Abstract
The ability of 21 C3 and C4 monocot and dicot species to rapidly export newly fixed C in the light at both ambient and enriched CO2 levels was compared. Photosynthesis and concurrent export rates were estimated during isotopic equilibrium of the transport sugars using a steady-state 14CO2-labeling procedure. At ambient CO2 photosynthesis and export rates for C3 species were 5 to 15 and 1 to 10 μmol C m−2 s−1, respectively, and 20 to 30 and 15 to 22 μmol C m−2 s−1, respectively, for C4 species. A linear regression plot of export on photosynthesis rate of all species had a correlation coefficient of 0.87. When concurrent export was expressed as a percentage of photosynthesis, several C3 dicots that produced transport sugars other than Suc had high efflux rates relative to photosynthesis, comparable to those of C4 species. At high CO2 photosynthetic and export rates were only slightly altered in C4 species, and photosynthesis increased but export rates did not in all C3 species. The C3 species that had high efflux rates relative to photosynthesis at ambient CO2 exported at rates comparable to those of C4 species on both an absolute basis and as a percentage of photosynthesis. At ambient CO2 there were strong linear relationships between photosynthesis, sugar synthesis, and concurrent export. However, at high CO2 the relationships between photosynthesis and export rate and between sugar synthesis and export rate were not as strong because sugars and starch were accumulated.
Photosynthesis involves both the light-trapping reactions and the dark reactions associated with C assimilation that are localized in cells containing chloroplasts. However, it is clear that drawing any correlation between photosynthesis and plant productivity depends on a more complete understanding of C partitioning within the leaf and of the subsequent translocation of assimilates via the phloem to sinks (Gordon, 1986; Farrar, 1988; Wardlaw, 1990; Geiger and Servaites, 1994). C partitioning and allocation are processes that occur in both the light and dark. What controls the initial partitioning of photosynthates to temporary storage pools within the leaf (Lunn and Hatch, 1995) or to immediate export remains unresolved (Gordon, 1986). An important first step in answering this fundamental question is to develop a reasonable measurement of the rate of immediate export.
Most researchers (Canny, 1973; Zimmermann and Ziegler, 1975; Stitt et al., 1987; Van Bel, 1993; Geiger and Servaites, 1994; Turgeon, 1995) acknowledge that it is very difficult to quantify simultaneously C assimilation by the leaf, C recycling within the leaf, temporary C storage within the leaf, and immediate C efflux rate via the phloem. In the present study a steady-state 14CO2-feeding technique originally developed by Geiger and Fondy (1979) and modified by Jiao and Grodzinski (1996) was used to estimate concurrent (i.e. immediate) export during photosynthesis. Our protocol is based on determining the period in which the transport pools of sugars appear to be in isotopic equilibrium before equating the photosynthetic rate with the concurrent efflux of 14C from the leaf.
Using this protocol, errors associated with non-steady-state labeling and pulse-chase experiments were reduced. For example, in a study of bean (Phaseolus vulgaris) leaves, which export primarily Suc, bacterial infection with Xanthamonas campestris cv Phaseoli was observed to affect export from the host leaves (Jiao et al., 1996). This conclusion was not evident from pulse-chase experiments. Similarly, it was shown that when photorespiration was suppressed by CO2 enrichment, both photosynthesis and concurrent export increased (Grodzinski et al., 1995; Jiao and Grodzinski, 1996; Leonardos et al., 1996). Furthermore, warming the leaf reduced the concurrent export rate more than photosynthesis. The measurements of concurrent export obtained from our steady-state labeling procedure clearly demonstrated that leaf warming resulted in a reduction in the export rate prior to an inhibition of the operation of the photosystems and/or C-fixation processes (Jiao and Grodzinski, 1996).
Using a pulse-chase procedure, Hofstra and Nelson (1969) compared the rate of disappearance of label (14C) during a 6-h chase period for leaves of six C3 dicots: castor bean (Ricinus communis), radish (Raphanus sativus), soybean (Glycine max), sunflower (Helianthus annuus), tobacco (Nicotiana tabacum), and tomato (Lycopersicon esculentum) and four C4 monocots: maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor), and sugarcane (Saccharum officinarum). Although no C4 dicots were examined, this early study of export provided a comparison among C3- and C4-fixation types. Gordon (1986) observed that maize and sorghum, two C4 plants, accumulated Suc during the photoperiod at lower rates than C3 plants of the same family that also had parallel venation patterns.
In the present study, steady-state photosynthesis rates were established and concurrent export rates from mature, attached leaves of 21 species were measured. Initially, we attempted to address two questions: First, what is the probable maximum rate of export of the newly formed photoassimilates (i.e. efflux of C) in leaves of species with different capacities to fix the C (e.g. C3 and C4 species)? Second, what happens to the rate of export during photosynthesis in response to CO2 enrichment of the leaf atmosphere? After addressing these questions it was possible to pose a new set of questions relating to the existence of any general relationship between the capacity to export C during photosynthesis and either the mechanisms of CO2 assimilation (i.e. C3 and C4 species) or of the leaf anatomy (e.g. the venation patterns of monocots and dicots), or the type of sugars being formed in the leaf and translocated.
MATERIALS AND METHODS
Four C4 species, including two monocots, maize (Zea mays) and sorghum (Sorghum bicolor cv Sudan), and two dicots, pigweed (Amaranthus retroflexus) and gomphrena (Gomphrena globosa), were grown from seeds. Three C3 monocots, oat (Avena sativa L. cv Elgin), barley (Hordeum vulgare L.), and wheat (Triticum aestivum L. cv Karat), were also grown from seeds. Two other monocots, alstroemeria (Alstroemeria sp. cv Jacqueline) and sandersonia (Sandersonia aurantiaca), were grown from rhizomes and tubers, respectively.
The current survey also included 12 C3 dicots. Seven of these transport primarily Suc: sweet pepper (Capsicum annuum cv Cubico), chrysanthemum (Chrysanthemum morifolium), sunflower (Helianthus annuus), tobacco (Nicotiana tabacum), bean (Phaseolus vulgaris L.), pea (Pisum sativum cv Improved Laxton's Progress), and rose (Rosa hybrida cv Samantha). All were grown from seed except chrysanthemum and rose, which were grown from rooted cuttings.
