Abstract
This study investigates differential multi-scale structure and function relationships of the outer and inner annulus fibrosus (AF) to osmotic swelling in different buffer solutions by quantifying tensile mechanics, GAG content, water content and tissue swelling, and collagen fibril ultrastructure. In the outer AF, the tensile modulus decreased by over 70% with 0.15M PBS treatment but was unchanged with 2M PBS treatment. Moreover, the modulus loss following 0.15M PBS treatment was reversed when followed by 2M PBS treatment, potentially from increased interfibrillar and interlamellar shearing associated with fibril swelling. In contrast, the inner AF tensile modulus was unchanged by 0.15M PBS treatment and increased following 2M treatment. Transmission electron microscopy revealed that the mean collagen fibril diameters of the untreated outer and inner AF were 87.8 ± 27.9 and 71.0 ± 26.9 nm, respectively. In the outer AF, collagen fibril swelling was observed with both 0.15M and 2M PBS treatments, but inherently low GAG content remained unchanged. In the inner AF, 2M PBS treatment caused fibril swelling and GAG loss, suggesting that GAG plays a role in maintaining the structure of collagen fibrils leading to modulation of the native tissue mechanical properties. These results demonstrate important regional variations in structure and composition, and their influence on the heterogeneous mechanics of the AF. Moreover, because the composition and structure is altered as a consequence of progressive disc degeneration, quantification of these interactions is critical for study of the AF pathogenesis of degeneration and tissue engineering.
Keywords: tensile properties, collagen fibril diameter, extrafibrillar matrix, annulus fibrosus, ultrastructure, glycosaminoglycan
INTRODUCTION
The annulus fibrosus (AF) is a multi-lamellar fibrocartilagenous ring in the intervertebral disc. The AF composition and structure are critical to its physiological role, which is mechanical. The AF, comprised primarily of collagens and proteoglycans, possesses a highly ordered structure: concentric lamellae are composed of collagen bundles arranged at alternating angles of approximately ±30° to the transverse axis, to form an angle-ply architecture.13 The collagen network of the AF consists mainly of types I and II. The relative ratio of type I to type II collagen gradually decreases from the outer to inner AF; the outer AF contains mostly type I collagen16 and innermost AF contains only type II collagen.17 Aggrecan, a high molecular weight aggregating proteoglycan, and hyaluronan, a non-sulfated glycoasaminoglycan (GAG), contents gradually increase from the outer to inner AF.3,25,27,37,44 Furthermore, some members of small leucine rich proteoglycan such as decorin is found at a higher concentration in the outer AF compared to the inner AF.19,49 This variation biochemical content within the AF is in large part responsible for the heterogeneous AF mechanical properties.1,17,39 As disc degeneration progresses, many biochemical and structural changes occur, such as loss of proteoglycan, an increase in the collagen type I to II ratio, and eventually both structural and functional degradation.3,37,43,49 While AF degenerative changes are well-documented, how these manifest in the functional decline of AF mechanics is not fully understood. Therefore, it is crucial to investigate the relationships between structure, composition, and mechanical function.
In load-bearing soft tissues, it is generally accepted that collagen resists tension and proteoglycan resists compression, however the relationships between structure, composition, and tensile mechanical function remain poorly understood. In tissues with high GAG content (>25% dry weight) such as articular cartilage, enzymatic removal of GAG increases tensile modulus.4,5,45 Similar findings have been reported in various engineered cartilage, fibrocartilage, and the inner AF.32–34 In contrast, in tissues with relatively low GAG content such as tendon and ligament (<1% dry weight), depletion of GAG has little or no effect on tensile modulus.29,46,54 Because GAG content increases radially from the outer AF inward,3,25,37 the relationship between AF mechanics, GAG content, and potential GAG loss by diffusion following prolonged periods in PBS remains unknown.
In vitro tissue-level studies require mechanical testing in aqueous buffers such as PBS to prevent tissue dehydration. It is therefore important to examine how PBS buffer affects tissue function. Moreover, the varying GAG content, and associated fixed charge density, from outer to inner AF suggest that the response to high and low PBS osmolarity may also be different with AF radial position. Previous studies in tendon and ligament have been conflicting: soaking tendon fascicles in PBS decreased tensile modulus,46,47 testing patellar tendon in PBS bath increased strength and modulus,22 and treating ligament in buffer had no effect on modulus.29 The influence of aqueous in vitro testing environment is further confounded by the contrasting findings on the relationship of collagen fibril tensile modulus and environmental salt concentration.20,51 Soaking ligament in buffer solutions, as a control for enzymatic digestion, alters the mechanics and the tissue water content.14,24,52
Collagen ultrastructure, specifically fibril diameter, has been suggested as a crucial determinant of tissue tensile modulus.2,12,40–42,48 In addition, collagen cross-links, fibril length, and concentration within matrix have been implied to have significant roles in force transmission in ligament, tendon, and engineered extracellular matrix.8,11,40–42 Differences in collagen fibril ultrastructure may exist across AF regions, and it is possible that tissue treatments prior to and during mechanical testing can alter this ultrastructure, affecting tissue mechanics in a region-specific manner.20,24,46 Therefore, tissue ultrastructure should be considered in the investigation of the composition, structure, and function relationships.
