Abstract
Human butyrylcholinesterase (hBChE) is currently being developed as a detoxication enzyme for the catalytic hydrolysis or stoichiometric binding of organophosphates (OPs). Previously, rationally designed hBChE mutants (G117H and E197Q) were reported in the literature and showed the feasibility of engineering OP hydrolytic functional activity into hBChE. However, the OP hydrolysis rate for G117H is too low for clinical utility. Additional OP-resistant hBChE variants with greater hydrolysis rates are needed as OP nerve-agent countermeasures for therapeutic utility. As described herein, a directed molecular evolution process was used to identify amino acid residues that contribute to OP-resistant functional activity of hBChE variants. In this article, we describe the development and validation of a novel method to identify hBChE variants with OP-resistant functional activity (decreased rate of OP inhibition). The method reported herein used an adenoviral protein expression system combined with a functional screening protocol of OP nerve-agent model compounds that have been shown to have functional properties similar to authentic OP nerve-agent compounds. The hBChE screening method was robust for transfection efficiency, library diversity, and reproducibility of positive signals. The screening approach not only identified the previously reported hBChE G117H variant, but also identified a series of additional hBChE variants, including hBChE G117N, G117R, E197C, and L125V, that exhibited OP-resistant functional activities not reported previously. The mammalian functional screening approach can serve as a cornerstone for further optimization and screening for OP-resistant hBChEs for potential therapeutic applications.
Introduction
The primary physiologically relevant target of organophosphate (OP) nerve agents and pesticides in the central nervous system is acetylcholinesterase (AChE), and a prominent target in the periphery is butyrylcholinesterase (BChE) (Gupta, 2006). Currently available treatment for OP nerve-agent poisoning includes combined administration of a cholinesterase (ChE) reactivator (an oxime), a muscarinic receptor antagonist (atropine), and an anticonvulsant (diazepam) (Fleisher and Harris, 1965; Harris et al., 1966; Munro et al., 1990). These treatments only act in a competitive fashion and are not adequate because they do not prevent brain damage and incapacitation. One attractive approach is to administer a detoxication enzyme that not only binds the OP but also hydrolyzes OP nerve agents before they reach the central nervous system and cause irreversible damage. Human BChE (hBChE) represents an ideal enzyme for enzyme supplementation therapy, because it does not require cofactors and is soluble and highly functional at the pH of the plasma, and the products of its action are generally nontoxic. Furthermore, wild-type (WT) hBChE has been developed as a catalytic or stoichiometric OP detoxication scavenger (Lenz et al., 2010). hBChE scavenges low doses of OP nerve agents by forming covalent bonds with these agents by using a similar mechanism as AChE. Rationally designed OP-resistant hBChE variants (G117H and E197Q) were reported previously and showed the feasibility of engineering OP hydrolytic functional activity into hBChE (Masson et al., 2010) However, the OP hydrolysis rate of hBChE G117H is too low for clinical utility. OP-resistant hBChE variants with greater hydrolysis rates are needed as nerve-agent countermeasures.
Previous biochemical efforts used to search for a ChE designed to efficiently facilitate OP nerve-agent hydrolysis have focused mainly on the mutagenesis of selected amino acids based on X-ray structure analysis (Millard et al., 1995, 1998; Shafferman et al., 1996; Lockridge et al., 1997; Masson et al., 1997; Schopfer et al., 2004). Those studies have provided insight regarding the interactions between OP compounds and cholinesterase enzymes. For example, introduction of a histidine side chain (G117H) near the active-site Ser198 increased spontaneous enzyme dephosphorylation (Millard et al., 1995; Lockridge et al., 1997). Introduction of E197Q into an hBChE variant was shown to slow down enzyme aging, a process where OP adducts lose one or more of their alkyl moieties by spontaneous hydrolysis (Millard et al., 1998; Li et al., 2007). The hydrolysis rate for soman, sarin, and S-[2-(diisopropylamino)ethyl]-O-ethyl methylphosphonothioate (VX) is increased 200-, 1400-, and 4700-fold, respectively, for the G117H/E197Q double mutant variant (Millard et al., 1998). In addition, both Asp70 and Trp82 of hBChE were found to be important for irreversible aging (Masson et al., 1997). It was proposed that Trp86 of AChE contributes to the aging process by stabilizing the putative carbonium ion on the 1,2,2-trimethylpropyl moiety of soman, and Glu202 and Phe338 apparently contribute to the aging process by stabilizing the imidazolium ion of the catalytic triad His447 (Shafferman et al., 1996). Mutations of Phe338, Trp86, and Asn74 and mutants of the hydrogen-bond network, Glu202, Glu450, and Tyr133, in AChE all showed resistance to aging (Shafferman et al., 1996). Apparently the molecular basis for efficient ChE OP hydrolytic functional activity is distributed throughout a number of residues in the primary sequence of the protein.
As described herein, we used a directed molecular evolution approach to identify residues of hBChE that can markedly improve its OP hydrolytic functional activity. In this article, we describe the development and validation of a novel screening method that identified hBChE variants with a decreased rate of inhibition by OPs. Because the use of chemical warfare agents is strictly regulated, we used nerve-agent model compounds that have been shown to have similar functional properties to authentic OP compounds (Barakat et al., 2009; Gilley et al., 2009). OP model compounds not only served as a useful means for screening hBChE variants in molecular evolution screening, but also were used in kinetic studies of variants identified (Ralph et al., 2011). The hBChE screening method was assessed for its robustness, including transfection efficiency, library diversity, and reproducibility of positive signals. The method not only could identify the known hBChE G117H variant from among the library members, but also identified G117N, G117R, E197C, and L125V hBChE variants that have not been reported previously. With the help of a newly developed His-tag protein purification procedure (Ralph et al., 2011), we investigated the kinetics of many identified hBChE variants. The molecular evolution screening method described herein thus can serve as a cornerstone for further optimization and screening for OP-resistant hBChEs in a mammalian protein expression system.
Materials and Methods
Biological and Chemical Reagents.
Butyrylthiocholine (BTC) iodide, acetylthiocholine (ATC), and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma-Aldrich (St. Louis, MO). BES-Thio (2,4-dinitrobenzenesulfonyl fluorescein) was synthesized following procedures described previously (Maeda et al., 2005). Goat anti-rabbit antibodies conjugated with horseradish peroxidase (HRP) enzyme and Supersignal West Pico Chemiluminescent substrate were purchased from Thermo Fisher Scientific (Waltham, MA). HRP-conjugated goat antiadenovirus serum was purchased from ViroStat (Portland, ME). Buffers and solvents were purchased from VWR (West Chester, PA) in the highest purity commercially available. Molecular biology reagent was purchased from Invitrogen (Carlsbad, CA) unless otherwise specified. Highly purified hBChE, anti-BChE polyclonal antibodies, echothiophate (ETP) iodide [2-(diethoxyphosphinyl)-thio-N,N,N-trimethylethanaminium iodide], and original wild-type hBChE cDNA constructs in pRC/CMV encoding the full-length wild-type hBChE, G117H single mutant, and G117H/E197Q double mutants were generously provided by Dr. Oksana Lockridge (University of Nebraska Medical Center, Omaha, NE). The nerve-agent model compounds Sp-O-cyclohexyl S-methyl methylphosphonothioate hydrochloride (SpGF-Met), Rp-O-cyclohexyl S-methyl methylphosphonothioate hydrochloride (RpGF-Met), Sp-O-isopropyl S-(2-trimethylammoniumethyl) methylphosphonothioate iodide (SpGB-C), and Sp-2-((dimethylamino)(ethoxy)phosphorylthio)-N,N,N-trimethylethane aminium iodide (SpGA-C) were synthesized as described previously (Berman and Leonard, 1989; Barakat et al., 2009). The nerve-agent model compounds are toxic and must be handled with extreme care. Chemical waste containing these nerve-agent model compounds was degraded by hydrolysis by overnight incubation with 2.5 M NaOH and 10% ethanol before disposal.
