Abstract
Variation in pharmacology and function of ligands at species orthologs can be a confounding feature in understanding the biology and role of poorly characterized receptors. Substantial selectivity in potency of a number of GPR35 agonists has previously been demonstrated between human and rat orthologs of this G protein-coupled receptor. Via a bioluminescence resonance energy transfer-based assay of induced interactions between GPR35 and β-arrestin-2, addition of the mouse ortholog to such studies indicated that, as for the rat ortholog, murine GPR35 displayed very low potency for pamoate, whereas potency for the reference GPR35 agonist zaprinast was intermediate between the rat and human orthologs. This pattern was replicated in receptor internalization and G protein activation assays. The effectiveness and mode of action of two recently reported GPR35 antagonists, methyl-5-[(tert-butylcarbamothioylhydrazinylidene)methyl]-1-(2,4-difluorophenyl)pyrazole-4-carboxylate (CID-2745687) and 2-hydroxy-4-[4-(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]butanoylamino)benzoic acid (ML-145), were investigated. Both CID-2745687 and ML-145 competitively inhibited the effects at human GPR35 of cromolyn disodium and zaprinast, two agonists that share an overlapping binding site. By contrast, although ML-145 also competitively antagonized the effects of pamoate, CID-2745687 acted in a noncompetitive fashion. Neither ML-145 nor CID-2745687 was able to effectively antagonize the agonist effects of either zaprinast or cromolyn disodium at either rodent ortholog of GPR35. These studies demonstrate that marked species selectivity of ligands at GPR35 is not restricted to agonists and considerable care is required to select appropriate ligands to explore the function of GPR35 in nonhuman cells and tissues.
Introduction
GPR35 is a poorly characterized member of the rhodopsin-like, class A subfamily of G protein-coupled receptors (GPCRs), which, based in part on expression pattern, has attracted attention as a possible therapeutic target in conditions ranging from diabetes and cardiovascular disease to inflammation and pain (MacKenzie et al., 2011; Milligan, 2011). There are, however, major challenges in efforts to understand the role of this receptor. These include that kynurenic acid, the first endogenously produced molecule identified as being able to activate GPR35 (Wang et al., 2006), is substantially more potent at the rat ortholog than the human form (Jenkins et al., 2010). This has resulted in suggestions that kynurenic acid may not be a true endogenous regulator of at least the human ortholog, leaving GPR35 as an “orphan” receptor (MacKenzie et al., 2011). Although a number of synthetic GPR35 agonists have been identified via various screens (Jenkins et al., 2010; Zhao et al., 2010), some of them also display marked variations in potency to activate the human and rat orthologs (Jenkins et al., 2010; Milligan, 2011). Both drug discovery programs and the use of animal models to explore receptor function marked variation in ligand activity and/or ligand structure-activity relationships pose important challenges, particularly for poorly characterized receptors in which the orthosteric binding site remains undefined. Moreover, although the available literature on ligand activation of this receptor is relatively limited, it is also not entirely consistent. For example, the most potent agonist reported to date at human GPR35 is pamoate (Fig. 1) (Jenkins et al., 2010; Zhao et al., 2010). However, although pamoate has been reported to also be an effective agonist at mouse GPR35 (Zhao et al., 2010), it has been reported to show very low activity at the rat ortholog (Jenkins et al., 2010). A further limitation in efforts to understand the function of GPR35 is that, until recently, no antagonist ligands had been described. Two reports have, however, demonstrated that both methyl-5-[(tert-butylcarbamothioylhydrazinylidene)methyl]-1-(2,4-difluorophenyl)pyrazole-4-carboxylate (CID-2745687) (Fig. 1) (Zhao et al., 2010) and 2-hydroxy-4-[4-(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]butanoylamino)benzoic acid (ML-145) (Fig. 1) (Heynen-Genel et al., 2010) are antagonists.
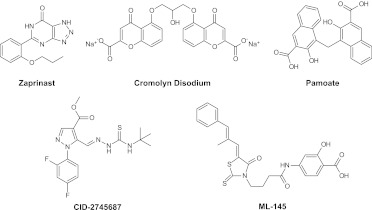
Fig. 1.
Structures of the three agonist ligands, zaprinast, cromolyn disodium, and pamoate, and the two previously reported antagonists, CID-2745687 and ML-145, are shown.
Because of the potential differences in the responses of rat and mouse GPR35 to pamoate (Jenkins et al., 2010; Zhao et al., 2010) and the high potency of this ligand at the human ortholog, we have reassessed ligand selectivity at these orthologs and considered the potential usefulness of the reported GPR35 antagonists in unraveling the physiological roles of this receptor. Using a bioluminescence resonance energy transfer (BRET)-based GPR35-β-arrestin-2 interaction assay, a receptor internalization assay, and generation of intracellular inositol phosphates via coexpression of chimeric G proteins we demonstrate that pamoate is highly selective for human GPR35 compared with either of the rodent orthologs. Furthermore, although CID-2745687 (Zhao et al., 2010) and ML-145 (Heynen-Genel et al., 2010) act as effective antagonists at human GPR35, neither displays significant activity at either rodent ortholog. Finally, although ML-145 acts as a competitive antagonist for a number of agonists at human GPR35, CID-2745687, a competitive antagonist of the agonist actions of both zaprinast and cromolyn disodium, antagonizes the agonist effect of pamoate at human GPR35 in a noncompetitive manner.
These studies highlight a number of the challenges in exploring both the basis of ligand binding and potentially understanding the function of poorly characterized receptors such as GPR35 and demonstrate that considerable care is required to select appropriate ligands to explore the function of GPR35 in nonhuman cells and tissues.
Materials and Methods
Materials.
Materials for cell culture were from Sigma Chemical (Poole, Dorset, UK), Invitrogen (Paisley, UK), or PAA Laboratories Ltd (Yeovil, Somerset, UK). Polyethylenimine (PEI) [linear poly(vinyl alcohol) (MW-25000)] was from Polysciences (Warrington, PA). Zaprinast, CID-2745687, and ML-145 were from Tocris Bioscience (Bristol, UK). Cromolyn disodium, pamoate, and Hoechst 33258 solution were from Sigma Chemical. Ligands were initially dissolved in dimethyl sulfoxide and then diluted in assay buffer.
GPR35 Constructs.
A FLAG epitope (DYKDDDDK) was introduced in the N-terminal ends of human, rat, and mouse GPR35 cDNA by polymerase chain reaction using the following primers: human sense, 5′-GCGAAGCTTGCCACCATGGATTACAAGGATGACGACGATAAGAATGGCACCTACAACACC-3′; rat sense, 5′-CGCGGATCCGCCACCATGGATTACAAGGATGACGACGATAAGAACAATACAAATTGTAGCATC-3′; mouse sense, 5′-TTTTGGATCCGCCACCATGGATTACAAGGATGACGACGATAAGATGAATAGTACAACCTGTAACAGCACC-3′; human antisense, 5′-GGCCGCGGCCGCCTCTAGAATTAGGCGAGGG-3′; rat antisense, 5′-GGCCGCGGCCGCCGGTGAGGCTCAGGCTCTG-3′; and mouse antisense, 5′-TTTTGCGGCCGCGGTGAGGCTCAGGATCTGG-3′.
The HindIII, BamHI, and NotI restriction sites used for cloning are underlined. The resulting cDNA was subsequently cloned in-frame into the HindIII/BamHI and NotI sites of an eYFP-pcDNA5/FRT/TO plasmid, yielding the final N-terminal epitope and C-terminal fluorescence-tagged constructs human FLAG-GPR35-eYFP-pcDNA5/FRT/TO, rat FLAG-GPR35-eYFP-pcDNA5/FRT/TO, and mouse FLAG-GPR35-eYFP-pcDNA5/FRT/TO. The integrity of each fusion was checked by DNA sequencing.