The objective of this study was to investigate multi-scale structure and function relationships of the outer and inner AF in response to osmotic loading in isotonic (0.15M) and hypertonic (2M) buffer solutions by quantifying tensile mechanics, GAG content, water content, tissue swelling, and collagen fibril ultrastructure. Because the mechanical properties, biochemical composition, and structure vary noticeably between the inner and outer AF,3,16,17,25,37 it was hypothesized that the functional contributions of treatment would differ between the inner and outer AF. A secondary objective was to address the implication for the effect of the PBS baths when interpreting mechanical studies of many fibrous soft tissues. The multi-scale AF structure and function relationships quantified here are necessary to understand pathogenesis of degeneration and develop engineered and biological AF treatments.
MATERIALS AND METHODS
Sample Preparation and Treatment
Intervertebral discs were isolated from adult bovine caudal spines. Bovine caudal discs exhibit similar structure and mechanics to human discs, thus making them a reliable and accessible model for intervertebral disc research.9,26,35 Inner and outer AF tissue was excised from the harvested discs using a scalpel. Each sample was sectioned to 1.5 mm thickness on a freezing-stage microtome at −20°C. Samples were further cut to dimensions of 14 × 3 × 1.5 mm (circumferential length × axial width × radial thickness) while frozen. Untreated control samples were wrapped in gauze and stored immediately at −20°C until mechanical testing.
Samples subject to treatments were introduced to both isotonic (0.15M) and hypertonic (2M) PBS (pH 7.2) to investigate differential multi-scale structure and function relationships in response to osmotic loading by quantifying tensile mechanics, GAG content, water content, tissue swelling, and collagen fibril ultrastructure. Treating samples in a solution with high salt concentration (2M PBS) creates an osmotic gradient that decreases tissue water content and prevent swelling. Osmolarity of PBS was determined based on NaCl concentration (2M PBS: 2M NaCl, 118.26 mM Na2HPO4, 39.42 mM KCl, 21.9 mM KH2PO4). For both outer and inner AF, samples were classified into 6 groups: samples treated in 1) 0.15M PBS for 6-hours (6h-0.15M), 2) 2M PBS for 6-hours (6h-2M), 3) 0.15M PBS for 12-hours (12h-0.15M), 4) 2M PBS for 12-hours (12h-2M), 5) 0.15M PBS for the first 6-hours, then subsequently treated in 2M PBS for additional 6-hours (0.15→2M), 6) and untreated control samples (untreated). All treatments were performed at 37°C under gentle agitation. After treatment, sample was gently blotted and wet weight was measured using an analytical balance. Cross-sectional area was measured with a custom non-contact laser device that recorded five width and thickness measurements to determine the mean cross-sectional area.29,34,38 For samples treated for 12 hours, cross-sectional area and wet weight were measured at 0, 6, and 12 hour time points to determine change in these parameters on a matched-sample basis. After these measurements the sample was wrapped in gauze and stored at −20°C for no longer than 3 days until mechanical testing.
Uniaxial Tensile Testing
6 samples per group and region were mechanically tested. Prior to mechanical testing, sample was thawed at room temperature for 30 minutes. Each sample was lightly speckle-coated with black enamel paint using an airbrush for optical surface strain analysis.15,34 Uniaxial tensile testing was performed using an Instron 5542 (Instron, Norwood, MA). Samples in groups 1 and 3 were tested in a 0.15M PBS, and samples in groups 2, 4 and 5 were tested in a 2M PBS bath. Samples were placed into custom-made serrated grips and loaded into the Instron. A 5 min preload of 0.1N was applied. Samples were then preconditioned for 15 cycles to 0.1% strain at a rate of 0.5%/sec to minimize grip effects. Samples were tested to failure at a strain rate of 0.1%/sec. Digital images of the sample mid-substance were captured every 5 sec (1 image per 0.5% strain increment).
Stress was calculated as load divided by the initial cross-sectional area. Lagrangian strain was calculated from acquired images using Vic-2D texture correlation software (Correlated Solutions Inc., Columbia, SC). Linear modulus was calculated using a custom-written MATLAB (The Mathworks Inc., Natick, MA) program that performed a regression to the linear region of stress-strain curve.
Glycosaminoglycan Content
After mechanical testing, each sample was dried overnight in a 60°C oven and dry weight was measured (n=6 per group). Percent water content was determined by subtracting sample dry weight from wet weight and dividing by wet weight. The dried sample was then digested with papain solution at 60°C overnight. Total GAG content was determined via the 1,9-dimethylmethylene blue dye binding (DMMB) assay and normalized by dry weight.28,34 Dry weights from 0, 6, and 12 hour time points were compared on a sample-by-sample basis to determine potential salt migrating into the tissue.
Electron Microscopy
The cross-sectional plane of the collagen fibrils in both inner and outer AF were imaged via transmission electron microscopy (TEM) to determine the fibril diameter distribution and percent extrafibrillar space of the untreated (n=1) and 6 hr treated groups (0.15M and 2M; n=1 each). Two adjacent caudal discs were dissected from an initially frozen bovine tail. Samples were prepared under a dissecting microscope to aid visualization of fiber alignment, and then trimmed to rectangular blocks (4 × 3 mm) with the long axis parallel to one of the fiber directions using a scalpel. Samples did not undergo any freeze/thaw cycles upon dissection.