Cell Culture.
HEK 293A cells were purchased from the American Type Culture Collection (Manassas, VA). HEK 293A cells were cultured in DMEM (Invitrogen) containing 8% FBS (Invitrogen) during passage and seeding. The media were exchanged to serum-free media when culture media were used for enzyme assays to avoid interference from bovine AChE from the serum. The adenovirus vector system is based on the adenovirus serotype 5 genome that contains no E1 and E2A regions (Gorziglia et al., 1996). For recombinant virus packaging, two cell lines, 293A and EC7, were used, both of which carry the E1 and E2A genes. These features allowed the production of functional infective, yet replication-defective adenoviral vectors. The CHO-cTA-CAR suspension cell line was kindly provided by Dr. Ronald Gilbert (Biotechnology Research Institute, National Research Council, Montreal, Canada) (Gaillet et al., 2007). CHO-CAR suspension cells were cultured in CD-CHO medium (Invitrogen).
Construction of hBChE Mutation Libraries.
One site-saturation mutation library (library 0) and six random mutation libraries (libraries 1–6) were constructed for the current study by using PCR, cloning, and transfection procedures. First, mutations were introduced through PCR amplification using mutation primers (Supplemental Table 1). Site-saturation mutagenesis at DNA corresponding to amino acids Gly117 and Glu197 of hBChE was achieved through PCR by using the oligonucleotide primers listed in Supplemental Table 1. Three separate PCRs were done by using the primer pairs KpnI_F/117_R, 117_F/197_R, and 197_F/XhoI_R. The PCR products obtained were extracted from an agarose gel, and the purified fragments were combined and used as templates in PCRs by using the primer pair KpnI_F/XhoI_R to produce full-length hBChE genes. For random mutation library 1, two separate PCRs using primer pairs Kpn_F/I_R, and I_F/Xho_R were used. The PCR products obtained were extracted from the agarose gel and combined as a template in PCRs using primer pair KpnI_F/XhoI_R to produce a full-length hBChE gene. Random mutation libraries 2 to 6 were generated through PCR as described above by using the corresponding primers listed in Supplemental Table 1.
The full-length PCR fragments from each library were cloned into pENTR1A vector (Invitrogen) through KpnI and XhoI sites and transformed into the electro-competent 10B cells (Bio-Rad Laboratories, Hercules, CA). Twenty random clones were sequenced to verify the introduction of the mutation. Approximately 5000 colonies from the pENTR1A cloning transformation were pooled for plasmid preparation to obtain mutation libraries in pENTR1A. A LR Clonase II (Invitrogen) recombination reaction was done between the pENTR1A-BChE library and the pAd/CMV/V5-DEST vector following the manufacturer's instructions (Invitrogen). Recombination reaction products were transformed into electro-competent 10B cells (Bio-Rad Laboratories). Twenty clones were randomly selected and sequenced to verify the presence of the mutation. Approximately 105 colonies from the recombination transformation were pooled for plasmid preparation to obtain the mutation library in pAD.
Generation of recombinant adenovirus using the ViraPower Adenovirual Gateway Expression system was achieved following the manufacturer's instructions (Invitrogen). In brief, adenovirus plasmids encoding hBChE genes were linearized with PacI digestion and transfected into EC7 cells preseeded in 10-cm culture plates using Lipofectamine 2000. Recombinant virus generation was monitored based on the appearance of a cytopathic effect. Cells were lysed with three freeze-thaw cycles, and recombinant viruses released into the medium were collected by filtration through a 0.22-μm filter. The primary recombinant AD library stock was stored in −80°C in small aliquots.
Recombinant virus encoding WT hBChE and G117H variant enzymes were generated through subcloning hBChE gene fragments into KpnI and XhoI sites of pENTR1A, recombination into pAd/CMV/V5-DEST, and transfection after PacI linearization was done as described for the library construction except that single colonies were isolated at each stage of the cloning process.
Virus Titer Determination and Amplification.
For determination of virus titer, HEK 293A cells were seeded into 48-well plates and incubated overnight at 2 × 104/well in 0.2 ml of 7% FBS/DMEM/well. Viral stocks were serial-diluted in 7% FBS/DMEM and used to infect HEK 293A cells. At 48 h postinfection, the cells were rinsed with PBS and fixed by incubation with cold MeOH at −20°C for 20 min. Fixed cells were probed with HRP-conjugated goat antiadenovirus serum (ViroStat) diluted 1:2000 in PBS containing 1% bovine serum albumin. After 1-h incubation, the plate was thoroughly washed with PBS and stained by using a Metal Enhanced DAB Substrate Kit (Thermo Fisher Scientific) following the manufacturer's instructions. The number of stained cells was counted with a microscope to calculate the virus titer. Virus collected post-transfection was generally in the range of 107 to 108 infection units/ml.
Solid-Phase Screen for OP-Resistant Variants.
To screen for OP-resistant hBChE variants, HEK 293A cells were infected with a 0.02 to 0.002 multiplicity of infection recombinant AD library. At 24 h postinfection, the cells were washed with PBS to remove unbound virus and serum. The cells were then coated with 1% agarose in serum-free minimal essential medium premelted and cooled to 50°C. The agarose coating was allowed to solidify at room temperature, and the culture plates were incubated for 3 days (37°C) to allow local virus amplification and virus-mediated hBChE library expression. Culture plates were incubated with 20 to 200 μM of a specific OP for 2 to 6 h. After OP inhibition, substrate BTC (200 μM) and detection reagent (1 μM DTNB or 1 μM Bes-Thio) were applied in a top layer of the 1% agarose plates. Enzymes not inhibited by OP incubation that hydrolyzed BTC produced a yellow color signal. Single spots were readily identified by visualization by eye for the yellow color developed from the Ellman reagent-based detection. Agarose plugs containing adenovirus were cored out by using a wide-orifice pipette tip and stored in PBS. Gel plugs served as a starting point for material in the after liquid-phase screening.
Liquid-Phase Screen for OP-Resistant Variants.
For confirmation of OP-resistant hBChE variants identified from the solid-phase screens, isolated virus was amplified by infecting HEK 293A cells. The hBChE gene products from the virus identified in the screen as hits were amplified through PCR using primer pairs KpnI_F/Xho_R and then prepared for sequence analysis. The enzyme variant that was identified as a hit was expressed through infection in 500 μl of CHO-CAR suspension cells at a density of 5 × 105 cells/ml in a 96-well deep-well format using amplified virus. Culture medium that contained expressed enzyme variants was assayed for BTC functional activity and OP inhibition-resistant functional activity in clear-bottom 96-well plates. In brief, 5 μl of medium was incubated with a specified OP at different concentrations for different lengths of time. Residual enzyme activity was determined by the addition of Ellman reaction mix to provide 1 mM BTC and 200 μM DTNB in 50 mM sodium phosphate buffer, pH 7.2. The incubation was followed photometrically. The slope of the absorbance change over 10 min for each incubation was monitored by using a Victor2 plate reader (PerkinElmer Life and Analytical Sciences, Waltham, MA) at 405 nm through continuous consecutive readings. For each OP-resistant variant candidate, the enzyme activities (OD405/min) with and without OP treatment were compared. Variants showing no changes or slight changes (usually wild-type hBChE was completely inhibited within 20 min in the presence of OP) were picked up as OP-resistant variants and were elected to proceed to the next “purification” step.