The following C3 dicots that transport Suc and other sugars were also examined: celery (Apium graveolens L.), which translocates mannitol (Everard et al., 1994; Loescher and Everard, 1996); cucumber (Cucumis sativus cv Revenue); and several representative species of the mint family (Labiatae), including coleus (Coleus blumei), catnip (Nepeta faassenii), and salvia (Salvia splendens cv Bonfire), which translocate raffinose series sugars (Zimmermann and Ziegler, 1975; Madore and Grodzinski, 1984, 1985; Turgeon and Wimmers, 1988).
All C3 and C4 plants were grown in a similar manner in our greenhouse in an attempt to avoid differences in photosynthesis, Suc synthesis, and export attributable to growing plants in different conditions (Huber et al., 1985). Plants were grown in Promix-BX (Les tourbières Premier LTÉE, Rivière du Loup, Quebec, Canada) in 14-cm (1.4-L) pots in a research greenhouse maintained at 25 ± 3°C day and 18 ± 1°C night temperatures. Plants were watered and fertilized regularly. Gas-exchange and C-export studies were conducted on a recently expanded mature leaf or leaflet. All plants were studied intact. No attempt was made to remove other source leaves or to manipulate sink number. Generally, the plants used were about 8 to 12 weeks old and flowering, thus providing active sinks for the source leaf, which was the object of our measurements.
Steady-State 14CO2 Labeling and Export of 14C Photoassimilates
Steady-state gas exchange of leaves was measured using an open-flow, steady-state, 14CO2-labeling gas-analysis system described previously (Jiao and Grodzinski, 1996). The specific activity of the 14CO2, which was supplied by a precision syringe pump, was constant during a single experiment but varied among experiments from 1 to 5 kBq μg−1 C, depending on the photosynthetic rate and the CO2 concentration. A GM detector (window area 6.8 cm2, model EWGM, Bicron, Newbury, OH) positioned under the leaf surface was used to monitor the radioactivity accumulated in the source leaf during the 2-h feeding period. The GM tube output through a ratemeter (model 8731–32, Nuclear Chicago, Des Plaines, IL) was recorded, and the counts were corrected for the total radioactivity recovered in the leaf fed with 14CO2 at the end of the feeding period.
Export was calculated as the difference between the C-assimilation rate measured continuously by the IRGA, and the 14C-retention rate estimated by the GM trace after correcting for the CE for that leaf. Calculations of 14CO2 losses during photorespiration and dark respiration were not needed to estimate the rate of export, since the IRGA, only measured net CO2 exchange by the leaf (Geiger and Fondy, 1979; Jiao and Grodzinski, 1996). If the rate of net photosynthesis at a high light level (i.e. 1200 μmol m−2 s−1 PAR, 400–700 nm) was not constant for a period of 2 to 3 h, we did not attempt to evaluate the concurrent export flux. With each of the species used in this study, we confirmed that the light-saturated photosynthesis rate and the concurrent export rate did not vary significantly for labeling studies conducted between 10 am and 4 pm EST. Within 90 min, isotopic equilibrium of the transport sugars was obtained (see data below).
Partitioning of 14C Photoassimilates
Partitioning of the recently fixed 14C in the source leaf was determined in a manner similar to that described previously (Jiao and Grodzinski, 1996). After rapid extraction of the leaf tissue in boiling ethanol (80%), the leaf extract was vacuum dried, rehydrated, and partitioned against chloroform (chloroform:water, 2:1; v/v). Ion-exchange chromatography (AG50-X8 and AG1-X8, Bio-Rad) was used to separate the aqueous phase into neutral (sugars), acidic (organic acids), and basic (amino acids) fractions. Individual sugars were separated by HPLC (Beckman) using a μ-Spherogel carbohydrate (7.5% cross-linked) column at 85°C, and quantified using a refractive index detector (model 156, Beckman). Each peak was calculated using an integrator (model HP3390A, Hewlett-Packard). Fractions of each sugar were pooled and radioactivity was determined by liquid-scintillation counting (model LS-6800, Beckman). The radioactivity remaining in the ethanol-insoluble fraction (95% starch) was counted by liquid scintillation in a gel suspension (1 mL water:1.5 mL Cytoscint [ICN]) of the oven-dried tissue.
RESULTS
Estimating Steady-State Photosynthesis and the Concurrent Export Rate
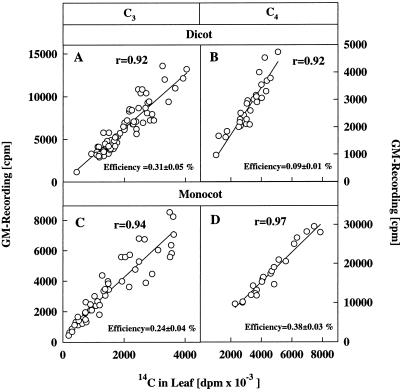
Figure 1 shows typical estimates of the CE of the GM tubes in leaves of four species. Because of the differences in leaf characteristics (i.e. leaf thickness and venation pattern) among the species, CE varied between 0.1 and 0.4%. Nevertheless, for each species and for each leaf type there was a linear correlation between the radioactivity determined by destructive analysis and that counted by the GM tube. Figure 2, A through C, shows that a constant photosynthetic rate was recorded with the IRGA throughout the feeding period for each species. In each case the photosynthetic rate was constant for at least 30 min before 14CO2 was supplied at a constant specific activity throughout the feeding period. Although the CE of the GM detector was low and varied with species (Fig. 1), the retention of 14C measured nondestructively with the GM detector was in close agreement with measurements of radioactivity made by destructive sampling (Fig. 2, A–C). Export was calculated as the difference between the data derived from the IRGA and from the GM detector; however, we were very selective in choosing the appropriate time to calculate the concurrent export rate.
Figure 1.
A comparison of radioactivity measured nondestructively by monitoring 14C accumulation in the leaf with a GM detector and by scintillation counting after destructive sampling of the leaf of salvia (A), gomphrena (B), alstroemeria (C), and maize (D). Measurements were made in leaf cuvettes as described in Methods. Each point is the measurement of one leaf under different environmental conditions. Values on the x axis were multiplied by 10−3 (i.e. a value of 5000 dpm is 5 × 106 dpm). CE is the value of radioactivity obtained by the GM detector divided by the radioactivity determined after destructive analysis of the leaf tissue multiplied by 100.
