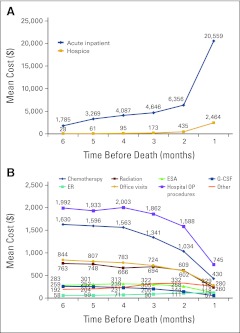
Oncology costs increase in the last 6 months before death largely because of increased inpatient costs, whereas outpatient costs decrease.
Abstract
Purpose:
With rising health care costs in the United States, clearly defined end-of-life (EOL) cancer costs are needed to help health administrators proactively manage this important care. Our objective was to examine EOL health care resource costs among oncology patients in a US commercial insurance population.
Methods:
A retrospective claims database affiliated with OptumInsight was analyzed. Included patients had: a medical claim with cancer diagnosis between July 1, 2002, and December 31, 2009; death on or before December 31, 2009; continuous enrollment with medical/pharmacy benefits from diagnosis until death; ≥ 180 follow-up days; and active cancer in the last 6 months before death (MBD). Death was captured from facility discharge codes or Social Security Administration death files. Costs were determined by summing paid amounts on all services utilized within the last 6 MBD: cancer-related inpatient (IP) stays, cancer- related hospice care, and cancer-related outpatient (OP) services (ie, chemotherapy, erythropoiesis-stimulating agents, granulocyte colony-stimulating factors, radiation, cancer-related office or emergency room visits, cancer-related hospital OP procedures, and other services with cancer diagnosis).
Results:
A total of 28,530 patients met inclusion criteria. Mean total cancer-related costs in the last 6 MBD were $74,212 (standard deviation, $112,740), comprising IP costs of $40,702 (55%), OP costs of $30,254 (41%), and hospice costs of $3,256 (4%). OP costs decreased from $6,021 in the sixth MBD to $2,238 in the last MBD, whereas IP care costs increased from $1,785 to $20,559. Hospice utilization increased from 0.7% in the sixth MBD to 35.6% in the last MBD.
Conclusion:
Oncology costs increase in the last 6 MBD largely because of increased IP costs, whereas OP costs decrease.
Introduction
Cancer in the United States has been identified as the second most costly medical condition after heart disease.1 As a result of the dramatic increase in cost and extent of care,2,3 annual direct cancer costs are projected to rise from $104 billion in 20064 to > $173 billion in 2020 and beyond.5 Total cancer-related costs vary by tumor type and stage at diagnosis,6,7 with the highest costs incurred in the last year of life.8 Oncology treatment and care have been rapidly changing over the past two decades, and this study characterizes which services the current cancer care dollar is being spent on immediately before patient death.
Medicare studies using data from the 1970s and 1980s7,9 demonstrated that cancer end-of-life (EOL) costs are burdensome because the 5% to 6% of beneficiaries who died each year consumed 27% to 30% of the annual Medicare payments (mean cost, $13,316 per beneficiary death per year).7 Most of these costs were the result of life-sustaining care, with 78% of costs accrued from acute care in the final 30 days of life.9 Carlson et al10 found that patients who disenrolled from hospice were more likely to be hospitalized, admitted to the emergency room (ER) or intensive care unit, and die in the hospital compared with patients who remained enrolled in hospice until death; significantly higher ($124 per day more; P < .01) Medicare expenditures were found for the hospice disenrollees.
Costs of EOL cancer care in the United States have been well defined in the Medicare population, although with relatively older historical data. These costs have been found to be substantial and to vary by tumor type, site of malignancy and metastasis, phase of care, stage at diagnosis, and survival. Little research on EOL cancer care costs has been conducted in the commercially insured population. This study examines EOL costs and related health care resource utilization among a commercially insured oncology patient population who died between July 1, 2002, and December 31, 2009. The analysis of cost was completed from the perspective of the payers (ie, the patient and the health plan).
Methods
Data Sources
Medical and pharmacy claims and enrollment information from the Life Sciences Research Database, a large, geographically diverse, proprietary research database affiliated with OptumInsight, were accessed for this study. The study data were de-identified and accessed in accordance with the Health Insurance Portability and Accountability Act of 1996,11 and therefore, institutional review board or privacy board approval was not required.