G Protein Chimeras.
The chimeric Gqi5 or Gq135 Gα subunits in which the C-terminal five amino acids of Gαq were replaced by the corresponding amino acids from either Gαi1/2 or Gα13 have been described previously (Jenkins et al., 2011).
Cell Culture and Transfection.
Flp-In TREx 293 cells (Invitrogen) were maintained in Dulbecco's modified Eagle's medium without sodium pyruvate (Invitrogen), supplemented with 10% (v/v) fetal calf serum, 1% penicillin/streptomycin mixture, and 10 μg/ml blasticidin at 37°C in a 5% CO2 humidified atmosphere. HEK293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 0.292 g/liter l-glutamine and 10% (v/v) newborn calf serum at 37°C in a 5% CO2 humidified atmosphere. Transfection was performed by using 1 mg/ml PEI (linear MW-25000). Cells were plated until 60 to 80% confluent then transfected with 5 μg of required plasmid DNA and PEI (ratio 1:6 DNA/PEI), diluted in 150 mM NaCl, pH 7.4. After incubation at room temperature for 10 min, the mixture was added to HEK293T cells. Cells were incubated 24 h then transferred to 96-well plates coated with poly-d-lysine. In all experiments, the total amount of DNA transfected was equalized between constructs by the addition of the empty expression vector pcDNA3.
Generation of Flp-In TREx 293 Cells Stably Expressing Forms of GPR35.
To generate Flp-In TREx 293 cells able to inducibly express human FLAG-GPR35-eYFP, rat FLAG-GPR35-eYFP, or mouse FLAG-GPR35-eYFP, the cells were transfected with a mixture containing the desired cDNA in pcDNA5/FRT/TO vector and the pOG44 vector (1:9) by using Effectene (QIAGEN, Dorking, Surrey, UK), according to the manufacturer's instructions. After 48 h, the medium was changed to medium supplemented with 200 μg/ml hygromycin B to initiate the selection of stably transfected cells. After isolation of resistant cells, expression of the appropriate construct from the Flp-In TREx locus was induced by treatment with up to 100 ng/ml doxycycline for 24 h.
BRET.
HEK293T cells were cotransfected with the required ortholog of FLAG-GPR35 tagged with eYFP and β-arrestin-2-Renilla luciferase 6 (ratio 4:1), using PEI. An additional set of transfections used only the Renilla luciferase construct and empty expression vector pcDNA3. From 10-cm dishes, 50,000 cells were seeded per well into poly-d-lysine-coated 96-well plates. After 24 h cells were washed twice with Hanks' balanced salt solution, pH 7.4, and coelentrazine-h (Promega, Southampton, UK) was added to a final concentration of 5 μM. Cells were incubated in darkness for 10 min at 37°C before the addition of ligands. Cells were incubated an additional 5 min at 37°C before being read on a PHERAstar FS reader (BMG Labtech, Durham, NC). The BRET ratio was then calculated as emission at 530 nm/emission at 485 nm. Net BRET was defined as the 530/485-nm ratio of cells coexpressing the Renilla luciferase and eYFP constructs minus the BRET ratio of cells expressing only the Renilla luciferase construct in the same experiment. This value was multiplied by 1000 to obtain mBRET units.
Internalization and Live Cell Epifluorescence Microscopy.
Cells expressing human or rat FLAG-GPR35-eYFP were grown on poly-d-lysine-coated coverslips. The coverslips were placed into a microscope chamber containing physiological saline solution (130 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, and 10 mM d-glucose, pH 7.4). For internalization studies, ligands were added to the microscope chamber, and fluorescent images were acquired at 5-min intervals by using a spinning disk structured illumination Viva Tome device attached to the bottom port of a Zeiss Axio Observer.Z1 invert microscope (Carl Zeiss, Inc., Thornwood, NY). Narrow-band 490/20-nm excitation light was reflected through a 63×, oil immersion Plan-Apochromat objective lens to excite eYFP, and the resulting emitted light was detected at 536/40 nm by using an Axiocam MRm charge-coupled device camera (Carl Zeiss, Inc.).
ArrayScan High Content Analysis of GPR35 Internalization.
Flp-In T-Rex 293 cells harboring human FLAG-GPR35-eYFP, rat FLAG-GPR35-eYFP, or mouse FLAG-GPR35-eYFP were seeded into poly-d-lysine-coated, clear-view 96-well plates at a density of 60,000 cells per well and treated with up to 100 ng/ml doxycycline to induce receptor construct expression. After 24 h cells were washed twice with Hanks' balanced salt solution and incubated with ligands for 1 h at 37°C. Cells were then incubated for another 30 min with ligands and 10 μg/ml Hoechst nuclear stain at 37°C. Images were acquired immediately by using a Cellomics ArrayScan II high content imager (Thermo Fisher Scientific, Waltham, MA), and internalized receptors were quantified by using a proprietary algorithm designed to identify the number of endosomal recycling compartments per cell.
Inositol Phosphate Accumulation Assays.
HEK293T cells were transiently cotransfected with the human, rat, or mouse orthologs of FLAG-GPR35-eYFP and a chimeric G protein (either Gαqi5 or Gαq135) by using PEI. After 16- to 24-h incubation at 37°C + 5% CO2, cells were resuspended in IP-One stimulation buffer (10 mM HEPES, 1 mM CaCl2, 0.5 mM MgCl2, 4.2 mM KCl, 146 mM NaCl, 5.5 mM glucose, and 50 mM LiCl, pH 7.4) and seeded at 10,000 cells/well in white, solid-bottom, 384-well plates. Ligands were diluted in IP-One stimulation buffer according to the manufacturer's instructions (Cisbio HTRF IP-One Tb kit; Cisbio Bioassays, Bedford, MA). Antagonist compounds were preincubated with cells for 15 min at 37°C before the addition of agonist. Cells were incubated with agonist for 2 h at 37°C, before the addition of d2-conjugated inositol monophosphate (IP1) and anti-IP1 Lumi4-Tb cryptate diluted in lysis buffer according to the manufacturer's instructions. After incubation at room temperature for 1 h, homogeneous time-resolved fluorescence was measured by using a PHERAstar FS plate reader.
Data Analysis and Curve Fitting.
All data represent mean ± S.E. of at least three independent experiments. Data analysis and curve fitting was carried out by using the GraphPad Prism software package version 5.0b (GraphPad Software, Inc., San Diego, CA). Concentration-response data were fit to three-parameter sigmoidal concentration-response curves. All statistical analysis of curve fit parameters was carried out by independently fitting the data from triplicate experiments and comparing the resulting curve fit values by t test or one-way analysis of variance as appropriate.
Results
Pamoate Is a Highly Selective but Partial Agonist for Human GPR35 in a β-Arrestin-2 Recruitment Assay.
