Abstract
We reported previously that natriuretic peptides, including brain natriuretic peptide (BNP), promote norepinephrine release from cardiac sympathetic nerves and dopamine release from differentiated pheochromocytoma PC12 cells. These proexocytotic effects are mediated by an increase in intracellular calcium secondary to cAMP/protein kinase A (PKA) activation caused by a protein kinase G (PKG)-mediated inhibition of phosphodiesterase type 3 (PDE3). The purpose of the present study was to search for novel means to prevent the proadrenergic effects of natriuretic peptides. For this, we focused our attention on neuronal inhibitory Gαi/o-coupled histamine H3 and H4 receptors. Our findings show that activation of neuronal H3 and H4 receptors inhibits the release of catecholamines elicited by BNP in cardiac synaptosomes and differentiated PC12 cells. This effect results from a decrease in intracellular Ca2+ due to reduced intracellular cAMP/PKA activity, caused by H3 and H4 receptor-mediated PKG inhibition and consequent PDE3-induced increase in cAMP metabolism. Indeed, selective H3 and H4 receptor agonists each synergized with a PKG inhibitor and a PDE3 activator in attenuating BNP-induced norepinephrine release from cardiac sympathetic nerve endings. This indicates that PKG inhibition and PDE3 stimulation are pivotal for the H3 and H4 receptor-mediated attenuation of BNP-induced catecholamine release. Cardiac sympathetic overstimulation is characteristic of advanced heart failure, which was recently found not to be improved by the administration of recombinant BNP (nesiritide), despite the predicated beneficial effects of natriuretic peptides. Because excessive catecholamine release is likely to offset the desirable effects of natriuretic peptides, our findings suggest novel means to alleviate their adverse effects and improve their therapeutic potential.
Introduction
Although natriuretic peptides have been viewed as a compensatory neurohormonal system that is up-regulated in the setting of heart failure, affording beneficial cardiac and hemodynamic effects via particulate guanylyl cyclase stimulation and increased cGMP formation (Molkentin, 2003; Munagala et al., 2004), their role in alleviating cardiac ailments has been challenged (Wang et al., 2004; Simon et al., 2008). Indeed, in a recent large clinical trial, the administration of nesiritide [recombinant brain natriuretic peptide (BNP)] was found not to protect patients with acute heart failure (O'Connor et al., 2011).
We had reported previously that BNP promotes norepinephrine (NE) release in the guinea pig heart ex vivo, an effect that is further enhanced in ischemia/reperfusion (Chan et al., 2012). We also found that natriuretic peptides, sodium nitroprusside, and cell-permeable cGMP analogs all elicit catecholamine exocytosis in sympathetic nerves isolated from the guinea pig heart (i.e., cardiac synaptosomes) and in nerve growth factor (NGF)-differentiated PC12 cells, which bear a sympathetic nerve-ending phenotype (Chan et al., 2012). This proexocytotic effect results from an increase in intracellular calcium (Ca2+). The process involves a protein kinase G (PKG)-mediated inhibition of phosphodiesterase type 3 (PDE3), which increases cAMP and protein kinase A (PKA) activity (Chan et al., 2012).
More recently, it was reported that BNP increases heart rate in mice by activating the guanylyl cyclase-linked natriuretic peptide A and B receptors and inhibiting PDE3 activity, resulting in an increase in L-type Ca2+ current (Springer et al., 2012). An association of BNP with cardiac sympathetic overdrive, originating from altered Ca2+ handling and culminating in ventricular arrhythmia, was also recently described in mice (Thireau et al., 2012).
Thus, it is conceivable that the proadrenergic effects of natriuretic peptides may offset their beneficial hemodynamic effects, as implied by the findings that β-adrenoceptor blockade protects the heart from the deleterious effects of BNP (Fujimura et al., 2009; Thireau et al., 2012). Given that an enhanced NE release bears dysfunctional and arrhythmogenic consequences (Schömig, 1990; Meredith et al., 1991; Levi and Smith, 2000; Grassi et al., 2009), we investigated novel means to reduce the NE-releasing effect of natriuretic peptides, hoping that they might eventually enable a safe and effective treatment of congestive heart failure with natriuretic peptides. For this, we focused our attention on neuronal histamine H3 receptors, which are Gαi/o-coupled and effectively inhibit physiologic and pathophysiologic NE release (Imamura et al., 1995; Seyedi et al., 1997; Levi and Smith, 2000). Likewise, histamine H4 receptors are also Gαi/o-coupled (Nijmeijer et al., 2012) and seem to be present in central and peripheral neurons (Nakaya et al., 2004; Connelly et al., 2009). Therefore, we ascertained the presence of H4 receptors in cardiac sympathetic nerve terminals and investigated their possible modulation of BNP-induced NE release.
We report that the activation of neuronal H3 and H4 receptors inhibits the release of catecholamines elicited by BNP, and this effect results from a decrease in intracellular Ca2+. This process involves a decrease in intracellular cAMP and PKA activity, based on H3 and H4 receptor-mediated PKG inhibition and consequent PDE3-induced increase in cAMP metabolism.
Materials and Methods
NE Release from Cardiac Synaptosomes.
Male Hartley guinea pigs weighing 300 to 350 g (Charles River Laboratories, Inc., Wilmington, MA) were killed by cervical dislocation under light anesthesia with CO2 vapor in accordance with institutional guidelines. The ribcage was dissected away, and the heart was rapidly excised, freed from fat and connective tissue, and transferred to a Langendorff apparatus. Spontaneously beating hearts were perfused through the aorta for 15 min at constant pressure (40 cm of H2O) with Ringer's solution at 37°C saturated with 5% CO2 and 95% O2. Ringer's solution composition was 154 mM NaCl, 5.6 mM KCl, 2.2 mM CaCl2, 6.0 mM NaHCO3, and 5.6 mM dextrose. This procedure ensured that no blood traces remained in the coronary circulation. At the end of the perfusion, the hearts were minced in ice-cold HEPES-buffered saline solution (HBS), which contained 50 mM HEPES, pH 7.4, 144 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, and 10 mM glucose. Synaptosomes were isolated as described previously (Seyedi et al., 1997). Minced tissue was digested with 40 mg of collagenase (type II; Worthington Biochemicals, Freehold, NJ) per 10 ml of HBS per gram of wet heart weight for 1 h at 37°C. HBS contained 1 mM pargyline to prevent enzymatic destruction of NE. After low-speed centrifugation (10 min at 120g and 4°C), the resulting pellet was suspended in 10 volumes of 0.32 M sucrose and homogenized with a Teflon/glass homogenizer. The homogenate was spun at 650g for 10 min at 4°C, and the pellet was then rehomogenized and respun. The pellet containing cellular debris was discarded, and the supernatants from the last two spins were combined and equally subdivided into tubes. Each tube was centrifuged for 20 min at 20,000g at 4°C. This pellet, which contained cardiac synaptosomes, was resuspended in HBS to a final volume of 1 ml in a water bath at 37°C. Each suspension functioned as an independent sample and was used only once. In every experiment, one sample was untreated (control, basal NE release), and others were incubated with BNP for 10 min. When drugs were used, synaptosomes were preincubated with drugs for 10 min. When antagonists were used, samples were incubated with the antagonists before incubation with the agonist. Controls were incubated for an equivalent length of time without drugs. At the end of the incubation period, each sample was centrifuged (20 min, 20,000g, 4°C). The supernatant was assayed for NE content by high-pressure liquid chromatography with electrochemical detection (Seyedi et al., 1997). The pellet was assayed for protein content by a modified Lowry procedure (Seyedi et al., 1997).
Cell Culture.
