Abstract
Background
Genomic testing to identify driver mutations that enable targeted therapy is emerging for patients with non-small cell lung cancer (NSCLC). We report the implementation of systematic prospective genotyping for somatic alterations in BRAF, PIK3CA, HER2, and ALK, in addition to EGFR and KRAS, in NSCLC patients at the Dana-Farber Cancer Institute.
Methods
Patients with NSCLC were prospectively referred by their providers for clinical genotyping. Formalin-fixed, paraffin embedded tumor samples were analyzed by Sanger sequencing for mutations in selected exons of EGFR, KRAS, BRAF, PIK3CA, and HER2. ALK rearrangements were detected by FISH or immunohistochemistry.
Results
Between 7/1/2009 and 8/1/2010, 427 specimens from 419 patients were referred for genomic characterization; 344 (81%) specimens were successfully genotyped with a median turnaround time of 31 days (range, 9-155). Of the 344 specimens, 185 (54%) had at least one identifiable somatic alteration (KRAS: 24%, EGFR: 17%, ALK: 5%, BRAF: 5%, HER2: 4%, PIK3CA: 2%). As of 8/1/2011, 63/288 (22%) advanced NSCLC patients had received molecularly targeted therapy based on their genotypic results, including 34/42 (81%) patients with EGFR mutations, 12/15 (80%) with ALK rearrangements, and 17/95 (18%) with KRAS, BRAF or HER2 mutations.
Conclusions
Large scale testing for somatic alterations in EGFR, KRAS, BRAF, PIK3CA, HER2 and ALK is feasible and impacts therapeutic decisions. As the repertoire for personalized therapies expands in lung cancer and other malignancies, there is a need to develop new genomics technologies that can generate a comprehensive genetic profile of tumor specimens in a time and cost effective manner.
Keywords: Lung cancer, cancer genomics, molecular targeted therapy
Introduction
Targeted cancer therapy is transforming the care of patients with non-small cell lung cancer (NSCLC) and driving efforts to incorporate tumor genotyping into clinical decision-making. For example, it is now standard care to examine tumor specimens from patients with NSCLC for somatic alterations in EGFR to identify those with sensitizing mutations for initial therapy with gefitinib or erlotinib.1 Similarly, the ALK tyrosine kinase inhibitor, crizotinib, has shown response rates in excess of 60%, progression-free survival greater than 10 months, and median survival in excess of two years from the start of crizotinib therapy in patients with advanced NSCLC bearing ALK rearrangements.2, 3 Crizotinib is recommended as initial therapy for ALK-rearranged NSCLC in the National Comprehensive Cancer Network guidelines.
Thus far, approximately 15 to 20% of NSCLC patients from Europe and North America have tumors bearing EGFR mutations or ALK rearrangements, with drugs available to treat these genomic changes. Other potential therapeutic targets emerging in 2009 in patients with NSCLC included activating mutations in KRAS, BRAF, HER2, and PIK3CA. KRAS is the RAS family member most frequently mutated in lung adenocarcinomas, with mutations in codons 12, 13, and 61 detected in approximately 20% of cases. KRAS mutations are a negative predictive marker for response to EGFR tyrosine kinase inhibitors, as well as a potential therapeutic target.4 Activating mutations in BRAF, HER2, and PIK3CA have each been reported at lower frequencies in NSCLC, ranging from 1% to 3%.5-8 Ongoing research at our institution and others is attempting to determine whether therapeutic inhibition of KRAS, BRAF, HER2, and PIK3CA will be an effective strategy in NSCLC, and to identify additional driver mutations that can be successfully targeted with existing or novel compounds. Therefore, consistent multiplex genotyping is needed for patients with NSCLC to inform therapeutic choices and to expand the possible candidates for personalized lung cancer therapies.
The Lowe Center for Thoracic Oncology at the Dana-Farber Cancer Institute, in conjunction with the Center for Advanced Molecular Diagnostics in the Pathology Department at the Brigham and Women’s Hospital and the Laboratory for Molecular Medicine at the Partners Healthcare Center for Personalized Genetic Medicine, introduced prospective genotyping of advanced NSCLC for somatic alterations in BRAF, HER2, PIK3CA, and ALK in July 2009, in addition to routine mutational analysis of EGFR and KRAS, which had been ongoing since 2004. Three years have passed since we initiated this expanded genomic testing, allowing adequate time for determining the success of genomic characterization and assigning patients to therapies based on the molecular findings. The purpose of this report is to provide data on the ability of our center to generate this information and to guide other institutions as they develop and modify their procedures for multiplex genomic characterization of lung cancers and other solid tumors.