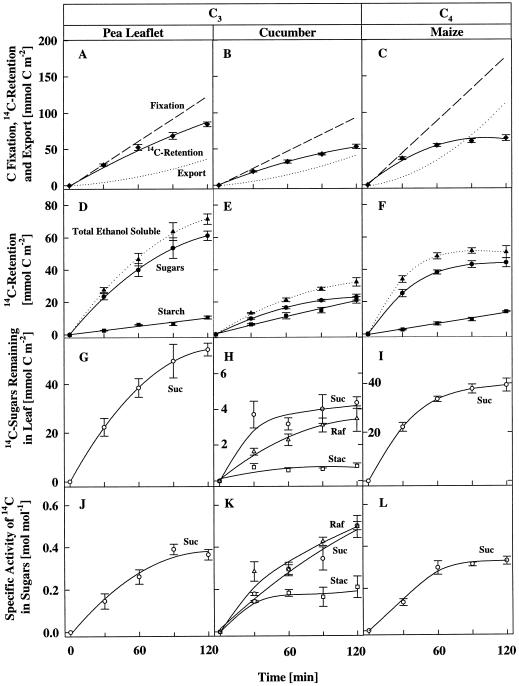
Figure 2.
Total C fixation and 14C retention, export, and partitioning in major intermediates during a 2-h 14CO2 feeding of the source leaf of pea (A, D, G, and J), cucumber (B, E, H, and K), and maize (C, F, I, and L) under 35 Pa CO2, 21 kPa O2, and 25°C. Cumulative net C fixation (dashed line in A, B, and C) was calculated from IRGA data, whereas 14C retention in the leaf was measured both nondestructively by monitoring 14C with a GM detector continuously (solid line in A, B, and C) and in a parallel set of leaves by destructive analysis (•). Export (dotted line in A, B, and C) was estimated as the difference between total fixation (dashed line) and 14C retention in the leaf (solid line). D, E, and F show partitioning of total 14C in the ethanol-soluble fraction (▴), the total sugar fraction (•), and in starch (▪). G, H, and I, Partitioning of total 14C. J, K and L, Specific activity of the major sugars: Suc (○), raffinose (Raf, ▵), and stachyose (Stac, □). Each point is the average of at least four leaves on four different plants, and each error bar represents the se of the mean.
When the leaf was sampled during a typical 2-h feeding period, the pattern of 14C-partitioning in the transport sugars indicated that isotopic equilibrium between the 14CO2 in the air stream and the major 14C translocates was generally not achieved in the 1st h (Fig. 2, J–L). For example, in pea, a C3 dicot (Fig. 2J), and in maize, a C4 monocot (Fig. 2L), 60 to 90 min was required before the specific activity of the major sugars reached a steady level. Data for leaves of other species are not shown here, but the labeling patterns were similar to those in pea (Fig. 2, G and J), maize (Fig. 2, I and L), and bean (Jiao et al., 1996).
We used data between 90 and 120 min to calculate values for photosynthesis and the corresponding concurrent export rate. During this period there appeared to be steady-state labeling of the sugar pools. The labeling patterns in species that transport sugars other than Suc confirm the value of using an estimate of the concurrent export rate calculated from data obtained after at least 90 min of labeling under steady-state photosynthetic conditions. Figure 2K shows that in cucumber, for example, the specific activity of the stachyose pool was essentially unchanged after 30 min. Labeling was similar in leaves of coleus, catnip, and salvia, all members of the mint family, which also export sugars of the raffinose series (Jiao and Grodzinski, 1996). We assumed that these sugars are produced in the phloem (see Turgeon, 1995) and therefore are suitable endogenous markers of export.
Photosynthesis versus Concurrent Export at Ambient CO2
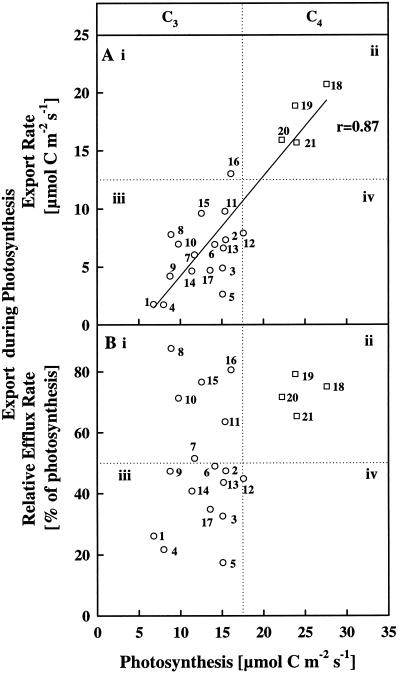
Figure 3A shows photosynthetic and concurrent export rates for all 21 species expressed as micromoles of C (fixed and exported) on a leaf-area basis. Among the very different C3 and C4 species studied, a general pattern of export during photosynthesis clearly existed. A linear regression line using all of the values in Figure 3A produced a high correlation coefficient of 0.87. At ambient CO2, the greater the rate of photosynthesis, the greater the rate of concurrent export. The species with the highest rates of photosynthesis and export on a leaf-area basis were the C4 species maize, sorghum, pigweed, and gomphrena (Fig. 3A). Leaves of all C3 species had much lower rates of both photosynthesis and export.
Figure 3.
Net photosynthesis of leaves of 21 species plotted against their concurrent export rates, expressed as absolute values (A) and as relative C-efflux rate calculated as a percentage of the net CO2-influx rate (B). A and B were each divided into four quadrants labeled i, ii, iii, and iv, so that the data could be compared easily with those in Figure 4. The exact intersection point of the vertical and horizontal lines on the axes (i.e. relative to specific values of photosynthesis and export rates) must be viewed as arbitrary. The division of the graphs into quadrants was strictly for convenience in developing an overview of the relationships among the following species: 1, alstroemeria; 2, barley; 3, oat; 4, sandersonia; 5, wheat; 6, bean; 7, catnip; 8, celery; 9, chrysanthemum; 10, coleus; 11, cucumber; 12, pea; 13, pepper; 14, rose; 15, salvia; 16, sunflower; 17, tobacco; 18, maize; 19, sorghum; 20, pigweed; and 21, gomphrena. The C3 and C4 species are indicated by circular and square symbols, respectively. Photosynthesis and export rates were determined as outlined in Methods using mature leaves of each species exposed to light-saturating conditions, ambient CO2 concentrations (inlet air of 35 Pa CO2), and 25°C (the daytime growth temperature). Each point is an average of at least four leaves on four different plants. The se of the means are not shown for graphical clarity.