Medical claims, sourced from industry-standard forms (eg, UB-92 and HCFA1500), were collected from all available health care sites for all types of provided services; they included: multiple diagnosis codes recorded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), procedures recorded with ICD-9-CM procedure codes, Current Procedural Terminology codes, or Healthcare Common Procedure Coding System codes; site of service codes; paid amounts; and other information.
Claims for pharmacy services were submitted electronically by various pharmacies. The pharmacy claims history is a profile of all outpatient prescription pharmacy services provided and covered by a health plan, including: drug name, dosage form, drug strength, fill date, number of days of supply, and paid amounts.
Date of death was obtained from the Social Security Administration master death file or facility discharge codes and linked to claims data based on a patient's social security number, name, and birth date. To ensure that data remained de-identified, only date of death without any other personal identifiable information was retained in this study.
Study Patient Identification
Patients were considered for inclusion if they had a medical claim with a cancer diagnosis between July 1, 2002, and December 31, 2009. The index date was defined as the service date from the first medical claim with a cancer diagnosis during this period. All patients were required to have non–rule-out cancer, defined as ≥ two separate medical claims with a cancer diagnosis, with service dates ≥ 42 days apart. Cancer diagnosis codes could occur in either a primary or secondary position on the claim and included ICD-9 codes: 140.xx to 172.xx, 174.xx to 209.xx, 230.xx to 231.xx, 233.xx to 234.xx, and 238.7x. Alternatively, if a second claim with a cancer diagnosis was not identified, patients were considered to have non–rule-out cancer if there was evidence in the medical claim that they were receiving chemotherapy, radiation, or cancer-related surgery.
In addition to the requirements for identification of a malignancy, study patients had to have evidence of death on or before December 31, 2009, continuous enrollment with both medical and pharmacy benefits from the index date until the date of death, at least 180 days of follow-up from index cancer diagnosis before death, and evidence of non–rule-out cancer during the last 6 months of life. Female patients with diagnosis codes for prostate cancer and men with diagnosis codes for breast, uterine, ovarian, or cervical cancer were excluded.
Study Measures
Outcomes.
All cost measures were computed as the combined health plan– and patient-paid amounts for each claim and were adjusted for inflation to 2009 US dollars by the medical component of the Consumer Price Index. The cost of cancer-related services comprised: medical and surgical acute cancer-related inpatient stays, cancer-related hospice care (inpatient or outpatient), outpatient chemotherapy, supportive care, cancer-related office or ER visits, cancer-related hospital outpatient procedures, and other services with a diagnosis of cancer. Cancer-related inpatient stays were defined as all inpatient stays with a cancer diagnosis or administration of chemotherapy and supportive care or radiation therapy at some time during the hospitalization. Supportive care was defined as the use of erythropoiesis-stimulating agents (ESAs) and/or granulocyte colony-stimulating factors (G-CSFs). Office visits, ER visits, outpatient services, hospice care, and other services were considered cancer related if a cancer diagnosis appeared in the primary or secondary position of the claim. Outpatient chemotherapy, supportive care, cancer-related office or ER visits, cancer-related hospital outpatient procedures, and other services with a diagnosis of cancer were further combined and categorized as cancer-related outpatient services.
The cost of cancer-related services (including subcategories) was identified during each of the last 6 months before death (MBD). One month was defined as 30 days. Costs for each month were mutually exclusive, and as such, the cost of inpatient stays that spanned ≥ 2 months was attributed to the month in which the patient was discharged from the facility.
Demographic and clinical characteristics.