Materials and Methods
Study population
Patients eligible for this analysis included those with histologically or cytologically confirmed NSCLC who underwent genomic characterization of BRAF, PIK3CA and HER2 when added to the ongoing standard characterization of EGFR and KRAS, from July 1, 2009 until August 1, 2010. Specific genotyping studies were ordered at the discretion of the treating provider; in a majority of cases, ALK testing was also performed. Patients could be genotyped anytime during the course of their therapy. Patients were identified through a query of patient information for subjects prospectively enrolled in the Clinical Research Information System (CRIS) within the Lowe Center for Thoracic Oncology at the Dana-Farber Cancer Institute that tracks all of the patients referred for genomic testing from our center. This patient information has been used for previous reports.9-11 Patients studied during a period of 13 months were evaluated to include a full year of data, including those during the first month start-up phase. Patients whose tumors were tested after August 1, 2010 were excluded from this analysis to assure at least one year of clinical follow-up after testing. During the study period, EGFR and KRAS tumor genotyping were considered routine clinical tests without the need for patient consent. Patients provided written informed consent for BRAF, PIK3CA, HER2 and ALK testing, as well as for the collection of baseline information, details on their treatments, clinical outcomes information, and ability to contact them for potential trials. The collection of clinical information on patients referred for genotyping was approved by the local institutional review board at the Dana-Farber Cancer Institute.
Genomic characterization
Tumor specimens submitted for genomic characterization consisted of formalin-fixed paraffin-embedded (FFPE) material and were pre-screened by a board-certified pathologist (NIL) to confirm adequate tumor material for testing. Specimens were analyzed for the presence of somatic mutations of EGFR (exons 18 to 21), KRAS (exons 2 and 3), BRAF (exons 11 and 15), PIK3CA (exons 8, 10 and 21), and HER2 (exon 20) by bidirectional Sanger dideoxyterminator sequencing according to described methods.12 This method allows detection of expected key driver mutations in the genes tested as well as other genetic changes that may have clinical significance. Mutation analysis was performed at the Laboratory for Molecular Medicine at the Partners Healthcare Center for Personalized Genetic Medicine under conditions certified according to the Clinical Laboratory Improvement Amendments. Only mutations detected on both forward and reverse strands and confirmed by testing a second aliquot of DNA were reported as positive. Sequences were independently interpreted by two technologists, a molecular geneticist (VAJ), and a pathologist (NIL).
Fluorescence in situ hybridization (FISH) was performed on 4-micron sections of FFPE tumor samples cut onto glass slides using an ALK break-apart probe (Abbott Vysis, Abbott Park, IL), according to previously described methods.2, 3 FISH-positive specimens were defined as separated orange and green signals, with a split distance of at least 2 probe diameters, in greater than 15% of tumor cells. FISH slides were independently interpreted by two technologists, a cytogeneticist (VAJ), and a pathologist (NIL). In some cases, immunohistochemistry (IHC) was initially performed, and samples scored as positive or equivocal for ALK expression were confirmed by FISH analysis as previously reported.13 Before the laboratory became more efficient at handling high throughput, a number of samples were referred to a commercial laboratory for ALK FISH testing. Other samples were prospectively assessed for ALK rearrangements at a central laboratory as part of eligibility screening for clinical trials.
Statistical methods
Baseline clinical characteristics were determined by prospective collection from a patient-administered questionnaire. Smoking status was categorized as never (<100 lifetime cigarettes), light (≤10 pack-years) or heavy smoker (>10 pack-years). Tumor histology was classified using the 2004 WHO criteria.14
Patients who had a somatic alteration in at least one of the six genes were categorized as being treated with a molecularly targeted therapy if they received an agent targeted against that genomic change or closely related downstream pathway. Patients were also considered to have been assigned to a molecularly targeted therapy if they participated in a trial where prospective identification of a mutation was required for enrollment. For instance, the patients with KRAS mutations who were entered on the trial where they were assigned either to docetaxel or docetaxel plus selumetinib were categorized as being treated with targeted therapy (NCT00890825).