In Figure 3B concurrent export is expressed as a percentage of the CO2 uptake rate rather than as an absolute value. When this relative C-flux rate through the leaf was plotted against the photosynthesis rate, a slightly different picture of the ability of a source leaf to export C emerges (Fig. 3B). First, the strong linear relationship between the fixation rate and the concurrent export rate offered in Figure 3A was not obtained. Both A and B in Figure 3 are divided by vertical and horizontal dotted lines to help compare the distribution of the data obtained under both ambient (Fig. 3) and enriched CO2 (Fig. 4) conditions. All C3 species had lower photosynthetic rates than did the C4 species. The values for the C4 plants were in quadrant ii. Most of the data for the C3 plants remained in quadrant iii in Figure 3B, but six of the C3 species (i.e. catnip, celery, coleus, cucumber, salvia, and sunflower) were segregated into quadrant i in Figure 3B. These six species displayed a relatively high capacity for exporting newly fixed C. For example, compared with the monocot and dicot C4 species, which exported approximately 75 to 80% of newly fixed C when exposed to ambient CO2, salvia, a C3 dicot, exported over 75% of the C being assimilated (Fig. 3B). Salvia, catnip, coleus, and cucumber translocated sugars of the raffinose series in addition to Suc (Fig. 2, H and K); celery also translocated mannitol, whereas sunflower translocated primarily Suc (data not shown).
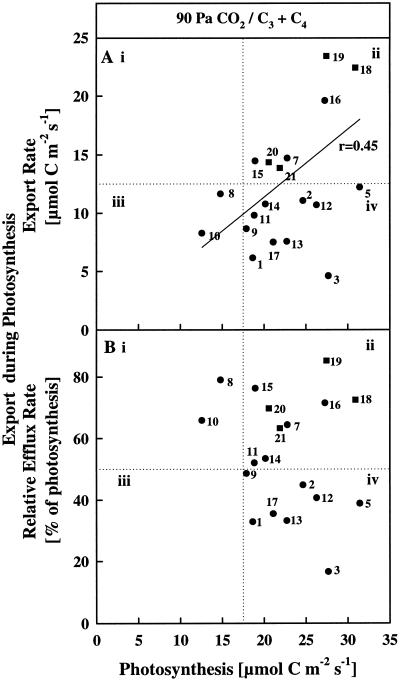
Figure 4.
The effect of short-term exposure of the source leaves to CO2 enrichment on net photosynthesis and concurrent export rates expressed as absolute values (A) and as a percentage of photosynthesis (B). The species tested and assay conditions were those outlined in Figure 3, except that the source leaf was exposed to high CO2 concentrations (inlet air of 90 Pa CO2). Each point is an average of at least four leaves on four different plants. The se of the means are not shown for graphical clarity.
Photosynthesis versus Concurrent Export during Short-Term CO2 Enrichment
When the CO2 level of the gas stream entering the leaf cuvette was increased from 35 to 90 Pa, the photosynthetic rates of the two C4 monocots increased slightly, whereas those of the two C4 dicots decreased slightly (Fig. 4, A and B). The export rates changed similarly. Overall, the data points for the four C4 species were in quadrant ii in Figure 4A as they were in Figure 3A. As a group, the C4 species continued to export a high percentage of recently fixed C (Fig. 4B). However, during CO2 enrichment (Fig. 4, A and B) photosynthetic rates of the C3 species increased dramatically because photorespiration was reduced. As a consequence, the data points for the C3 species were shifted to the right in Figure 4, A and B, relative to the positions they occupied in Figure 3, A and B, respectively. The C3 species appeared to segregate into two subgroups with respect to their capacity to export the increased amount of newly reduced C (Fig. 4B).
One subgroup, which included species such as sunflower, catnip, cucumber, and salvia, and seemed to export C rapidly at ambient CO2 on a percentage basis relative to C fixation (Fig. 3B), continued under CO2 enrichment to export C readily on both a percentage (Fig. 4B) and an absolute basis (Fig. 4A). At high CO2 the photosynthetic rate and the export capacity of these species were comparable to those of the C4 species (Fig. 4A). In contrast, even though photosynthetic rates were increased by CO2 enrichment in all C3 species, many merely retained more label and did not achieve similarly high, concurrent export rates (Fig. 4A). These species are represented by data sets found in quadrant iv (Fig. 4, A and B). The export data expressed as C efflux relative to C fixation and the distribution of 14C remaining in the leaf tissue in the primary storage pools at the end of the feeding periods are summarized in Table I.
Table I.
