following guidelines do you use frequently for making treatment decisions? NCCN, National Comprehensive Cancer Network.
Discussion
One of ASCO's primary missions is to improve cancer care. Guidelines can aid the implementation of evidence-based patient care. A rigorous and transparent guideline development process within ASCO should result in a guideline product that practitioners regularly use in the routine care of patients. Guidelines should be user-friendly, relevant, and up-to-date in order to ensure their use. ASCO guidelines are viewed by clinicians, policy makers, and third-party payers as important resources for evidence-based guidance on specific topics. A negative gap between ratings of guideline importance and ratings of satisfaction with ASCO guidelines was identified in 2009 through a member survey and two focus groups at that year's ASCO Breast Cancer Symposium. In response, ASCO created the PCO and endorsed guidelines generated by other organizations that utilize similarly rigorous guideline development methodology. In addition, the ASCO guideline Web page was redesigned to improve ease of navigation, and the guidelines include executive summaries and are disseminated through JCO and JOP. The guidelines also are accessible through slide sets available on the ASCO Clinical Tools and Resource Web site, and patient educational guideline materials are available on Cancer.net. However, recent survey data indicate that a need remains for ASCO guidelines to cover more clinical scenarios and to be more readily accessible during a busy patient encounter. Survey data demonstrate that additional steps are needed to assist oncologists in offering patients the best possible treatment.
Critique of the Current Guidelines
1. Limited scope.
ASCO guidelines presently cover limited indications and focus largely on clinical situations for which level I evidence exists. There are notable exceptions, including the ASCO recommendations for fertility preservation.7
Potential solutions.
We view this as the most challenging step in the guideline development process. There is no question that patient care decisions should be based on the strongest level of evidence available. However, in this rapidly changing field, many clinical situations lack robust evidence on which to base decisions. In such situations, practicing oncologists clearly prefer an expert opinion for guidance to no guideline. It will be necessary for ASCO to adopt a pragmatic approach to balance between requiring level I evidence for guidelines and using consensus opinions if the needs of the membership are to be fully met. We recommend the appointment of standing panels of experts for each disease site that will be charged with reviewing the literature and developing new guidelines and/or consensus statements for management of common clinical situations.
2. Accessibility.
The ASCO documents are available in print and online; however, the presentation format does not permit rapid and focused reading. During a patient visit it is not practicable to access the guidelines.
Potential solutions.
The guidelines are promoted in ASCO media such as ASCO Connection. The ASCO Express is e-mailed to all ASCO members and announces new guidelines, PCOs, and other guidance documents. In addition, the ASCO Annual Meeting may have educational sessions or other symposia focusing on new guidelines. ASCO has established a working group within the CPGC to create a more accessible and nimble approach to guidelines dissemination for practicing ASCO members via www.asco.org. These approaches are being fully vetted by the CPGC with active support from ASCO's Board of Directors and include Web-based platforms that may allow for input from general ASCO membership and stakeholders during guideline creation. These actions increase the dissemination of information, but the ASCO guidelines remain dense documents that may not be suitable for use “on the go” because the current online format is essentially the same as the printed materials. There is a clear need to develop more user-friendly presentation of the guidelines for the Web, with appropriate linkage to ongoing clinical trials, references, and other resources that providers could use to improve patient care.
3. Generating new guidelines is a slow process.
Populating the guideline panel of experts on the subject matter while balancing potential COIs is time consuming, and generating the protocol to guide the literature search and literature review is laborious. Arriving at consensus on the interpretation of the data and writing the guideline require highly coordinated efforts and time. Until recently, the systematic review process used by ASCO, while highly valued, required that there be sufficient high-quality evidence on which to base recommendations. It also required a great deal of work on the part of from ASCO staff and volunteers, which limited the number of guidelines that could be produced. Because ASCO policies mandate that 51% of an expert panel have no COI, this has further limited the ability of ASCO to create these groups, increasing the time required to create each individual guideline.
Potential solutions.