Rat pheochromocytoma PC12 cells were transfected with the human histamine H3 receptor (donated by Dr. T. W. Lovenberg, Johnson and Johnson Pharmaceutical R&D, LLC, San Diego, CA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. PC12-H3 cell lines were selected and maintained in selection media containing 500 μg/ml G-418 sulfate (Mediatech, Herndon, VA). PC12 and PC12-H3 cells were maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum, 5% donor horse serum, 1% l-glutamine, and antibiotics at 37°C in 5% CO2. The differentiating protocol involved plating PC12 and PC12-H3 cells on tissue culture plates coated with collagen (rat tail type VII; Sigma-Aldrich, St. Louis, MO) combined with exposure to low serum medium containing 1% fetal bovine serum, 0.5% donor horse serum, 1% l-glutamine, and antibiotics supplemented with 7S-NGF (BD Biosciences Discovery Labware, Bedford, MA). For each experiment, the culture medium was aspirated and cells were washed twice with Na-Ringer's (140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM MgCl2, 2 mM glucose, and 2 mM CaCl2), then incubated with BNP (100 nM), for 20 min in an incubator at 37°C either in the absence or presence of methimepip (histamine H3 receptor agonist; 1 nM) (Kitbunnadaj et al., 2005), 4-methylhistamine (histamine H4 receptor agonist; 20 μM) (Lim et al., 2005), 1-{3-[4-(piperidin-1-ylmethyl)phenoxy]propyl}piperidine (JNJ5207852) (histamine H3 receptor antagonist; 30 nM) (Barbier et al., 2004), or 4-((3R)-3-amino-pyrrolidin-1-yl)-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine (A943931) (histamine H4 receptor antagonist; 300 nM) (Cowart et al., 2008). When these drugs were used, PC12-H3 cells were preincubated with them for 10 min. Controls were incubated for an equivalent length of time without drugs. At the end of each experiment aliquots of the supernatant and cell lysates (after a 30-min treatment with Triton X-100) were taken from each well and analyzed for dopamine (DA) content by high-performance liquid chromatography with electrochemical detection with a 6-min retention time. Other cell lysates were analyzed for histamine H3 and H4 receptor expression by Western blotting, intracellular cAMP levels, PKA activity, intracellular Ca2+, PKG activity, or PDE3 activity.
Intracellular Ca2+ Assay.
Cells were washed twice with Na-Ringer's, and then treated with potassium (100 mM; 3 min) or BNP (100 nM; 10 min) in the presence or absence of methimepip (histamine H3 receptor agonist; 1 nM), 4-methylhistamine (histamine H4 receptor agonist; 20 μM), JNJ5207852 (histamine H3 receptor antagonist; 30 nM), or A943931 (histamine H4 receptor antagonist; 300 nM). Controls were incubated for an equivalent length of time without drugs. At the end of each experiment, cells were washed with Dulbecco's phosphate-buffered saline containing 10 mM EGTA (to chelate external Ca2+) and then with normal phosphate-buffered saline to remove the remaining EGTA. Cells were then lysed with the addition of water and harvested with a scraper. Ca2+ content was determined by using a Ca2+ assay kit (QuantiChrom Ca2+ Assay Kit; BioAssay Systems, Hayward, CA). The Ca2+ content was adjusted by the protein content of the cells and expressed as milligrams of Ca2+ per milligram of protein.
cAMP Assay.
Cells were treated and lysed as described above. Intracellular cAMP levels were determined by using a cAMP Biotrak EIA kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) following the manufacturer's protocol. This cAMP assay is highly specific and based on competition between unlabeled cAMP and a fixed quantity of peroxidase-labeled cAMP for a limited number of binding sites on a cAMP-specific antibody. The cross-reactivity for cGMP, AMP, ADP, and ATP is below 0.01%, whereas cAMP is 100%.
PKA Activity.
PKA phosphorylation (an indication of PKA activation) was measured by using a phosphorylated-PKAα antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in Western blot. Methods for Western blot analysis were as described previously (Chan et al., 2012).
PKG Activity.
Phosphorylated vasodilator-stimulated phosphoprotein (VASP; a major substrate for PKG) at Ser239 is a sensitive biochemical marker for monitoring the activity of PKG (Gill et al., 2007). VASP phosphorylation (i.e., PKG activity) was measured by using a phosphorylated-VASP antibody (Santa Cruz Biotechnology, Inc.) in Western blot. Methods for Western blot analysis were as described previously (Chan et al., 2012).
PDE3 Activity.
PDE3 activity was measured by using a commercially available colorimetric PDE assay kit (Enzo Life Sciences, Farmingdale, NY) as described previously (Chan et al., 2012). Cell lysates were prepared and then total protein concentration was measured as described above. Free phosphate contamination was removed according to the manufacturer's protocol. Samples were incubated for 10 min at 37°C, and reactions were stopped with Biomol Green (Enzo). Samples were then put on a shaker for 20 min at room temperature. Results were measured by using a microplate reader (Molecular Devices, Sunnyvale, CA). PDE3-specific cAMP-hydrolytic activity was expressed as the difference between cAMP hydrolyzed (expressed as nmol/min/mg protein) in the presence and absence of the specific PDE3 inhibitor cilostamide.
Drugs and Chemicals.
BNP was purchased from AnaSpec, Inc. (Fremont, CA); 8-Br-cGMP, forskolin, Rp-8-Br-cGMPS, insulin, and cilostamide were purchased from Sigma-Aldrich. Methimepip, JNJ5207852, 4-methyl histamine, and A943931 were purchased from Tocris Bioscience (Ellisville, MO).
Statistics.
Data are presented as mean ± S.E.M. Parametric tests were used throughout the study. Either unpaired t test or one-way ANOVA followed by post hoc Dunnett's test was used in all figures. GraphPad Prism version 4.03 for Windows (GraphPad Software, Inc., San Diego, CA) was used. Values of P < 0.05 were considered statistically significant.
Results
K+- and BNP-Induced Norepinephrine Release from Cardiac Sympathetic Nerve Endings: Attenuation by Histamine H3 and H4 Receptor Activation.
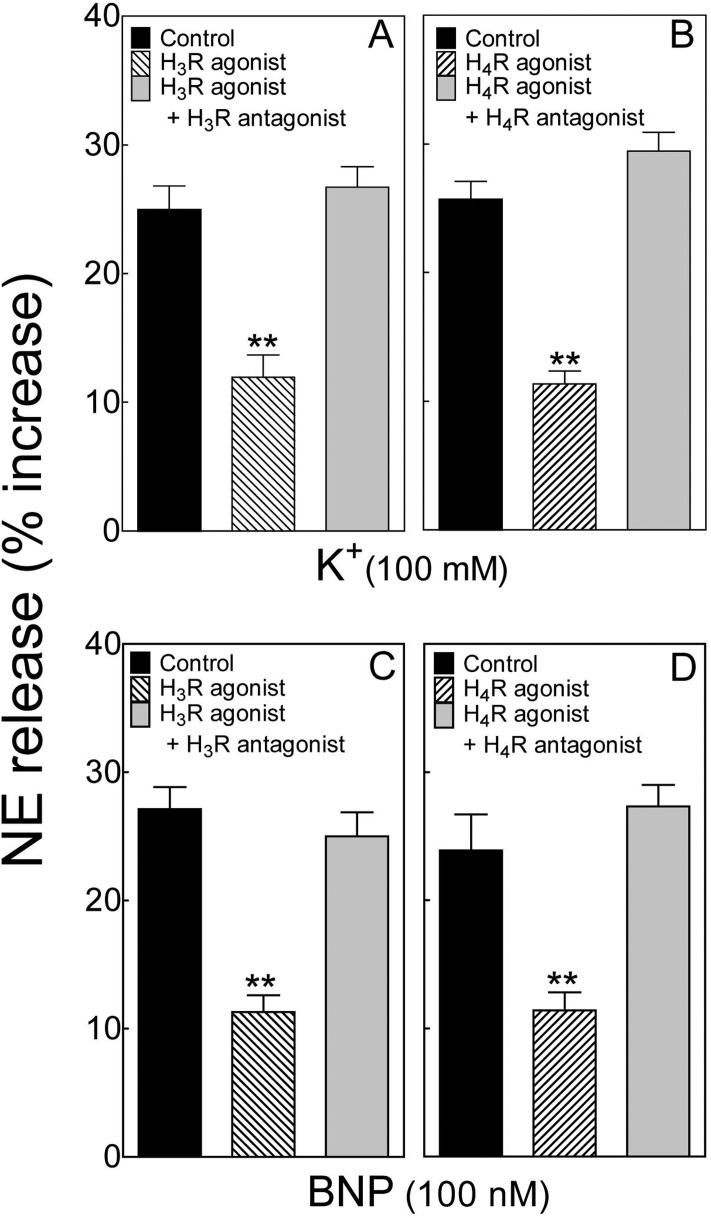
Depolarization of isolated cardiac synaptosomes with extracellular potassium (100 mM) elicited a ∼25% increase in NE release (Fig. 1, A and B). In the presence of the histamine H3 receptor agonist methimepip (1 nM) (Kitbunnadaj et al., 2005) the K+-induced increase in NE release was reduced by ∼50%, an effect that was abolished by the selective H3 receptor antagonist JNJ5207852 (30 nM) (Barbier et al., 2004) (Fig. 1A). The K+-induced increase in NE release was also attenuated by ∼54% by the selective H4 receptor agonist 4-methylhistamine (20 μM) (Lim et al., 2005) (Fig. 1B). This effect was abolished by the selective H4 receptor antagonist A943931 (300 nM) (Cowart et al., 2008) (Fig. 1B).