Samples were fixed overnight at 4°C in 2.5% glutaraldehyde, 2.0% paraformaldehyde in 0.1M sodium cacodylate buffer (pH 7.4), then washed several times in buffer and post-fixed for 1 hour in 2% osmium tetroxide. After brief washes with deionized water, samples were stained en bloc in 2% aqueous uranyl acetate for 30 minutes, rinsed again in deionized water prior to dehydration in a graded ethanol series, and finally infiltrated and embedded in EMbed-812 (Electron Microscopy Sciences, Hatfield, PA). Sections were stained with uranyl acetate and lead citrate prior to examination in a JEOL 1010 electron microscope (JEOL Ltd., Peabody, MA) fitted with a Hamamatsu digital camera (Hamamatsu Corp., Japan) and AMT advantage image capture software (Advanced Microscopy Techniques Corp., Woburn, MA).
Ten images at 60,000X magnification were chosen randomly from different locations within the sample. ImageJ (NIH, Bethesda, MD) was used to measure collagen fibril diameters (n=300). Fibril diameter distribution was binned in 20 nm intervals and normalized to the total number of measured fibrils. Ten images at 20,000X magnification were used to determine percent extrafibrillar space (n=10), calculated using a custom-written MATLAB program that converted each gray scale TEM image to a binary image and calculated the ratio of the white pixel area (no fibrils) to the total pixel area.
Statistical Analysis
For all statistics, significance was set at the 95 percent confidence level (p < 0.05). For GAG content and tensile modulus, a one-way ANOVA with Tukey’s post-hoc analysis was used to test for significant difference between groups (factor: treatment, levels: untreated, 0.15M, 2M for the 6-hour groups, levels: untreated, 0.15M, 0.15→2M, 2M for the 12-hour groups). Statistics were performed separately for the outer and inner AF. The untreated inner and outer AF groups were compared with two-tailed Student’s t-test. For the change in cross-sectional area and water content, a one-factor repeated measure ANOVA (factor: time, levels: 0, 6, 12 hours) with Tukey’s post-hoc was applied to test for significant difference within each treatment group.
To compare fibril diameter distributions between groups in each of the outer and inner AF, statistical analyses were performed as previously described.18 For each fibril diameter distribution, sample median, median-to-first quartile distance, and median-to-third quartile distance were calculated (n=10). These values assess the center, the spread, and the degree of skewness of the distributions.18 Distributions of the sample medians and median-to-quartile distances were compared to the corresponding untreated group via the Kolmogorov-Smirnov test. For percent extrafibrillar space, a one-way ANOVA with Tukey’s post-hoc analysis was performed. The untreated inner and outer AF groups were compared with two-tailed Student’s t-test.
RESULTS
Mechanical Properties
Linear modulus was significantly greater for the outer AF than the inner (17.22±7.69 and 2.65±1.03 MPa respectively, p<0.05, Fig. 1). In the outer AF, treatment in 0.15M PBS for both 6 and 12 hours decreased the tensile modulus by 89 and 74%, respectively, compared to untreated (p<0.05, Fig. 1). However, 2M PBS treatment for both 6 and 12 hours maintained the modulus at the untreated levels (Fig. 1). The reduction in modulus observed with 0.15M PBS treatment was reversed if followed by 2M PBS (0.15→2M), where no significant difference was observed relative to either untreated or 2M PBS groups (Fig. 1).
Figure 1.
Linear-region tensile modulus (MPa) of the untreated and treatment groups of both outer and inner AF. Statistical analysis performed on groups within each time point plus the respective untreated group. 0.15→2M = 6h 0.15M PBS + 6h 2M PBS. * = p < 0.05 compared to untreated. ★ = p < 0.05 compared to 0.15M. # = p < 0.05 compared to the inner AF untreated.
In contrast to the outer AF, tensile modulus of the inner AF samples treated in 0.15M PBS for 6 and 12 hours remained unchanged compared to untreated (Fig. 1). However, 2M PBS treatment for 6 and 12 hours increased the modulus 250 and 130%, respectively, compared to untreated (p<0.05, Fig. 1).
GAG Content, Cross-Sectional Area and Water Content
The mean dry weights of untreated and 0.15M, 2M PBS treated groups were not statistically different, ensuring insignificant effect of salt solution confounding water and GAG content measurements (data not shown). The GAG content in the untreated outer AF was 6.65 ± 3.48% dry weight and the inner AF was 19.75 ± 4.85% dry weight (p < 0.05, Fig. 2), similar to previously reported findings.25 In the outer AF, no significant change in GAG content was observed following any PBS treatment (Fig. 2). In the inner AF, treatment with 2M PBS resulted in a 43–60% loss of GAG compared to the untreated group (p<0.05, Fig. 2). No significant changes were observed following 0.15M and 0.15→2M PBS treatments.
Figure 2.