Purification and Functional Activity of hBChE Variants.
Each enzyme variant with OP inhibition-resistant functional activity was verified by an aqueous-phase assay. Further validation and confirmation of the functional activity was done by cloning and expression of the desired gene in pAD. Specific enzyme variants were expressed through AD infection of 50 ml of CHO-CAR cells as described above. The level of enzyme expression was determined by BTC activity measurement. To harvest the secreted protein, cell pellet was removed by centrifugation at 30,000g for 30 min. The supernatant was concentrated by using a 10-kDa MWCO filter (Millipore Corporation, Bedford, MA) and dialyzed with buffer A (20 mM sodium phosphate, pH 8 and 1 mM EDTA) two to three times. The proteins were concentrated through filtration and then used for kinetic analysis using BTC as a substrate.
Adenovirus-Mediated hBChE Protein Expression.
Human WT BChE and G117H variant enzymes were expressed in CHO adherent or CHO-cTA-CAR suspension cells cultured in the CD-CHO medium including 50 μg/ml dextran sulfate and 5 mM glutamine (Ralph et al., 2011). Cells were cultured at a density of 5 × 105 cells/ml and infected with recombinant adenovirus at 100 multiplicities of infection. Infected CHO-cTA-CAR cells were incubated at 37°C for 2 days, and then transferred to 30°C for 7 days. The functional activity of the expressed enzyme was monitored photometrically with an Ellman assay by examining aliquots from the medium. The Ellman assay consisted of incubation in the presence of 1 mM BTC and 200 μM DTNB in 50 mM sodium phosphate buffer, pH 7.2 (Ellman et al., 1961). Supernatant-containing secreted enzyme was separated from cells and cell debris through centrifugation at 30,000g for 30 min. The supernatant was concentrated by using a 50-kDa Amicon ultra centrifugal filter (Millipore Corporation) according to the manufacturer's protocol by using a J2-21M Induction Drive Centrifuge (Beckman Coulter, Fullerton, CA). The concentrated enzyme was washed three times with Tris-HCl buffer (40 mM, pH 7.4 at 25°C). Enzyme functional activity of the concentrated enzyme was determined by an Ellman assay as described above. Details about protein purification were previously reported (Ralph et al., 2011). In brief, His tag-truncated hBChE variants were purified by using a Ni-NTA Superflow Resin (50% slurry) following protocols from the manufacturer (QIAGEN, Valencia, CA).
Enzyme Assays.
As described previously (Barakat et al., 2009), AChE and BChE functional activity was measured spectrophotometrically (Lambda 25; PerkinElmer Life and Analytical Sciences) with an Ellman assay (Ellman et al., 1961). In brief, ATC or BTC was used as a substrate for AChE and BChE, respectively, at 1 mM final concentration. The incubations were carried out in 50 mM potassium phosphate buffer, pH 7.2, at 25°C in the presence of 0.2 mM DTNB. Hydrolysis was followed continuously by monitoring absorbance at 412 nm. Functional activity was calculated by using the molar extinction coefficient of 13,600 M−1·cm−1 (Ellman et al., 1961).
Determination of Inhibition Rate Constants (kinh) for AChE and BChE.
To prevent the loss of ChE enzyme activity during incubation, 30 μg/ml of bovine milk β-lactoglobulin was included in the enzyme-inhibitor incubation mixture. Nerve-agent model compounds were placed in acetonitrile (ETP and paraoxon) or dimethyl sulfoxide (SpGD, SpGB, SpGF, or SpGAc) and then diluted in H2O before use. Final solvent concentration in the inhibition mixtures was <5%. Control experiments showed no impact on enzyme activity with 5% solvent or less in the incubation mixture (BChE or AChE incubated with 30 μg/ml bovine milk β-lactoglobulin in 10 mM Tris buffer, pH 7.6 at 25°C plus 5% acetonitrile or dimethyl sulfoxide). The inhibition mixtures were incubated at 25°C, and aliquots were withdrawn at defined times (every 2 min) to determine functional activity. The rates of BTC hydrolysis were measured by using the Ellman assay described above for enzyme assays. The observed apparent rates of BTC hydrolysis were fit to a single-phase decay by using Prism software (GraphPad Software, Inc., San Diego, CA) as a function of the incubation time with OP to yield apparent rates of inhibition (kinh).
Data Analysis.
In the enzyme kinetic studies, the data were analyzed with GraphPad Prism version 5.01.
Results
Design and Construction of Highly Diversified Mutation Libraries of Human BChE.
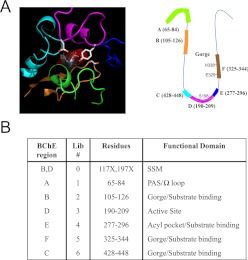
As shown in the cartoon model (Fig. 1A), the active site of hBChE contains a traditional esterase catalytic triad Ser198-Glu325-His438 that lies near the bottom of a deep and narrow gorge (Fig. 1A). Because a successful molecular evolution approach depends on the diversity and size of the random mutagenesis library, six critical structurally relevant regions were selected for targeted random mutagenesis (Fig. 1B). The design of the libraries incorporated structural information known about hBChE (Cokugras, 2003) and allowed the generation of a set of focused mutation libraries. Together with previously identified mutation sites (Gly117 and Glu197), that were used as a positive control (library 0, an independent library distinct from libraries 1–6; Fig. 1B) to validate the overall functional screening process, we designed seven libraries in total (Fig. 1B) that covered different regions of the active-site pocket of hBChE as shown in Fig. 1B.
Fig. 1.
A depiction of mutation libraries of hBChE. A, depiction of each designed mutation library around the active site of hBChE. Left, three-dimensional view of the active site of hBChE. Right, a cartoon model showing the active site of hBChE. Each color represents a corresponding peptide sequence that was covered by each individual library: green, library 1; orange, library 2; pink, library 3; blue, library 4; brown, library 5; aqua, library 6. B, list of libraries and their corresponding function. Library 0 is a site-saturated mutation library. Libraries 1 to 6 are randomized mutation libraries designed to cover six structurally relevant regions around the active pocket of hBChE.
PCR Mutagenesis and Library Diversity Validation.