Summary statistics are provided regarding the demographic and disease characteristics of the 419 patients tested and are summarized by mutation status. Wilcoxon rank sum and Fisher’s exact tests were used to compare the characteristics between patients with genomic changes and wild-type tumors. Turnaround time was calculated as the interval from the time of test requisition until genotyping report finalization and included the time required to obtain the FFPE tumor material, which had to be requested from outside hospitals in some cases; to section and review the submitted material for adequacy; to extract, amplify and sequence DNA, and to re-extract DNA in the case of inadequate malignant tissue amount or integrity in the original tested sample; to transport the sample to and from the laboratory with genomic characterization capability; and to interpret and sign out the results. The turnaround time was calculated for BRAF, HER2 and PIK3CA mutational analysis; specimens were most frequently also tested for mutations in EGFR and KRAS, although in some cases, mutational analysis of EGFR and KRAS had previously been performed. The turnaround time could not be reliably assessed for specimens referred for ALK testing as different laboratories were involved. All reported P values are based on two-sided hypothesis tests. The statistical analysis was performed using SAS 9.2.
Results
Patient characteristics
Between July 1, 2009 and August 1, 2010, 427 specimens from 419 patients with NSCLC were prospectively referred for clinical genotyping for mutations in BRAF, PIK3CA, and HER2. Table 1 shows the demographic and clinical characteristics of these 419 patients. The median age of the study cohort was 61 years (range, 24-95 years). There were 173 (41%) men and 246 (59%) women, and 36% of patients were never or light smokers (≤10 pack-years). Most patients were White, non-Hispanic, reflecting the predominant ethnic background of our clinic population. The majority of patients had stage IV or relapsed NSCLC at the time of genetic test requisition (80%). Histology was predominantly adenocarcinoma (87%), reflecting the patient population primarily targeted by clinical genotyping at our institution. Eight patients had two different samples tested; 4 of these patients had a second specimen submitted because the original sample contained insufficient tumor material for genetic analysis, two patients had samples from two different body sites tested, and two patients underwent surgical resection of synchronous primary tumors and each tumor was successfully genotyped.
Table 1.
Baseline patient characteristics
| Total, N | 419 |
|---|---|
| Median age -- yrs (range) | 61 (24-95) |
| Gender -- no. (%) | |
| Male | 173 (41) |
| Female | 246 (59) |
| Race -- no. (%) | |
| White, non-Hispanic | 371 (89) |
| White, Hispanic | 6 (1) |
| Asian | 12 (3) |
| Black | 28 (7) |
| Other | 2 (<1) |
| Smoking Status -- no. (%)* | |
| Never-smoker | 97 (23) |
| ≤ 10 pack-years | 55 (13) |
| > 10 pack-years | 262 (63) |
| Histology -- no. (%) | |
| Adenocarcinoma | 363 (87) |
| Adenosquamous | 12 (3) |
| Squamous | 7 (2) |
| Large cell carcinoma | 4 (1) |
| NSCLC NOS | 33 (8) |
| Stage† | |
| I / II / IIIA | 31 (7) / 14 (2) / 30 (7) |
| IIIB | 9 (2) |
| IV | 217 (52) |
| Relapsed‡ | 118 (28) |
Data not available for 5 patients
AJCC staging system, 7th edition
Patients with stage I-IIIA NSCLC with relapse following definitive therapy
Abbreviations: NOS, not otherwise specified
Genomic characterization
Of the 427 specimens referred for genomic characterization of BRAF, HER2 and PIK3CA, 344 (81%) specimens from 341 patients were successfully tested. Table 2 outlines the reasons for the 83 samples that did not complete genetic analysis. Genotyping failures occurred more commonly in specimens obtained from bone (5 of 9 samples did not complete genetic analysis). Correspondingly, recent reports have shown that fixatives with a low pH, such as bone decalcifying solutions, can affect the quality and quantity of DNA in the samples, thereby interfering with molecular testing.15 Therefore, subsequent bone samples were excluded from this study and genomic testing on bone specimens is no longer routinely performed at our center. Of the 344 samples successfully tested for BRAF, HER2 and PIK3CA mutations, 336 (98%) samples were also successfully tested for EGFR mutations, 328 (95%) were successfully tested for mutations in KRAS, and 310 (90%) underwent successful ALK testing.
Table 2.
Specimens referred for clinical genotyping
| No. (%) | |
|---|---|
| Specimens referred for BRAF, HER2 and PIKC3CA mutational analysis | 427 (100) |
| Specimens that did not complete genetic analysis | 83 (19) |
| Insufficient tumor material for genotyping; testing not performed* | 34 (8) |
| Insufficient tumor material for conclusive results; testing performed† | 22 (5) |
| Failed PCR amplification | 15 (4) |
| Incomplete testing at all predefined exons of BRAF, HER2 and/or PIK3CA | 6 (1) |
| Specimen could not be located | 6 (1) |
| Specimens successfully tested for BRAF, HER2 and PIK3CA mutations | 344 (81) |
Insufficient tumor material for genotyping identified during pathology pre-review.