The desire of the membership for broadening the scope of ASCO guidelines has been previously documented.4 ASCO has responded by increasing the number of guidelines and by giving official endorsement to systematic review guidelines of other organizations, when appropriate. In addition, ASCO is diversifying the types of guidance documents being made, such as the new format of the PCO. If ASCO were to move toward the creation of standing committees in each disease area, while adhering to the ASCO COI policy, these expert panels might be poised to move more rapidly forward in creating the desired document. The standing subcommittees of the CPGC might also facilitate the initial assignment of a proposed topic to the most appropriate type of ASCO document (guideline v PCO v other). These standing subcommittees could help facilitate the building of consensus when evidence of the highest level is not available in the literature. Producing guidelines is labor intensive, and both ASCO staff and ASCO volunteer resources must be available to complete the task.
4. Process to request new guidelines is cumbersome.
The process by which guidelines are requested may not be apparent to the general membership and leaves the CPGC, in collaboration with the ASCO Board, to make most recommendations for guideline topics. The suggested guideline topic must be drafted within a detailed ASCO template document, and the cumbersome paperwork may be a deterrent to some members.
Potential solutions.
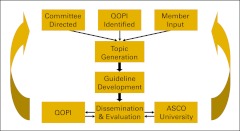
Revising the guidelines request process for the membership would increase participation and facilitate the creation of desired guidelines. This process could be simplified by modifications to the ASCO Web site that provide additional transparency to the ASCO guideline process and the status of current guidelines under development (Figure 2). The need for a new guideline could also be prompted by the Quality Oncology Practice Initiative (QOPI). When practice patterns show divergence within the oncology community, the perceived lack of clear guidance on a clinical issue can identify the need for a new guideline.
Figure 2.
Proposed algorithm for guideline development. QOPI, Quality Oncology Practice Initiative.
5. Hurdles to engagement by membership.
ASCO members are not able to provide feedback on current guidelines or evaluate the impact of guidelines on patient care. This might result in guidelines being perceived as somewhat remote, and less relevant to their day-to-day practice.
Potential solutions.
After the creation of a new guideline, subsequent QOPI data could then be used to assess the utilization of those guidelines. The 2011 IOM report2 recommends the implementation of guidelines developed by systematic review and advocates for rigorously developed clinical practice guidelines that have the power to translate the complexity of scientific research into clinical recommendations and potentially enhance health care quality and outcomes. Evaluating the success of clinical practice guidelines goes beyond measuring how frequently such documents are accessed; rather, it requires determining whether the guidelines improve patient care.8,9 QOPI assesses clinical practice for concordance with established ASCO guidelines and provides metrics to oncologists regarding their performance. As of spring 2012, 117 oncology practices across the country had been certified, and many more were undergoing the certification process. Implementation of QOPI has now been shown in some realms—specifically end-of-life care—to improve practice patterns and thus improve treatment.10
ASCO is poised to provide clinical practice guidance and feedback to the clinicians on the successful implementation of best care practices. ASCO University and the Self Evaluation Program of the American Board of Internal Medicine utilize ASCO guidelines and provide direct feedback to the clinical care providers.
A proposed simplified algorithm for ASCO guideline development is shown in Figure 2. This process is rooted in ASCO membership participation, starting with input on guideline topic selection and going full circle with QOPI metrics to assess how well the guidance document aided in the delivery of patient care. The creation of disease-based standing committees would allow for volunteer members to become well versed in the guideline process and could speed the development and dissemination of new guidance documents. A regular evaluation process should be incorporated to ensure a timely and iterative improvement in these guidelines as new evidence becomes available.