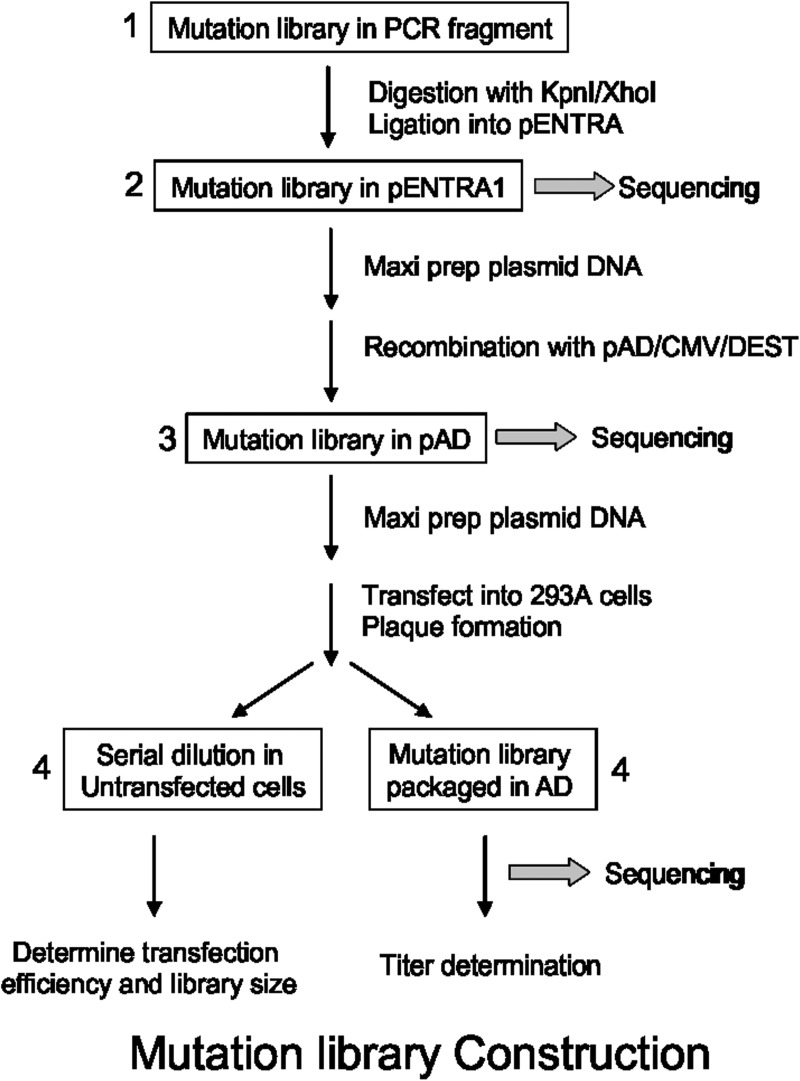
As shown in Fig. 2, the hBChE library construction involved four stages: 1) introduction of mutations through PCR using doped oligonucleotides; 2) subcloning of PCR library fragments into the shuttle vector pENTR1A; 3) transfer of the mutation library from pENTR1A to pAD through recombination; and 4) packaging of the mutation libraries in pAD in recombinant AD particles through transfection. In stage 1, mutagenesis of the hBChE gene was done through PCR with primers that had proportionally doped nucleotides (96% wild type and 4% NNK) to introduce random amino acid substitutions into the target regions. Mutagenesis primers used are listed in Supplemental Table 1. As indicated in Supplemental Table 1, oligonucleotides synthesized were 96% wild type and 4% doped with NNK for each amino acid codon (i.e., MNN for antisense primers). In practice, the doped primers contained nucleotide changes that could be translated into approximately one to two random amino acid substitutions among the 120 targeted residues within each of the six primers. The diversity of library 0 was defined to be 1024 (saturation mutagenesis at two positions by incorporating NNK was 32 × 32 = 1024). The target diversity of mutation libraries 1 to 6 was also ∼1000 (randomization of 20 residues through 1–1.5 mutations). The number of colonies recovered at each stage of the subcloning steps (stages 2 and 3) was >5000 colonies for library 0 and >104 colonies for libraries 1 to 6 and ensured good statistical coverage of the diversity of the libraries.
Fig. 2.
Overview of the hBChE library construction. The mutation library construction was divided into four stages. Stage 1, both site-saturation mutagenesis (library 0) and randomized mutagenesis (libraries 1–6) were done through PCR using designed primers. Stage 2, PCR products were then cloned into a pENTR1A vector to establish a mutation library. Twenty clones from the library were randomly picked and sequenced to verify the diversity of the library (Table 1). Stage 3, the mutation library in pENTR1A plasmid was then exchanged to destination vector pAD/CMV-DEST to establish an adenoviral library by conducting a clonase reaction. Stage 4, after transfection of HEK 293A cells with a pAD library, the adenovirus library stock was prepared after packaging in HEK 293A cells.
At stage 4, for adenovirus packaging, a titer of >107 infection units was routinely achieved at 4 days post-transfection for a typical 10-μg linearized pAD library DNA. The results of parallel control transfection studies with a spike of 0.1% (g/g) of a pAD-LacZ construct allowed consistent recovery of pAD-LacZ. This result confirmed that >1000 virus packaging events/transfection was achieved with HEK 293A cells to ensure that the complexity of the library was maintained at the recombinant virus stage. The diversity of the mutation library was also verified based on DNA sequence analysis of stage 1 PCR products by sequencing random colonies from stage 2 and 3 subclones and from sequencing DNA from random virus after limited dilution. Representative sequencing results (Table 1) showed that the mutation rate was 1.8% in nucleotides and 3.8% in amino acids.
TABLE 1.
Results of sequencing pAD library 1
| Amino Acid Position |
Mutant No. |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 105/Lys | 106/Asn | 107/Ala | 108/Thr | 109/Val | 110/Leu | 111/Ile | 112/Trp | 113/Ile | 114/Tyr | 115/Gly | 116/Gly | 117/Gly | 118/Phe | 119/Gln | 120/Thr | 121/Gly | 122/Thr | 123/Ser | 124/Ser | 125/Leu | 126/His | DNA | AA | |
| Codon | AAA | AAT | GCC | ACT | GTA | TTG | ATA | TGG | ATT | TAT | GGT | GGT | GGT | TTT | CAA | ACT | GGA | ACA | TCA | TCT | TTA | CAT | ||
| Clone 1 | 0 | 0 | ||||||||||||||||||||||
| Clone 2 | TCC | GCA | GCT | 3 | 3 | |||||||||||||||||||
| Ser | Ala | Ala | ||||||||||||||||||||||
| Clone 3 | AAA | GTT | GCT | 3 | 2 | |||||||||||||||||||
| Lys | Val | Ala | ||||||||||||||||||||||
| Clone 4 | GCT | 1 | 0 | |||||||||||||||||||||
| Ala | ||||||||||||||||||||||||
| Clone 5 | AAG | ACG | 3 | 1 | ||||||||||||||||||||
| Lys | Thr | |||||||||||||||||||||||
| Clone 6 | CGG | AGT | 2 | 2 | ||||||||||||||||||||
| Arg | Ser | |||||||||||||||||||||||
| Clone 7 | 0 | 0 | ||||||||||||||||||||||
| Clone 8 | IAI | 2 | 1 | |||||||||||||||||||||
| Tyr | ||||||||||||||||||||||||
| Clone 9 | AAG | 1 | 1 | |||||||||||||||||||||
| Lys | ||||||||||||||||||||||||
| Clone 10 | ATT | 1 | 1 | |||||||||||||||||||||
| Ile | ||||||||||||||||||||||||
| Clone 11 | GAT | 1 | 1 | |||||||||||||||||||||
| Asp | ||||||||||||||||||||||||
| Clone 12 | 0 | 0 | ||||||||||||||||||||||
| Clone 13 | 0 | 0 | ||||||||||||||||||||||
| Clone 14 | GGG | 1 | 0 | |||||||||||||||||||||
| Gly | ||||||||||||||||||||||||
| Clone 15 | ATA | 1 | 1 | |||||||||||||||||||||
| Ile | ||||||||||||||||||||||||
| Clone 16 | TGT | 2 | 2 | |||||||||||||||||||||
| Cys | ||||||||||||||||||||||||
| Clone 17 | TTA | TTCA | 2 | 1 | ||||||||||||||||||||
| Leu | Fram shift* | |||||||||||||||||||||||
| Clone 18 | CTT | 1 | 1 | |||||||||||||||||||||
| Leu | ||||||||||||||||||||||||
| Clone 19 | 0 | 0 | ||||||||||||||||||||||
| Clone 20a | - | - | ||||||||||||||||||||||
| 24 | 17 | |||||||||||||||||||||||
Clone 20 had poor sequence quality.