GAG content (% DW) of both outer and inner AF. Statistical analysis performed on groups within each time point plus the respective untreated group. DW = dry weight. 0.15→2M = 6h 0.15M PBS + 6h 2M PBS. * = p < 0.05 compared to untreated. # = p < 0.05 compared to the inner AF untreated.
In the outer AF, treatment in 0.15M PBS for 6 and 12 hours resulted in a significant 40% increase in cross-sectional area from the 0-hour initial state (Fig. 3, p < 0.05). After 12 hours in 2M PBS, a significant decrease in cross-sectional area and water was observed relative to the 6-hour state (Fig. 3 and 4). Inner AF water content decreased after 6 and 12 hours of treatment in 0.15M PBS from the initial state (p < 0.05, Fig. 4), however area was unchanged. After 6 and 12 hours of treatment in 2M PBS, a significant decrease in cross-sectional area from the initial state and in water content from the 6-hour state was observed (Fig. 3 and 4). The changes observed with 0.15M PBS treatment were generally reversed if followed by 2M PBS (0.15→2M), where the final values were consistent with 2M PBS groups (Fig. 3 and 4).
Figure 3.
Percent change in cross-sectional area normalized to 0-hour time point. 0.15→2M = 6h 0.15M PBS + 6h 2M PBS. * = p < 0.05 compared to respective 0-hour measurements. # = p < 0.05 compared to respective 6-hour measurements.
Figure 4.
Percent change in water content normalized to 0-hour time point. 0.15→2M = 6h 0.15M PBS + 6h 2M PBS. * = p < 0.05 compared to respective 0-hour measurements. # = p < 0.05 compared to respective 6-hour measurements.
Electron Microscopy
Electron microscopy was performed on the 6 hr treatment groups. The mean collagen fibril diameter of the untreated outer AF (87.8 ± 27.9 nm, Fig. 5A, Table 1) was larger than the collagen fibril diameter of the inner AF (71.0 ± 26.9 nm, Fig. 5D, Table 1). The median of the outer AF fibril diameter was significantly greater than the fibril diameter of the inner AF, whereas both median-to-first and median-to-third quartile distances were similar (p > 0.05), suggesting that the outer AF has comparable fibril distribution than the inner AF, but larger fibril diameter (Table 1).
Figure 5.
Distribution of collagen fibril diameter (nm) in the AF. A: Outer AF untreated, B: Outer AF 0.15M, C: Outer AF 2M, D: Inner AF untreated, E: Inner AF 0.15M, F: Inner 2M AF. Black lines represent means of respective untreated groups.
Table 1.
Summary of mean fibril diameter, median, median-to-first quartile distance (Median-Q1), and median-to-third quartile distance (Median-Q3) of collagen fibril diameter distributions of different groups (nm).
| Mean Diameter | Median | Median-Q1 | Median-Q3 | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | |
| Outer AF | |||||||
| Untreated# | 87.8 ± 27.9 | 85.1 ± 7.0 | 0.03 | 17.7 ± 4.9 | n.s | 25.2 ± 7.0 | n.s |
| 6h 0.15M | 97.9 ± 25.8 | 100.2 ± 6.8 | 0.001 | 19.4 ± 7.2 | n.s | 16.9 ± 5.0 | 0.03 |
| 6h 2M | 123.3 ± 39.6 | 130.3 ± 14.1 | <0.001 | 33.6 ± 17.0 | 0.007 | 24.1 ± 10.3 | n.s |
| Inner AF | |||||||
| Untreated | 71.0 ± 26.9 | 71.2 ± 8.4 | - | 20.5 ± 7.1 | - | 18.9 ± 6.1 | - |
| 6h 0.15M | 80.6 ± 27.2 | 82.2 ± 9.1 | n.s | 22.7 ± 7.2 | n.s | 20.0 ± 7.4 | n.s |
| 6h 2M | 105.5 ± 26.0 | 104.5 ± 7.1 | <0.001 | 17.0 ± 6.5 | n.s | 20.3 ± 9.6 | n.s |
p values are obtained from two-sample Kolmogorov-Smirnov tests against the corresponding native values.
= compared to the untreated inner AF.