Summary of 14C partitioning in the total sugar and starch pools after a 2-h feeding under ambient (35 Pa) and high (90 Pa) CO2 and comparison with export
| Plant | Ambient
CO2
|
High CO2
|
||||||
|---|---|---|---|---|---|---|---|---|
| Sugars | Starch | Ratio | Exporta | Sugars | Starch | Ratio | Export | |
| mmol C m−2 | mmol C m−2 | |||||||
| C4 Dicots | ||||||||
| Pigweed | 31.0 ± 3.6 | 13.0 ± 2.2 | 2.4 | 29.5 ± 2.2 | 17.3 ± 2.3 | 1.7 | ||
| Gomphrena | 36.0 ± 4.3 | 11.5 ± 1.4 | 3.1 | 45.4 ± 5.8 | 11.5 ± 0.7 | 3.9 | ||
| Average | 2.8 | 15.8 (69%) | 2.8 | 14.2 (67%) | ||||
| C4 Monocots | ||||||||
| Maize | 41.8 ± 1.4 | 9.4 ± 1.4 | 4.5 | 50.4 ± 4.3 | 16.6 ± 2.2 | 3.0 | ||
| Sorghum | 33.1 ± 4.3 | 10.8 ± 0.7 | 3.1 | 23.0 ± 0.7 | 10.1 ± 0.7 | 2.3 | ||
| Average | 3.8 | 19.8 (77%) | 2.7 | 22.9 (79%) | ||||
| Average of all C4 | 3.3 | 17.8 (73%) | 2.8 | 18.6 (73%) | ||||
| C3 Dicots | ||||||||
| Subgroup 1 | ||||||||
| Celery | 1.4 ± 0.0 | 4.3 ± 0.7 | 0.3 | 2.9 ± 0.0 | 21.6 ± 5.0 | 0.1 | ||
| Catnip | 23.0 ± 0.7 | 12.9 ± 0.7 | 1.8 | 49.7 ± 5.8 | 20.9 ± 2.2 | 2.4 | ||
| Coleus | 7.9 ± 0.7 | 10.8 ± 0.7 | 0.7 | 12.2 ± 0.7 | 17.3 ± 2.9 | 0.7 | ||
| Cucumber | 17.3 ± 0.7 | 23.0 ± 1.4 | 0.8 | 21.6 ± 0.7 | 30.9 ± 0.7 | 0.7 | ||
| Salvia | 13.0 ± 0.7 | 5.0 ± 0.7 | 2.6 | 35.3 ± 2.9 | 21.6 ± 1.4 | 1.6 | ||
| Sunflower | 17.3 ± 1.4 | 7.2 ± 0.7 | 2.4 | 38.2 ± 5.8 | 14.4 ± 4.0 | 2.7 | ||
| Average | 1.4 | 8.9 (72%) | 1.4 | 13.1 (68%) | ||||
| Subgroup 2 | ||||||||
| Chrysanthemum | 18.7 ± 2.2 | 7.9 ± 0.7 | 2.4 | 48.2 ± 3.6 | 13.7 ± 1.4 | 3.5 | ||
| Pepper | 29.5 ± 1.4 | 33.1 ± 5.0 | 0.9 | 51.8 ± 0.7 | 61.9 ± 17 | 0.8 | ||
| Tobacco | 30.2 ± 2.9 | 27.4 ± 3.6 | 1.1 | 47.5 ± 4.3 | 43.2 ± 5.8 | 1.1 | ||
| Bean | 27.4 ± 2.2 | 21.6 ± 3.6 | 1.3 | NAb | NA | NA | ||
| Pea | 66.9 ± 3.6 | 10.8 ± 0.7 | 6.2 | 102 ± 6.5 | 17.3 ± 1.4 | 5.9 | ||
| Rose | 36.0 ± 2.9 | 5.8 ± 0.7 | 6.3 | 51.1 ± 3.6 | 15.1 ± 0.7 | 3.4 | ||
| Average | 3.0 | 5.8 (44%) | 2.9 | 9.1 (42%) | ||||
| C3 Monocots | ||||||||
| Alstroemeria | 42.4 ± 2.5 | 5.8 ± 1.2 | 7.3 | 83.7 ± 5.1 | 36.1 ± 2.6 | 2.3 | ||
| Sandersonia | 43.7 ± 0.6 | 6.5 ± 0.6 | 6.7 | NA | NA | NA | ||
| Barley | 54.7 ± 6.4 | 7.9 ± 0.7 | 6.9 | 101 ± 16 | 11.5 ± 1.4 | 8.8 | ||
| Oat | 77.0 ± 2.9 | 5.0 ± 0.7 | 15.3 | 148 ± 7.0 | 11.5 ± 0.7 | 12.9 | ||
| Wheat | 61.9 ± 12 | 7.2 ± 0.7 | 8.6 | 133 ± 9.0 | 20.2 ± 2.2 | 6.6 | ||
| Average | 9.0 | 3.7 (29%) | 7.7 | 8.5 (33%) | ||||
| Average of all C3 | 4.5 | 6.1 (48%) | 4.0 | 10.2 (48%) | ||||
14C Partitioning in the Leaf
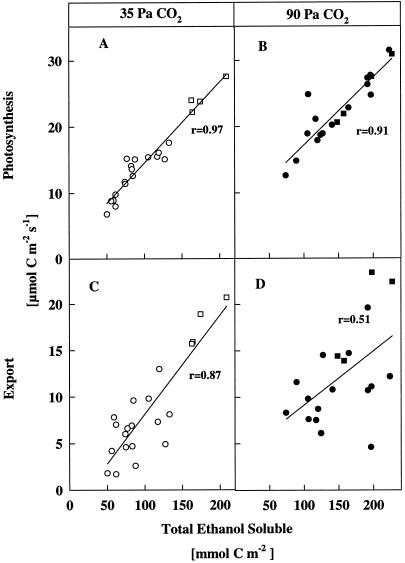
Photosynthesis and 14C partitioning into the ethanol-soluble fraction (primarily sugars) were positively correlated at both ambient (Fig. 5A) and high CO2 (Fig. 5B). However, export and 14C partitioning into the solubles were more strongly correlated at ambient CO2 (Fig. 5C). The linear regression line between export and synthesis of translocates in all species assayed at ambient CO2 had a correlation coefficient of 0.87 (Fig. 5C). The same value was obtained when the concurrent export rate was plotted against photosynthesis (Fig. 3A). At high CO2 the linear correlation coefficent between photosynthesis and synthesis of 14C translocates in all species was only 0.53 (Fig. 5B), similar to that between photosynthesis and the concurrent export rate at high CO2 (r = 0.45; Fig. 4A). In most C3 species the absolute rates of export during photosynthesis increased during CO2 enrichment (Fig. 4A); however, at high CO2 the export fluxes relative to fixation did not increase proportionally to the photosynthesis rates, and a much greater amount of 14C was retained in all species.
Figure 5.
Photosynthesis rates (A and B) and concurrent export rates (C and D) at ambient and high CO2 plotted against the total partitioning of label into the ethanol-soluble fraction of the leaf, which primarily consisted of retained sugars but also contained other potentially phloem-mobile intermediates.