Fig. 1.
Activation of histamine H3 and H4 receptors attenuates K+- and BNP-induced NE release from cardiac sympathetic nerve endings. A and B, release of endogenous NE from guinea pig heart synaptosomes by depolarization with K+ (100 mM) in the absence and presence of the selective H3 receptor agonist methimepip (1 nM) or the selective H4 receptor agonist 4-methylhistamine (20 μM), respectively. Each agonist was used either alone or together with the respective selective antagonist, JNJ5207852 (H3 receptor antagonist; 30 nM) and A943931 (H4 receptor antagonist; 300 nM). Bars represent mean increases in NE release above basal level (± S.E.M.; n = 8 and 12 for A and B, respectively). Basal NE level was 255.4 ± 16.8 pmol/mg of protein (n = 36). **, P < 0.01 from corresponding control by one-way ANOVA followed by post hoc Dunnett's test. C and D, release of endogenous NE from guinea pig heart synaptosomes by human BNP (100 nM; 10-min exposure) in the absence and presence of the selective H3 receptor agonist methimepip (1 nM) or the selective H4 receptor agonist 4-methylhistamine (20 μM), respectively. Each agonist was used either alone or together with the respective selective antagonist as in A and B. Bars represent mean increases in NE release above basal level (± S.E.M.; n = 12 for C and D, respectively). Basal NE level was 279.6 ± 9.3 pmol/mg of protein (n = 36). **, P < 0.01 from corresponding control by one-way ANOVA followed by post hoc Dunnett's test.
Incubation of isolated cardiac synaptosomes with BNP (100 nM; 10 min) elicited a ∼25 to 28% increase in NE release (Fig. 1, C and D). In the presence of the selective H3 receptor agonist methimepip (1 nM) (Fig. 1C) or the selective H4 receptor agonist 4-methylhistamine (20 μM) (Fig. 1D) the BNP-induced increase in NE release was reduced by ∼58 and 52%, respectively (Fig. 1, C and D). These effects were abolished by the selective H3 receptor antagonist JNJ5207852 (30 nM) (Fig. 1C) and the selective H4 receptor antagonist A943931 (300 nM) (Fig. 1D), respectively.
These findings indicated the presence not only of histamine H3 receptors in cardiac sympathetic nerve endings (Seyedi et al., 2005) but also H4 receptors, both of which are capable of attenuating the release of NE elicited by K+-induced depolarization or the administration of a natriuretic peptide.
BNP-Induced Dopamine Release from PC12 and PC12-H3 Cells: Attenuation by Histamine H3 and H4 Receptor Activation.
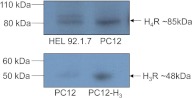
To investigate possible mechanisms of the H3 and H4 receptor-mediated attenuation of the NE-releasing effect of natriuretic peptides, we used the rat pheochromocytoma PC12 cell line. These cells, once differentiated with NGF, exhibit a sympathetic nerve-ending phenotype (Chan et al., 2012) and constitutively express only the H4 receptor (Fig. 2). We also used a PC12 cell line stably transfected with the H3 receptor (PC12-H3) (Morrey et al., 2008) (Fig. 2). Dopamine is the endogenous catecholamine in both cell types (Morrey et al., 2008).
Fig. 2.
Expression of histamine H3 and H4 receptors in PC12 and PC12-H3 cells. Top, Western blotting analysis of HEL 92.1.7 cells (Liu et al., 2001; Zhu et al., 2001) (HEL 92.1.7 are human erythroleukemia cells, in which H4 receptors are highly expressed, and thus serve as positive control) and PC12 cells, both expressing histamine H4 receptors. Bottom, Western blotting analysis of PC12 and PC12-H3 cells showing expression of histamine H3 receptors in PC12-H3 cells but not in PC12 cells. Twenty micrograms of total proteins were loaded in each lane.
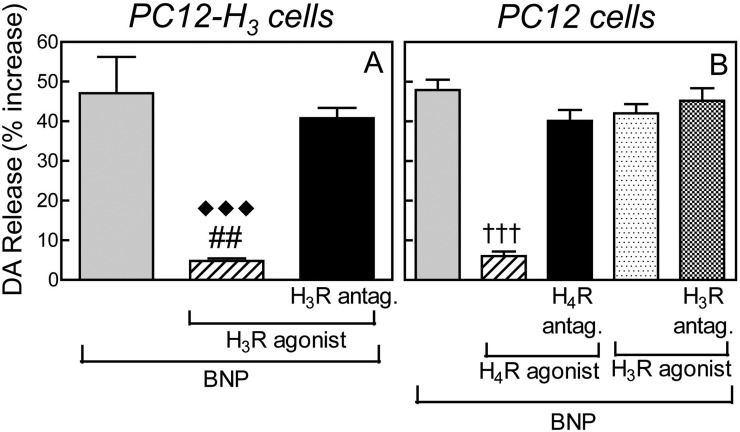
Incubation of PC12 and PC12-H3 cells with BNP (100 nM, 20 min) elicited a ∼48% increase in endogenous DA release (Fig. 3). In the presence of the selective H3 receptor agonist methimepip (1 nM) (Fig. 3A) or the selective H4 receptor agonist 4-methylhistamine (20 μM) (Fig. 3B), the BNP-induced increase in DA release was inhibited by ∼90% in each case (Fig. 3). This inhibition was abolished by the selective H3 receptor antagonist JNJ5207852 in PC12-H3 cells (Fig. 3A) and the selective H4 receptor antagonist A943931 in PC12 cells (Fig. 3B). In contrast, the H3 receptor agonist methimepip, either alone or in the presence of the H3 receptor antagonist JNJ5207852, failed to modify the BNP-induced increase in DA release in PC12 cells, which do not express H3 receptors (i.e., negative control; Fig. 3B).
Fig. 3.
BNP-induced dopamine release in PC12 cells: inhibition by histamine H3 and H4 receptor activation. A, dopamine release elicited by BNP (100 nM) in PC12-H3 cells, in the absence or presence of the selective H3 receptor agonist methimepip (1 nM), either alone or in combination with the selective H3 receptor antagonist JNJ5207852 (30 nM). Columns are means ± S.E.M. (n = 7–10). Significantly different from BNP and BNP + H3 receptor agonist + H3 receptor antagonist: ##, P < 0.01 and ♦♦♦, P < 0.0001, respectively, by unpaired t test. B, dopamine release elicited by BNP (100 nM) in PC12 cells, in the absence or presence of the selective H4 receptor agonist 4-methyl histamine (20 μM), either alone or in combination with the selective H4 receptor antagonist A943931 (300 nM). As a negative control, BNP-induced dopamine was also evaluated in PC12 cells (i.e., lacking constitutive H3 receptors) in the absence or presence of the selective H3 receptor agonist methimepip (1 nM), either alone or in combination with the selective H3 receptor antagonist JNJ5207852 (30 nM). Columns are means (± S.E.M.; n = 8). †††, significantly different from BNP and BNP + H4 receptor agonist + H4 receptor antagonist (P < 0.0001 by unpaired t test).
BNP Increases cAMP and Activates PKA in PC12 and PC12-H3 Cells: Attenuation by Histamine H3 and H4 Receptor Activation.