Twenty-two specimens were found to be wild-type for BRAF, HER2, and PIK3CA but were classified as inconclusive because there was less than 50% malignant tissue in the specimen.
Among the 344 genotyped specimens, the median turnaround time was 31 days (range, 9-155 days). The turnaround time was significantly longer when pathology specimens were obtained from outside institutions (n=138) compared with specimens available in the Department of Pathology at the Brigham and Women’s Hospital (n=206) (median 36 v 28 days, respectively; p<0.001). Sixty-four specimens had a turnaround time greater than 50 days, including 3 specimens with a turnaround time greater than 100 days. Of those 3 specimens, two required DNA re-extraction to meet quality standards, which significantly prolonged the turnaround time, and were subsequently successfully tested. The remaining specimen was obtained from an outside hospital; 127 days elapsed before the tumor block was received, after which genomic testing was successfully completed and reported in 28 days.
A somatic alteration in at least one of the 6 genes was identified in 185 of the 344 (54%) genotyped specimens; seven of the 185 specimens harbored two mutations. Table 3 lists the somatic mutations identified. Overall, we identified 60 EGFR mutations in 56/336 (17%) specimens, 79 KRAS mutations in 79/328 (24%) specimens, 16 BRAF mutations in 16/344 (5%) specimens, 15 HER2 mutations in 15/344 (4%) specimens, and 6 PIK3CA mutations in 6/344 (2%) specimens. A rearrangement of the ALK gene was detected in 16 of the 310 (5%) successfully tested samples (FISH-positive: 12; IHC-only positive: 4). Three specimens harbored concurrent mutations in PIK3CA and either KRAS (n=2) or EGFR (n=1). Two specimens with EGFR T790M had concurrent sensitizing EGFR mutations in exon 19. Two additional specimens harbored two EGFR mutations involving G719. Two novel BRAF mutations not previously reported in NSCLC patients were identified. BRAF p.1794_1796dupTAC (c.T599_V600insT), a somatic change previously described in a female patient with pancreatic cancer, was identified in one sample.16 Another specimen harbored BRAF p.1405_1407delGGA (c.G469del), a somatic change not previously reported in the literature; other missense mutations in BRAF codon 469 have been detected in solid tumors. One hundred and twenty eight of 313 (41%) specimens were wild-type at all predefined exons and negative for the ALK rearrangement.
Table 3.
Somatic gene alterations identified
| Gene | Exon | Mutations identified -- no. (%) |
|---|---|---|
| EGFR a | --- | 60 (100)b |
| 18 | G719A: 2 (3); G719C: 1 (2); G719S: 1 (2) | |
| 19 | deletions: 13 (22); deletion-insertions: 8 (13) L747P (cis) or L747L/L747S (trans)c: 2 (3) |
|
| 20 | duplications: 4 (7); insertions: 2 (3); deletion-insertions: 1 (2); deletions: 1 (2) T790M: 2 (3); S768I: 1 (2) |
|
| 21 | L858R: 20 (33); L861Q: 2 (3) | |
| KRAS a | --- | 79 (100)d |
| 2 | G12C: 30 (38); G12D: 18 (23); G12V: 11 (14); G12A: 7 (9); G12S: 3 (4) G12F (cis) or G12C/G12V (trans)e: 1 (1); G12L (cis) or G12R/G12V (trans)f: 1 (1) G13C: 1 (1); G13D: 1 (1) |
|
| 3 | Q61H: 4 (5); Q61K: 1 (1) | |
| BRAF | --- | 16 (100) |
| 11 | G466V: 1 (6); G466R: 1 (6); G469A: 1 (6); G469del: 1 (6) | |
| 15 | V600E: 9 (56); D594N: 1 (6); D594G: 1 (6) T599_V600insT: 1 (6) |
|
| HER2 | --- | 15 (100) |
| 20 | duplications: 9 (60); insertions: 3 (20): deletion-insertions: 2 (13) V777L: 1 (7) |
|
| PIK3CA a | --- | 6 (100) |
| 8 | E453_P458delinsD: 1 (17) | |
| 10 | E542K: 3 (50) | |
| 21 | H1047R: 2 (33) | |
| ALK | --- | 16 (100) Rearrangement: FISH-positive, 12 (75); IHC-only positive, 4 (25) |
Three specimens harbored concurrent mutations in PIK3CA and either KRAS (n=2) or EGFR (n=1).
Four specimens had two concurrent EGFR mutations.
