Abstract
Selegiline, the R-enantiomer of deprenyl, is used in the treatment of Parkinson's disease. Bupropion, an antidepressant, often used to treat patients in conjunction with selegiline, is metabolized primarily by CYP2B6. The effect of selegiline on the enzymatic activity of human cytochrome CYP2B6 in a reconstituted system and its effect on the metabolism of bupropion were examined. Selegiline was found to be a mechanism-based inactivator of the 7-ethoxy-4-(trifluoromethyl)coumarin O-deethylation (7-EFC) activity of CYP2B6 as well as bupropion metabolism. The inactivations were time-, concentration-, and NADPH-dependent and were characterized by KI values of 0.14 and 0.6 μM, kinact values of 0.022 and 0.029 min−1, and t1/2 values of 31.5 and 24 min, respectively. In standard inhibition assays, selegiline increased the Km of CYP2B6 for bupropion from 10 to 92 μM and decreased the kcat by ∼50%. The reduced carbon-monoxide difference spectrum revealed over a 50% loss in the cytochrome P450 spectrum in the inactivated sample, with no loss in heme, and there was ∼70% loss in enzyme activity. Trapping of the reactive metabolite using GSH led to the identification of a GSH-selegiline conjugate with a m/z 528 that could be explained by hydroxylation of selegiline followed by the addition of glutathione to the propargyl moiety after oxygenation to form the ketene intermediate. Liquid chromatography-tandem mass spectrometry analysis of the labeled protein following digestion with trypsin revealed the peptide 64DVFTVHLGPR73 as the peptide modified by the reactive metabolite of selegiline and the site of adduct formation is Asp64.
Introduction
The cytochrome P450 (P450) enzymes are a superfamily of oxidative enzymes that are involved in the metabolism of a number of structurally diverse xenobiotics. In humans, total hepatic CYP2B6 content ranges from 2 to 10% of total P450 content (Stresser and Kupfer, 1999). The enzyme is responsible for the metabolism of approximately 3 to 8% of marketed pharmaceuticals, including bupropion, efavirenz, and cyclophosphamide (Chang et al., 1993; Faucette et al., 2000; Hesse et al., 2000; Miksys et al., 2003). To determine the role of specific P450s in the clearance of various drugs and to prevent drug-drug interactions caused by the inhibition of metabolic clearance, the prediction and identification of compounds that act as mechanism-based inactivators have become an important factor in the drug discovery process. Mechanism-based inactivation has also been used to study the structure-function relationship of individual P450 proteins because the active site residues on the apoprotein modified by the reactive species can be identified and the mechanisms by which activity is lost can be determined (Hollenberg et al., 2008).
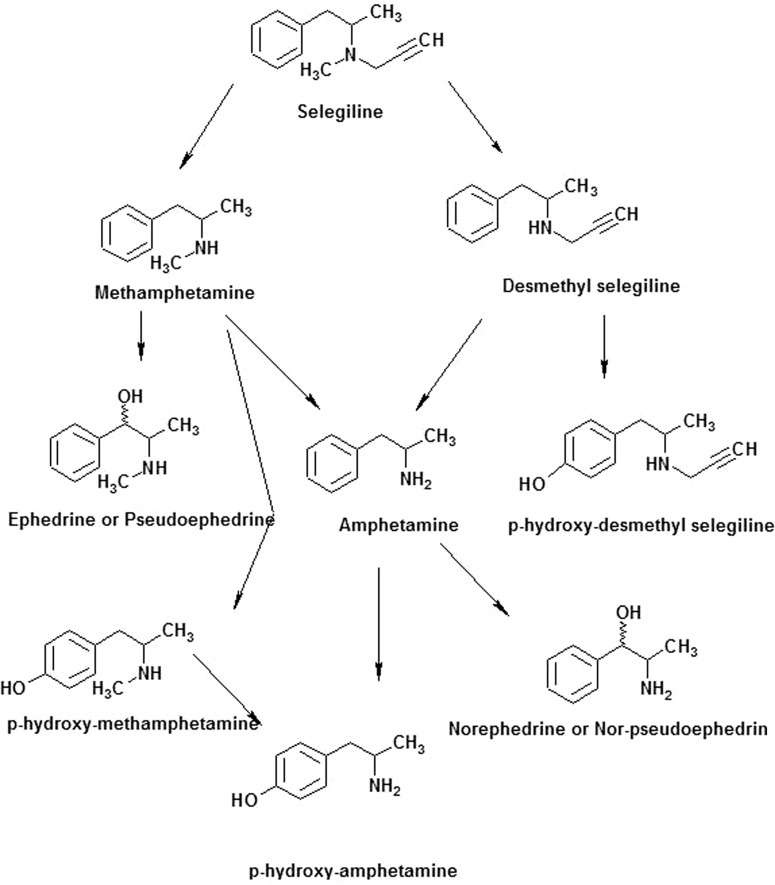
Selegiline, [(−)-(R)-N,α-dimethyl-N-2-propynylphenethylamine hydrochloride)], is an optically active compound where the isomers exhibit different pharmacological activities. The R(−)-isomer of selegiline, also called deprenyl, is used clinically for the treatment of patients with Parkinson's disease. The efficacy of the d(+)-isomer is only 1/150 that of the R-isomer. The half-life of selegiline in humans is ∼0.15 h, and it is quickly metabolized to give desmethylselegiline and methamphetamine, both of which can be further metabolized to amphetamine as shown in Fig. 1 (Gerlach et al., 1996). Only