The type of malignancy on the index date was identified from diagnosis codes (excluding diagnoses on laboratory and radiology claims). Malignancy types included: lung (162.xx, 231.2x); breast (174.xx, 175.xx, 233.0x); colorectal (153.xx, 154.xx, 230.3x, 230.4x); prostate (185.xx, 233.4x); uterine, cervical, and ovarian (179.xx, 180.xx, 182.xx, 183.xx, 233.1x, 233. 2x); lymphoma (200.xx to 202.xx); leukemia (204.xx to 208.xx); bladder (188.xx, 233.7x); and other (all other cancer codes not classified here). Patients with evidence of more than one type of malignancy on the index date were categorized into only one group by first choosing the malignancy type that was not recorded as other. If there were multiple malignancy types that were not recorded as other, the one in the highest position on the medical claim was used. Patients were further categorized as having either a hematologic malignancy (lymphoma or leukemia) or solid tumor (lung, breast, colorectal, prostate, uterine, cervical, ovarian, or bladder cancer). Patients classified as having an other malignancy type included those with all other tumors not specified here.
Metastatic sites were identified from diagnosis codes for patients with evidence of metastases during the follow-up period. Five mutually exclusive groupings were created: brain metastases: all patients with brain metastases (198.3x to 198.4x) with or without evidence of other metastasis; liver metastases: no evidence of brain but with liver metastases (197.7x); lung metastases: no evidence of brain or liver but with lung metastases (197.0x to 197.3x); bone metastases: no evidence of brain, liver, or lung but with bone metastases (198.5x); and other metastases: no evidence of brain, liver, lung, or bone but with other metastases (196.xx to 198.xx excluding brain, liver, lung, and bone metastases).
Statistical Analyses
Means, standard deviations (SDs), and medians, were calculated for continuous variables, and frequencies and percentages were calculated for categorical variables. The analysis of cost over time was conducted by examining costs in each of the last 6 MBD.
Costs for patients who did not have at least 6 months of data before death were also examined before exclusion from analysis. These comprised patients who either died within 6 months of index diagnosis or those who died within 6 months of health plan enrollment. Mean total costs in the last 6 months were slightly lower ($65,302) in this subsample compared with patients who met all inclusion criteria ($74,212). However, because patients had < 6 months of data, the cost per month was greater. Also, the distribution of costs was similar to that found in our study sample; therefore, detailed costs among patients in this subsample are not included.
Results
Sample Identification
There were a total of 912,712 patients with cancer who had medical claims between July 1, 2002, and December 31, 2009. After applying inclusion and exclusion criteria, the final sample consisted of 28,530 patients. Mean age was 61.5 years (SD, 13.3), and 54.3% were male (Table 1). Without considering the other classification, the most frequent individual type of malignancy was lung cancer (n = 5,115; 17.9%), followed by breast (n = 3,491; 12.2%) and colorectal cancers (n = 2,623; 9.2%). There were 2,581 patients (9.0%) with a hematologic malignancy, 15,736 patients (55.2%) with a solid tumor, and 10,213 patients (35.8%) with an other tumor type. Just less than one quarter of patients (n = 6,431; 22.5%) did not have evidence of metastasis.
Table 1.