Conclusions
The establishment of clinical practice guidelines aids in addressing clinical questions and informing the patient and provider of their treatment choices. Oncologists are committed to providing excellent care, and ASCO is uniquely positioned to take a lead role in developing state-of-the-art oncology clinical practice guidelines. As cancer care continues to become more sophisticated and personalized, the need for evidence-based guidelines will increase exponentially, as will the potential speed with which practitioners demand such guidance. ASCO serves as a forum for dissemination of high-quality research and has the breadth and depth of interdisciplinary experts to generate guidelines developed in accordance with the IOM report. In addition, QOPI, ASCO University, and the Self Evaluation Program may provide clinicians with the metrics to evaluate implementation of best practices. ASCO resources need to be utilized effectively so that high-quality care for patients with cancer can be delivered universally, but this will require the development of a more facile approach to guidelines development that may include standing panels of experts in addition to the linkage with quality metrics evaluations. Because membership has ranked guidelines as the third most important benefit of their membership, and recognizing that members look to other organizations for their guideline programs, ASCO needs to reevaluate their dedication of resources to this process. ASCO must also leverage its unique ability to conduct rigorous evaluations of the literature by highly trained staff and volunteers with clearly documented COIs and to tie its guidelines to a trusted quality management program.
Acknowledgment
We thank the ASCO Leadership and Development staff.
Appendix
Survey Methodology
A paper survey to be completed “by hand” was distributed to participants of the CPC Fall 2010 meeting (Alexandria, VA) and an electronic format for the survey was distributed at the Georgia State Society meeting in the fall of 2010. The results of all surveys were compiled and are reported in a descriptive manner. No formal statistical methods were used.
Survey Questions
- (1) What is your professional background?
- Medical oncology/hematology
- Physician-other specialties
- Pharmacist
- RN/NP/PA
- Laboratory-based researcher
- Patient advocate
- Other
- (2) Which of the following guidelines do you use frequently for making treatment decisions?
- NCCN guidelines
- ASCO guidelines
- Cancer care pathways developed by own practice group
- A combination of the above
- (3) When you refer to a guideline do you choose that guideline because of it is:
- Accessible on the web
- Easy to navigate through
- Tied to reimbursement
- Evidence based
- Comprehensive
- Multidisciplinary
- (4) When you refer to a guideline what is the next most important reason you choose that guideline:
- Accessible on the web
- Easy to navigate through
- Tied to reimbursement
- Evidence based
- Comprehensive
- Multidisciplinary
- (5) How often do you use cancer treatment guidelines in a week?
- Fewer than 5 times
- 5 to 10 times
- 10 to 20 times
- More than 20 times
- (6) How many times in the past 1 month have you turned to ASCO for guidance on patient care?
- 0
- 1 to 4
- 5 to 10
- More than 10
- (7) Which of the following do you prefer regarding guidelines?
- They should be strictly evidence based
- They should be based on best possible information and expert opinion
- (8) When definitive evidence does not support making a recommendation, which of the following do you prefer?
- If there is no proven evidence-based on objective trials data, there should not be any formal recommendation
- I would like a recommendation from a panel of experts based on their practice
- Any help is appreciated
- (9) Which of the following ASCO resources do you use to provide patient care?
- Practice Guidelines (rigorous, evidence-based review of the literature)
- Provisional Clinical Opinion (a rapid response to emerging data in clinical oncology)
- Clinical Evidence Review (examines areas of interest to community where there is clinical uncertainly and practice variation, and insufficient data to inform practice recommendations.)
- Clinical Tools and Resources (slide sets, tables, surveillance flow sheets)
- All of the above
- None of the above
- (10) Which of the following ASCO resources would you want to see more of?
- Practice Guidelines
- Provisional Clinical Opinion (a rapid response to emerging data in clinical oncology)
- Clinical Evidence Review (examines areas of interest to community where there is clinical uncertainly and practice variation, and insufficient data to inform practice recommendations.)
- Clinical Tools and Resources (slide sets, tables, surveillance flow sheets)
- 1 and 2
- 1 and 3
- 2 and 3
- 1 and 4
- All of the above
Table A1.