R(−) derivatives have been identified in the urine, plasma, and tissue samples of animals treated with selegiline, indicating that no racemization or inversion happened during metabolism (Magyar et al., 1996). Selegiline is also a suicide inhibitor of MAO-B resulting in irreversible inhibition of the enzyme. Selegiline first forms a reversible noncovalent complex with MAO-B, and subsequent oxidation by MAO-B leads to formation of a covalent bond with the FAD cofactor of the enzyme (Gerlach et al., 1996). Selegiline is used alone or in combination with levodopa in the treatment of Parkinson's disease. Levodopa is converted to dopamine, a natural substance that is needed to control movement, thus rendering a therapeutic effect. The dopamine is further metabolized by MAO-B, and selegiline prolongs the anti-Parkinson effects of levodopa treatment by inhibiting MAO-B. The products of metabolism of selegiline are amphetamine, N-methylamphetamine, and N-propargylamphetamine. The dealkylated metabolites may undergo further hydroxylation at the para-position of the benzene ring and on the alkyl group at the β-position as shown in Fig. 1.
Fig. 1.
Major pathways for the metabolism of selegiline.
Previous metabolism studies performed in vitro have shown that CYP2B6 and CYP2C19 are the major enzymes responsible for the metabolism of selegiline in human liver (Hidestrand et al., 2001; Benetton et al., 2007). The involvement of CYP3A4 and CYP1A2 in selegiline metabolism in humans has also been suggested by Taavitsainen et al. (2000). We have previously demonstrated that selegiline is a mechanism-based inactivator of rat CYP2B1. Kinetic parameters for the inactivation of CYP2B1 were determined. Our studies demonstrated that inactivation occurred because of protein destruction rather than heme damage (Sharma et al., 1996). Selegiline has been shown to be a mechanism-based inactivator of CYP2A6 and inhibits the metabolism of nicotine in humans and mice (Siu and Tyndale, 2008).
Bupropion is an antidepressant drug that is used for the treatment of patients with Parkinson's disease. Clearance of bupropion occurs through hydroxylation and CYP2B6 has been identified as the major enzyme catalyzing this metabolic reaction (Ascher et al., 1995). Limited studies have been performed concerning the impact of bupropion use in patients with Parkinson's disease given the wide use of this drug as a pharmacotherapy in people with Parkinson's disease. In this study, we have investigated the metabolism of bupropion in the presence and absence of selegiline. During these studies, we found that selegiline is also a mechanism-based inactivator of CYP2B6. The study provides evidence for the formation of a ketene intermediate during P450-mediated bioactivation of selegiline that could be trapped in the presence of GSH in the reconstituted system. LC-MS studies led to identification of an adduct that revealed the mass of the reactive intermediate responsible for the loss of the enzymatic activity. Trypsin digestion of the selegiline-modified protein revealed that selegiline is first hydroxylated by CYP2B6, and then it binds covalently to the Asp64 of the P450 leading to its inactivation. The mechanism-based inactivation of CYP2B6 by selegiline may have significant neuropsychopharmacological implications for patients with Parkinson's disease being treated concomitantly with bupropion or other drugs metabolized primarily by CYP2B6.