Demographic and Clinical Characteristics by Malignancy Type
| Characteristic | Total (N = 28,530) |
Lung (n = 5,115) |
Breast* (n = 3,491) |
Colorectal (n = 2,623) |
Prostate (n = 2,225) |
Uterine, Cervical, or Ovarian (n = 1,496) |
Lymphoma (n = 1,459) |
Leukemia (n = 1,122) |
Bladder (n = 786) |
Other (n = 10,213) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years (continuous) | ||||||||||||||||||||
| Mean | 61.50 | 62.47 | 58.64 | 61.28 | 73.83 | 58.14 | 60.85 | 58.70 | 69.08 | 59.69 | ||||||||||
| SD | 13.31 | 10.35 | 12.39 | 12.41 | 10.19 | 11.88 | 15.15 | 18.35 | 11.49 | 13.40 | ||||||||||
| Sex | ||||||||||||||||||||
| Male | 15,499 | 54.33 | 2,926 | 57.20 | 0 | 0.00 | 1,598 | 60.92 | 2,225 | 100.0 | 0 | 0.00 | 941 | 64.50 | 714 | 63.64 | 622 | 79.13 | 6,473 | 63.38 |
| Female | 13,031 | 45.67 | 2,189 | 42.80 | 3,491 | 100.0 | 1,025 | 39.08 | 0 | 0.00 | 1,496 | 100.0 | 518 | 35.50 | 408 | 36.36 | 164 | 20.87 | 3740 | 36.62 |
| Region | ||||||||||||||||||||
| Northeast | 3,018 | 10.58 | 472 | 9.23 | 380 | 10.89 | 276 | 10.52 | 297 | 13.35 | 152 | 10.16 | 160 | 10.97 | 120 | 10.70 | 104 | 13.23 | 1,057 | 10.35 |
| South | 12,776 | 44.78 | 2,421 | 47.33 | 1,591 | 45.57 | 1,219 | 46.47 | 898 | 40.36 | 672 | 44.92 | 614 | 42.08 | 478 | 42.60 | 319 | 40.59 | 4,564 | 44.69 |
| Midwest | 8,831 | 30.95 | 1,657 | 32.39 | 1,057 | 30.28 | 781 | 29.78 | 644 | 28.94 | 479 | 32.02 | 465 | 31.87 | 363 | 32.35 | 248 | 31.55 | 3,137 | 30.72 |
| West | 3,905 | 13.69 | 565 | 11.05 | 463 | 13.26 | 347 | 13.23 | 386 | 17.35 | 193 | 12.90 | 220 | 15.08 | 161 | 14.35 | 115 | 14.63 | 1,455 | 14.25 |
| Metastasis type | ||||||||||||||||||||
| Brain | 6,877 | 24.10 | 2,191 | 42.83 | 1,429 | 40.93 | 293 | 11.17 | 312 | 14.02 | 180 | 12.03 | 181 | 12.41 | 53 | 4.72 | 89 | 11.32 | 2,149 | 21.04 |
| Liver | 6,808 | 23.86 | 668 | 13.06 | 962 | 27.56 | 1,508 | 57.49 | 252 | 11.33 | 468 | 31.28 | 101 | 6.92 | 29 | 2.58 | 189 | 24.05 | 2,631 | 25.76 |
| Lung | 3,536 | 12.39 | 1,040 | 20.33 | 411 | 11.77 | 197 | 7.51 | 164 | 7.37 | 270 | 18.05 | 107 | 7.33 | 27 | 2.41 | 90 | 11.45 | 1,230 | 12.04 |
| Bone | 2,164 | 7.58 | 278 | 5.43 | 218 | 6.24 | 69 | 2.63 | 606 | 27.24 | 64 | 4.28 | 108 | 7.40 | 33 | 2.94 | 74 | 9.41 | 714 | 6.99 |
| Other | 2,714 | 9.51 | 250 | 4.89 | 180 | 5.16 | 320 | 12.20 | 116 | 5.21 | 425 | 28.41 | 123 | 8.43 | 34 | 3.03 | 134 | 17.05 | 1,132 | 11.08 |
| None | 6,431 | 22.54 | 688 | 13.45 | 291 | 8.34 | 236 | 9.00 | 775 | 34.83 | 89 | 5.95 | 839 | 57.51 | 946 | 84.31 | 210 | 26.72 | 2,357 | 23.08 |
Abbreviation: SD, standard deviation.
Men with breast cancer were excluded from the study.
Outcomes
Cancer-related health care costs.
Patients incurred a mean of $74,212 (SD, $112,740) in cancer-related expenses during the 6 MBD, with the majority attributed to acute inpatient care ($40,702; 55%; SD, $98,478) followed by outpatient services ($30,254; 41%; SD, $38,881) and lastly hospice ($3,256; 4%; SD, $10,570). The mean cost of acute inpatient care increased steadily from $1,785 (SD, $8,823) in the sixth MBD to $6,356 (SD, $27,683) in the second MBD and then rose sharply to $20,559 (SD, $73,714) in the last MBD (Fig 1A). Hospice care followed a similar pattern, with low costs in the sixth MBD ($28; SD, $619) and a sharp rise in the last MBD ($2,464; SD, $8,518). Conversely, the mean cost of outpatient services exhibited a decreasing trend from $6,021 (SD, $10,524) in the sixth MBD to $2,238 (SD, $5,947) in the last MBD (Fig 1B).