Two specimens had two DNA sequence variants in EGFR: 2239T>C and 2240T>C. If these two variants occur on the same allele (cis), the expected amino acid change is L747P. If these two variants occur on separate alleles (trans), the expected amino acid changes are L747L and L747S.
One specimen was tested at an outside facility and reported as positive for a KRAS mutation but information on the specific amino acid change was not available.
Two DNA sequence variants were detected in KRAS: 34G>T and 35G>T. If these variants occur on the same allele, the expected amino acid change is G12F. If these variants occur on separate alleles, the expected amino acid changes are G12C and G12V.
Two DNA sequence variants were detected in KRAS: 34G>C and 35G>T. If these variants occur on the same allele, the expected amino acid change is G12L. If these variants occur on separate alleles, the expected amino acid changes are G12R and G12V.
The demographic and clinical characteristics of the patients with tumors bearing an identifiable oncogenic driver mutation or pan-negative (wild-type) tumors are shown in Table 4. Consistent with previous reports, patients with ALK-rearranged tumors were significantly younger than patients with wild-type tumors (p=0.005).17 Further comparative analyses showed that never or light smoking history (≤10 pack-years) was significantly associated with mutations in EGFR (p<0.001), BRAF (p=0.073), PIK3CA (p=0.048), and HER2 (p<0.001), as well as ALK rearrangements (p<0.001), while heavier smoking was significantly correlated with KRAS mutations (p=0.007). The two patients with tumors bearing concurrent mutations in PIK3CA and KRAS were a 30-year-old white female never smoker with adenocarcinoma and a 58-year-old white male former heavy smoker with adenosquamous carcinoma. An 81-year-old white female, never smoker, had adenocarcinoma that harbored an EGFR exon 19 deletion and a PIK3CA mutation before exposure to any systemic therapy.
Table 4.
Demographics of the patients successfully genotyped by mutation status*
| EGFR † | KRAS † | ALK | BRAF | HER2 | pik3ca † | Wild-type | |
|---|---|---|---|---|---|---|---|
| Proportion mutated (%) | 55‡/334 (16) | 79/325 (24) | 16/309 (5) | 16/341 (5) | 15/341 (4) | 6/341 (2) | 128/313 (41) |
| Median age (range) | 58 (34-81) | 63 (30-82) | 54 (24-79) | 61 (41-77) | 61 (44-75) | 66 (30-81) | 63 (26-87) |
| Gender -- no. (%) | |||||||
| Male | 21 (38) | 28 (35) | 4 (25) | 7 (44) | 6 (40) | 3 (50) | 68 (53) |
| Female | 34 (62) | 51 (65) | 12 (75) | 9 (56) | 9 (60) | 3 (50) | 60 (47) |
| Race -- no. (%) | |||||||
| White, non-Hispanic | 51 (93) | 74 (94) | 15 (94) | 15 (94) | 14 (93) | 5 (83) | 107 (84) |
| White, Hispanic | 0 | 1 (1) | 1 (6) | 0 | 0 | 0 | 4 (3) |
| Asian | 1 (2) | 1 (1) | 0 | 0 | 1 (7) | 0 | 6 (5) |
| Black | 3 (5) | 2 (3) | 0 | 1 (6) | 0 | 1 (17) | 10 (8) |
| Other | 0 | 1 (1) | 0 | 0 | 0 | 0 | 1 (1) |
| Smoking Status -- no. (%)§ | |||||||
| Never-smoker | 29 (53) | 5 (6) | 6 (38) | 5 (31) | 10 (67) | 4 (67) | 24 (19) |
| ≤ 10 pack-years | 12 (22) | 3 (4) | 6 (38) | 3 (19) | 4 (27) | 0 | 8 (6) |
| ≥ 10 pack-years | 14 (25) | 71 (90) | 4 (25) | 8 (50) | 1 (7) | 2 (33) | 93 (73) |
| Histology -- no. (%) | |||||||
| Adenocarcinoma | 53 (96) | 73 (92) | 14 (88) | 12 (75) | 13 (87) | 4 (67) | 107 (84) |
| Adenosquamous | 2 (4) | 3 (4) | 0 | 1 (6) | 1 (7) | 1 (17) | 5 (4) |
| Squamous | 0 | 0 | 0 | 0 | 0 | 0 | 3 (2) |
| Large cell carcinoma | 0 | 0 | 1 (6) | 0 | 0 | 0 | 1 (1) |
| NSCLC NOS | 0 | 3 (4) | 1 (6) | 3 (19) | 1 (7) | 1 (17) | 12 (9) |
Three patients with two separate tumor specimens successfully genotyped were accounted for only once in the respective genotype cohort.