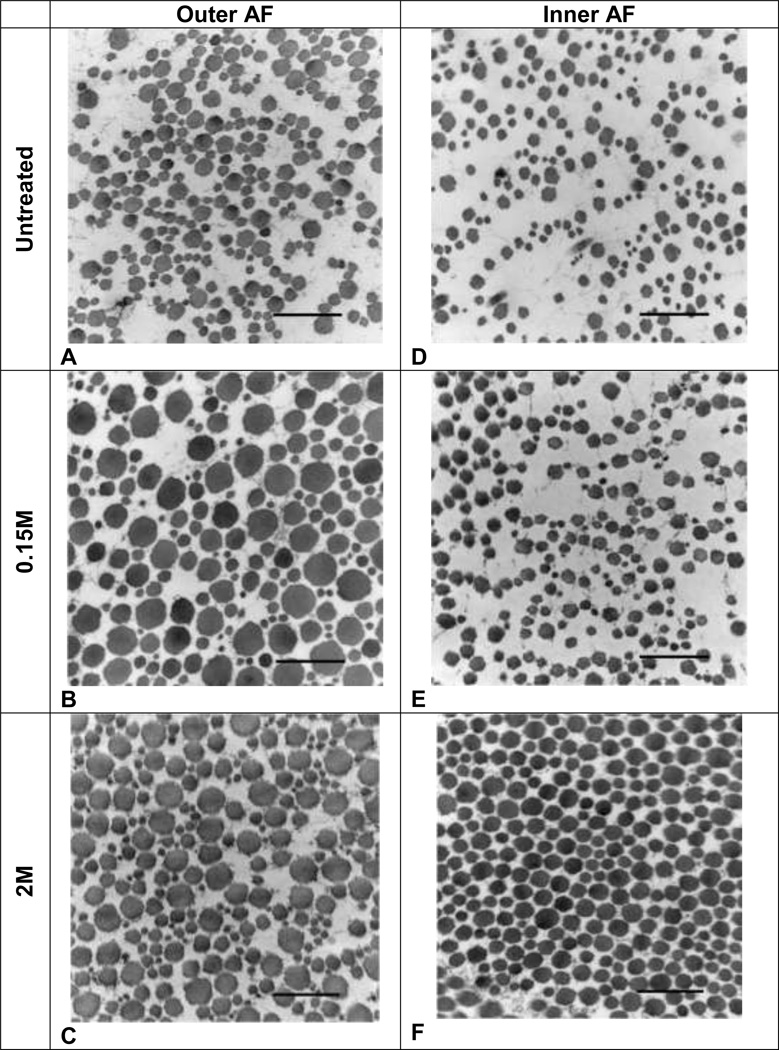
High magnification images of the outer AF revealed apparent swollen fibrils in the 0.15M and 2M PBS samples compared to the untreated (Fig. 6A–C). The inner AF showed no evident morphological differences between the untreated and 0.15M PBS samples (Fig. 6D–E). However, the fibril organization of the 2M PBS sample appeared denser than the other groups (Fig. 6F). Similar observations were made in low magnification images of the outer and inner AF (Fig. 7A–F). In both outer and inner AF, percent extrafibrillar space of the 2M sample was significantly reduced from the untreated (Fig. 8). Percent extrafibrillar spaces of the 0.15M PBS group was unaffected compared to untreated.
Figure 6.
Representative TEM images of collagen fibrils in the outer and inner AF taken at 60000X magnification. A: Outer AF untreated, B: Outer AF 0.15M, C: Outer AF 2M, D: Inner AF untreated, E: Inner AF 0.15M, F: Inner AF 2M. Bars = 500 nm.
Figure 7.
Representative TEM images of collagen fibrils in the outer and inner AF taken at 20000X magnification. A: Outer AF untreated, B: Outer AF 0.15M, C: Outer AF 2M, D: Inner AF untreated, E: Inner AF 0.15M, F: Inner AF 2M. Bars = 500 nm.
Figure 8.
Percent extrafibrillar space of the untreated, 0.15M, and 2M outer/inner AF groups. * = p < 0.05 compared to untreated.
For the outer AF, the distributions of both treatment groups (0.15M and 2M) had larger fibril diameters, as indicated by significant increases in medians, compared to the untreated (Fig. 5A–C, Table 1). The mean fibril diameters of the samples treated in 0.15M and 2M PBS increased by 10.1 and 35.5 nm, respectively (Table 1). The median-to-first quartile distances of the 2M PBS group significantly increased from the untreated, and the 0.15M group had significantly decreased median-to-third quartile distance compared to the untreated, collectively supporting the overall shift in distribution toward larger fibril diameter with both treatments (Table 1).
For the inner AF, the collagen fibril distribution of 0.15M was unchanged compared to the untreated (Fig. 5E, Table 1). On the other hand, the mean fibril diameter increased by 34.5 nm for 2M PBS group from the untreated (Fig. 5F, Table 1). Significantly increased median values of the 2M PBS group support increased fibril diameter with treatments (Table 1). The distribution was generally unaffected by treatments (Table 1).
DISCUSSION
This study demonstrated different multi-scale structure and function response of the outer and inner AF to osmotic treatments in isotonic (0.15M) and hypertonic (2M) buffer solutions, including the tensile modulus, GAG content, water content and tissue swelling, and collagen fibril ultrastructure. For the first time, to the best of authors’ knowledge, collagen fibril diameters of inner and outer AF were measured. The mechanical function results in the study can be interpreted as due to the effects of three measured factors: GAG content, tissue swelling, and collagen ultrastructure, as discussed below for the inner and outer AF.
The Inner AF
Role of GAG and Tissue Swelling
The inner AF experienced both an increased modulus and GAG loss with 2M PBS treatment (Fig. 1 and 2), which is consistent with the observed effect of GAG loss due to chondroitinase ABC treatment in articular cartilage,4,5,45 GAG-rich engineered constructs,32–34 and inner AF.34 High native GAG content (~25% dry weight) is a similarity across these tissues. It is theorized that the inner AF becomes stiffer with GAG depletion because the rotation and recruitment of collagen fibers in the direction of loading becomes less constricted.6,34
The inner AF tissue did not swell during treatments, rather 0.15M PBS treatment resulted in a lower water content and 12-hour 2M PBS treatment resulted in both lower cross-sectional area and water content (Fig. 3 and 4). One possible explanation for the absence of swelling with 0.15M PBS treatment is that the inner AF is highly hydrated in situ, as it lies in direct apposition to the nucleus pulposus, which has a very high GAG and water content with osmolality of approximately 0.45 to 0.55 Osm.53 The tissue expelled water during 2M PBS treatment to maintain osmotic equilibrium ex vivo.