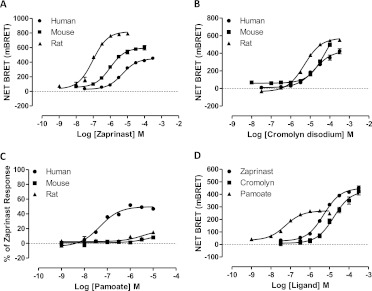
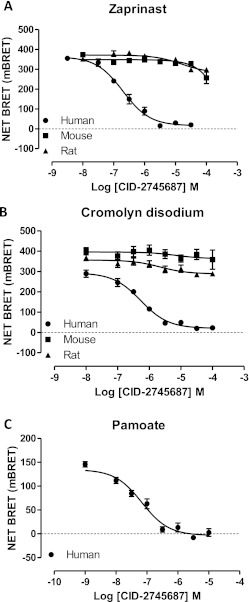
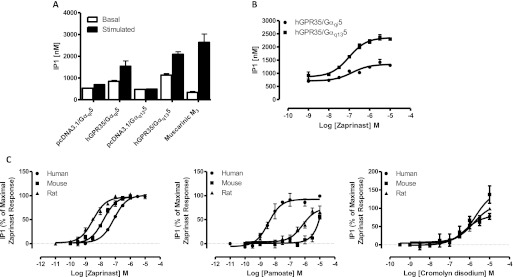
BRET-based GPR35-β-arrestin-2 interaction assays were established in HEK293T cells after cotransfection of species orthologs of FLAG-GPR35-eYFP and β-arrestin-2 Renilla luciferase. As reported previously (Jenkins et al., 2010, 2011), zaprinast (Fig. 1), which in the absence of a clearly defined, high-potency endogenous agonist (Milligan, 2011) is often used as a reference ligand at GPR35, produced robust, concentration-dependent BRET signals. Zaprinast was significantly (p < 0.001) more potent (pEC50 = 7.02 ± 0.05, mean ± S.E.M.; n = 7) in producing this effect at rat GPR35 than at the human ortholog (pEC50 = 5.30 ± 0.03, mean ± S.E.M.; n = 6). Equivalent studies with mouse GPR35 confirmed that zaprinast also acted as an agonist at this ortholog with potency (pEC50 = 6.01 ± 0.06; n = 6) intermediate between the two other species (Fig. 2A). The antiasthma medicine and histamine sensitizer cromolyn disodium (Fig. 1) (Jenkins et al., 2010; Yang et al., 2010) also acted as an agonist with efficacy equal to that of zaprinast at all three species orthologs. In this case ligand potency was lower, but was relatively similar between the human and rat orthologs with pEC50 = 4.78 ± 0.10 (mean ± S.E.M.; n = 7) at the human receptor, 5.30 ± 0.03 (n = 3) at rat, and somewhat lower, 4.24 ± 0.06 (n = 9) at mouse GPR35 (Fig. 2B). We have also previously reported that although pamoate (Fig. 1) is a relatively high-potency (pEC50 = 7.28 ± 0.07, mean ± S.E.M.; n = 7) but partial (Emax = 54.8 ± 0.8%) agonist at human GPR35 compared with zaprinast in such BRET-based GPR35-β-arrestin-2 interaction assays (Fig. 2, C and D) it has little agonist (Fig. 2C) or antagonist (Jenkins et al., 2010) activity at rat GPR35. This was also the case when examining mouse GPR35, with only a hint of agonism detected in this assay at concentrations up to 1 × 10−5 M (Fig. 2C). This was somewhat surprising because Zhao et al. (2010) have reported pamoate had agonist function at mouse GPR35 in a distinct β-arrestin-2 interaction assay.
Fig. 2.
Pamoate is a GPR35 partial agonist with high selectivity for the human ortholog. A to C, BRET-based GPR35-β-arrestin-2 interaction assays were performed in HEK293T cells using human (●), mouse (■), or rat (▴) FLAG-GPR35-eYFP constructs. The effects of varying concentrations of zaprinast (A), cromolyn disodium (B), and pamoate (C) were assessed. D, the potency and relative efficacy of these ligands at human GPR35 is displayed. Data are representative of at least six different experiments.
Pamoate Is Also Highly Species Ortholog Selective in an Assay of Ligand-Induced Internalization of GPR35.
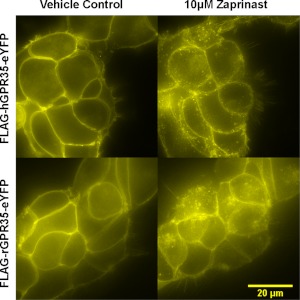
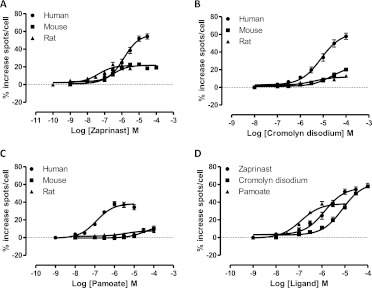
Because of these differences, we also assessed the function of these ligands in receptor internalization assays. In addition to interacting strongly with β-arrestin-2 in an agonist-dependent manner, human GPR35 has been reported to internalize from the surface of cells upon exposure to agonist ligands (Zhao et al., 2010; Jenkins et al., 2011; Deng et al., 2012). Human FLAG-GPR35-eYFP was cloned into the Flp-In locus of Flp-In TREx 293 cells and a pool of cells isolated that were able to express this construct upon addition of the antibiotic doxycycline. Induction of expression resulted in plasma membrane delivery of the receptor (Fig. 3), and addition of zaprinast (1 × 10−5 M) resulted in marked internalization of the eYFP-tagged receptor into punctate intracellular vesicles (Fig. 3). Similar effects were noted in cells induced to express rat FLAG-GPR35-eYFP (Fig. 3). Such effects were quantified in cell populations by using an ArrayScan high content imager. Zaprinast (pEC50 = 6.01 ± 0.07) (Fig. 4A), cromolyn disodium (pEC50 = 5.19 ± 0.10) (Fig. 4B), and pamoate (pEC50 = 6.86 ± 0.11) (Fig. 4C) (each n = 3) all promoted internalization of human FLAG-GPR35-eYFP in a concentration-dependent fashion with EC50 values similar to and with the same rank order of potency (pamoate > zaprinast > cromolyn disodium) (Fig. 4D) as obtained in the BRET-based β-arrestin-2 interaction assays (Table 1). However, when similar studies were performed on equivalent cells able to induce the expression of mouse FLAG-GPR35-eYFP, zaprinast produced internalization with pEC50 = 6.63 ± 0.13 (Fig. 4A) similar to that observed in the BRET-based β-arrestin-2 interaction assay, and pamoate was completely without effect at concentrations up to 1 × 10−4 M (Fig. 4C). Although cromolyn disodium did promote internalization of mouse FLAG-GPR35-eYFP at concentrations above 1 × 10 −5 M, an EC50 value could not be estimated accurately (Fig. 4B). In cells induced to express rat FLAG-GPR35-eYFP zaprinast promoted internalization of the receptor with pEC50 = 7.41 ± 0.11 (Fig. 4A), pamoate produced no significant effect at concentrations below 1 × 10−5 M (Fig. 4C), and cromolyn disodium produced only a small effect (Fig. 4B).
Fig. 3.
Zaprinast promotes internalization of both human and rat GPR35-eYFP. Flp-In TREx 293 cells able to express either human (top) or rat (bottom) FLAG-GPR35-eYFP upon addition of doxycycline were induced with this antibiotic for 24 h. Such cells were then exposed to vehicle (left) or zaprinast (1 × 10−5 M) (right) for 30 min before imaging.
Fig. 4.
Pamoate-mediated internalization of GPR35 is restricted to the human ortholog. A to C, Flp-In TREx 293 cells able to express human (●), mouse (■), or rat (▴) FLAG-GPR35-eYFP constructs upon addition of doxycycline were induced with this antibiotic for 24 h. The capacity of varying concentrations of zaprinast (A), cromolyn disodium (B), or pamoate (C) to promote internalization of the receptor constructs was then assessed and quantified by using an ArrayScan high content imager. D, the potency and relative efficacy of these ligands at human GPR35 is displayed. Data are representative of at least six different experiments.
TABLE 1.