Functional Screening for OP-Resistant hBChE Variants.
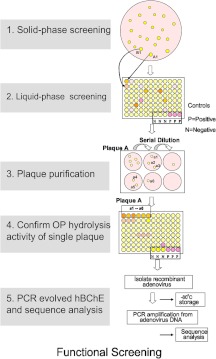
In the functional screening assay for hBChE variants possessing OP resistance (decreased rate of inhibition by OP) (Fig. 3), both solid-phase screening and liquid-phase screening techniques were used with full-length hBChE. In solid-phase screening (Fig. 3, step 1), gel plugs that gave bright yellow positive signals from the Ellman assay (plates were incubated with OP for 2–6 h, followed by incubation with 1 mM BTC and 1 mM DTNB, that gave rise to the yellow color of 2-nitro-5-thiobenzoate when the disulfide bond of DTNB was cleaved by the thiol group of BTC) were identified and isolated by punching a hole on the agarose gel with large pore pipettes. To confirm the positive Ellman assay results from solid-phase screening, a liquid-based method was developed. Virus particles isolated from gel plugs were used to infect cells cultured in 96-well plates to get amplified virus and then used to confirm the observed OP resistance in an Ellman assay. This method was referred to as liquid screening (Fig. 3, step 2). In liquid-phase screening, amplified virus was serially diluted and used for functional BTC hydrolysis activity assays. The assays were done in the presence of 1 mM BTC and 1 mM DTNB for 10 min in 50 mM PBS buffer, pH 7.4. Virus from the incubation where enzyme was expressed that showed OP inhibition on the basis of the BTC assay was diluted and reinfected, and amplified cells were used to isolate DNA. The viral DNA was sequenced to identify the sites where mutagenesis was observed (Fig. 3, steps 3–5).
Fig. 3.
Functional screening of hBChE variants. In functional screening of hBChE variants, both solid-phase and liquid-phase screening were used. In solid-phase screening, a gel plug that had a positive signal (a yellow color, indicating a positive Ellman reaction) was isolated. To confirm the results from solid-phase screening, virus particles isolated from gel plugs were used to infect cells cultured in 96-well plates to confirm OP resistance. Viruses that were confirmed to be OP-resistant variants were further purified and sequenced to identify the site of mutation. Variants were subcloned, expressed, purified, and studied further in in vitro incubations to characterize their kinetic parameters.
Mutagenesis Studies.
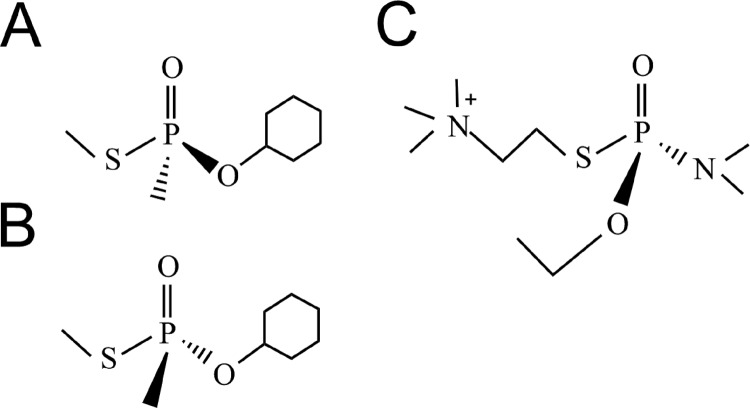
The initial hBChE molecular evolution library constructed (library 0) was a site-saturated mutagenesis control library targeting two residues simultaneously (Gly117 and Glu197 were mutagenized at the same time). Amino acid substitutions at these positions of hBChE, including G117H and G117H/E197Q, have previously been shown to be associated with acquired OP-resistant activity and a low level of OP hydrolysis functional activity (Lockridge et al., 1997; Millard et al., 1998). Thus, positive identification of known variants from library 0 served as a validation of the entire design process. Two compounds used for screening of library 0 were nerve-agent GF model compound enantiomers (SpGF-Met and RpGF-Met) (Fig. 4, A and B) that were described previously (Barakat et al., 2009; Gilley et al., 2009). These nerve-agent model compounds recapitulate the behavior of authentic GF quite well with the exception that they are less toxic. In good agreement with the actual nerve-agent enantiomers, the SpGF-Met isomer preferentially inhibited hBChE compared with the RpGF-Met enantiomer (Barakat et al., 2009). In brief, the solid-phase screen was done as follows: 20 μM SpGF-Met was mixed with agarose and placed on a 10-cm plate (distributed uniformly over the plate) and incubated with the library for 0 for 30 min, followed by incubation in the presence of 1 mM BTC and 1 mM DTNB for 2 to 6 h. Gel spots showing a bright yellow color were judged to be positive, and yellow plugs were isolated with large pore pipettes. The virus DNA was extracted from the plugs with a ViroStat kit and prepared for sequence analysis. After confirmation by sequence analysis of the viral DNA from the yellow plugs a few hits were identified (Table 2). Based on sequence analysis, some of the hits contained G117H mutations. To identify additional variants that were resistant to nerve-agent model compound inhibition, libraries 1 to 6 were screened with SpGAc and the Sp enantiomer of a nerve-agent model compound for tabun (Fig. 4C). In addition to some previously identified variants such as G117H, new variants such as G117N, G117R, E197C, and L125V were identified during the screening of libraries 1 to 6 (Table 2).
Fig. 4.
Chemical structures of model compounds used in screening hBChE variants. A, SpGF-Met. B, RpGF-Met. C, SpGAc.
TABLE 2.
Human BChE variants identified in their respective libraries
| Variant | Library |
|---|---|
| G117H | 0,1 |
| G117R | 0 |
| G117N | 0 |
| E197C | 0 |
| L125V | 1 |
To investigate the kinetics of the variants identified, significant amounts of hBChE variant protein were isolated and purified. However, purification of hBChE has been confounded because of low yields using currently available methodology that use procainamide as the binding ligand in affinity chromatography (Masson and Lockridge, 2010). In addition, because hBChE variants can possess even lower affinity to procainamide than wild-type enzyme a new expression and purification procedure was developed (Ralph et al., 2011). Thus, positive variants identified in the screens were cloned into pENTR1A and pAD vectors by using a truncated version with a His tag at the C terminus. As reported previously, utilization of the His tag facilitated protein production, purification, and robust kinetic studies (Ralph et al., 2011). By using this approach, relatively large amounts of highly purified hBChE variant protein were obtained and used in kinetic studies.