Although cognizant of the pitfalls inherent in examining the 14C-partitioning data obtained after a 2-h labeling period, we nevertheless created Table I from these data. The rationale for including these data here is that these data were collected for attached leaves for which we have values for steady-state photosynthesis and concurrent export rates (Figs. 3 and 4). As pointed out by Lunn and Hatch (1995), there are very few studies of immediate partitioning of 14C under steady-state conditions using attached leaves. Table I was structured to assess whether there was any pattern in the ability to maintain a high rate of concurrent export during photosynthesis and the partitioning of label into sugars versus starch (over 95% of the labeled aqueous-ethanol insolubles), among several recognizable groups of plants (e.g. C3 and C4, or monocots and dicots, or species transporting sugars other than Suc).
All species tested appeared to accumulate both sugars and starch. Which sugars were formed at elevated CO2 depended on the individual species. For example, in pea and maize, Suc was the dominant storage sugar, whereas in cucumber and other mints, raffinose and verbascose accumulated in addition to Suc and starch (e.g. Fig. 2, H and K). Table I shows that on average the sugar-to-starch ratios for different groups of plants (e.g. C3) were similar under ambient and high CO2, as was the proportional export rate when expressed as a percentage of the photosynthetic rate. When the average values for export flux were expressed as a percentage of photosynthesis and compared within groups with the accumulation of sugars or starch, it was clear that C4 dicots and monocots displayed similar export capacities and similar 14C-partitioning into sugars versus starch. On average, the sugar-to-starch ratio was approximately 3 at both ambient and high CO2.
Among the C3 dicots, two subgroups were distinguishable based on the rates of concurrent export flux. The first subgroup was comprised of those species (i.e. celery, catnip, coleus, cucumber, salvia, and sunflower) with relatively high C-efflux rates (Fig. 3B) compared with the C4 species. The average relative export fluxes of this subgroup were about 72% at ambient CO2 and 68% at elevated CO2 (Table I). Among this subgroup the sugar-to-starch ratio in the leaf was rather low, averaging 1.4 at both CO2 levels. This subgroup seemed to export newly fixed C rapidly and to store proportionally less as sugars and more as starch (Table I). However, as shown in Table I, it is incorrect to equate a rapid export rate during photosynthesis with low storage rates of sugar versus starch. When data for the second subgroup of C3 dicots were averaged, the sugar-to-starch ratios were about 3 at both CO2 levels (Table I). Upon closer examination of this second subgroup of C3 dicots, the ratio for pea (6.2) and for rose (6.3) tended to raise the average ratio for all C3 dicots. In total, 10 of the 12 C3 dicots examined (i.e. 6 in the first subgroup plus chrysanthemums, pepper, tobacco, and bean) had an average sugar-to-starch ratio of less than 1.5. The export rates of the first subgroup were of the order of 70% exported versus 42% exported for the second subgroup of C3 dicots.
The monocots as a group also illustrate the problem associated with drawing general conclusions from the data. First, there was a very high ratio of sugar accumulation relative to starch synthesis; at ambient CO2 the average sugar-to-starch ratio was 9.0, and at high CO2 the average ratio among the C3 monocots was 7.7. Second, the efflux rates were the lowest recorded among the 21 species. In contrast to the export characteristics of C3 monocots, the C4 monocots had the highest export rates in the light.
DISCUSSION
Our data confirm the conclusions of others (Hofstra and Nelson, 1969) that, as a general rule, at ambient CO2 and O2 conditions C4 species such as maize fix and export more C per unit leaf area than do leaves of C3 species (Fig. 3A). However, the concept that C4 species export newly fixed C more readily than C3 species is challenged. The concurrent export rates of many C3 species at ambient CO2 were equal to or greater than those of C4 species when concurrent export was expressed as a percentage of the rate of CO2 assimilation (Fig. 3B). In particular, a number of C3 species that produce auxiliary phloem mobile-transport sugars (e.g. raffinose sugars) appear to be able to maintain a relatively high C export rate relative to the rate of C fixation.
Reducing photorespiration by CO2 enrichment generally increased photosynthesis in C3 species relative to the CO2 fixation rates observed in the C4 species (Fig. 4A). However, relative to the C fixation rate, the concurrent export rates from the leaves did not increase proportionally in all C3 species (Fig. 4B). At high CO2 a number of C3 species that transport sugars other than Suc were capable of maintaining a high export flux rate both in absolute amounts of C being exported per unit leaf area and in terms of export flux relative to the rate of C fixation. A notable exception was sunflower. Hofstra and Nelson (1969) noted previously that sunflower displayed an unusually high rate of photosynthesis and export relative to other C3 and C4 species. When chrysanthemum, another member of the family Compositae, was tested, a relatively low rate of export was measured compared with that of sunflower. Our survey of other members of this family or of other members of the same genus is ongoing. It remains a curiosity why within the subgroup of C3 dicots that showed very high rates of export during photosynthesis (Table I; Figs. 3 and 4), sunflower, which translocates no special sugars other than Suc, should have such a high relative export rate at both ambient and high CO2 compared with the C4 plants.
The high export rates of C4 species such as gomphrena, maize, pigweed, and sorghum may be due to a combination of the differences between C3 and C4 plants, with the most prominent being the lower photosynthetic and higher photorespiratory rates of C3 plants (Hofstra and Nelson, 1969; Berry and Björkman, 1980; Zelitch, 1992). Huber et al. (1985) have shown that leaves of maize have higher amounts of Suc-phosphate synthase relative to several C3 species. A faster rate of Suc synthesis in maize is certainly consistent with the view that leaves exhibiting C4 metabolism export C more rapidly than do leaves with C3 metabolism (Fig. 3A), but these observations do not explain why many species accumulate sugars in their leaves during the photoperiod (e.g. Table I) and yet do not export these sugars immediately (Gordon, 1986; Geiger and Servaites, 1994).
There are certainly many potential sites of temporary storage of sugars in the leaf, which may become even more important when export is blocked temporarily (Robards and Lucas, 1990; Jiao and Grodzinski, 1996). At high CO2 all sites of storage in the leaf tissue have the potential to become filled more readily. It is well known that sugars and starch act as temporary storage pools in a wide variety of C3 and C4 leaf types (Stitt et al., 1987; Geiger and Servaites, 1994; Lunn and Hatch, 1995). An examination of the 14C intermediates remaining in the leaves at the end of the 2-h feeding periods indicated that at both CO2 levels, label accumulated in both sugar- and starch-storage pools (Table I). There was no discernable pattern in the labeling of sugar and starch among the C3 and C4 species tested, which would address specifically the question of whether the type of storage product would predict if a species had a propensity to export C readily in the light.