Figure 1.
Mean total cancer-related costs for each of the last 6 months of life for (A) inpatient and hospice and (B) outpatient (OP) services. ER, emergency room; ESA, erythropoiesis-stimulating agent; G-CSF, granulocyte colony-stimulating factor.
Outpatient service costs in the last six MBD by cost category were: chemotherapy ($7,594; 25%), ESAs ($1,579; 5%), G-CSFs ($1,149; 5%), radiation ($3,709; 12%), emergency room ($507; 2%), office visits ($4,040; 13%), hospital outpatient procedures ($10,123; 33%), and other services ($1,549; 5%). Hospital outpatient procedures, chemotherapy, ESA, and G-CSF costs all decreased from the sixth MBD to the last MDB (Fig 1B). Chemotherapy and supportive care comprised a low percentage of the total cost of EOL care; chemotherapy accounted for only 10% of total cost, with ESAs at 2% and G-CSFs at 1.5%.
Patients with a hematologic malignancy had higher mean EOL cancer-related costs ($160,361; SD, $235,756) compared with patients with a solid tumor ($59,822; SD, $78,421). Acute inpatient costs were also higher for patients with a hematologic malignancy than a solid tumor ($121,651; SD, $216,042 v $27,778; SD, $62,570). In contrast, mean EOL hospice care costs were higher for patients with a solid tumor than for those with a hematologic malignancy ($3,092; SD, $8,831 v $2,050; SD, $15,732).
Patients with leukemia had the highest mean total EOL cancer-related costs ($197,676; SD, $267,886), whereas those with prostate cancer had the lowest ($29,962; SD, $58,177). Acute inpatient costs were the primary contributor to higher total costs for patients with leukemia ($157,638; SD, $246,862). Patients with prostate cancer had the lowest cancer-related costs in all categories except radiation, in which those with leukemia had the lowest costs ($1,094; SD, $5,242; Table 2).
Table 2.
Cancer-Related Costs Stratified by Tumor Type
| Service ($) | Lung (n = 5,115) |
Breast (n = 3,491) |
Colorectal (n = 2,623) |
Prostate (n = 2,225) |
Uterine, Cervical, or Ovarian (n = 1,496) |
Lymphoma (n = 1,459) |
Leukemia (n = 1,122) |
Bladder (n = 786) |
Other (n = 10,213) |
Total (N = 28,530) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Inpatient care | 25,529 | 57,602 | 28,242 | 59,544 | 32,441 | 75,886 | 16,087 | 47,025 | 44,358 | 75,640 | 93,977 | 184,321 | 157,638 | 246,862 | 26,338 | 60,669 | 40,157 | 85,758 | 40,702 | 98,478 |
| Hospice (inpatient and outpatient | 2,916 | 7,647 | 3,151 | 9,061 | 3,732 | 9,828 | 1,605 | 6,334 | 5,372 | 12,811 | 2,051 | 9,156 | 2,048 | 21,460 | 1,707 | 6,961 | 3,813 | 11,345 | 3,256 | 10,570 |
| Chemotherapy | 8,280 | 19,332 | 9,389 | 17,524 | 11,417 | 22,050 | 2,827 | 6,813 | 7,766 | 21,583 | 5,838 | 12,490 | 4,556 | 25,643 | 3,707 | 17,025 | 7,553 | 15,597 | 7,594 | 17,626 |
| ESAs | 1,822 | 4,323 | 2,034 | 4,561 | 1,338 | 3,626 | 925 | 3,159 | 2,171 | 4,587 | 1,709 | 4,366 | 998 | 3,208 | 1,499 | 4,139 | 1,471 | 3,941 | 1,579 | 4,063 |
| G-CSFs | 1,193 | 3,533 | 1,618 | 4,380 | 928 | 3,635 | 384 | 2,006 | 1,559 | 4,070 | 2,355 | 5,072 | 1,401 | 3,997 | 902 | 3,293 | 949 | 3,281 | 1,149 | 3,641 |