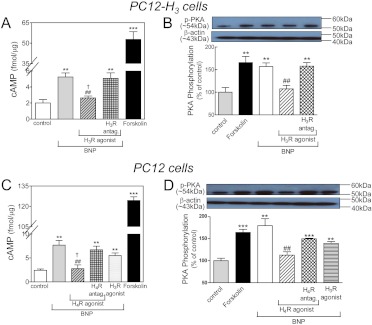
Incubation of PC12-H3 cells with BNP (100 nM) caused a >2-fold increase in the intracellular concentration of cAMP (compared with a ∼10-fold increase by 10 μM forskolin, positive control). The BNP-induced increase in cAMP was inhibited by ∼50% by the selective H3 receptor agonist methimepip (1 nM), a result that was abolished by the selective H3 receptor antagonist JNJ5207852 (30 nM) (Fig. 4A). BNP also activated PKA, as evidenced by a ∼60% increase in PKA phosphorylation (similar to that elicited by forskolin used as positive control; Fig. 4B). The BNP-induced increase in PKA activity was also inhibited by ∼50% by the selective H3 receptor agonist methimepip (1 nM), a result that was abolished by the selective H3 receptor antagonist JNJ5207852 (30 nM) (Fig. 4B).
Fig. 4.
Histamine H3 receptor activation attenuates the increase in intracellular cAMP and phosphorylation of PKA (PKA activity) elicited by BNP in PC12-H3 cells. Histamine H4 receptor activation attenuates the increase in intracellular cAMP and phosphorylation of PKA elicited by BNP in PC12 cells. A, intracellular cAMP levels in PC12-H3 cells treated with forskolin (10 μM; positive control) or BNP (100 nM) in the absence or presence of the selective H3 receptor agonist methimepip (1 nM), either alone or in combination with the selective H3 receptor antagonist JNJ5207852 (30 nM). Columns are means ± S.E.M. (n = 4). Significantly different from control: **, P < 0.01 and ***, P < 0.001 by unpaired t test. Significantly different from BNP: ##, P < 0.01 by unpaired t test. Significantly different from BNP + H3 receptor agonist + H3 receptor antagonist: †, P < 0.05 by unpaired t test. B, PKA activity in PC12-H3 cells treated with forskolin (10 μM; positive control) or BNP (100 nM) in the absence or presence of the H3 receptor agonist methimepip (1 nM), either alone or in combination with the H3 receptor antagonist JNJ5207852 (30 nM). Top band, representative immunoblot of PC12-H3 cell lysate probed with antiphosphorylated PKA antibody. Bottom band, same immunoblot probed with anti-β-actin antibody. Bars represent mean quantitative values (± S.E.M.; n = 4). Significantly different from control: **, P < 0.01 by unpaired t test. Significantly different from BNP and BNP + H3 receptor agonist + H3 receptor antagonist: ##, P < 0.01 by unpaired t test. C, intracellular cAMP levels in PC12 cells treated with forskolin (10 μM; positive control) or BNP (100 nM) in the absence or presence of the H3 receptor agonist methimepip (1 nM; negative control) or H4 receptor agonist 4-methylhistamine (20 μM), either alone or in combination with the H4 receptor antagonist A943931 (300 nM). Columns are means ± S.E.M. (n = 4). Significantly different from control: **, P < 0.01 and ***, P < 0.0001 by unpaired t test. Significantly different from BNP: ##, P < 0.01 by unpaired t test. Significantly different from BNP + H4 receptor agonist + H4 receptor antagonist: †, P < 0.05 by unpaired t test. D, PKA activity in PC12 cells treated with forskolin (10 μM; positive control) or BNP (100 nM) in the absence or presence of the H3 receptor agonist methimepip (1 nM) or H4 receptor agonist 4-methylhistamine (20 μM), either alone or in combination with the H4 receptor antagonist A943931 (300 nM). Top band, representative immunoblot of PC12 cell lysate probed with antiphosphorylated PKA antibody. Bottom band, same immunoblot probed with anti-β-actin antibody. Bars represent mean quantitative values (± S.E.M.; n = 4). Significantly different from control: ***, P < 0.001 and **, P < 0.01 by unpaired t test. Significantly different from BNP and BNP + H4 receptor agonist + H4 receptor antagonist: ##, P < 0.01 by unpaired t test.
Incubation of PC12 cells with BNP (100 nM) caused a ∼3-fold increase in the intracellular concentration of cAMP (compared with a ∼15-fold increase by 10 μM forskolin, positive control). The BNP-induced increase in cAMP was inhibited by ∼60% by the selective H4 receptor agonist 4-methylhistamine (20 μM), a result that was abolished by the selective H4 receptor antagonist A943931 (300 nM) (Fig. 4C). In contrast, H3 receptor activation with methimepip (1 nM) did not affect the BNP-induced increase in cAMP in these cells, which did not express H3 receptors (negative control) (Fig. 4C). The BNP-induced increase in PKA activity was also inhibited by ∼50% by the selective H4 receptor agonist 4-methylhistamine (20 μM), an effect that was abolished by the selective H4 receptor antagonist A943931 (300 nM) (Fig. 4D). H3 receptor activation with methimepip did not affect the BNP-induced increase in PKA activity (negative control) (Fig. 4D).
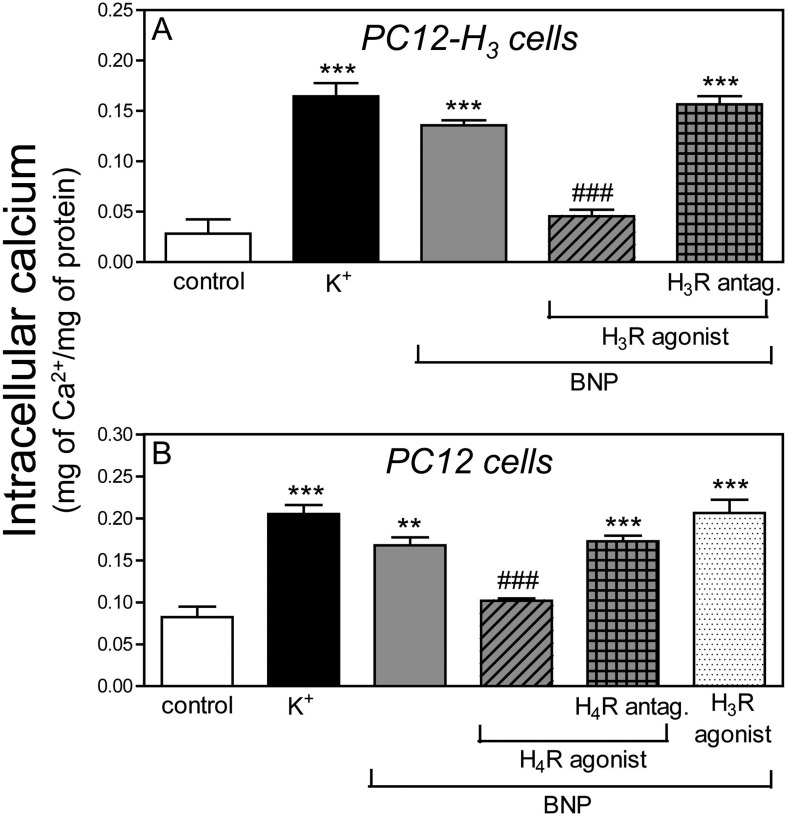
BNP Increases Intracellular Ca2+ in PC12 and PC12-H3 Cells: Attenuation by Histamine H3 and H4 Receptor Activation.
Depolarization of PC12 and PC12-H3 cells with K+ (100 mM) increased intracellular Ca2+ concentration ∼2.5- and 5-fold, respectively (positive control). Incubation with BNP (100 nM) also increased intracellular Ca2+ concentration 2- and 4-fold, respectively (Fig. 5). The effect of BNP was reduced by ∼40 and ∼65% in the presence of the H4 receptor agonist 4-methylhistamine (20 μM) and the H3 receptor agonist methimepip (1 nM) in PC12 and PC12-H3 cells, respectively (Fig. 5). Methimepip (negative control) did not affect the BNP-induced increase in intracellular Ca2+ in PC12 cells (Fig. 5B). The H3 and H4 receptor-mediated inhibition of the BNP-induced increase in intracellular Ca2+ was abolished by the respective H3 and H4 receptor antagonists [JNJ5207852 (30 nM) and A943931 (300 nM)] (Fig. 5).