Comparison of the potency of agonist ligands at human GPR35
The potency of ligands at human GPR35 is compared in BRET-based β-arrestin-2 recruitment and receptor internalization assays. Results are pEC50 mean ± S.E.M.
| Ligand | β-Arrestin-2 Recruitment | Internalization |
|---|---|---|
| Zaprinast | 5.30 ± 0.03 | 6.01 ± 0.07 |
| Cromolyn disodium | 4.78 ± 0.10 | 5.19 ± 0.10 |
| Pamoate | 7.28 ± 0.07* | 6.86 ± 0.11* |
indicates partial agonist compared with zaprinast.
Antagonists of GPR35 Also Display Marked Species Ortholog Selectivity.
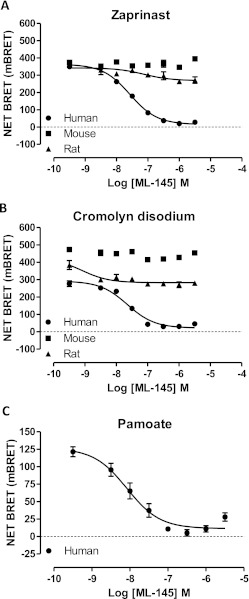
Based on the marked species selectivity of certain agonist ligands, we next considered whether species selectivity might also be true of reported antagonists. CID-2745687 (Fig. 1) is the only GPR35 antagonist detailed so far in the primary scientific literature (Zhao et al., 2010). Using the BRET-based GPR35-β-arrestin-2 interaction assay and an EC80 concentration of zaprinast (2 × 10−5 M) as agonist, CID-2745687 behaved as a moderately potent, concentration-dependent antagonist at human GPR35 with pIC50 = 6.70 ± 0.09 (mean ± S.E.M.; n = 9) (Fig. 5A). By contrast, even at 1 × 10−4 M CID-2745687 did not substantially block the agonist action of an EC80 concentration of zaprinast (4 × 10−6 M) at mouse GPR35 (Fig. 5A), and a similar inability to antagonize the effect of an EC80 concentration of zaprinast (4 × 10−7 M) was observed with rat GPR35 (Fig. 5A). This again was unexpected because Zhao et al. (2010) have reported CID-2745687 blocks the effects of zaprinast in a mouse pain model. Equivalent results were obtained with cromolyn disodium as agonist. Once again, a concentration-dependent inhibitory activity of CID-2745687 (pIC50 = 6.27 ± 0.08, mean ± S.E.M.; n = 9) to fully reverse the agonist action of an EC80 concentration of cromolyn disodium was observed only at the human ortholog (Fig. 5B). Although we were only able to explore the ability to inhibit the agonist action of pamoate at human GPR35, CID-2745687 did so fully and in a concentration-dependent manner with pIC50 = 7.16 ± 0.12 (mean ± S.E.M.; n = 9) (Fig. 5C).
Fig. 5.
CID-2745687 is a potent antagonist in β-arrestin-2 interaction assays only at human GPR35. A to C, BRET-based GPR35-β-arrestin-2 interaction assays as in Fig. 2 were performed by using human (●), mouse (■), or rat (▴) FLAG-GPR35-eYFP. In each case an EC80 concentration of zaprinast (A), cromolyn disodium (B), or pamoate (only at human GPR35) (C) was added along with varying concentrations of CID-2745687.
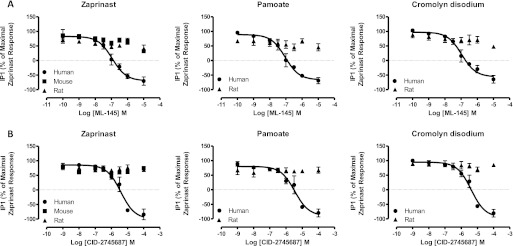
ML-145 (Fig. 1), identified in a β-arrestin-dependent high content screen, has also been described as an antagonist of GPR35 (Heynen-Genel et al., 2010). Like CID-2745687, ML-145 fully inhibited the agonist effects of EC80 concentrations of zaprinast (Fig. 6A), cromolyn disodium (Fig. 6B), and pamoate (Fig. 6C) at human GPR35 in a concentration-dependent manner. Consistent with the reported higher affinity of ML-145 (Heynen-Genel et al., 2010), in each case this ligand displayed higher potency than CID-2745687 (Table 2). Once again, however, ML-145 was either without effect (mouse) or displayed only a small and apparently noncompetitive inhibitory effect (rat) at the rodent orthologs (Fig. 6, A and B).
Fig. 6.
ML-145 is also a highly selective human GPR35 antagonist in β-arrestin-2 interaction assays. A to C, experiments were conducted as in Fig. 5 except that ML-145 replaced CID-2745687. A, zaprinast. B, cromolyn disodium. C, pamoate.
TABLE 2.
ML-145 displays greater potency as an antagonist at human GPR35 than CID-2745687
Human GPR35-β-arrestin-2 interaction assays were conducted by using EC80 amounts of the indicated agonists and varying concentrations of either ML-145 or CID-2745687. In all cases a maximally effective concentration of either antagonist ligand was able to fully reverse the effects of the agonist ligand. Data are means ± S.E.M. from nine independent experiments.
| Agonist | Antagonist |
|
|---|---|---|
| ML-145 | CID-2745687 | |
| Zaprinast | 7.57 ± 0.07 | 6.70 ± 0.09 |
| Cromolyn disodium | 7.65 ± 0.07 | 6.27 ± 0.08 |
| Pamoate | 8.08 ± 0.12 | 7.16 ± 0.12 |
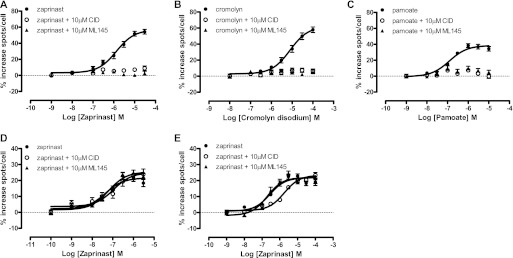
Both CID-2745687 and ML-145 Can Also Prevent Agonist-Induced Internalization of Human GPR35.
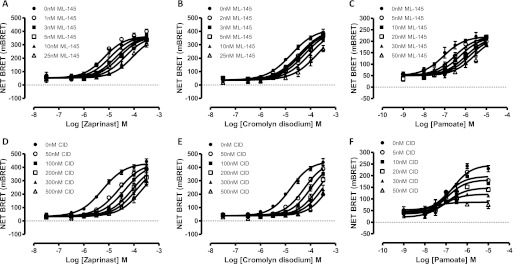
It is noteworthy that both CID-2745687 and ML-145 (1 × 10−5 M) also fully blocked internalization of human FLAG-GPR35-eYFP in response to varying concentrations of zaprinast, cromolyn disodium, and pamoate (Fig. 7, A–C). This was not the case for either CID-2745687 or ML-145 when tested against zaprinast at rat FLAG-GPR35-eYFP (Fig. 7D). At mouse FLAG-GPR35-eYFP, although ML-145 was without effect on the potency or effect of zaprinast (Fig. 7E), at 1 × 10−5 M CID-2745687 consistently produced a modest, but not statistically significant, decrease in potency but not a maximal effect of zaprinast (Fig. 7E). To explore this more fully, the ability of varying concentrations of ML-145 or CID-2745687 to prevent internalization of the human or mouse orthologs of GPR35 in response to an EC80 concentration of zaprinast was assessed. Here, both ML-145 (Fig. 8A) and CID-2745687 (Fig. 8B) were effective inhibitors of zaprinast at the human ortholog but neither produced a substantial effect at the mouse ortholog (Fig. 8, C and D). Equivalent results for both ML-145 (Fig. 8, A, and C) and CID-2745687 (Fig. 8, B and D) were produced when EC80 concentrations of cromolyn disodium (at both human and mouse GPR35) or pamoate (at the human ortholog) were used.