| Radiation | 5,373 | 12,048 | 4,199 | 10,532 | 2,390 | 8,952 | 1,949 | 7,064 | 2,737 | 9,515 | 2,732 | 9,158 | 1,094 | 5,242 | 2,521 | 8,098 | 4,093 | 12,527 | 3,710 | 10,969 |
| Emergency room visits | 525 | 1,792 | 515 | 1,914 | 433 | 1,623 | 209 | 917 | 492 | 1,720 | 651 | 3,686 | 872 | 6,890 | 273 | 1,060 | 541 | 2,058 | 506 | 2,368 |
| Office visits | 4,436 | 6,673 | 5,416 | 7,292 | 5,678 | 9,877 | 1,681 | 3,673 | 3,827 | 6,151 | 4,095 | 7,725 | 3,564 | 8,467 | 2,625 | 4,678 | 3,649 | 6,178 | 4,040 | 6,905 |
| Hospital outpatient procedures | 7,714 | 18,029 | 10,117 | 22,466 | 10,838 | 27,605 | 3,715 | 12,964 | 10,223 | 19,355 | 16,565 | 36,775 | 22,449 | 43,309 | 5,508 | 12,981 | 10,610 | 23,227 | 10,123 | 24,008 |
| Other | 1,031 | 3,763 | 1,099 | 3,443 | 2,007 | 6,599 | 581 | 2,771 | 2,303 | 8,422 | 1,692 | 9,507 | 3,057 | 11,388 | 1,423 | 10,033 | 1,772 | 7,384 | 1,550 | 6,679 |
| Total cancer-related costs | 58,818 | 74,271 | 65,780 | 74,564 | 71,201 | 92,404 | 29,962 | 58,177 | 80,807 | 88,092 | 131,664 | 203,178 | 197,676 | 267,886 | 46,504 | 74,759 | 74,609 | 98,972 | 74,212 | 112,740 |
Abbreviations: ESA, erythropoiesis-stimulating agent; G-CSF, granulocyte colony-stimulating factor; SD, standard deviation.
Health care resource utilization.
Patients receiving acute inpatient care increased from 12.2% in the sixth MBD (mean cost, $1,785) to 43.8% in the last MBD (mean cost, $20,559). Similarly, the percentage of patients who received hospice care increased from 0.7% (mean cost, $28) in the sixth MBD to 35.6% in the last MBD (mean cost, $2,464). In contrast, the percentage of patients who received outpatient chemotherapy decreased from 44.6% in the sixth MBD (mean cost, $2,172) to 20.4% in the last MBD (mean cost, $606). Outpatient ESA (15.1% to 7.6%) and G-CSF (8.5% to 2.3%) usage decreased as well. The percentage of patients who received chemotherapy in an inpatient setting remained low throughout the end of life, rising from 1.7% of patients in the sixth MBD to 3.2% in the last MDB. Similarly, the use of radiation (1.0% to 4.7%), ESAs (0.0% to 0.5%), and G-CSFs (0.0% to 0.1%) were low, increasing slightly from the sixth to last MBD.
Discussion
Although death is inevitable, its timing is not certain. Families and physicians wrestle with decisions regarding aspects of continued care, including hospice care, EOL planning, and limiting the use of medical interventions. Proactive management of unrealistic expectations and current and factual information about EOL care is essential for making the best individualized decisions regarding EOL care.
Harrington et al12 noted that at least 20% of patients with solid tumors receive chemotherapy within 2 weeks of dying. Furthermore, it was recently proposed that expensive chemotherapy treatments drive cancer EOL costs,13 which is contrary to the findings of this analysis. Treatment with chemotherapy as well as supportive care decreased from the sixth to last MBD and accounted for a small percentage of the total cost of EOL care (chemotherapy, 10%; ESAs, 2%; G-CSFs, 1.5%).