Fig. 5.
Histamine H3 and H4 receptor activation attenuates the increase in intracellular Ca2+ elicited by BNP in PC12-H3 and PC12 cells, respectively. A, intracellular Ca2+ content of PC12-H3 cells depolarized with K+ (100 mM; positive control) or BNP (100 nM) in the absence or presence of the H3 receptor agonist methimepip (1 nM), either alone or in combination with the H3 receptor antagonist JNJ5207852 (30 nM). Bars are means ± S.E.M. (n = 4). Significantly different from control: ***, P < 0.001 by unpaired t test. Significantly different from BNP and BNP + H3 receptor agonist + H3 receptor antagonist: ###, P < 0.001 by unpaired t test. B, intracellular Ca2+ content of PC12 cells depolarized with K+ (100 mM; positive control) or BNP (100 nM) in the absence or presence of the H4 receptor agonist 4-methyl histamine (20 μM), either alone or in combination with the H4 receptor antagonist A943931 (300 nM). The H3 receptor agonist methimepip (1 nM) was used as a negative control. Bars are means ± S.E.M. (n = 4). Significantly different from control: **, P < 0.01 and ***, P < 0.001 by unpaired t test. Significantly different from BNP and BNP + H4R agonist + H4R antagonist: ###, P < 0.001 by unpaired t test.
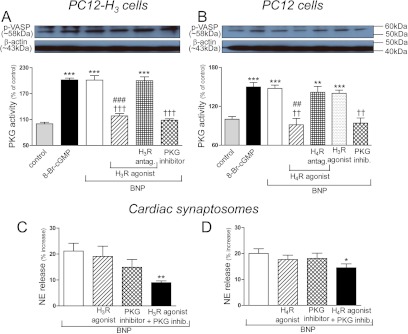
BNP-Induced Increase in PKG Activity in PC12 and PC12-H3 Cells: Attenuation by Histamine H3 and H4 Receptor Activation.
Incubation of PC12-H3 cells with either 8-Br-cGMP (1 μM; positive control) or BNP (100 nM) elicited a 2-fold increase in PKG activity, which was prevented either by the PKG inhibitor Rp-8-Br-cGMPS (0.5 μM) (Moretto et al., 1993) or the H3 receptor agonist methimepip (1 nM); methimepip's effect was abolished by the H3 receptor antagonist JNJ5207852 (30 nM) (Fig. 6A). Incubation of PC12 cells with either 8-Br-cGMP (1 μM; positive control) or BNP (100 nM) elicited a ∼50% increase in PKG activity, which was prevented either by the PKG inhibitor Rp-8-Br-cGMPS (0.5 μM) or the H4 receptor agonist 4-methylhistamine (20 μM); 4-methylhistamine's effect was abolished by the H4 receptor antagonist A943931 (300 nM) (Fig. 6B). Methimepip did not affect the BNP-induced increase in PKG activity in PC12 cells, which do not constitutively express H3 receptors (negative control) (Fig. 6B).
Fig. 6.
A and B, histamine H3- and H4 receptor activation inhibits the increase in PKG activity elicited by BNP in PC12-H3 and PC12 cells, respectively. C and D, H3 and H4 receptor activation synergizes with PKG inhibition in attenuating BNP-induced NE release in cardiac synaptosomes, respectively. A, PKG activity in PC12-H3 cells treated with 8-Br-cGMP (1 μM; positive control) or BNP (100 nM) in the absence or presence of the PKG inhibitor Rp-8-Br-cGMPS (0.5 μM) or the H3 receptor agonist methimepip (1 nM), either alone or in combination with the H3 receptor antagonist JNJ5207852 (30 nM). Top band, representative immunoblot of PC12-H3 cell lysate probed with antiphosphorylated VASP (p-VASP; a major PKG substrate) antibody. Bottom band, same immunoblot probed with anti-β-actin antibody. Bars represent mean quantitative values (± S.E.M.; n = 4). Significantly different from control: ***, P < 0.0001 by unpaired t test. Significantly different from BNP: †††, P < 0.001 by unpaired t test. Significantly different from BNP + H3 receptor agonist + H3 receptor antagonist: ###, P < 0.001 by unpaired t test. B, PKG activity in PC12 cells treated with 8-Br-cGMP (1 μM; positive control) or BNP (100 nM) in the absence or presence of the PKG inhibitor Rp-8-Br-cGMPS (0.5 μM) or the H4 receptor agonist (4-methylhistamine; 20 μM), either alone or in combination with the H4 receptor antagonist (A943931; 300 nM). The H3 receptor agonist methimepip (1 nM; negative control) failed to modify the response to BNP in PC12 cells, which do not constitutionally express H3 receptors. Top band, representative immunoblot of PC12 cell lysate probed with antiphosphorylated VASP antibody. Bottom band, same immunoblot probed with anti-β-actin antibody. Bars represent mean quantitative values (± S.E.M.; n = 4). Significantly different from control: ***, P < 0.001 and **, P < 0.01 by unpaired t test. Significantly different from BNP: ††, P < 0.01 by unpaired t test. Significantly different from BNP + H4R agonist + H4R antagonist: ##, P < 0.01 by unpaired t test. C, inhibition of BNP (100 nM)-induced NE release in cardiac synaptosomes by subthreshold concentrations of the PKG inhibitor Rp-8-Br-cGMPS (0.3 μM) and the H3 receptor agonist methimepip (0.03 nM), administered either alone or in combination. D, inhibition of BNP-induced NE release in cardiac synaptosomes by subthreshold concentrations of the PKG inhibitor Rp-8-Br-cGMPS and the H4 receptor agonist 4-methyl histamine (0.03 μM), administered either alone or in combination. Note in C and D that a significant attenuation of NE release occurs when the PKG inhibitor is combined either with the H3 or the H4 receptor agonist (*, P < 0.05 and **, P < 0.005 by unpaired t test). Bars, means ± S.E.M. (n = 8–18), represent the BNP-induced increase in NE release above the basal level of 232.1 ± 8.9 pmol/mg (n = 25).
To further assess the role of diminished PKG activity in the H3 and H4 receptor-mediated attenuation of BNP-induced catecholamine exocytosis, we next determined whether a synergistic effect could be seen when H3 and H4 receptor activation was combined with PKG inhibition. As shown in Fig. 6C, when either methimepip or Rp-8-Br-cGMPS was used at subthreshold concentrations (0.03 nM and 0.3 μM, respectively), neither caused a significant diminution of BNP-induced (100 nM) NE release in cardiac synaptosomes. In contrast, a significant attenuation occurred when the same subthreshold concentrations of methimepip and Rp-8-Br-cGMPS were combined (Fig. 6C). Likewise, when either 4-methylhistamine or Rp-8-Br-cGMPS was used at subthreshold concentrations (0.03 and 0.3 μM, respectively), neither caused a significant diminution of BNP-induced (100 nM) NE release in cardiac synaptosomes. In contrast, a significant attenuation occurred when the same subthreshold concentrations of 4-methylhistamine and Rp-8-Br-cGMPS were combined (Fig. 6D). These synergistic responses suggested that a decrease in PKG activity is likely to be involved in the H3 and H4 receptor-mediated attenuation of BNP-induced catecholamine exocytosis.
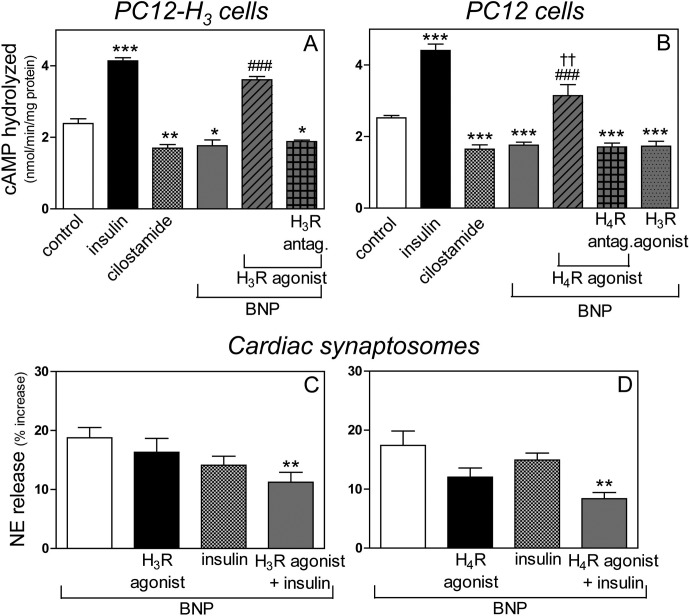
Histamine H3 and H4 Receptor Activation Prevents the BNP-Induced Inhibition of PDE3 Activity in PC12 Cells.