Fig. 7.
CID-2745687 and ML-145 both block agonist-mediated internalization of human but not rodent forms of GPR35. A to C, the ability of varying concentrations of zaprinast (A), cromolyn disodium (B), and pamoate (C) to promote internalization of human GPR35-eYFP and the capacity of either CID-2745687 (○) or ML-145 (▴) (1 × 10 −5 M) to prevent this was assessed via high content imaging using an ArrayScan high content imager. D and E, in equivalent studies the capacity of varying concentrations of zaprinast to cause internalization of rat (D) and mouse (E) GPR35-eYFP and any capacity of CID-2745687 and ML-145 (1 × 10−5 M) to prevent this was assessed.
Fig. 8.
CID-2745687 and ML-145 block agonist-mediated internalization of human GPR35 in a concentration-dependent fashion. Experiments akin to those of Fig. 7 examined the ability of varying concentrations of ML-145 (A and C) or CID-2745687 (B and D) to prevent internalization of human (A and B) or mouse (C and D) GPR35 promoted by an EC80 concentration of the identified agonist ligands.
Ligand Selectivity at GPR35 Orthologs Is Also Observed in G Protein-Dependent Assays.
Each of the assays described above is G protein-independent. GPR35 has been indicated to interact, however, with both pertussis toxin-sensitive Gi family G proteins (Wang et al., 2006) and the nonpertussis toxin-sensitive G protein Gα13 (Jenkins et al., 2011). We took advantage of that to establish IP1 accumulation assays after transfection of HEK293T cells with an ortholog of FLAG-GPR35-eYFP and either chimeric Gqi5 or Gq135 Gα subunits (Kostenis et al., 2005; Milligan and Kostenis, 2006). A positive control for IP1 production was provided by transfecting cells with the muscarinic M3 acetylcholine receptor and adding the synthetic acetylcholine mimetic carbachol (1 × 10−3 M) (Fig. 9A), because this receptor is known to couple effectively to inositol phosphate production and the subsequent elevation of intracellular calcium concentration. In preliminary experiments we demonstrated that zaprinast (1 × 10 −5 M) increased the levels of IP1 when human FLAG-GPR35-eYFP and either of these G protein chimeras were coexpressed (Fig. 9A). Such an effect of zaprinast was not observed when either human FLAG-GPR35-eYFP was coexpressed with full-length Gq (data not shown) or the G protein chimeras were expressed without GPR35 (Fig. 9A). It is noteworthy that in the absence of zaprinast coexpression of human FLAG-GPR35-eYFP with either of the G protein chimeras resulted in greater accumulation of IP1 than in the absence of the receptor, which is potentially consistent with a level of receptor constitutive activity in this assay (Fig. 9A). Subsequently, we expressed human FLAG-GPR35-eYFP with either Gqi5 or Gq135 and explored the concentration dependence for zaprinast induction of IP1 generation (Fig. 9B). Zaprinast was significantly more potent (pEC50 = 6.98 ± 0.14, Gqi5; pEC50 = 7.08 ± 0.12, Gq135; n = 3) in these assays than in either of the non-G protein-dependent assays, but was not different between the two chimeric G proteins. Similar results were obtained with rat FLAG-GPR35-eYFP except, as in the other assays, zaprinast was significantly more potent (pEC50 = 8.47 ± 0.09, Gqi5; and pEC50 = 8.51 ± 0.05, Gq135) there than at the human ortholog (Fig. 9C). When using mouse FLAG-GPR35-eYFP the equivalent values were pEC50 = 8.32 ± 0.24 (Gqi5) and 7.80 ± 0.04 (Gq135) (Fig. 9C). Subsequent studies predominantly used the Gq135 chimera. As in the β-arrestin-2 interaction assays, cromolyn disodium was as efficacious as zaprinast and more potent in this assay (pEC50 = 6.32 at human, 5.82 at rat, and 5.55 at mouse) (Fig. 9C). The enhanced potency of ligands in this assay allowed reassessment of potential agonism of pamoate at the rodent orthologs of GPR35 (Fig. 9C). At human GPR35 pamoate displayed high potency (pEC50 = 8.44 ± 0.13) and was some 30-fold more potent than zaprinast (Fig. 9C). It is noteworthy that in this assay pamoate acted as a full agonist with respect to zaprinast (Fig. 9C). Despite this, at mouse GPR35 pamoate remained virtually inactive, with no significant agonist function uncovered at concentrations below 1 × 10−5 M (Fig. 9C). As such, pamoate displays at least 3000-fold selectivity for human over murine GPR35 in this assay. At the rat ortholog pamoate displayed some activity (pEC50 = 6.15 ± 0.18) (Fig. 9C) but remained some 200-fold less potent than at human GPR35.
Fig. 9.
GPR35 stimulates IP1 accumulation when coexpressed with suitable chimeric G protein α subunits. A, HEK293T cells were transfected with or without human FLAG-GPR35-eYFP alongside either Gqi5 or Gq135 Gα subunits. Basal (open bars) IP1 accumulation and effects of zaprinast (1 × 10 −5 M) on IP1 accumulation (filled bars) were measured. A positive control was provided after transfection of the M3 muscarinic acetylcholine receptor and addition of the agonist carbachol (1 × 10 −3 M). B, after cotransfection of human FLAG-GPR35-eYFP with either Gqi5 (●) or Gq135 (■) Gα subunits the effect of various concentrations of zaprinast were assessed. C, after transfection of human (●), mouse (■), or rat (▴) FLAG-GPR35-eYFP with Gq135 the ability of varying concentrations of zaprinast (left), pamoate (center), or cromolyn disodium (right) to promote IP1 accumulation was assessed.
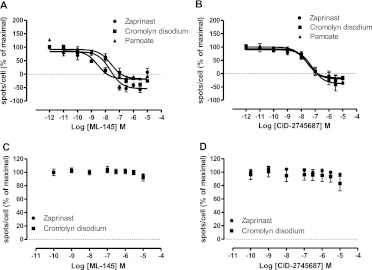
As anticipated from the other assays, ML-145 inhibited the effect of EC80 concentrations of zaprinast (3 × 10−7 M), cromolyn disodium (3 × 10−6 M), and pamoate (1 × 10−8 M) at human GPR35 in the IP1 accumulation assays with pIC50 close to 7.4 in these conditions (Fig. 10A). Furthermore, ML-145 also decreased basal IP1 production in a concentration-dependent manner (Fig. 10A), consistent with this ligand behaving as an inverse agonist and suppressing the constitutive activity of human GPR35. By contrast, ML-145 displayed little capacity to inhibit the agonist action of an EC80 concentration of zaprinast at either mouse or rat GPR35 in this assay (Fig. 10A). Although it was necessary to use pamoate at 3 × 10−6 M to generate a substantial signal via rat GPR35, ML-145 was entirely without effect at concentrations up to 1 × 10−5 M (Fig. 10A). CID-2745687 also inhibited the effect of zaprinast, cromolyn disodium, and pamoate at human GPR35 in a concentration-dependent manner but was more than 10-fold less potent than ML-145 (Fig. 10B). As for ML-145, CID-2745687 also acted as an inverse agonist and was able to reduce basal IP1 generation in a concentration-dependent manner (Fig. 10B). As noted for ML-145, CID-2745687 was without significant effect at either rat or mouse GPR35 in these assays (Fig. 10B).
Fig. 10.