Taken together, the data show that at ambient CO2 there was a strong linear relationship between photosynthesis, synthesis of translocates, and concurrent export (Figs. 3A and 5, A and B). These data are consistent with a feed-forward relationship among photosynthesis, sugar synthesis, and immediate export (Stitt et al., 1987; Geiger and Servaites, 1994). However, at high CO2 the relationship between photosynthesis and export rate, or between immediate export and sugar synthesis was not as strong because sugars and starch were being stored.
It has been suggested that the distance that photoassimilates may travel before being loaded into the vascular system of C4 species may be shorter than in C3 species (Wardlaw, 1990, and refs. therein). Although it seems that a C4 leaf might have an advantage over a C3 leaf in concentrating CO2 at the site of Rubisco in the bundle sheath and in exporting reduced forms of C between fixation sites and the phloem, C4 species have additional C transport steps that may negate such advantages. In maize, for example, the Suc-synthesis enzymes are mainly located in the mesophyll cells (Ohsugi and Huber, 1987). Thus, trioses produced in bundle-sheath cells must be transferred to mesophyll cells before being converted to Suc. Newly formed Suc must then be returned to the bundle sheath, presumably via a symplastic route, before it can be loaded into the phloem. Whether Suc is then loaded into the phloem via a symplastic or an apoplastic mechanism is not known. Even among C3 species the movement of sugars from the mesophyll to the vascular tissue is not well defined.
Several review articles have pointed out that loading of sugars can occur by both apoplastic and symplastic routes (Robards and Lucas, 1990; Van Bel, 1993; Turgeon, 1995). In plants that export photoassimilates in the form of Suc, transfer of photoassimilates to the phloem region may be primarily symplastic, whereas phloem loading (sieve element loading) is apoplastic (Giaquinta, 1983; Van Bel, 1993). In plants that export Suc and auxiliary sugars of the raffinose series, vein loading appears to involve a symplastic mechanism (Turgeon, 1991, 1995). However, it is unclear whether there is any relationship between the mechanism of vein loading and the immediate allocation of C to export during photosynthesis. Gamalei (1991) and Van Bel (1993) have hypothesized that from an evolutionary standpoint, symplastic loading mechanisms may be less efficient than apoplastic loading mechanisms.
Whatever the energy requirements of symplastic loading mechanisms, our data show that under light-saturated photosynthetic conditions, the efflux of C (proportional to the rate of C reduction) in these C3 species is as great as in C4 plants. Species such as cucumber, coleus, catnip, and salvia, which might be classified as symplastic loaders, displayed rapid rates of C export relative to their fixation rates. In both ambient (Fig. 3B) and enriched (Fig. 4B) CO2 atmospheres, intact, attached leaves of these species exported C more readily under steady-state labeling conditions than did leaves of other C3 species such as pea that are apoplastic loaders (Giaquinta, 1983; Turgeon and Wimmers, 1988; Van Bel, 1993). Celery, which translocated mannitol, and sunflower, which translocated Suc, both exported newly fixed C as readily as the group making the raffinose sugars (Figs. 3B and 4B).
Our data are restricted to a comparison of export during photosynthesis among 21 species under conditions in which the light levels did not limit the uptake of C. These experiments do not provide an accurate estimate of daytime versus nighttime C export, which certainly reflect diurnal patterns of control (Geiger and Servaites, 1994). When the leaf is in the dark, both C skeletons for the synthesis of the translocates and the energy generated for translocation processes must be derived exclusively from previously stored carbohydrate reserves with no real opportunity to refix the CO2 lost during respiration (Grange, 1985; Gordon, 1986; Côté et al., 1992; Geiger and Servaites, 1994; Bouma et al., 1995; Grodzinski et al., 1995). Although no clear pattern of partitioning of 14C into major storage pools of sugars and starch that could be related to export during photosynthesis was derived (Table I), the nature of the storage products may affect nighttime export and the total net export of reduced C from the leaf.
We are currently investigating daytime and nighttime export patterns in a wider range of leaf forms (e.g. isogenic lines of peas) that have different export abilities under varying O2 levels (Côté et al., 1992), as well as examining a number of C4 subtypes, including C3-C4 intermediate species (Leonardos and Grodzinski, 1997). More experiments are clearly required to determine whether (and under what environmental conditions) the mechanism of loading or the utilization of auxiliary translocates (Zimmermann and Ziegler, 1975) is a factor affecting export rate during photosynthesis and the storage and subsequent export of these assimilates during the night.
ACKNOWLEDGMENTS
The authors appreciate the technical assistance of Bernie Watts and George Lin in the study of the pepper leaves and in the analysis of 14C photoassimilates, respectively. The comments of Dr. Donald Collier and Arende Librande during the preparation of an early version of this manuscript are also gratefully acknowledged.
Abbreviations:
- CE
counting efficiency
- GM
Geiger-Müller
- IRGA
IR gas analyzer
Footnotes
This research was supported by grants to B.G. from the Natural Sciences and Engineering Research Council of Canada; the Ontario Ministry of Agriculture, Food, and Rural Affairs; Flowers Canada, Ltd. (Ontario); the Cecil Delworth Foundation; and the Center for Research in Earth and Space Technology; and by an Ontario-Québec Exchange Fellowship (B.G.). E.D.L. was a recipient of an Ontario graduate fellowship.
This paper is dedicated to our friend the late David Strathern Fenson, Professor Emeritus, Mount Allison University, New Brunswick, Canada, who helped to stimulate much of the thinking about the problems associated with the measurement of export fluxes from leaves.