Ultrastructure
Fibril diameter and extrafibrillar space were consistent with observations of the modulus and cross-sectional area. 2M PBS treatment increased the mean fibril diameter by 50% and reduced extrafibrillar space by 50% (Fig. 8, Table 1). As the tissue compacts in 2M hypertonic solution, it is likely that some water leaves the tissue (water content decreased) while some water enters the fibrils (diameter increased and extrafibrillar space decreased). As 6-hour 2M PBS treatment also lost GAG, it is possible that GAG is an extrafibrillar matrix molecule that contributes to preventing collagen fibril swelling. For instance, it has been shown that decorin-deficiency during tendon development causes distorted collagen fibrils with increase fibril diameter.56 In addition a structural investigation on SLRP revealed that collagen II and aggrecan are joined to collagen VI via SLRP, providing interconnected mesh-like networks within the ECM.55
The Outer AF
Role of GAG and Tissue Swelling
The outer AF exhibited significantly different mechanical responses to treatments than the inner AF. In the outer AF, modulus decreased 80–90% with 0.15M PBS compared to the untreated (Fig. 1). Treatment in 2M PBS did not affect tensile modulus (Fig. 1). Past studies on tendon soaked in PBS recorded similar observations, where the tensile modulus of tendon fascicles in 0.15M PBS decreased significantly.46,47 Electron microscopy suggested that swelling increased the distance between collagen fibrils, potentially affecting the hypothesized mechanical load transfer interactions between collagen and GAG.46,47 A similar phenomenon was observed in the outer AF in shear, where PBS treatment decreased the shear modulus.28 Because the GAG content is relatively low in the outer AF (~7% DW) and no significant change in GAG content was observed, the role of GAG in the outer AF is difficult to interpret.
Maintenance of the outer AF modulus with 2M PBS treatment suggests that 2M PBS reduces or prevents tissue swelling, which is supported by decreased cross-sectional area and water content (Fig. 3 and 4). Analogously, samples soaked in 0.15M PBS increased cross-sectional area from the original state, suggesting bulk tissue swelling (Fig. 3). Samples that have been swollen and subsequently un-swollen (0.15→2M) also were able to recover their tensile modulus, suggesting that the dramatic loss in tensile modulus that occurs with PBS is in fact reversible when the sample is subjected to hypertonic conditions. Osmolarity of buffer solution influences the water content of the tissue, which subsequently has an effect on mechanical properties.20,24,52 For instance, increased dilatational stress generated from tissue hydration is positively related to creep, stress and cyclic relaxation responses, giving dry tissues stiffer properties compared to hydrated tissues.7,14,23,24,30,31,36,52
Ultrastructure
Both osmolarity treatments resulted in greater mean fibril diameter relative to the untreated for the outer AF. Unlike the shift in fibril diameter distribution with swelling in 2M PBS treated inner AF (Fig. 5F), the distributions of treated outer AF groups did not shift to the right in a uniform manner. It is postulated that smaller fibrils in the outer AF swelled more extensively compared to the larger fibrils. The samples following 0.15M PBS treatment resulted in a reduction in the modulus, suggesting that larger fibril diameter is related to lower tensile modulus. A previous finding that compared the ultrastructure of individual collagen fibrils with mechanical properties of the nucleus pulposus demonstrated that the compressive modulus linearly increases with the mean fibril diameter.2 Similarly, a three-dimensional matrix prepared from type I collagen with smaller fibril diameters exhibited increased tensile linear modulus and failure stress compared to the matrix made up of fibrils with larger diameter, further highlighting the importance of fibril diameter on tissue mechanical function.41 Multi-scale models of collagen fibrils have suggested that tensile stiffness of collagen fibrils is inversely related to the number of tropocollagen molecules in a fibril’s cross-section, supporting that tensile stiffness decreases as fibril diameter increases.12 The mechanical behavior of collagen fibrils is dependent on collagen fibril geometry (diameter) as well as structural features (spatial variation) within the fibrils, which may exhibit different fibril crimping characteristics and shear behavior.48
Despite overall increased fibril diameters in the 2M PBS group compared to the untreated (Fig. 5C), the modulus was maintained compared to the untreated (Fig. 1). Possibly, more compact fibril organization induced with hypertonic solution, along with decreased water content, may have prevented additional reduction of the modulus from increased interfibrillar and interlamellar shearing (Fig. 8).21,28
Additional Factors and Limitations
A limitation in the current study was that only one tissue sample per group underwent TEM imaging, although 10 individual regions of the sample were analyzed and 300 fibril diameters were measured. In addition, the TEM samples were prepared from fresh-frozen tissue. While this may alter the ultrastructure, no effects of freezing were apparent. Moreover, the data analysis was comparative with respect to osmotic treatment and AF region, so any effect of freezing should carry across all analyses.