CID-2745687 and ML-145 block both constitutive and agonist-mediated IP1 accumulation only at the human ortholog of GPR35. The capacity of varying concentrations of ML-145 (A) or CID-2745687 (B) to limit IP1 accumulation in response to EC80 concentrations of zaprinast (left), pamoate (center), or cromolyn (right) was assessed in cells transfected to express the noted ortholog of FLAG-GPR35-eYFP with Gq135. Note the marked reduction of IP1 below basal levels (defined as 0% on y-axis), which is indicative of inverse agonism, produced by higher concentrations of ML-145 (A) and CID-2745687 (B) at the human ortholog.
ML-145 Is a Competitive Antagonist at Human GPR35.
To define the mode of action of ML-145 at human GPR35 we returned to the BRET-based β-arrestin-2 interaction assay and performed a series of agonist concentration-response curves to zaprinast (Fig. 11A), cromolyn disodium (Fig. 11B), and pamoate (Fig. 11C) in the presence of concentrations of ML-145 ranging from 1 to 50 nM. In all cases, global analysis of the data was consistent with ML-145 acting as a competitive and surmountable antagonist, although the relatively low potency of cromolyn disodium meant that sufficient agonist could not be used to fully overcome the effect of the highest concentration of ML-145 used. Such studies produced estimates of the pA2 for ML-145 between 8.67 and 8.83 (1.48–2.13 × 10−9 M).
Fig. 11.
ML-145 is a competitive antagonist at human GPR35, whereas the nature of antagonism by CID-2745687 depends on the identity of the agonist. BRET-based GPR35-β-arrestin-2 interaction assays were performed in HEK293T cells as in Fig. 2 by using human FLAG-GPR35-eYFP. The effects of varying concentrations of ML-145 (A-C) or CID-2745687 (D-F) on concentration-response curves to zaprinast (A and D), cromolyn disodium (B and E), and pamoate (C and F) are shown in representative experiments.
CID-2745687 Inhibits the Action of Agonists at Human GPR35 by Distinct Mechanisms.
To assess whether CID-2745687 also acted as a competitive antagonist, the ability of a range of concentrations of this ligand to alter the concentration-response curves of zaprinast, cromolyn disodium, and pamoate was also examined at human GPR35. For both zaprinast (Fig. 11D) and cromolyn disodium (Fig. 11E) such studies produced surmountable shifts in the potency of the agonist to higher concentrations. Global-fit analyses of such curves were also consistent with a competitive mode of antagonism with pA2 affinity values for CID-2745687 of 7.7 to 7.8 (1.6–1.9 × 10−8 M) and slope factor close to 1.0. By contrast, equivalent studies with pamoate produced very different results (Fig. 11F). Increasing concentrations of CID-2745687 had little effect on the EC50 of pamoate but rather decreased the maximal effect of the agonist, which is indicative of a noncompetitive mode of action.
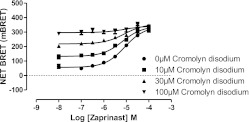
Consistent with the observations that both zaprinast and cromolyn disodium were antagonized competitively by CID-2745687 and ML-145 and are full agonists in the β-arrestin-2 interaction assay, the addition of a series of submaximally effective concentrations of cromolyn disodium did not alter the observed EC50 of zaprinast at human GPR35 (Fig. 12), suggesting the two agonists share a common or overlapping binding site (Jenkins et al., 2011).
Fig. 12.
Zaprinast and cromolyn disodium probably share a common binding site on human GPR35. BRET-based GPR35-β-arrestin-2 interaction assays were performed in HEK293T cells as in Fig. 11 by using human FLAG-GPR35-eYFP. Concentration-response curves to zaprinast were performed in the presence of a range of submaximally effective concentrations of cromolyn disodium. The EC50 of zaprinast was unaffected by the copresence of cromolyn disodium.
Discussion
In recent times the poorly characterized GPCR GPR35 has been suggested to be a potential therapeutic target in areas ranging from diabetes, cardiovascular disease, and inflammation to pain (MacKenzie et al., 2011; Milligan, 2011). In part, this has been based simply on the expression patterns of GPR35. However, because a GPR35 knockout line of mice has been reported to have markedly elevated blood pressure (Min et al., 2010), whereas blockade of acetic acid-induced writhing in mice has been shown to be limited by certain ligands, including pamoate (Zhao et al., 2010) and zaprinast (Cosi et al., 2011) that are reported to have agonist potency at GPR35, there are a developing number of supporting physiological studies. Despite this, to date only the studies of Min et al. (2010) have used GPR35 knockout animals. This means that detailed pharmacological analysis of proposed ligands at GPR35 must be performed and understood with multiple species orthologs of the receptor before linking other potential physiological functions to the regulation of GPR35. Many of the ligands reported to have agonist action at GPR35 display modest potency (Jenkins et al., 2010; Yang et al., 2010, 2012; Deng et al., 2011, 2012; Deng and Fang, 2012; Hu et al., 2012) and frequently are also known to have other molecular targets. For example, despite both kynurenic acid (Wang et al., 2006) and lysophosphatidic acid (Oka et al., 2010) being proposed as endogenous activators of GPR35, the synthetic ligand zaprinast has become the standard reference agonist. Zaprinast, however, is better appreciated as an inhibitor of cGMP phosphodiesterases (Taniguchi et al., 2006), and other recently reported GPR35 agonists are generally either very simple molecules with modest potency/affinity and known activity at multiple other targets (Yang et al., 2010, 2012; Deng and Fang, 2012; Deng et al., 2012; Hu et al., 2012) or were identified via screens of libraries of known therapeutic ligands (Jenkins et al., 2010). As such, the identification of pamoate as a relatively potent GPR35 agonist (Jenkins et al., 2010; Zhao et al., 2010) potentially offers a more useful tool.
Potential species ortholog selectivity of ligands is a major issue for target validation and candidate selection in the drug discovery arena, and even one of the initial reports of the activity of pamoate as a GPR35 agonist noted that although identified in a screen against human GPR35 this was not replicated when rat GPR35 was the target (Jenkins et al., 2010). Furthermore, in the current studies we have extended such studies to demonstrate that pamoate has no substantial agonist action at mouse GPR35. Observations of marked variation in the pharmacology of species orthologs of GPCRs are well known but are of particular relevance in the early stages of receptor characterization. For example, the differences in ligand affinity at the orthologs of the histamine H4 receptor (Liu et al., 2001) even led to suggestions that there might be other receptors for histamine and further detailed pharmacological characterization of various orthologs of this receptor (Lim et al., 2010; Schnell et al., 2011), and the development of early-generation β3-adrenoceptor selective agonists was severely compromised by, at that time unappreciated, ortholog selectivity (de Souza and Burkey, 2001; Arch, 2008).
Reports of the identification of a pair of chemically distinct GPR35 antagonists with nanomolar potency at this receptor (Heynen-Genel et al., 2010; Zhao et al., 2010) also potentially offered an excellent avenue for exploring the biology and physiological function of GPR35. However, based on the issues with species selectivity of agonists at this receptor, we wanted to explore both the mode of action and any potential species selectivity of these ligands. Although we have been able to confirm that CID-2745687 and ML-145 are high-affinity antagonists and inverse agonists at human GPR35, they are both close to inactive at the rat and mouse orthologs. This means that they can be of no use in assessing the role of GPR35 in either cell or in vivo models based on these species. It is, therefore, most likely that the reported capacity of CID-2745687 to block pamoate-induced effects on writhing in a mouse model of pain (Zhao et al., 2010) reflects non-GPR35-mediated mechanisms for both the purported agonist and antagonist, although what the molecular target was in these studies remains unclear. Despite this, the activity of the antagonist ligands at human GPR35 should be of considerable use for assessing, for example, whether the reported ability of kynurenic acid to enhance interactions of human leukocytes with vascular endothelium under flow conditions (Barth et al., 2009) is truly a GPR35-mediated effect. Although short hairpin RNA-mediated silencing of GPR35 was certainly consistent with this, the high potency of kynurenic acid reported in experiments using human umbilical vein endothelial cells (Barth et al., 2009) was surprising given the low potency of this ligand noted at human GPR35 in in vitro assays (Jenkins et al., 2011; Deng et al., 2012).