Incubation of PC12 and PC12-H3 cells with BNP (100 nM) significantly decreased the rate of cAMP hydrolysis (an index of PDE3 activity). Insulin (100 nM) (Watanabe et al., 2004) and cilostamide (10 μM) (Hidaka et al., 1979), PDE3 activator and inhibitor, respectively, served as controls (Fig. 7, A and B). In the presence of the selective H3 receptor agonist methimepip (1 nM), the BNP-induced decrease in PDE3 activity was reversed in PC12-H3 cells, and this action was abolished by the selective H3 receptor antagonist JNJ5207852 (30 nM) (Fig. 7A). Likewise, in the presence of the selective H4 receptor agonist 4-methylhistamine (20 μM), the BNP-induced decrease in PDE3 activity cells was reversed in PC12 cells, and this action was abolished by the selective H4 receptor antagonist A943931 (300 nM) (Fig. 7B). In contrast, methimepip did not modify the BNP-induced decrease in PDE3 activity in PC12 cells, because these cells do not constitutively express H3 receptors (negative control) (Fig. 7B).
Fig. 7.
A and B, histamine H3 and H4 receptor activation inhibits the decrease in PDE3 activity (expressed as rate of cAMP hydrolyzed) elicited by BNP in PC12-H3 and PC12 cells. C and D, H3 and H4 receptor activation synergizes with PDE3 activation in attenuating BNP-induced NE release in cardiac synaptosomes. A, BNP (100 nM) decreases the rate of cAMP hydrolyzed (i.e., a decrease in PDE3 activity) in PC12-H3 cells. The H3 receptor agonist methimepip (1 nM) reverses the PDE3-inhibiting effect of BNP. Pretreatment with the H3 receptor antagonist JNJ5207852 (30 nM) restores the PDE3-inhibiting effect of BNP. The PDE3 activator insulin (100 nM) and the PDE3 inhibitor cilostamide (10 μM) serve as controls. Columns are means ± S.E.M. (n = 4). Significantly different from control: ***, P < 0.001; **, P < 0.01; and*, P < 0.05 by unpaired t test. Significantly different from BNP and BNP + H3 receptor agonist + H3 receptor antagonist: ###, P < 0.0001 by unpaired t test. B, BNP (100 nM) decreases PDE3 activity in PC12 cells. The H4 receptor agonist 4-methylhistamine (20 μM) reverses the PDE3-inhibiting effect of BNP. Pretreatment with the H4 receptor antagonist A943931 (300 nM) restores the PDE3-inhibiting effect of BNP. Note that the H3R agonist methimepip (1 nM) does not affect the PDE3-inhibiting effect of BNP, because PC12 cells do not constitutively express H3 receptors (negative control). As in A, the PDE3 activator insulin (100 nM) and the PDE3 inhibitor cilostamide (10 μM) serve as controls. Bars are means ± S.E.M. (n = 4–14). Significantly different from control: ***, P < 0.0001 by unpaired t test. Significantly different from BNP: ###, P < 0.0001 by unpaired t test. Significantly different from BNP + H4 receptor agonist + H4 receptor antagonist: ††, P < 0.01 by unpaired t test. C, inhibition of NE release induced by BNP (100 nM) in cardiac synaptosomes by subthreshold concentrations of the PDE3 activator insulin (10 nM) and the H3 receptor agonist methimepip (0.03 nM). D, inhibition of NE release induced by BNP (100 nM) in cardiac synaptosomes by subthreshold concentrations of the PDE3 activator insulin (10 nM) and the H4 receptor agonist 4-methylhistamine (0.03 μM) administered either alone or in combination. Note in C and D that a significant attenuation of NE release occurs when insulin is combined either with the H3 or the H4 receptor agonist (**, P < 0.005 by unpaired t test). Bars, means ± S.E.M. (n = 8–19), represent BNP-induced increase in NE release above the basal level of 226.9 ± 12.9 pmol/mg (n = 21).
To further assess the role of an increased PDE3 activity in the H3 and H4 receptor-mediated attenuation of BNP-induced catecholamine exocytosis, we next determined whether a synergistic effect could be seen when H3 and H4 receptor activation was combined with PDE3 stimulation. As shown in Fig. 7C, when either methimepip or insulin was used at subthreshold concentrations (0.03 and 10 nM, respectively), neither caused a significant diminution of BNP-induced (100 nM) NE release in cardiac synaptosomes. In contrast, a significant attenuation occurred when the same subthreshold concentrations of methimepip and insulin were combined (Fig. 7C). Likewise, when either 4-methylhistamine or insulin was used at subthreshold concentrations (0.03 μM and 10 nM, respectively), neither caused a significant diminution of BNP-induced (100 nM) NE release in cardiac synaptosomes. In contrast, a significant attenuation occurred when the same subthreshold concentrations of 4-methylhistamine and insulin were combined (Fig. 7D). These synergistic responses suggested that an increase in PDE3 activity is likely to be involved in the H3 and H4 receptor-mediated attenuation of BNP-induced catecholamine exocytosis.
Discussion
The purpose of our study was to search for novel means to prevent the recently uncovered proadrenergic effects of natriuretic peptides. Our findings indicate that the activation of neuronal histamine H3 and H4 receptors attenuates BNP-induced catecholamine release by inhibiting PKG, thus enhancing PDE3-mediated cAMP metabolism culminating in a decrease in intracellular Ca2+.
Although H4 receptors are expressed predominantly in hematopoietic cells (Nijmeijer et al., 2012), their presence had been reported in the brain (Zhu et al., 2001; Connelly et al., 2009) and peripheral neurons of the nasal mucosa (Nakaya et al., 2004). Here, we functionally identified H4 receptors in cardiac sympathetic neurons and demonstrated their protein expression in NGF-differentiated PC12 cells exhibiting a sympathetic neuron phenotype. It is noteworthy that differentiated PC12 cells constitutively expressed only H4 receptors. We further demonstrated that, similar to H3 receptors, these neuronal H4 receptors negatively modulate catecholamine exocytosis elicited by K+-induced depolarization or BNP. Given that H4 receptors are highly homologous to H3 receptors and that, like H3 receptors, are coupled to inhibitory Gi/o proteins (Oda et al., 2000; Liu et al., 2001; Zhu et al., 2001), it was not surprising to find that H4 receptors attenuate catecholamine exocytosis elicited by K+ depolarization. As is the case for H3 receptors, the antiexocytotic action of H4 receptors could result from a Gαi-mediated impairment of the adenylyl cyclase-cAMP-PKA pathway leading to a decrease in intraneuronal Ca2+ (Silver et al., 2002; Seyedi et al., 2005). A direct Gβγ-induced attenuation of Ca2+ current (ICa) could also play a role (Morrey et al., 2008). On the other hand, regarding the H3 and H4 receptor-mediated attenuation of catecholamine release elicited by BNP, our findings suggest that the reduction in intracellular Ca2+ derives primarily from PKG inhibition and a consequent enhancement in PDE3-mediated cAMP metabolism, rather than a Gαi-mediated decrease in adenylyl cyclase activity.
We found that H3 and H4 receptor activation synergized with PKG inhibition and PDE3 stimulation, respectively, in inhibiting the BNP-induced promotion of catecholamine release. These synergistic responses strongly suggest that a decrease in PKG activity and an increase in PDE3 activity are both pivotal in the H3 and H4 receptor-mediated attenuation of BNP-induced catecholamine exocytosis. Whether PDE3 activation indirectly results from PKG inhibition because of H3 and H4 receptor activation or is a direct and independent target of H3 and H4 receptor activation remains to be understood.