Three patients had tumors bearing concurrent mutations in PIK3CA and either KRAS (n=2) or EGFR (n=1); the demographics of the patient corresponding with each of the 3 tumor specimens were included and accounted for in the respective genotype cohort.
One patient had samples from two different body sites tested, both of which showed an exon 20 insertion mutation of EGFR. The demographics of the patient were accounted for only once in the EGFR cohort.
Data not available for 3 patients in the wild-type cohort.
Clinical and therapeutic implications
As of August 1, 2011, 288 of the 341 patients (84%) were diagnosed with stage IV or relapsed metastatic NSCLC. Of the 288 patients, 152 (53%) had at least one identifiable somatic alteration (EGFR: 41, EGFR + PIK3CA: 1, KRAS: 62, KRAS + PIK3CA: 2, BRAF: 15, HER2: 13, PIK3CA: 3, ALK: 15). Thirty-five of the 152 (23%) patients enrolled in a study of molecularly targeted therapy, including 9 of 42 patients with EGFR mutations. An additional 25 EGFR mutant advanced NSCLC patients were treated with erlotinib off protocol. Eight patients with EGFR mutations were not treated with an EGFR tyrosine kinase inhibitor. Of those 8 patients, two were treated at outside institutions with no therapeutic information available; three had exon 20 insertion mutations, which are not sensitive to treatment with erlotinib or gefitinib; two were asymptomatic requiring no systemic therapy for their advanced NSCLC; and one died shortly after the identification of a sensitizing mutation of EGFR. Twelve of 15 patients with ALK-rearranged advanced NSCLC enrolled in a clinical trial, including 2 patients who were treated at outside institutions. Three patients with ALK rearrangements were not treated with crizotinib. Of the 3 patients, one remained on maintenance pemetrexed with prolonged stable disease at the time of this analysis; the remaining two patients were not eligible for trial enrollment due to poor performance status and died shortly after the identification of an ALK rearrangement. Finally, three patients with HER2 mutations received a trastuzumab-containing regimen off protocol. Thus, a total of 63 of 152 (41%) patients received molecularly tailored therapy based on their genetic alterations (Figure 1).
Figure 1.
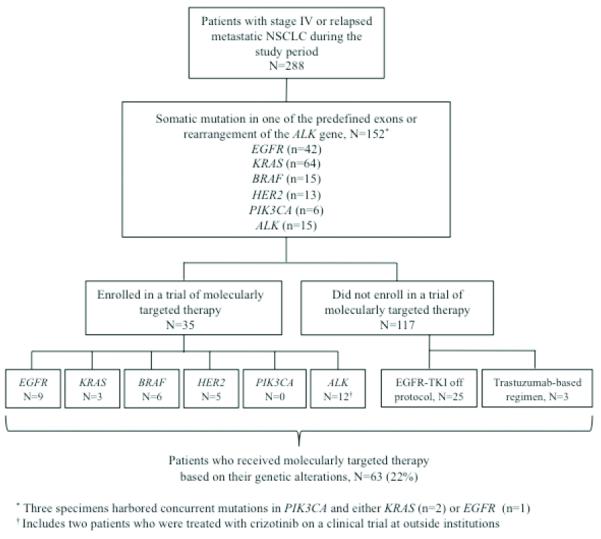
Flowchart of patients with stage IV or relapsed metastatic NSCLC onto molecularly targeted therapy during the study period
Finally, one patient in our cohort had profiling of metachronous resected bilateral T2 tumors that revealed two distinct genotypes (KRAS G12C in one case, and wild-type for all tested sites in the other), suggesting two early stage primary tumors as opposed to metastatic disease. Another patient had a similar scenario, but the two specimens were wild type for all tested sites.
Discussion
Over the past two decades, modifications of chemotherapy combinations and the addition of the antiangiogenic agent, bevacizumab, have brought about modest gains in survival for patients with advanced NSCLC.18-21 Treatment with erlotinib or gefitinib for NSCLC patients with EGFR mutations and crizotinib for patients with ALK rearrangements has transformed therapy for these patient subsets. Other promising agents for lung cancers that harbor uncommon genomic changes are under development and have prompted the need for extensive genomic characterization of advanced NSCLC. Research efforts published in 2010 and 2011 performed genetic profiling of resected NSCLC and defined the frequency and types of somatic mutations of EGFR, KRAS, NRAS, HRAS, HER2, BRAF, PIK3CA, ALK rearrangements, and ROS1 fusions.12, 22 Although resected lung cancers provide an abundant source of DNA for molecular profiling, the patients who more urgently need to undergo genomic characterization are those with advanced or relapsed NSCLC to guide the selection of initial systemic therapy and to identify candidates for investigational therapy.