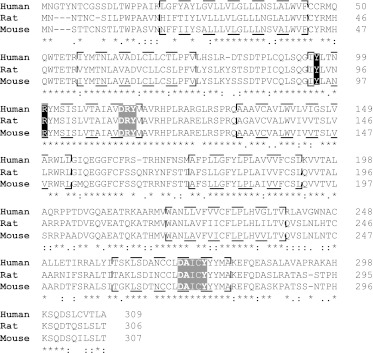
ML-145 acted as a competitive and surmountable antagonist against each of the three selected agonists at human GPR35, and this was also true for CID-2745687 against zaprinast and cromolyn disodium. Consistent with this, additivity studies using combinations of zaprinast and cromolyn disodium suggested that these two agonists bind to a common site. By contrast, CID-2745687 displayed noncompetitive inhibition of the effect of pamoate, suggesting that pamoate, which was clearly a partial agonist at human GPR35 in β-arrestin-2 interaction assays, may bind in a distinct manner. Furthermore, detailed analysis will be required to define this more fully. Because the endogenous ligands that activate GPR35 remain to be fully defined, then definition of the orthosteric binding pocket is challenging. However, previous mutagenesis studies have suggested a key role of arginine 3.36 (position 108 in the short isoform of human GPR35) (Milligan, 2011) (Fig. 13). Many of the reported GPR35 agonists, including the purported endogenous acid kynurenic acid and pamoate, have a carboxylate moiety that could provide an ionic interaction with the side chain of this residue. Alignment of the human, mouse, and rat orthologs of GPR35 shows the conversation of this residue (Fig. 13). However, because GPR35 is distant phylogenetically from any of the GPCRs for which atomic-level crystal structures are currently available, efforts to use homology modeling, for example, for understanding the variations in pharmacology between species remain extremely challenging.
Fig. 13.
Sequence alignment of human, mouse, and rat orthologs of GPR35. The sequence of the short isoform of human GPR35 is aligned with the mouse and rat orthologs. Asterisks indicate amino acid identity across these orthologs, and colons indicate conservative and full-stop semiconservative substitutions between species. Potential transmembrane domains are boxed. The DRY domain and the NPXXY region (light shading), two defining sequence elements of rhodopsin-like, class A GPCRs are highlighted, as are arginine (R) 3.36 and tyrosine (Y) 3.32 (dark shading). Mutation of either of these residues to alanine (Jenkins et al., 2011) eliminates the function of all GPR35 agonists for which this has been assessed (MacKenzie et al., 2011). As such, these residues probably contribute to the ligand binding pocket.
A potentially important point is that many of the efforts to identify novel GPR35 agonists have been based on G protein-independent assays. In part, this reflects that in both academic and industrial settings GPCR agonist screens based on interactions with a β-arrestin have become more dominant (Hamdan et al., 2005), because of a combination of the relative ease of performing such studies and the argument that they are more “universal” (Verkaar et al., 2008). In the case of poorly characterized GPCRs this has an abiding attraction if the G protein-coupling profile of the receptor is unknown. Specifically, in relation to GPR35, various studies have indicated roles for both pertussis toxin-sensitive Gi-family G proteins (Wang et al., 2006; Ohshiro et al., 2008; Barth et al., 2009) and Gα13 (Jenkins et al., 2010, 2011) in signal transduction. It was certainly possible that the structure-activity relationship of certain GPR35 agonists might be distinct in a G protein-dependent assay compared with a G protein-independent assay, a feature usually described as bias (DeWire et al., 2007; Luttrell and Kenakin, 2011). Chimeric G protein α subunits, in which the extreme C-terminal receptor recognition element of one G proteins is introduced into the sequence of a second G protein in which the effector recognition element provides an easy-to-automate functional assay (Coward et al., 1999; Milligan and Kostenis, 2006), have been a mainstay of industrial-level GPCR-ligand screening programs (Milligan and Rees, 1999; Milligan and Kostenis, 2006), particularly in allowing linkage to the elevation of intracellular Ca2+ (Emkey and Rankl, 2009). Although before these studies there was limited available information on whether chimeric G proteins could be used to study GPR35 or on potential ligand bias we now show clearly that in assays in which chimeric G proteins are used to couple the species orthologs of GPR35 to the production of inositol phosphates, the same basic pharmacology is observed as in the β-arrestin-2 recruitment assay, in that although pamoate is a potent activator of human GPR35 this is not the case at either the rat or mouse orthologs. Although the relatively high potency of GPR35 ligands in the inositol phosphate assays and the apparent full agonism of pamoate at the human receptor were useful in allowing detailed analysis of the selectivity of ligands between the species orthologs, these features may reflect receptor and G protein reserve because expression levels in such transient assays were not clearly defined. Furthermore, although restricted to studies on the human ortholog, it is noteworthy that Deng et al. (2012) recently noted that the rank order of potency of a group of previously characterized GPR35 agonists including pamoate, zaprinast, and cromolyn disodium was the same in both a GPR35-β-arrestin interaction assay and dynamic mass redistribution, a “label-free” approach that integrates signals from all G protein-mediated pathways (Schröder et al., 2011) in human HT-29 cells that express this receptor endogenously. Overall, these sets of studies suggest that at least for the ligands used herein it is unlikely that marked bias exists.
Although it is also possible that nonreceptor accessory proteins might alter the pharmacology of GPR35 in a species-dependent manner, the current studies re-emphasize the need to perform standard but insightful pharmacological analyses to fully understand both the potential and the potential limitations of novel ligands identified via various screens and their detailed mode of action. The current studies define that, despite their high affinity at human GPR35, neither CID-2745687 nor ML-145 are useful pharmacological antagonists for probing the functions of GPR35 in either mouse or rat models of physiology and disease, and pamoate is not a high-potency agonist at rodent orthologs of this receptor.
Acknowledgments
A.E.M. thanks the Biotechnology and Biosciences Research Council and J.E.L. thanks the British Heart Foundation for studentship support.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- GPCR
- G protein-coupled receptor
- BRET
- bioluminescence resonance energy transfer
- mBRET
- milliBRET
- eYFP
- enhanced yellow fluorescent protein
- FRT
- FLP recombination target
- HEK
- human embryonic kidney
- IP1
- inositol monophosphate
- PEI
- polyethylenimine
- CID-2745687
- methyl-5-[(tert-butylcarbamothioylhydrazinylidene)methyl]-1-(2,4-difluorophenyl)pyrazole-4-carboxylate
- ML-145
- 2-hydroxy-4-[4-(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]butanoylamino)benzoic acid
- TO
- tetracycline operator.
Authorship Contributions
Participated in research design: Jenkins, Harries, Lappin, Mackenzie, Neetoo-Isseljee, Southern, McIver, Nicklin, Taylor, and Milligan.
Conducted experiments: Jenkins, Harries, Lappin, Mackenzie, and Neetoo-Isseljee.
Contributed new reagents or analytic tools: Southern, McIver, and Taylor.
Performed data analysis: Jenkins, Harries, Lappin, Mackenzie, Nicklin, and Milligan.
Wrote or contributed to the writing of the manuscript: Jenkins, Harries, Lappin, MacKenzie, Neetoo-Isseljee, Southern, McIver, Nicklin, Taylor, and Milligan.