Summary of the Guideline Development Process Adopted by Key Organizations
| Agency | Method/Basis | No. of Guidelines | Scope of Guidelines | Method of Evaluation |
|---|---|---|---|---|
| ASCO | Expert panel and CGPC | < 50 | Exhaustive on selected topics | Quality Indicators (QOPI) |
| NCCN | Expert panel | 50-100 | Bulleted algorithms for most cancer scenarios | Undefined |
| Cancer Care Ontario | Expert panel | > 100 | Concise summary of systematic review | Quality Indicators |
| ESMO | Expert panel | 50-100 | Review paper | Undefined |
| American Urologic Association | Expert panel | < 50 | Bulleted outlines | Undefined |
| American College of Cardiology | Expert panel | < 50 | Exhaustive on selected topics | Reporting mechanism, but not built into guidelines |
| World Health Organization | Department-produced guidelines | 50-100 | Exhaustive on selected topics | Desired outcomes are identified but not tested |
Abbreviations: ASCO, American Society of Clinical Oncology; CGPC, Clinical Guideline Practice Committee; ESMO, European Society of Medical Oncology; NCCN, National Comprehensive Cancer Network; QOPI, Quality Oncology Practice Initiative.
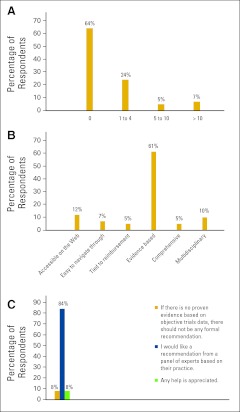
Figure A1.
Percentage responses to survey questions (A) How many times in the past 1 month have you turned to ASCO for guidance on patient care? (B) When you refer to a guideline do you choose that guideline because it is ___? and (C) When definitive evidence does not support making a recommendation, which of the following do you prefer?
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Melissa Dillmon, John M. Goldberg, Suresh S. Ramalingam, Robert J. Mayer, Catherine Van Poznak
Administrative support: Robert J. Mayer
Collection and assembly of data: Melissa Dillmon, John M. Goldberg, Suresh S. Ramalingam, Catherine Van Poznak
Data analysis and interpretation: John M. Goldberg, Suresh S. Ramalingam, Robert J. Mayer, Catherine Van Poznak
Manuscript writing: Melissa Dillmon, John M. Goldberg, Suresh S. Ramalingam, Patrick Loehrer, Catherine Van Poznak
Final approval of manuscript: All authors
References
- 1.Bennett CL, Somerfield MR, Pfister DG, et al. Perspectives on the value of American Society of Clinical Oncology clinical guidelines as reported by oncologists and health maintenance organizations. J Clin Oncol. 2003;21:937–941. doi: 10.1200/JCO.2003.07.165. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Clinical practice guidelines we can trust. http://www.iom.edu/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx. [Google Scholar]
- 3.Balaban EP, et al. Issues in clinical practice guideline implementation and utilization. Am Soc Clin Oncol Ed Book. 2009:338–344. [Google Scholar]
- 4.Somerfield MR, Einhaus K, Hagerty KL, et al. American Society of Clinical Oncology clinical practice guidelines: Opportunities and challenges. J Clin Oncol. 2008;26:4022–4026. doi: 10.1200/JCO.2008.17.7139. [DOI] [PubMed] [Google Scholar]
- 5.Danielson E, Demartino J, Mullen JA. Managed care and medical oncology: The focus is on value. J Natl Compr Canc Netw. 2010;8:S28–S37. doi: 10.6004/jnccn.2010.0132. [DOI] [PubMed] [Google Scholar]
- 6.Poonacha TK, Go RS. Level of scientific evidence underlying recommendations arising from the National Comprehensive Cancer Network clinical practice guidelines. J Clin Oncol. 2011;29:186–191. doi: 10.1200/JCO.2010.31.6414. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in people treated for cancer. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DW, Craig W, Brant R, et al. A cluster randomized controlled trial comparing three methods of disseminating practice guidelines for children with croup [ISRCTN73394937] Implement Sci. 2006;1:10. doi: 10.1186/1748-5908-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimshaw J, Shirran L, Thomas R, et al. Changing provider behavior: An overview of systematic reviews of interventions. Med Care. 2001;39(suppl 2)(8):2-II. [PubMed] [Google Scholar]
- 10.Campion FX, Larson LR, Kadlubek PJ, et al. Advancing performance measurement in oncology: Quality Oncology Practice Initiative participation and quality outcomes. J Oncol Pract. 2011;7(suppl):31s–35s. doi: 10.1200/JOP.2011.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]