Collagen cross-links may also be involved in tissue swelling and tensile properties, although they were not quantified in this study. Single fibrils from an adult human patellar tendon with a high content of mature cross-links had no relationship between tensile properties and environment salts,50 while a fibril from a reconstituted bovine Achilles tendon with little or no mature cross-links had a twofold increase in tensile modulus with increased salt concentration in a phosphate buffer.20 The importance of mature cross-links for mechanical stability also has been established in rat aorta, where a 49% decrease in pyridinoline resulted in a significantly reduced maximum load and stiffness.10
The outer and inner AF are clearly distinguished by their collagen organization as well by collagen type. The AF collagen network becomes more organized from the nucleus pulposus radially outward and the ratio of type I to type II increases.16,17 The collagen fibrils of the inner AF (20–140nm) were smaller than the collagen fibrils of the outer AF (20–160nm; Fig. 5). It is possible that varying fibril diameters in the outer and inner AF arise from different types of collagen. It is likely that different types of collagen with varying geometry have distinct mechanical roles.2,12,48
Conclusion
The inner and outer AF mechanical behaviors are distinct and their responses to osmotic loading in isotonic and hypertonic buffers vary significantly due to regional variations in disc ultrastructure and composition. The present work demonstrates inhomogeneous multi-scale relationships between AF composition, structure and tensile mechanical function, and how aqueous environment can significantly alter the tissue properties. These observations show the importance of considering the effect of PBS baths when interpreting fibrous soft tissue mechanical studies. Moreover, because the composition and structure of the AF is altered as a consequence of progressive disc degeneration, quantification of these interactions are critical for study of pathogenesis of degeneration and development of engineered and biological treatments for the AF.
ACKNOWLEDGEMENTS
Funded by the National Institutes of Health EB02425 and the Penn Center for Musculoskeletal Disorders.
REFERENCES
- 1.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20:2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Aladin DMK, Cheung KMC, Ngan AHW, Chan D, Leung VYL, Lim CT, Luk KDK, Lu WW. Nanostructure of collagen fibrils in human nucleus pulposus and its correlation with macroscale tissue mechanics. J. Orthop. Res. 2010;28:497–502. doi: 10.1002/jor.21010. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asanbaeva A, Masuda K, Thonar EJ-MA, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188–198. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 5.Asanbaeva A, Masuda K, Thonar EJ-MA, Klisch SM, Sah RL. Cartilage growth and remodeling: modulation of balance between proteoglycan and collagen network in vitro with beta-aminopropionitrile. Osteoarthr. Cartil. 2008;16:1–11. doi: 10.1016/j.joca.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 2009;42:1163–1176. doi: 10.1016/j.jbiomech.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson TS, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 8.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 9.Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine. 2008;33:E166–E173. doi: 10.1097/BRS.0b013e318166e001. [DOI] [PubMed] [Google Scholar]
- 10.Brüel A, Ortoft G, Oxlund H. Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis. 1998;140:135–145. doi: 10.1016/s0021-9150(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 11.Buehler MJ. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater. 2008;1:59–67. doi: 10.1016/j.jmbbm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Buehler MJ, Wong SY. Entropic elasticity controls nanomechanics of single tropocollagen molecules. Biophys. J. 2007;93:37–43. doi: 10.1529/biophysj.106.102616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989;23:75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 14.Chimich D, Shrive N, Frank C, Marchuk L, Bray R. Water content alters viscoelastic behaviour of the normal adolescent rabbit medial collateral ligament. J Biomech. 1992;25:831–837. doi: 10.1016/0021-9290(92)90223-n. [DOI] [PubMed] [Google Scholar]
- 15.Elliott DM, Setton LA. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: experimental measurement and material model predictions. J Biomech Eng. 2001;123:256–263. doi: 10.1115/1.1374202. [DOI] [PubMed] [Google Scholar]
- 16.Eyre DR. Biochemistry of the intervertebral disc. Int Rev Connect Tissue Res. 1979;8:227–291. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- 17.Eyre DR, Muir H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976;157:267–270. doi: 10.1042/bj1570267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Götz W, Barnert S, Bertagnoli R, Miosge N, Kresse H, Herken R. Immunohistochemical localization of the small proteoglycans decorin and biglycan in human intervertebral discs. Cell Tissue Res. 1997;289:185–190. doi: 10.1007/s004410050864. [DOI] [PubMed] [Google Scholar]
- 20.Grant CA, Brockwell DJ, Radford SE, Thomson NH. Tuning the elastic modulus of hydrated collagen fibrils. Biophys. J. 2009;97:2985–2992. doi: 10.1016/j.bpj.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerin HL, Elliott DM. Quantifying the contributions of structure to annulus fibrosus mechanical function using a nonlinear, anisotropic, hyperelastic model. J. Orthop. Res. 2007;25:508–516. doi: 10.1002/jor.20324. [DOI] [PubMed] [Google Scholar]
- 22.Haut RC, Powlison AC. The effects of test environment and cyclic stretching on the failure properties of human patellar tendons. J. Orthop. Res. 1990;8:532–540. doi: 10.1002/jor.1100080409. [DOI] [PubMed] [Google Scholar]