In our analysis, a majority of costs (55%) for patients with cancer at EOL were from acute inpatient care. Utilization of inpatient hospitalization (12.2% to 43.8%) and hospice care (0.7% to 35.6%) increased from 6 MBD to the last MBD, whereas administration of chemotherapy and supportive care declined (44.6% to 24.0%). These results demonstrate a shift in the type and frequency of services utilized at EOL.
Although we found that hospice use increased over the last 6 MBD, only 35.6% of patients utilized this service in the last month. An underuse of hospice services has been noted previously.14 Recent studies have reported that integration of palliative care with usual oncologic care was associated with equal15 or longer survival16 compared with usual care alone.17 The National Cancer Institute/National Institutes of Mental Health funded the Coping With Cancer project, examining cost differences in EOL care for patients who had EOL conversations with their physicians versus those who did not.18 EOL discussions, for the 31.2% who reported them, were associated with lower rates of aggressive interventions and 35.7% lower costs, compared with rates among those not having discussions.19 In addition, having EOL discussions was associated with entering hospice earlier, fewer intensive care unit admissions, greater likelihood of dying outside of the hospital, and better quality of life near death.
In this analysis, we were unable to distinguish the setting in which hospice services were delivered, and utilization of other palliative care services could not be analyzed in depth because of a lack of detailed claims coding. Understanding why patients are hospitalized at EOL rather than being cared for at home—which most patients prefer20,21—warrants further research. The American Society of Clinical Oncology recently released materials to help patients better communicate with their physicians.22 Realistic discussions of prognosis, potential benefits of both disease-directed and palliative therapy, and the effect of decisions on symptoms, quality of life, financial costs, and survival can help patients make decisions that best match their goals and preferences.23
There are several limitations to this study, including the degree to which claims data can accurately capture an individual's medical history, including comorbidities influencing treatment decisions, because claims data are collected for the purpose of payment and not specifically for clinical research. Furthermore, claims data contain the cost of billed services only, and as such, indirect costs reflecting loss of patient/caregiver productivity/wages, travel to/from treatment, over-the-counter medications, or other cancer-related expenses are not reflected in claims data. However, this study focused on health services reimbursed by commercial payers, which are within the purview of policymakers at health plan and national levels. In addition, this analysis focused only on a commercially insured population, and results may be limited to commercially insured groups. However, results of this study were consistent with research performed previously in Medicare populations, demonstrating high costs at EOL for many conditions, including cancer. Finally, patients who survived < 6 months after their cancer diagnosis were excluded from this study.
Because of the retrospective nature of the data, it is important to note that causal inferences cannot be made between the associations of EOL care and costs. Costs are often driven by acuity of care; that is, often, sick patients will have high costs, and sick patients often die as a result of exacerbations of their illness rather than of end-stage disease. Because this study examined a wide array of tumors types that have different disease histories, pathologies, and treatment regimens, we cannot conclude that EOL costs are uniform for all tumor types. However, we feel that this study provides the most current data on EOL costs for the leading cancers and provides important data for treatment providers, health care administrators, and future researchers.
This study demonstrated that EOL care is a significant cost to payers and patients. Although overall cancer-related health care costs increased at EOL, the largest contributor of increased costs was not aggressive systemic chemotherapy or novel targeted therapies but rather costs of acute inpatient care. This study also confirmed previous reports that hospice utilization rates in the United States are low. Understanding why patients are hospitalized at EOL rather than being cared for at home is an important topic for future research.