LITERATURE CITED
- Berry JA, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bouma TJ, De Visser R, Van Leeuwen PH, De Kock MJ, Lambers H. The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to leaf dark respiration. J Exp Bot. 1995;46:1185–1194. [Google Scholar]
- Canny M. Phloem Transport. Cambridge, UK: Cambridge University Press; 1973. [Google Scholar]
- Côté R, Thompson RG, Grodzinski B. Photosynthetic O2 production facilitates translocation of 11C-labelled photoassimilates from leaflets and tendrils of Pisum satium. J Exp Bot. 1992;23:819–829. [Google Scholar]
- Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH. Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol. 1994;106:281–292. doi: 10.1104/pp.106.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JF (1988) Temperature and the partitioning and translocation of carbon. In SP Long, FI Woodward, eds, Plants and Temperature. Symposium of the Society of Experimental Biology, Vol 42. Company of Biologists, Cambridge, UK, pp 203–235 [PubMed]
- Gamalei YV. Phloem loading and its development related to plant evolution from trees to herbs. Trees. 1991;5:50–63. [Google Scholar]
- Geiger DR, Fondy B. A method for continuous measurement of export from a leaf. Plant Physiol. 1979;64:361–365. doi: 10.1104/pp.64.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger DR, Servaites JC. Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:235–256. [Google Scholar]
- Giaquinta RT. Phloem loading of sucrose. Annu Rev Plant Physiol. 1983;34:347–387. [Google Scholar]
- Gordon AJ. Diurnal patterns of photosynthate allocation and partitioning among sinks. In: Cronshaw J, Lucas WJ, Giaquinta RT, editors. Phloem Transport. Plant Biology, Vol 1. Inc., New York: Alan R Liss; 1986. pp. 499–517. [Google Scholar]
- Grange RI. Carbon partitioning in mature leaves of pepper (Capsicum annuum) J Exp Bot. 1985;36:734–744. [Google Scholar]
- Grodzinski B, Jiao J, Lee Y. Effects of temperature on export and intracellular partitioning of 14C-photoassimilates in pea leaflets. In: Mathis P, editor. Photosynthesis: from Light to Biosphere, Vol V. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 471–474. [Google Scholar]
- Hofstra G, Nelson CD. A comparative study of translocation of assimilated 14C from leaves of different species. Planta. 1969;88:103–112. doi: 10.1007/BF01391116. [DOI] [PubMed] [Google Scholar]
- Huber SC, Kerr PS, Kalt-Torres W (1985) Regulation of sucrose formation and movement. In RL Heath, J Preiss, eds, Regulation of Carbon Partitioning in Photosynthetic Tissue. American Society of Plant Physiologists, Rockville, MD, pp 199–214
- Jiao J, Goodwin P, Grodzinski B. Photosynthesis and export during steady-state photosynthesis in bean leaves infected with the bacterium Xanthomonas campestris cv. phaseoli. Can J Bot. 1996;74:1–9. [Google Scholar]
- Jiao J, Grodzinski B. The effect of leaf temperature and photorespiratory conditions on export of sugars during steady-state photosynthesis in Salvia splendens. Plant Physiol. 1996;111:169–178. doi: 10.1104/pp.111.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardos ED, Grodzinski B. Photosynthesis, export and carbon partitioning in source leaves of C3, C3-C4 intermediates and C4 Panicum species at ambient and elevated CO2 (abstract no. 221) Plant Physiol. 1997;114:S-61. [Google Scholar]
- Leonardos ED, Tsujita JM, Grodzinski B. The effect of source or sink temperature on photosynthesis and 14C-partitioning in and export from a source leaf of Alstroemeria sp. cv. Jacqueline. Physiol Plant. 1996;79:563–575. [Google Scholar]
- Loescher WH, Everard JD. Sugar alcohol metabolism in sinks and sources. In: Zamski E, Schaffter AA, editors. Photoassimilate Distribution in Plants and Crops. Inc., New York: Marcel Dekker; 1996. pp. 185–207. [Google Scholar]
- Lunn JE, Hatch MD. Primary partitioning and storage of photosynthate in sucrose and starch in leaves of C4 plants. Planta. 1995;197:385–391. [Google Scholar]
- Madore M, Grodzinski B. Effect of oxygen concentration on 14C-photoassimilate transport from leaves of Salvia splendens L. Plant Physiol. 1984;76:782–786. doi: 10.1104/pp.76.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore M, Grodzinski B. Photosynthesis and transport of 14C-labelled photoassimilates in a dwarf cucumber cultivar under CO2 enrichment. J Plant Physiol. 1985;121:71–79. [Google Scholar]
- Ohsugi R, Huber SC. Light modulation and localization of sucrose phosphate synthase activity between mesophyll cells and bundle sheath cells in C4 species. Plant Physiol. 1987;84:1096–1101. doi: 10.1104/pp.84.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robards AW, Lucas WJ. Plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol. 1990;44:369–419. [Google Scholar]
- Stitt M, Huber S, Kerr P (1987) Control of photosynthetic sucrose formation. In MD Hatch, NK Boardman, eds, The Biochemistry of Plants. Photosynthesis, Vol 10. Academic Press, Inc., San Diego, CA, pp 327–409
- Turgeon R (1991) Symplastic phloem loading and sink-source transition in leaves: a model. In JL Bonnemain, S Delrot, J Dainty, WJ Lucas, eds, Recent Advances in Phloem Transport and Assimilate Compartmentation. Quest Editions, Nantes, France, pp 18–22
- Turgeon R (1995) The selection of raffinose family oligosaccharides as translocates in higher plants. In MA Madore, WJ Lucas, eds, Carbon Partitioning and Source Sink Interactions in Plants. Current Topics in Plant Physiology, Vol 13. American Society of Plant Physiologists, Rockville, MD, pp 195–203
- Turgeon R, Wimmers LE. Different patterns of vein loading of exogenous [14C]sucrose in leaves of Pisum sativum and Coleus blumei. Plant Physiol. 1988;87:179–182. doi: 10.1104/pp.87.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel AJE. Strategies of phloem loading. Annu Rev Plant Physiol Plant Mol Biol. 1993;41:369–419. [Google Scholar]
- Wardlaw IF. The control of carbon partitioning in plants: Tansley review no 27. New Phytol. 1990;116:341–381. doi: 10.1111/j.1469-8137.1990.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Control of plant productivity by regulation of photorespiration. BioScience. 1992;42:510–517. [Google Scholar]
- Zimmermann MH, Ziegler H. List of sugars and sugar alcohols in sieve-tube exudates. In: Zimmermann MH, Milburn JA, editors. Encyclopedia of Plant Physiology: New Series, Vol 1. Transport in Plants 1: Phloem Transport. New York: Springer-Verlag; 1975. pp. 480–503. [Google Scholar]