References
- Arch JR. (2008) The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from β3-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol 378:225–240 [DOI] [PubMed] [Google Scholar]
- Barth MC, Ahluwalia N, Anderson TJ, Hardy GJ, Sinha S, Alvarez-Cardona JA, Pruitt IE, Rhee EP, Colvin RA, Gerszten RE. (2009) Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem 284:19189–19195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosi C, Mannaioni G, Cozzi A, Carlà V, Sili M, Cavone L, Maratea D, Moroni F. (2011) G-protein coupled receptor 35 (GPR35) activation and inflammatory pain: Studies on the antinociceptive effects of kynurenic acid and zaprinast. Neuropharmacology 60:1227–1231 [DOI] [PubMed] [Google Scholar]
- Coward P, Chan SD, Wada HG, Humphries GM, Conklin BR. (1999) Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal Biochem 270:242–248 [DOI] [PubMed] [Google Scholar]
- Deng H, Fang Y. (2012) Aspirin metabolites are GPR35 agonists. Naunyn Schmiedebergs Arch Pharmacol 385:729–737 [DOI] [PubMed] [Google Scholar]
- Deng H, Hu H, Fang Y. (2011) Tyrphostin analogs are GPR35 agonists. FEBS Lett 585:1957–1962 [DOI] [PubMed] [Google Scholar]
- Deng H, Hu H, Fang Y. (2012) Multiple tyrosine metabolites are GPR35 agonists. Sci Rep 2:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza CJ, Burkey BF. (2001) β3-Adrenoceptor agonists as anti-diabetic and anti-obesity drugs in humans. Curr Pharm Des 7:1433–1449 [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. (2007) β-Arrestins and cell signaling. Annu Rev Physiol 69:483–510 [DOI] [PubMed] [Google Scholar]
- Emkey R, Rankl NB. (2009) Screening G protein-coupled receptors: measurement of intracellular calcium using the fluorometric imaging plate reader. Methods Mol Biol 565:145–158 [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Audet M, Garneau P, Pelletier J, Bouvier M. (2005) High-throughput screening of G protein-coupled receptor antagonists using a bioluminescence resonance energy transfer 1-based β-arrestin2 recruitment assay. J Biomol Screen 10:463–475 [DOI] [PubMed] [Google Scholar]
- Heynen-Genel S, Dahl R, Shi S, Sauer M, Hariharan S, Sergienko E, Dad S, Chung TDY, Stonich D, Su Y, et al. (2010) Selective GPR35 Antagonists: Probes 1 and 2. Probe Reports from the NIH Molecular Libraries Program, Bethesda, MD. National Center for Biotechnology Information, Bethesda, MD: [updated Oct 4 2010] [PubMed] [Google Scholar]
- Hu H, Deng H, Fang Y. (2012) Label-free phenotypic profiling identified d-luciferin as a GPR35 agonist. PLoS One 7:e34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L, Alvarez-Curto E, Campbell K, de Munnik S, Canals M, Schlyer S, Milligan G. (2011) Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα and β-arrestin-2. Br J Pharmacol 162:733–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L, Brea J, Smith NJ, Hudson BD, Reilly G, Bryant NJ, Castro M, Loza MI, Milligan G. (2010) Identification of novel species-selective agonists of the G-protein-coupled receptor GPR35 that promote recruitment of β-arrestin-2 and activate Gα13. Biochem J 432:451–459 [DOI] [PubMed] [Google Scholar]
- Kostenis E, Waelbroeck M, Milligan G. (2005) Techniques: promiscuous Gα proteins in basic research and drug discovery. Trends Pharmacol Sci 26:595–602 [DOI] [PubMed] [Google Scholar]
- Lim HD, de Graaf C, Jiang W, Sadek P, McGovern PM, Istyastono EP, Bakker RA, de Esch IJ, Thurmond RL, Leurs R. (2010) Molecular determinants of ligand binding to H4R species variants. Mol Pharmacol 77:734–743 [DOI] [PubMed] [Google Scholar]
- Liu C, Wilson SJ, Kuei C, Lovenberg TW. (2001) Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther 299:121–130 [PubMed] [Google Scholar]
- Luttrell LM, Kenakin TP. (2011) Refining efficacy: allosterism and bias in G protein-coupled receptor signaling. Methods Mol Biol 756:3–35 [DOI] [PubMed] [Google Scholar]
- MacKenzie AE, Lappin JE, Taylor DL, Nicklin SA, Milligan G. (2011) GPR35 as a novel therapeutic target. Front Endocrinol (Lausanne) 2:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. (2011) Orthologue selectivity and ligand bias: translating the pharmacology of GPR35. Trends Pharmacol Sci 32:317–325 [DOI] [PubMed] [Google Scholar]
- Milligan G, Kostenis E. (2006) Heterotrimeric G-proteins: a short history. Br J Pharmacol 147 (Suppl 1):S46–S55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Rees S. (1999) Chimaeric Gα proteins: their potential use in drug discovery. Trends Pharmacol Sci 20:118–124 [DOI] [PubMed] [Google Scholar]
- Min KD, Asakura M, Liao Y, Nakamaru K, Okazaki H, Takahashi T, Fujimoto K, Ito S, Takahashi A, Asanuma H, et al. (2010) Identification of genes related to heart failure using global gene expression profiling of human failing myocardium. Biochem Biophys Res Commun 393:55–60 [DOI] [PubMed] [Google Scholar]
- Ohshiro H, Tonai-Kachi H, Ichikawa K. (2008) GPR35 is a functional receptor in rat dorsal root ganglion neurons. Biochem Biophys Res Commun 365:344–348 [DOI] [PubMed] [Google Scholar]
- Oka S, Ota R, Shima M, Yamashita A, Sugiura T. (2010) GPR35 is a novel lysophosphatidic acid receptor. Biochem Biophys Res Commun 395:232–237 [DOI] [PubMed] [Google Scholar]
- Schnell D, Brunskole I, Ladova K, Schneider EH, Igel P, Dove S, Buschauer A, Seifert R. (2011) Expression and functional properties of canine, rat, and murine histamine H4 receptors in Sf9 insect cells. Naunyn Schmiedebergs Arch Pharmacol 383:457–470 [DOI] [PubMed] [Google Scholar]
- Schröder R, Schmidt J, Blättermann S, Peters L, Janssen N, Grundmann M, Seemann W, Kaufel D, Merten N, Drewke C, et al. (2011) Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors non-invasively in living cells. Nat Protoc 6:1748–1760 [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Tonai-Kachi H, Shinjo K. (2006) Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett 580:5003–5008 [DOI] [PubMed] [Google Scholar]
- Verkaar F, van Rosmalen JW, Blomenröhr M, van Koppen CJ, Blankesteijn WM, Smits JF, Zaman GJ. (2008) G protein-independent cell-based assays for drug discovery on seven-transmembrane receptors. Biotechnol Annu Rev 14:253–274 [DOI] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. (2006) Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 281:22021–22028 [DOI] [PubMed] [Google Scholar]
- Yang Y, Fu A, Wu X, Reagan JD. (2012) GPR35 is a target of the loop diuretic drugs bumetanide and furosemide. Pharmacology 89:13–17 [DOI] [PubMed] [Google Scholar]
- Yang Y, Lu JY, Wu X, Summer S, Whoriskey J, Saris C, Reagan JD. (2010) G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 86:1–5 [DOI] [PubMed] [Google Scholar]
- Zhao P, Sharir H, Kapur A, Cowan A, Geller EB, Adler MW, Seltzman HH, Reggio PH, Heynen-Genel S, Sauer M, et al. (2010) Targeting of the orphan receptor GPR35 by pamoic acid: a potent activator of extracellular signal-regulated kinase and β-arrestin2 with antinociceptive activity. Mol Pharmacol 78:560–568 [DOI] [PMC free article] [PubMed] [Google Scholar]