We can only speculate at this point on the molecular mechanisms possibly involved in H3 and H4 receptor-mediated PKG inhibition and PDE3 stimulation. We had shown previously that imetit, a mixed H3/H4 receptor agonist (Morse et al., 2001; Zhu et al., 2001), attenuates the phorbol ester-induced activation of PKC in NGF-differentiated PC12-H3 cells, an action prevented by the mixed H3/H4 receptor antagonist clobenpropit (Hashikawa-Hobara et al., 2012). Because PKC stimulation has been reported previously to activate PKG (Hou et al., 2003), it is conceivable that the H3/H4 receptor-induced decrease in PKC activity could in turn reduce PKG activity in cardiac sympathetic neurons. Reduced PKG activity would then alleviate PKG-mediated PDE3 inhibition, augment cAMP hydrolysis, and ultimately decrease intracellular Ca2+ and NE exocytosis. It is also possible that the activation of H3 and H4 receptors may lead to PDE3 stimulation independently of PKG inhibition. In fact, H3 receptors are known to activate the phosphatidylinositol 3-kinase pathway, which results in the phosphorylation/activation of Akt (Leurs et al., 2005). Akt is involved in the phosphorylation/activation of PDE3B (Wijkander et al., 1998), which was shown to be expressed in heart tissue with PDE3A (Liu and Maurice, 1998). Given the high homology of H3 receptors to H4 receptors, and the fact that they both are Gi/o-coupled (Nijmeijer et al., 2012), it is conceivable that H3 and H4 receptor activation may lead via the phosphatidylinositol 3-kinase pathway to PDE3 stimulation, increased cAMP hydrolysis, and decreased NE release.
It is noteworthy that PDE3 activity is significantly reduced in failing human hearts and murine hearts with chronic pressure overload (Ding et al., 2005). Moreover, long-term inhibition of PDE3 has been found to be associated with a 40% increase in mortality, primarily as a result of arrhythmias and sudden death (Packer et al., 1991; Nony et al., 1994). We had reported that inhibition of PDE3-mediated cAMP hydrolysis by natriuretic peptides, at concentrations likely to be reached in advanced congestive heart failure, promote excessive NE release (Chan et al., 2012), which we contend could explain at least in part why natriuretic peptides failed to correct the symptoms of congestive heart failure (O'Connor et al., 2011). Thus, we had advocated that agents that preserve PDE3 function, rather than inhibiting it, may be beneficial in the treatment of cardiac dysfunctions associated with excessive sympathetic activity (Chan et al., 2012). We report here that histamine H3 and H4 receptor activation stimulates PDE3 activity via PKG inhibition and/or directly. Accordingly, preserving and/or stimulating PDE3 function via H3 and H4 receptor activation could offer a useful new approach to the treatment of cardiac dysfunctions with natriuretic peptides. Indeed, although β-adrenoceptor blockade has been advocated to prevent the deleterious effects of chronic BNP exposure in congestive heart failure (Thireau et al., 2012), stimulation of PDE3 activity via H3 and H4 receptor activation might be preferable, given the notorious adverse effects of β-blockers (Lewis and McDevitt, 1986).
In conclusion, we had reported previously that natriuretic peptides augment NE exocytosis from cardiac sympathetic neurons by a PKG-mediated inhibition of PDE3 activity, which results sequentially in an increase in intraneuronal cAMP, augmented PKA activity, phosphorylation of Ca2+ channels, and increased intracellular Ca2+ (Chan et al., 2012). We present new evidence that this pathway can be effectively interrupted at the PKG and PDE3 levels. Indeed, our findings indicate that PKG and PDE3 are targeted for inhibition and stimulation, respectively, when histamine H3 and H4 receptors are activated (see Fig. 8).
Fig. 8.
Histamine H3 and H4 receptor activation inhibits Ca2+-dependent NE exocytosis from cardiac sympathetic nerves via inhibition of PKG and consequent reduction of PKG-dependent PDE3 inhibition. NP, natriuretic peptides; pGC, particulate guanylyl cyclase; cGMP, cyclic GMP; [Ca2+]i, intracellular calcium.
Cardiac sympathetic overstimulation is characteristic of advanced heart failure (Esler and Kaye, 2000; Braunwald, 2008; Grassi et al., 2009), which was recently found not to be improved by the administration of recombinant BNP (nesiritide) (O'Connor et al., 2011), despite the predicated beneficial effects of natriuretic peptides (Molkentin, 2003; Munagala et al., 2004). Because excessive NE release is likely to offset the desirable effects of natriuretic peptides, our findings suggest novel means to alleviate their adverse effects and improve their therapeutic potential.
Acknowledgments
The data presented in Fig. 1 were obtained in experiments performed by Dr. N. Seyedi. We thank Dr. Kenichi Takano for help with figure digitization.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL034215]; an American Heart Association Grant-in-Aid; the Caja Madrid Foundation; and a Pharmaceutical Research Manufacturers Association of America Foundation predoctoral fellowship.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- BNP
- brain natriuretic peptide
- ANOVA
- analysis of variance
- DA
- dopamine
- 8-Br-cGMP
- 8-bromoguanosine-3',5'-cyclic monophosphate
- H3R
- H3 receptor
- H4R
- H4 receptor
- HBS
- HEPES-buffered saline solution
- NE
- norepinephrine
- NGF
- nerve growth factor
- PDE3
- phosphodiesterase type 3
- PKA
- protein kinase A
- PKG
- protein kinase G
- Rp-8-Br-cGMPS
- 8-bromoguanosine-3′,5′-cyclic monophosphorothioate
- VASP
- vasodilator-stimulated phosphoprotein
- JNJ5207852
- 1-{3-[4-(piperidin-1-ylmethyl)phenoxy]propyl}piperidine
- A943931
- 4-((3R)-3-amino-pyrrolidin-1-yl)-6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-d]pyrimidin-2-ylamine.
Authorship Contributions
Participated in research design: Chan, Robador, and Levi.
Conducted experiments: Chan and Robador.
Performed data analysis: Chan, Robador, and Levi.
Wrote or contributed to the writing of the manuscript: Chan, Robador, and Levi.