- 23.Haut TL, Haut RC. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J Biomech. 1997;30:79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman AH, Robichaud DR, 2nd, Duquette JJ, Grigg P. Determining the effect of hydration upon the properties of ligaments using pseudo Gaussian stress stimuli. J Biomech. 2005;38:1636–1642. doi: 10.1016/j.jbiomech.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Iatridis JC, MacLean JJ, O’Brien M, Stokes IAF. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine. 2007;32:1493–1497. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iatridis JC, MaClean JJ, Ryan DA. Mechanical damage to the intervertebral disc annulus fibrosus subjected to tensile loading. J Biomech. 2005;38:557–565. doi: 10.1016/j.jbiomech.2004.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inkinen RI, Lammi MJ, Agren U, Tammi R, Puustjärvi K, Tammi MI. Hyaluronan distribution in the human and canine intervertebral disc and cartilage endplate. Histochem. J. 1999;31:579–587. doi: 10.1023/a:1003898923823. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs NT, Smith LJ, Han WM, Morelli J, Yoder JH, Elliott DM. Effect of orientation and targeted extracellular matrix degradation on the shear mechanical properties of the annulus fibrosus. J Mech Behav Biomed Mater. 2011;4:1611–1619. doi: 10.1016/j.jmbbm.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lujan TJ, Underwood CJ, Jacobs NT, Weiss JA. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J. Appl. Physiol. 2009;106:423–431. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maroudas A, Ziv I, Weisman N, Venn M. Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology. 1985;22:159–169. doi: 10.3233/bir-1985-22206. [DOI] [PubMed] [Google Scholar]
- 31.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 32.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. J. Orthop. Res. 2009;27:949–956. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A. 2009;15:3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nerurkar NL, Han W, Mauck RL, Elliott DM. Homologous structure-function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials. 2011;32:461–468. doi: 10.1016/j.biomaterials.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 36.Panagiotacopulos ND, Knauss WG, Bloch R. On the mechanical properties of human intervertebral disc material. Biorheology. 1979;16:317–330. doi: 10.3233/bir-1979-164-506. [DOI] [PubMed] [Google Scholar]
- 37.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J. Orthop. Res. 1987;5:198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 38.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. Mechanical properties of the long5 head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J. Orthop. Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perie DS, Maclean JJ, Owen JP, Iatridis JC. Correlating material properties with tissue composition in enzymatically digested bovine annulus fibrosus and nucleus pulposus tissue. Ann Biomed Eng. 2006;34:769–777. doi: 10.1007/s10439-006-9091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provenzano PP, Vanderby R., Jr Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25:71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124:214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 42.Roeder BA, Kokini K, Voytik-Harbin SL. Fibril microstructure affects strain transmission within collagen extracellular matrices. J Biomech Eng. 2009;131 doi: 10.1115/1.3005331. 031004. [DOI] [PubMed] [Google Scholar]
- 43.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem. Soc. Trans. 2002;30:869–874. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 44.Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S326–S332. doi: 10.1007/s00586-006-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J. Orthop. Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 46.Screen HRC, Chhaya VH, Greenwald SE, Bader DL, Lee DA, Shelton JC. The influence of swelling and matrix degradation on the microstructural integrity of tendon. Acta Biomater. 2006;2:505–513. doi: 10.1016/j.actbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Screen HRC, Shelton JC, Chhaya VH, Kayser MV, Bader DL, Lee DA. The influence of noncollagenous matrix components on the micromechanical environment of tendon fascicles. Ann Biomed Eng. 2005;33:1090–1099. doi: 10.1007/s10439-005-5777-9. [DOI] [PubMed] [Google Scholar]
- 48.Shen ZL, Dodge MR, Kahn H, Ballarini R, Eppell SJ. Stress-strain experiments on individual collagen fibrils. Biophys. J. 2008;95:3956–3963. doi: 10.1529/biophysj.107.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh K, Masuda K, Thonar EJ-MA, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine. 2009;34:10–16. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svensson RB, Hassenkam T, Grant CA, Magnusson SP. T ensile properties of human collagen fibrils and fascicles are insensitive to environmental salts. Biophys. J. 2010;99:4020–4027. doi: 10.1016/j.bpj.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Svensson RB, Hassenkam T, Hansen P, Kjaer M, Magnusson SP. Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect. Tissue Res. 2011;52:415–421. doi: 10.3109/03008207.2010.551569. [DOI] [PubMed] [Google Scholar]
- 52.Thornton GM, Shrive NG, Frank CB. Altering ligament water content affects ligament pre-stress and creep behaviour. J. Orthop. Res. 2001;19:845–851. doi: 10.1016/S0736-0266(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 53.Urban JPG. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002;30:858–864. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 54.Viidik A, Danielson CC, Oxlund H. On fundamental and phenomenological models, structure and mechanical properties of collagen, elastin and glycosaminoglycan complexes. Biorheology. 1982;19:437–451. doi: 10.3233/bir-1982-19305. [DOI] [PubMed] [Google Scholar]
- 55.Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegård D, Mörgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J. Biol. Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- 56.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]