Materials and Methods
Materials.
Selegiline, catalase, GSH, urea, and NADPH were obtained from Sigma-Aldrich (St. Louis, MO). 7-Ethoxy-4-(trifluoromethyl)coumarin (7-EFC) was obtained from Invitrogen (Carlsbad, CA). Trypsin was obtained from Promega (Madison, WI). All other chemicals were of highest quality available from commercial sources. Amphetamine and methamphetamine were generously donated by Dr. James H. Woods (Department of Pharmacology, University of Michigan).
Protein Purification.
CYP2B6 was expressed in Escherichia coli C41 DE3 cells and purified according to published protocols (Hanna et al., 2000). P450 reductase was purified as described previously (Hanna et al., 1998).
Inhibition of Bupropion Metabolism by Selegiline.
CYP2B6 was reconstituted with reductase at a ratio of 1:2 on ice for 45 min, with a final concentration of 1 μM P450 and 2 μM reductase. The reconstituted mixture was then diluted to a volume of 3 ml with 0.5 M potassium phosphate buffer, pH 7.4, catalase (100 units), and water. The mixture was then divided into two portions. One portion received selegiline (12 μM), and the other did not. Both of them were then measured in aliquots, and the samples received increasing concentrations of bupropion (40–400 μM). NADPH was added to initiate the reactions at a final concentration of 1.2 mM, and the reaction mixtures were incubated at 30°C for 30 min. The methods for incubating and determining product concentrations were followed as described in by Bumpus et al. (2005). Nonlinear regression analyses of the data were performed using Prism version 5.00 (GraphPad Software Inc., San Diego, CA).
Inactivation of CYP2B6 by Selegiline.
CYP2B6 (0.5 nmol) was reconstituted with reductase (1 nmol) on ice for 45 min with a final concentration of 0.8 μM P450 and 0.16 μM reductase. The reconstituted mixture was diluted to a volume of 500 μl with 0.5 M potassium phosphate buffer, pH 7.4, catalase (100 units), and water. The samples received increasing concentrations of selegiline (0.1 to 10 μM), with water added to the control samples, and they were then equilibrated at 30°C for 5 min. NADPH was added at a final concentration of 1.2 mM to initiate the reactions. At 0, 2, 5, 10, and 14 min, 9-pmol aliquots of the sample were transferred into secondary mixtures containing 100 μM 7-EFC, 1 mM NADPH, and 40 μg/ml bovine serum albumin in 50 mM potassium phosphate buffer, pH 7.4. The secondary reactions were incubated for 10 min and stopped by the addition of 334 μl of acetonitrile. The amount of 7-hydroxy-(4-trifluoromethyl)coumarin formed was measured using a Victor II fluorescence plate reader (PerkinElmer Life and Analytical Sciences, Waltham, MA), with excitation and emission wavelengths of 405 and 505 nm, respectively. The kinetic constants were calculated by linear regression using Prism (version 5; GraphPad Software Inc.). The addition of bovine serum albumin was based on the studies of Buters et al., 1993. For the inactivation of the CYP2B6-catalyzed metabolism of bupropion by selegiline, 5 nmol of CYP2B6 was reconstituted with 10 nmol of reductase to give a final concentration of 0.9 μM P450 and 0.18 μM reductase. The samples received increasing concentrations of selegiline (0.1–25 μM) and were equilibrated at 37°C for 5 min before the initiation of the inactivation reaction by the addition of NADPH. At 0, 2, 5, 8, and 10 min, 212-pmol aliquots of the sample were transferred into the secondary solution containing 200 μM bupropion, 50 mM potassium phosphate buffer, pH 7.4, 100 units of catalase, and 1.2 mM NADPH. The reaction mixtures were incubated at 37°C for 30 min. The methods for the incubation and determination of product formation were followed as described by Bumpus et al. (2005). Linear regression analyses of the data were performed using Prism version 5.0 (GraphPad Software Inc.).