References
- Barbier AJ, Berridge C, Dugovic C, Laposky AD, Wilson SJ, Boggs J, Aluisio L, Lord B, Mazur C, Pudiak CM, et al. (2004) Acute wake-promoting actions of JNJ-5207852, a novel, diamine-based H3 antagonist. Br J Pharmacol 143:649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E. (2008) Biomarkers in heart failure. N Engl J Med 358:2148–2159 [DOI] [PubMed] [Google Scholar]
- Chan NY, Seyedi N, Takano K, Levi R. (2012) An unsuspected property of natriuretic peptides: promotion of calcium-dependent catecholamine release via protein kinase G-mediated phosphodiesterase type 3 inhibition. Circulation 125:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly WM, Shenton FC, Lethbridge N, Leurs R, Waldvogel HJ, Faull RL, Lees G, Chazot PL. (2009) The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br J Pharmacol 157:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart MD, Altenbach RJ, Liu H, Hsieh GC, Drizin I, Milicic I, Miller TR, Witte DG, Wishart N, Fix-Stenzel SR, et al. (2008) Rotationally constrained 2,4-diamino-5,6-disubstituted pyrimidines: a new class of histamine H4 receptor antagonists with improved druglikeness and in vivo efficacy in pain and inflammation models. J Med Chem 51:6547–6557 [DOI] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, et al. (2005) Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation 111:2469–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler M, Kaye D. (2000) Measurement of sympathetic nervous system activity in heart failure: the role of norepinephrine kinetics. Heart Fail Rev 5:17–25 [DOI] [PubMed] [Google Scholar]
- Fujimura M, Akaike M, Iwase T, Yoshida S, Sumitomo Y, Yagi S, Ikeda Y, Hashizume S, Aihara K, Nishiuchi T, et al. (2009) Decrease in plasma brain natriuretic peptide level in the early phase after the start of carvedilol therapy is a novel predictor of long-term outcome in patients with chronic heart failure. Acta Cardiol 64:589–595 [DOI] [PubMed] [Google Scholar]
- Gill RM, Braz JC, Jin N, Etgen GJ, Shen W. (2007) Restoration of impaired endothelium-dependent coronary vasodilation in failing heart: role of eNOS phosphorylation and CGMP/cGK-I signaling. Am J Physiol Heart Circ Physiol 292:H2782–H2790 [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Quarti-Trevano F, Dell'oro R. (2009) Sympathetic activation in congestive heart failure: evidence, consequences and therapeutic implications. Curr Vasc Pharmacol 7:137–145 [DOI] [PubMed] [Google Scholar]
- Hashikawa-Hobara N, Chan NY, Levi R. (2012) Histamine 3 receptor activation reduces the expression of neuronal angiotensin II type 1 receptors in the heart. J Pharmacol Exp Ther 340:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H, Hayashi H, Kohri H, Kimura Y, Hosokawa T, Igawa T, Saitoh Y. (1979) Selective inhibitor of platelet cyclic adenosine monophosphate phosphodiesterase, cilostamide, inhibits platelet aggregation. J Pharmacol Exp Ther 211:26–30 [PubMed] [Google Scholar]
- Hou Y, Lascola J, Dulin NO, Ye RD, Browning DD. (2003) Activation of cGMP-dependent protein kinase by protein kinase C. J Biol Chem 278:16706–16712 [DOI] [PubMed] [Google Scholar]
- Imamura M, Seyedi N, Lander HM, Levi R. (1995) Functional identification of histamine H3 receptors in the human heart. Circ Res 77:206–210 [DOI] [PubMed] [Google Scholar]
- Kitbunnadaj R, Hashimoto T, Poli E, Zuiderveld OP, Menozzi A, Hidaka R, de Esch IJ, Bakker RA, Menge WM, Yamatodani A, et al. (2005) N-substituted piperidinyl alkyl imidazoles: discovery of methimepip as a potent and selective histamine H3 receptor agonist. J Med Chem 48:2100–2107 [DOI] [PubMed] [Google Scholar]
- Leurs R, Bakker RA, Timmerman H, de Esch IJ. (2005) The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov 4:107–120 [DOI] [PubMed] [Google Scholar]
- Levi R, Smith NC. (2000) Histamine H3 receptors: a new frontier in myocardial ischemia. J Pharmacol Exp Ther 292:825–830 [PubMed] [Google Scholar]
- Lewis RV, McDevitt DG. (1986) Adverse reactions and interactions with β-adrenoceptor blocking drugs. Med Toxicol 1:343–361 [DOI] [PubMed] [Google Scholar]
- Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R. (2005) Evaluation of histamine H1, H2, and H3 receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective histamine H4 receptor agonist. J Pharmacol Exp Ther 314:1310–1321 [DOI] [PubMed] [Google Scholar]
- Liu C, Ma X, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, Pyati J, Li X, Chai W, Carruthers N, et al. (2001) Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol 59:420–426 [DOI] [PubMed] [Google Scholar]
- Liu H, Maurice DH. (1998) Expression of cyclic GMP-inhibited phosphodiesterases 3A and 3B (PDE3A and PDE3B) in rat tissues: differential subcellular localization and regulated expression by cyclic AMP. Br J Pharmacol 125:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith IT, Broughton A, Jennings GL, Esler MD. (1991) Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med 325:618–624 [DOI] [PubMed] [Google Scholar]
- Molkentin JD. (2003) A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest 111:1275–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto M, López FJ, Negro-Vilar A. (1993) Nitric oxide regulates luteinizing hormone-releasing hormone secretion. Endocrinology 133:2399–2402 [DOI] [PubMed] [Google Scholar]
- Morrey C, Estephan R, Abbott GW, Levi R. (2008) Cardioprotective effect of histamine H3 receptor activation: pivotal role of Gβγ-dependent inhibition of voltage-operated Ca2+ channels. J Pharmacol Exp Ther 326:871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse KL, Behan J, Laz TM, West RE, Jr, Greenfeder SA, Anthes JC, Umland S, Wan Y, Hipkin RW, Gonsiorek W, et al. (2001) Cloning and characterization of a novel human histamine receptor. J Pharmacol Exp Ther 296:1058–1066 [PubMed] [Google Scholar]
- Munagala VK, Burnett JC, Jr, Redfield MM. (2004) The natriuretic peptides in cardiovascular medicine. Curr Probl Cardiol 29:707–769 [DOI] [PubMed] [Google Scholar]
- Nakaya M, Takeuchi N, Kondo K. (2004) Immunohistochemical localization of histamine receptor subtypes in human inferior turbinates. Ann Otol Rhinol Laryngol 113:552–557 [DOI] [PubMed] [Google Scholar]
- Nijmeijer S, de Graaf C, Leurs R, Vischer HF. (2012) Molecular pharmacology of histamine H4 receptors. Front Biosci 17:2089–2106 [DOI] [PubMed] [Google Scholar]
- Nony P, Boissel JP, Lievre M, Leizorovicz A, Haugh MC, Fareh S, de Breyne B. (1994) Evaluation of the effect of phosphodiesterase inhibitors on mortality in chronic heart failure patients. A meta-analysis. Eur J Clin Pharmacol 46:191–196 [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, et al. (2011) Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 365:32–43 [DOI] [PubMed] [Google Scholar]
- Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. (2000) Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem 275:36781–36786 [DOI] [PubMed] [Google Scholar]
- Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML. (1991) Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 325:1468–1475 [DOI] [PubMed] [Google Scholar]
- Schömig A. (1990) Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation 82:II13–II22 [PubMed] [Google Scholar]
- Seyedi N, Mackins CJ, Machida T, Reid AC, Silver RB, Levi R. (2005) Histamine H3 receptor-induced attenuation of norepinephrine exocytosis: a decreased PKA activity mediates a reduction in intracellular calcium. J Pharmacol Exp Ther 312:272–280 [DOI] [PubMed] [Google Scholar]
- Seyedi N, Win T, Lander HM, Levi R. (1997) Bradykinin B2 receptor activation augments norepinephrine exocytosis from cardiac sympathetic nerve endings. Mediation by autocrine/paracrine mechanisms. Circ Res 81:774–784 [DOI] [PubMed] [Google Scholar]
- Silver RB, Poonwasi KS, Seyedi N, Wilson SJ, Lovenberg TW, Levi R. (2002) Decreased intracellular calcium mediates the histamine H3 receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings. Proc Natl Acad Sci U S A 99:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T, Becker R, Voss F, Bikou O, Hauck M, Licka M, Katus HA, Bauer A. (2008) Elevated B-type natriuretic peptide levels in patients with nonischemic cardiomyopathy predict occurrence of arrhythmic events. Clin Res Cardiol 97:306–309 [DOI] [PubMed] [Google Scholar]
- Springer J, Azer J, Hua R, Robbins C, Adamczyk A, McBoyle S, Bissell MB, Rose RA. (2012) The natriuretic peptides BNP and CNP increase heart rate and electrical conduction by stimulating ionic currents in the sinoatrial node and atrial myocardium following activation of guanylyl cyclase-linked natriuretic peptide receptors. J Mol Cell Cardiol 52:1122–1134 [DOI] [PubMed] [Google Scholar]
- Thireau J, Karam S, Fauconnier J, Roberge S, Cassan C, Cazorla O, Aimond F, Lacampagne A, Babuty D, Richard S. (2012) Functional evidence for an active role of B-type natriuretic peptide in cardiac remodelling and pro-arrhythmogenicity. Cardiovasc Res 95:59–68 [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. (2004) Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 350:655–663 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Satoo H, Kohara K, Takami R, Motoyashiki T, Morita T, Ueki H. (2004) Orthovanadate stimulates cAMP phosphodiesterase 3 activity in isolated rat hepatocytes through mitogen-activated protein kinase activation dependent on cAMP-dependent protein kinase. Biol Pharm Bull 27:789–796 [DOI] [PubMed] [Google Scholar]
- Wijkander J, Landström TR, Manganiello V, Belfrage P, Degerman E. (1998) Insulin-induced phosphorylation and activation of phosphodiesterase 3B in rat adipocytes: possible role for protein kinase B but not mitogen-activated protein kinase or p70 S6 kinase. Endocrinology 139:219–227 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Michalovich D, Wu H, Tan KB, Dytko GM, Mannan IJ, Boyce R, Alston J, Tierney LA, Li X, et al. (2001) Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Mol Pharmacol 59:434–441 [DOI] [PubMed] [Google Scholar]