In the present study, we report on our experience prospectively screening 427 specimens from 419 patients for mutations in BRAF, HER2, and PIK3CA, in addition to routine mutational analysis of EGFR and KRAS, during an initial 13 months following implementation. In 90% of cases, ALK testing was also performed. Most specimens were successfully genotyped (344/427, 81%), and 185 of 344 specimens (54%) had an identifiable driver mutation. Drs. Dias-Santagata and Sequist previously reported on their ability to perform systematic genotyping of NSCLC.1 In their study, 552 of the 589 patients (94%) referred for clinical genotyping had their tumors successfully genotyped. Notably, their population included a greater number of patients with resected stage I/II NSCLC (197/546 or 37% v 45/419 or 11% in our cohort), offering a potential explanation for the higher proportion of specimens that were successfully genotyped in their study. Likewise, their group used a genotyping technique called SNaPshot that requires smaller amounts of DNA and a less pure population of tumor cells than the 50% malignant cells needed for direct DNA sequencing used in this report.1, 23 Correspondingly, we categorized 22 specimens that were found to be wild-type at all predefined exons of BRAF, HER2 and PIK3CA as “inconclusive” because they contained fewer than 50% malignant cells. As routine lung cancer care is redefined to incorporate tumor mutational profiling into clinical decision-making, the obstacles posed by limited tissue specimens stress the need to obtain adequate tumor material at the time of diagnostic sampling.
The goals of the genomic characterization of our NSCLC patients are to help guide therapy and ultimately lead to improved outcomes for those patients with specific genomic changes. Our group identified 152 of 288 (53%) patients with advanced or relapsed NSCLC with a somatic alteration of EGFR, KRAS, BRAF, PIK3CA, HER2, and/or ALK; 63 of the 152 (41%) patients began a molecularly tailored therapy in response to genotypic results. We expect that this proportion should increase as the scope of genotype-specific clinical trials is expanding at our institution and other centers. As was to be expected with proven effective therapies, the vast majority of EGFR mutant advanced NSCLC patients were treated with erlotinib (34/42, or 81%), either as part of a clinical trial or off protocol. Similarly, 12 of the 15 (80%) advanced NSCLC patients whose tumors harbored ALK rearrangements were treated with crizotinib. Research is ongoing to determine whether the outcomes of NSCLC patients with other genomic changes treated with targeted therapies are improved compared with those given empiric chemotherapy.
An important issue is the length of time needed to perform multiplex diagnostic testing, which is critical for purposes of clinical decision-making. In our study, the median turnaround time was 31 days (range, 9-155 days) for mutational analysis. This included the time required to obtain FFPE samples from outside hospitals in 40% of cases, and to transport specimens to and from the laboratory with genomic characterization capability for direct DNA sequencing. Other potential delays are technical. The Sanger sequencing assay involves multiple steps, including nested PCR, with quality control assessments at several stages of the process. Turnaround times are also extended by our laboratory’s policies of confirming positive results with a second amplification from a second aliquot of DNA, and independent sequential interpretations by a total of four reviewers. Nevertheless, there is a strong desire to shorten the time needed to generate the genotypic information in order to more rapidly implement appropriately targeted therapy. The International Association for the Study of Lung Cancer (IASLC) - European Thoracic Oncology Platform multidisciplinary workshop recommended that EGFR mutation testing be completed within 7 working days.24 Similarly, draft recommendations by the College of American Pathologists in conjunction with the IASLC and the Association for Molecular Pathology recommend that EGFR and ALK testing both be completed within two weeks (10 working days) of receiving the specimen in the testing laboratory. Therefore, retrieval and delivery of samples and testing within our center and others will need to take place more promptly. In September 2010, our molecular pathology laboratory introduced a rapid EGFR assay that provides genotype results for the two most common sensitizing mutations, exon 19 deletions and EGFR L858R, within 2 days (median) of receipt of the sample in the laboratory. This has led to a significant acceleration of the implementation of targeted EGFR therapy. If this could be achieved for all molecular testing, it would be a major advance.