Kinetic Studies of hBChE Variants Identified from Screening Libraries 0 to 6.
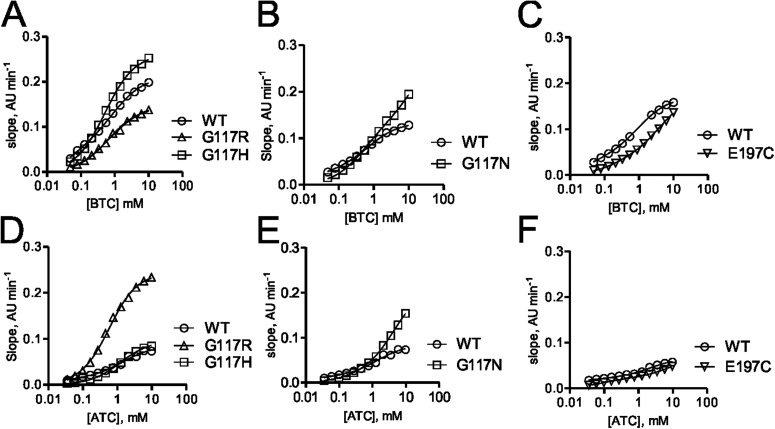
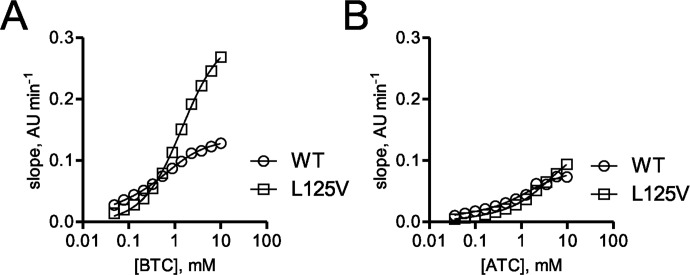
As shown on the basis of the data in Table 3, hBChE variants including G117H, G117R, G117N, E197C, and L125V that were identified by using the OP resistance functional screening method described above were evaluated by using in vitro kinetic studies. In contrast to the screening process described above, for kinetic analysis of the variants identified it was more expedient to use His tag-truncated hBChE variants. Highly purified His tag-truncated hBChE variants including G117H, G117R, G11N, and E197C from the site-saturation mutagenesis library 0 were analyzed for the rate of substrate hydrolysis by using a modified Ellman assay. Representative progress curves for hydrolysis of BTC for the identified variants G117H, G117R, G11N, and E197C are shown in Fig. 5, A to C, and representative progress curves for hydrolysis of ATC are shown in Fig. 5, D to F. Kinetic parameters for the variants identified were determined by fitting the observed rates of hydrolysis for both substrates to the Webb equation as described previously (Ralph et al., 2011). The hBChE variants G117R, G117N, and E197C (Km values = 490, 900, and 210 μM, respectively) showed a greater Km for BTC relative to wild-type hBChE (Km = 23 μM) and the previously characterized hBChE variant G117H (Km = 220 μM) (Table 3; Lockridge et al., 1997). Likewise, the Km for hydrolysis of ATC was greater for the hBChE variants G117R, G117N, and G117H (Km = 600, 190, and 1200 μM, respectively), but the Km for the E197C variant was somewhat similar (Km = 91 μM) to wild-type enzyme (Km = 25 μM). The larger Km observed for the hBChE variants with a mutation at 117 (G117R, G117H, and G117N) was probably caused by steric hindrance of the amino acid residue of the variants (Arg117, His117, and Asn117) that afforded a decreased binding affinity for both ATC and BTC. The kinetic parameters for the L125V hBChE variant identified from random mutagenesis library 2 (Figs. 1 and 6) were determined as described above for the site-saturation variants. Compared with wild-type hBChE (Km = 23 μM) the Km for BTC hydrolysis was 65-fold greater for hBChE L125V (Km = 1500 μM). In contrast, the Km for ATC hydrolysis by hBChE L125V was similar to that for wild-type hBChE (Table 3).
TABLE 3.
The effect of hBChE variants on kinetic parameters
Data were fit to the Webb equation (AKA modified Haldane equation) where B = 1 yields the Michaelis-Menten equation.
| WT | G117R | G117H | G117N | E197C | L125V | ||
|---|---|---|---|---|---|---|---|
| BTC | Km, μM | 23 ± 12 | 490 ± 130 | 220 ± 10 | 900 ± 100 | 210 ± 40 | 1500 ± 100 |
| B | 3.7 ± 0.7 | 1.3 ± 0.15 | 2.2 | 1* | 1* | 1* | |
| Kss, mM | 0.74 ± 0.09 | 1 | 0.55 ± 0.06 | N.A. | 4.4 ± 0.7 | N.A. | |
| ATC | Km, μM | 25 ± 17 | 600 ± 30 | 1200 ± 40 | 190 ± 90 | 91 ± 5 | 80 ± 20 |
| B | 5 ± 2 | 1 | 2.7 | 5.7 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 | |
| Kss, mM | 2.2 ± 0.6 | N.A. | 1.2 ± 0.2 | 12 ± 1 | 9 ± 1 | 9 ± 1 | |
| ETP | ki, min−1·μM−1 | 2.0 ± 0.1 | <0.02 | <0.02 | <0.02 | 1.5 ± 0.1 | 0.083 ± 0.004 |
| Paraoxon | ki, min−1·μM−1 | 1.2 ± 0.1 | 0.19 ± 0.01 | <0.01 | <0.01 | 0.7 ± 0.1 | 0.07 ± 0.01 |
| SpGD | ki, min−1·μM−1 | 0.20 ± 0.01 | ∼0.001 | <0.001 | <0.001 | 0.076 ± 0.005 | 0.012 ± 0.004 |
| SpGB | ki, min−1·μM−1 | 0.12 ± 0.01 | <0.004 | <0.004 | <0.004 | 0.22 ± 0.01 | (0.007 ± 0.003) |
| SpGAc | ki, min−1·μM−1 | 26 ± 1 | <1 | <1 | <1 | 16 ± 1 | 1.8 ± 0.3 |
| SpGF | ki, min−1·μM−1 | 2.4 ± 0.1 | <0.05 | <0.05 | <0.05 | 0.94 ± 0.02 | 0.15 ± 0.01 |
N.A., not available.
, value was assigned as a constant before analysis.
Fig. 5.
Substrate dependence for hBChE variants G117H, G117N, G117R, and E197C. A to C, substrate concentration-dependent rates of BTC hydrolysis for variants G117H and G117R (A), G117N (B), and E197C (C) monitored by UV-visible spectroscopy at 405 nm. D to F, substrate concentration-dependent rates of ATC hydrolysis for variants G117H and G117R (D), G117N (E), and E197C (F) monitored by UV-visible spectroscopy at 405 nm.
Fig. 6.
Kinetic studies of variant hBChE L125V. A, substrate concentration-dependent rates of BTC hydrolysis for the hBChE L125V variant. B, substrate concentration-dependent rates of ATC hydrolysis for the hBChE L125V variant.
hBChE variants identified in the screen (G117H, G117R, G117N, and L125V) were also evaluated for their resistance to inhibition by different OPs. Inhibition kinetics of hBChE variants (G117H, G117R, G117N, and L125V) were determined for several nerve-agent model compounds and two OP pesticide compounds (SpGD, SpGB, SpGAc, SpGF, echothiophate, and paraoxon) (Gilley et al., 2009). The hBChE 117 variants examined (G117H, G117N, and G117R) possessed inhibition rate constants below the limit of detection for the assay (0.02 min−1·μM−1). The hBChE variants L125V and E197C had measureable ki values that were lower than that for wild-type BChE (Table 3).