Acknowledgment
We thank Leigh Borton and Cynthia Taylor for their efforts in creating the database and conducting the statistical analysis, as well as Virginia M. Rosen, PhD, and Gretchen Parker, PhD, for their assistance in the writing of this article. We also thank Martin J. Zagari, MD, for insightful comments on the study and manuscript. Supported by Amgen, which contracted with OptumInsight to conduct this study. Presented in poster form at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Benjamin Chastek, OptumInsight; (C), Carolyn Harley, OptumInsight (C); Joel Kalich, OptumInsight (C); Lee Newcomer, UnitedHealthcare (C); Carly J. Paoli, Amgen (C); April H. Teitelbaum, OptumInsight (C) Consultant or Advisory Role: None Stock Ownership: Joel Kallich, Amgen; Lee Newcomer, UnitedHealthcare; Carly J. Paoli, Amgen Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Benjamin Chastek, Carolyn Harley, Joel Kallich, Lee Newcomer, April H. Teitelbaum
Financial support: Joel Kallich, Carly J. Paoli
Administrative support: Carly J. Paoli
Collection and assembly of data: Benjamin Chastek
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Centers for Disease Control and Prevention. Leading causes of death. www.cdc.gov/nchs/fastats/lcod.htm. [Google Scholar]
- 2.Elkin EB, Bach PB. Cancer's next frontier: Addressing high and increasing costs. JAMA. 2010;303:1086–1087. doi: 10.1001/jama.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Cancer trends progress report: 2009/2010 update. http://progressreport.cancer.gov. [Google Scholar]
- 5.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yabroff KR, Warren JL, Brown ML. Costs of cancer care in the USA: A descriptive review. Nat Clin Prac Oncol. 2007;4:643–656. doi: 10.1038/ncponc0978. [DOI] [PubMed] [Google Scholar]
- 7.Lubitz JD, Riley GF. Trends in Medicare payments in the last year of life. N Engl J Med. 1993;328:1092–1096. doi: 10.1056/NEJM199304153281506. [DOI] [PubMed] [Google Scholar]
- 8.Fireman BH, Quesenberry CP, Somkin CP, et al. Cost of care for cancer in a health maintenance organization. Health Care Financ Rev. 1997;18:51–76. [PMC free article] [PubMed] [Google Scholar]
- 9.McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22:329–342. doi: 10.1097/00005650-198404000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Carlson MD, Herrin J, Du Q, et al. Impact of hospice disenrollment on health care use and Medicare expenditures for patients with cancer. J Clin Oncol. 2010;28:4371–4375. doi: 10.1200/JCO.2009.26.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health Insurance Portability and Accountability Act of 1996. Pub L 104-191, 104th Congress, 2009. www.cms.hhs.gov/HIPAAGenInfo/Downloads/HIPAALaw.pdf. [Google Scholar]
- 12.Harrington SE, Smith TJ. The role of chemotherapy at the end of life: “When is enough, enough?”. JAMA. 2008;299:2667–2678. doi: 10.1001/jama.299.22.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith TJ, Hilner BE. Bending the cost curve in cancer care. N Engl J Med. 2001;364:2060–2065. doi: 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dartmouth Institute for Health Policy and Clinical Practice. Trends and variation in end-of-life care for Medicare beneficiaries with severe chronic illness: A report of the Dartmouth Atlas project. http://www.dartmouthatlas.org/downloads/reports/EOL_Trend_Report_0411.pdf. [PubMed] [Google Scholar]
- 15.Finn JW, Pienta KJ, Parzuchowski J, et al. Bridging cancer treatment and hospice care. Proc Am Soc Clin Oncol. 2002;21 abstr 1452. [Google Scholar]
- 16.Cowall DE, Yu BW, Heineken SL, et al. Evaluation of end of life (EOL) care at a comprehensive community cancer institute. J Clin Oncol. 2011;29(suppl) abstr e19702. [Google Scholar]
- 17.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright AA, Keating NL, Balboni TA, et al. Place of death: Correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28:4457–4464. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Society of Clinical Oncology. New booklet guides advanced cancer patients through tough conversations with physicians. www.asco.org/ASCOv2/Press+Center/Latest+News+Releases/New+Booklet+Guides+Advanced+Cancer+Patients+through+Tough+Conversations+with+Physicians. [Google Scholar]
- 23.Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology statement: Toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–60. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]