Similarly, there is a need for genotyping technology to evolve in order to generate a more comprehensive genetic analysis of routine clinical specimens, capturing point mutations, insertions, and deletions, as well as rearrangements and DNA copy number alterations in a single panel. This is especially relevant as we identify more non-mutational genomic targets in NSCLC, such as MET amplification, and ROS1 and RET rearrangements.25-27 Real-time prospective genotyping of NSCLC is currently challenged by the fact that different types of molecular alterations require different assays and necessitate tumor tissue to be processed properly in multiple ways. Moving forward, the adaptation of new technologies, such as next-generation sequencing, may offer a unifying approach to comprehensive profiling of tumor DNA, and potentially shorten the time of such analyses. Such targeted next-generation sequencing is clinically feasible and under development by commercial entities and academic centers.27
The primary limitations of our study are its retrospective observational design and the potential for selection bias introduced by the patients pursuing oncologic care at our tertiary referral center and in whom providers requested clinical genotyping. Nevertheless, our genotyping results are consistent with the documented mutational prevalence of the tested oncogenes.28 Similarly, although our findings support the clinical feasibility of broad prospective genotyping, the utility of this approach remains under investigation. Multiple agents targeting KRAS, BRAF, HER2, PIK3CA, and/or downstream pathways are in various phases of clinical development in patients with advanced NSCLC and prospectively identified mutations. These studies should help determine whether a targeted therapeutic strategy will result in improved outcomes for these patients, analogous to those observed for NSCLC patients with EGFR mutations or ALK translocations.
In summary, our experience implementing systematic prospective genotyping for somatic alterations in EGFR, KRAS, ALK, BRAF, HER2, and PIK3CA demonstrates the feasibility of this approach within the clinical workflow. The genotypic information supported diagnostic decisions, directed the administration of available targeted therapeutics, and facilitated enrollment of patients into clinical trials of molecularly tailored therapy. As the repertoire of mutations for which targeted therapy may be offered expands in lung cancer and other solid malignancies, strategies to enable rapid, accurate and comprehensive clinical genotyping will be essential to successfully integrate tumor molecular analysis into the fast pace of clinical decision-making and yield its greatest potential impact on patient management.
Acknowledgments
Conflicts of Interest and Source of Funding: V.A. Joshi: employment, consultant/advisory role and ownership interest (KEW Group, LLC). D.M. Jackman: consultant/advisory role (Foundation Medicine; Genentech). D.J. Kwiatkowski: consultant/advisory role (Millenium; Novartis). P.A. Jänne: consultant/advisory role (Genentech; Pfizer; AstraZeneca; Boehringer Ingelheim; Roche; Abbott Molecular; Sanofi); post marketing royalties for EGFR mutation testing. B.E. Johnson: consultant/advisory board (Bristol-Myers Squibb; Genentech; Pfizer; Millenium; AstraZeneca; Sanofi; Chugai); post marketing royalties for EGFR mutation testing.
This work was funded in part by the American Society of Clinical Oncology (ASCO) Conquer Cancer Foundation Translational Research Professorship (B.E. Johnson), NIH grant 5R01-CA114465 (B.E. Johnson, P.A. Jänne, and B.Y. Yeap), NIH grant 1RC2 CA148394-01 (D.J. Kwiatkowski), and the Alice and Stephen D. Cutler Investigator Fund in Thoracic Oncology at the Dana-Farber Cancer Institute (D.M. Jackman and S. Heon).
Footnotes
No potential conflicts of interest were disclosed by the other authors.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sequist LV, Heist RS, Shaw AT, et al. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22:2616–2624. doi: 10.1093/annonc/mdr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Naoki K, Chen TH, Richards WG, et al. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002;62:7001–7003. [PubMed] [Google Scholar]
- 7.Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Shigematsu H, Nomura M, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008;68:6913–6921. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa DB, Nguyen KS, Cho BC, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7067. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One. 2011;6:e28204. doi: 10.1371/journal.pone.0028204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–5223. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Baloglu G, Haholu A, Kucukodaci Z, et al. The effects of tissue fixation alternatives on DNA content: a study on normal colon tissue. Appl Immunohistochem Mol Morphol. 2008;16:485–492. doi: 10.1097/PAI.0b013e31815dffa6. [DOI] [PubMed] [Google Scholar]
- 16.Kubo T,, Kuroda Y,, Kokubu A,, et al. Resequencing analysis of the human tyrosine kinase gene family in pancreatic cancer. Pancreas. 2009;38:e200–206. doi: 10.1097/MPA.0b013e3181b8feb0. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breathnach OS, Freidlin B, Conley B, et al. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 21.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28:4616–4620. doi: 10.1200/JCO.2010.29.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirker R, Herth FJ, Kerr KM, et al. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol. 2010;5:1706–1713. doi: 10.1097/JTO.0b013e3181f1c8de. [DOI] [PubMed] [Google Scholar]
- 25.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 27.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]