Discussion
In a previously published study, it was reported that rationally designed variants of hBChE (G117H and E197Q) could hydrolyze PR/SCR, PSCS, and PRCS soman stereoisomers with apparent rate constants of 0.006, 0.077, and 0.128 min−1, respectively (Millard et al., 1998). Thus, although hBChE variants have the potential to show OP hydrolase functional activity (Millard et al., 1995, 1998), the OP hydrolysis rate for soman is too low for practical use or clinical utility. Previous efforts were focused on using a rational design based on the hBChE protein structure to design and develop an OP hydrolase (Shafferman et al., 1996; Lockridge et al., 1997; Saxena et al., 1997; Millard et al., 1998; Cokugras, 2003).
Using a molecular evolution approach to efficiently identify OP-resistant variants, a screening method was designed and developed to identify amino acid residues involved in hBChE OP hydrolase functional activity. As an unbiased approach, a directed molecular evolution screen is an ideal approach for identifying individual and multiple amino acid residues that are required to markedly improve OP hydrolytic functional activity of hBChE. In contrast to the evolution of bacterial OP hydrolase (Cho et al., 2002), molecular evolution of hBChE is more challenging because hBChE is a glycoprotein that requires mammalian cell expression for functional activity (Millard and Broomfield, 1992). Accordingly, an efficient expression system to identify OP-resistant variants using a functional screening approach was developed in mammalian cells.
Thus, we developed a procedure that included: 1) high-level protein expression in the intended screening system; 2) an efficient cloning method to construct the mutation library; and 3) an efficient gene recovery method so that after the functional screen the identification of the DNA sequence and any variants could be readily accomplished. Our screening method used an adenoviral protein expression system combined with nerve-agent model compound functional screening to identify OP-resistant hBChE variants. After the identification of positive variants, we used His tag-truncated versions of the variant hBChEs to facilitate the purification of mutant variants that are difficult to purify by using traditional procainamide column chromatography (Ralph et al., 2011). This method therefore met all of the conditions required for an efficient screening system. Successful expression of active hBChE with an adenoviral vector has been reported previously (Gao et al., 2005), and we optimized the use of an adenovirus system to develop the functional screening approach reported herein.
An analysis of the literature did not reveal any reports of any similar attempts to identify OP-resistant variants by using a screening procedure involving a mammalian protein expression system. Compared with a rational design approach, the molecular evolution approach is suitable for the screening of single or multiple amino acid variants that have novel functional activities. In mammalian protein expression systems, protein folding, post-translational modification, and glycosylation are efficient and correct, compared with a prokaryotic approach (Blagodatski and Katanaev, 2011). It is therefore a significant advantage to use a mammalian protein expression system and adenoviral approach to identify OP-resistant variants in a molecular evolution screen. However, according to our experience, library packaging efficiency is still not ideal, with capacity reaching to 1000 to 2000 variants/construction. This limitation limited the mutagenesis library diversity and therefore the final outcome of the mutation strategy.
One of the challenges in directed molecular evolution is that variants that have ideal functional activity are rarely present even with carefully designed libraries, because it is believed that novel function of a protein often occurs by incremental single amino acid changes (Tracewell and Arnold, 2009). Based on the work reported here, this seems to be particularly true in the case of the hBChE variants identified herein. For example, the well known variant G117H was identified not only from the site saturation mutagenesis library (library 0) as expected, but also from library 1, which was irrelevant to amino acid position 117 (Fig. 1).
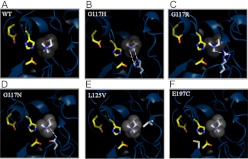
Combined with the available structural information for hBChE, we designed six libraries to cover most regions that were known to be important in the active site of hBChE or that impinged on the active site of hBChE (Fig. 1). These regions not only included the gorge/substrate binding regions, but also included the active site itself that has the catalytic triad Ser198-Asp325-His438 and regions that can facilitate substrate binding (PAS/omega loop and acyl pocket). However, after functional screening, we identified only a handful of OP-resistant variants (Table 2; Fig. 7). Furthermore, most of them (11 of 14) were recognized as previously reported mutation sites (Gly116, Gly117, or Glu197) (Millard et al., 1995; Lockridge et al., 1997), suggesting the importance of those single amino acids in mediating protein functional change during directed evolution. Amino acid 117 in hBChE is part of the oxyanion hole (Gly116, Gly117, and Ala199) that can stabilize the substrate or the transition state during ester hydrolysis (Lockridge et al., 1997).
Fig. 7.
Structural models of hBChE variants. Three-dimensional models of six hBChE variants identified in the molecular evolution screen and characterized by kinetics. A, wild-type hBChE. B, G117H. C, G117R. D, G117N. E, L125V. F, E197C. The catalytic triad of the hBChE substrate binding domain is shown as a yellow backbone on the left in every image. Substrate is shown as gray in the center. The amino acid that underwent mutation is shown with a white backbone near the bottom right corner (B–D); right middle (E), and bottom left (F). Hydrogen bonds are shown as dashed lines.
When Gly117 is replaced by Arg (G117R) or Asp (G117N), OP resistance is not as strong as when a His at position 117 is present. It is possible that G117H has stronger OP resistance because of its two hydrogen bonds with substrate instead of only one in the case of G117R or G117N (Lockridge et al., 1997; Schopfer et al., 2004) (Fig. 7, B–D). Although most variants identified in this study are well known hBChE variants, we did identify some new variants (L125V and N68D) that have not been reported before. Although L125V showed an increased Km when BTC was used as substrate compared with G117H (1500 versus 220 μM, respectively; Table 3), it showed a very low Km (80 versus 1200 μM, respectively; Table 3) when ATC was used as substrate (Table 3). This suggests that L125V has a different mechanism in mediating substrate hydrolysis compared with the G117H variant (Lockridge et al., 1997). The localization of Leu125 in hBChE close to the entry site to the gorge (Fig. 7E) suggests that it might function in facilitating the initial binding of substrate. The data suggest that the screening platform is valid for identifying new variants in addition to previously identified variants (G117H) and the new variants can also serve as intermediates for future screening and optimization to ultimately afford additional novel variants.
The method reported herein not only identified the previously reported hBChE G117H variant, but also identified G117N, G11R, E197C, and L125V hBChE variants that have not been reported before. The molecular evolution screening method can serve as a cornerstone for further optimization and screening for OP-resistant hBChEs in a mammalian protein expression system.
Supplementary Material
Acknowledgments
We thank Robert R. Reddy, Beilin Wang, Nora Barakat, and Longkuan Xiang for library screening and variant purification; and Cynthia B. Gilley and Mary MacDonald for chemical synthesis of OP.
This work was funded by the National Institutes of Health CounterACT Program through the National Institutes of Health National Institute of Neurological Disorders and Stroke awards [Grants NS058183, NS058038].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- OP
- organophosphate
- AChE
- acetylcholinesterase
- BChE
- butyrylcholinesterase
- hBChE
- human BChE
- ChE
- cholinesterase
- ETP
- echothiophate
- GB
- sarin
- GD
- soman
- GF
- cyclosarin
- GA
- tabun
- ATC
- acetylthiocholine
- BTC
- butyrylthiocholine iodide
- DTNB
- 5,5′-dithiobis(2-nitrobenzoic acid)
- SpGF-Met
- Sp-O-cyclohexyl S-methyl methylphosphonothioate hydrochloride
- RpGF-Met
- Rp-O-cyclohexyl S-methyl methylphosphonothioate hydrochloride
- AD
- adenovirus
- pAD
- AD plasmid vector
- PCR
- polymerase chain reaction
- CHO
- Chinese hamster ovary
- WT
- wild type
- CMV
- cytomegalovirus
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- PBS
- phosphate-buffered saline
- HRP
- horseradish peroxidase
- HEK
- human embryonic kidney
- NNK
- nitrosamine ketone
- AU
- arbitrary units.
Authorship Contributions
Participated in research design: Zhang and Cashman.
Conducted experiments: Zhang and Ralph.
Performed data analysis: Zhang, Chen, Ralph, and Dwyer.
Wrote or contributed to the writing of the manuscript: Chen, Dwyer, and Cashman.
References
- Barakat NH, Zheng X, Gilley CB, MacDonald M, Okolotowicz K, Cashman JR, Vyas S, Beck JM, Hadad CM, Zhang J. (2009) Chemical synthesis of two series of nerve agent model compounds and their stereoselective interaction with human acetylcholinesterase and human butyrylcholinesterase. Chem Res Toxicol 22:1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HA, Leonard K. (1989) Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothioates. Noncovalent determinants of enzyme chirality. J Biol Chem 264:3942–3950 [PubMed] [Google Scholar]
- Blagodatski A, Katanaev VL. (2011) Technologies of directed protein evolution in vivo. Cell Mol Life Sci 68:1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CM, Mulchandani A, Chen W. (2002) Bacterial cell surface display of organophosphorus hydrolase for selective screening of improved hydrolysis of organophosphate nerve agents. Appl Environ Microbiol 68:2026–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokugras AN. (2003) Butyrylcholinesterase: structure and physiological importance. Turk J Biochem 28:54–61 [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RML. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95 [DOI] [PubMed] [Google Scholar]
- Fleisher JH, Harris LW. (1965) Dealkylation as a mechanism for aging of cholinesterase after poisoning with pinacolyl methylphosphonofluoridate. Biochem Pharmacol 14:641–650 [DOI] [PubMed] [Google Scholar]
- Gaillet B, Gilbert R, Amziani R, Guilbault C, Gadoury C, Caron AW, Mullick A, Garnier A, Massie B. (2007) High-level recombinant protein production in CHO cells using an adenoviral vector and the cumate gene-switch. Biotechnol Prog 23:200–209 [DOI] [PubMed] [Google Scholar]
- Gao Y, Atanasova E, Sui N, Pancook JD, Watkins JD, Brimijoin S. (2005) Gene transfer of cocaine hydrolase suppresses cardiovascular responses to cocaine in rats. Mol Pharmacol 67:204–211 [DOI] [PubMed] [Google Scholar]
- Gilley C, MacDonald M, Nachon F, Schopfer LM, Zhang J, Cashman JR, Lockridge O. (2009) Nerve agent analogues that produce authentic soman, sarin, tabun, and cyclohexyl methylphosphonate-modified human butyrylcholinesterase. Chem Res Toxicol 22:1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia MI, Kadan MJ, Yei S, Lim J, Lee GM, Luthra R, Trapnell BC. (1996) Elimination of both E1 and E2 from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol 70:4173–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC. (2006) Toxicology of Organophosphate and Carbamate Compounds. Elsevier Academic Press, Boston [Google Scholar]
- Harris LW, Fleisher JH, Clark J, Cliff WJ. (1966) Dealkylation and loss of capacity for reactivation of cholinesterase inhibited by sarin. Science 154:404–407 [DOI] [PubMed] [Google Scholar]
- Lenz DE, Clarkson ED, Schulz SM, Cerasoli DM. (2010) Butyrylcholinesterase as a therapeutic drug for protection against percutaneous VX. Chem-Biol Interact 187:249–252 [DOI] [PubMed] [Google Scholar]
- Li H, Schopfer LM, Nachon F, Froment MT, Masson P, Lockridge O. (2007) Aging pathways for organophosphate-inhibited human butyrylcholinesterase, including novel pathways for isomalathion, resolved by mass spectrometry. Toxicol Sci 100:136–145 [DOI] [PubMed] [Google Scholar]
- Lockridge O, Blong RM, Masson P, Froment MT, Millard CB, Broomfield CA. (1997) A single amino acid substitution, Gly117His, confers phosphotriesterase (organophosphorus acid anhydride hydrolase) activity on human butyrylcholinesterase. Biochemistry 36:786–795 [DOI] [PubMed] [Google Scholar]
- Maeda H, Matsuno H, Ushida M, Katayama K, Saeki K, Itoh N. (2005) 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman's reagent in thiol-quantification enzyme assays. Angew Chem Int Ed Engl 44:2922–2925 [DOI] [PubMed] [Google Scholar]
- Masson P, Froment MT, Bartels CF, Lockridge O. (1997) Importance of aspartate-70 in organophosphate inhibition, oxime re-activation and aging of human butyrylcholinesterase. Biochem J 325:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P, Lockridge O. (2010) Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch Biochem Biophys 494:107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard CB, Broomfield CA. (1992) A computer model of glycosylated human butyrylcholinesterase. Biochem Biophys Res Commun 189:1280–1286 [DOI] [PubMed] [Google Scholar]
- Millard CB, Lockridge O, Broomfield CA. (1995) Design and expression of organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase. Biochemistry 34:15925–15933 [DOI] [PubMed] [Google Scholar]
- Millard CB, Lockridge O, Broomfield CA. (1998) Organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase: synergy results in a somanase. Biochemistry 37:237–247 [DOI] [PubMed] [Google Scholar]
- Munro NB, Watson AP, Ambrose KR, Griffin GD. (1990) Treating exposure to chemical warfare agents: implications for health care providers and community emergency planning. Environ Health Perspect 89:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph EC, Xiang L, Cashman JR, Zhang J. (2011) His-tag truncated butyrylcholinesterase as a useful construct for in vitro characterization of wild-type and variant butyrylcholinesterases. Protein Expr Purif 80:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Maxwell DM, Quinn DM, Radić Z, Taylor P, Doctor BP. (1997) Mutant acetylcholinesterases as potential detoxification agents for organophosphate poisoning. Biochem Pharmacol 54:269–274 [DOI] [PubMed] [Google Scholar]
- Schopfer LM, Ticu Boeck A, Broomfield CA, Lockridge O. (2004) Mutants of human butyrylcholinesterase with organophosphate hydrolase activity; evidence that His117 is a general base catalyst for hydrolysis of echothiophate. J Med Chem Def 2:1–21 [Google Scholar]
- Shafferman A, Ordentlich A, Barak D, Stein D, Ariel N, Velan B. (1996) Aging of phosphylated human acetylcholinesterase: catalytic processes mediated by aromatic and polar residues of the active centre. Biochem J 318:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracewell CA, Arnold FH. (2009) Directed enzyme evolution: climbing fitness peaks one amino acid at a time. Curr Opin Chem Biol 13:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.