Spectrophotometric and HPLC Analysis of Heme Remaining.
Reconstitution and inactivation of P4502B6 by selegiline were carried out as described above. After a-15 min incubation, a portion of the reconstituted mixture (control and inactivated samples) was injected into a C4 column (250 × 4.6 mm; Phenomenex, Torrance, CA) equilibrated with 30% acetonitrile containing 0.1% trifluoroacetic acid. The mobile phase consisted of solvent A (0.1% trifluoroacetic acid in water) and solvent B (0.1% trifluoroacetic acid in acetonitrile). The HPLC system was equipped with a Shimadzu Detector (SPA-M20A), Shimadzu pumps (LC 20 AB), and a Shimadzu autosampler (SIL-20 AC HT; Shimadzu, Kyoto, Japan). The samples were resolved using a linear gradient from 30% to 90% B over a 45-min period at a flow rate of 1 ml/min. The effluent was monitored at 405 nm for intact heme. The remaining portions of the samples were used to determine the reduced CO spectra. The samples were bubbled with CO, sodium dithionite was added, and the reduced CO spectra were recorded between 400 and 500 nm on a DW2 UV-visible spectrophotometer (SLM Aminco, Urbana, IL) equipped with an OLIS operating system (On-Line Instrument System, Inc., Bogart, GA). The absorbance difference between 450 and 490 nm was used to assess the extent of CO binding and heme remaining in the P450 samples.
Metabolite Identification by LC-ESI/MS.
Metabolite identification was accomplished by LC-ESI/MS. The wild-type and mutant P450 enzymes were reconstituted with reductase (1:2) and catalase and diluted to 550 μl with 0.5 M potassium phosphate buffer, pH 7.4, as described above. Each enzyme mixture was divided into two portions that received either 50 μM selegiline and water (control) or selegiline and NADPH. The samples were incubated at 30°C for 30 min. Two hundred and fifty microliter aliquots of 8% (w/v) sodium bicarbonate, pH 10, and 50 μl of acetic acid were added after 30 min to stop the reactions. The reactions were extracted twice with 2 ml of ethyl acetate. The organic phases were pooled, and 2 μl of dimethyl sulfoxide were added to each sample to facilitate the extraction of amine products. The samples were then dried under nitrogen and redissolved in 50% acetonitrile. The selegiline metabolites were separated using LC-ESI/MS on a LCQ classic Thermoquest mass spectrometer (Thermo Fisher Scientific, Waltham, MA) coupled to a Hewlett Packard 1100 series HPLC system. Metabolite separation was accomplished using a C8 reverse phase column (Zorbax 5 μm, 4.6 × 250 mm; DuPont, Wilmington, DE). The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), with a flow rate of 0.3 ml/min. Initial conditions were 30% B followed by a linear increase to 90% B in 20 min and then held at 90% B for 10 min before returning to equilibration conditions. The mass analyzer conditions were as follows: sheath gas (100 arbitrary units), auxiliary gas (40 arbitrary units), spray voltage (3 kV), capillary temperature (230°C), and capillary voltage (3.5 V).
In Vitro Trapping of the Reactive Metabolite with GSH.
The reaction mixture for trapping and identifying the reactive intermediate contained 0.25 nmol of recombinant purified CYP2B6 and 0.5 nmol of reductase, 50 units of catalase, and 10 mM GSH in 50 mM potassium phosphate buffer, pH 7.4, in a final volume of 200 μl. The final concentration of selegiline was 25 μM. The reaction was initiated with 1.2 mM NADPH, with control samples receiving the same volume of water. The reaction samples were incubated at 37°C for 45 min, and the reactions were terminated by the addition of 5 volumes of acetonitrile. The reaction mixtures were centrifuged at 13,200g at room temperature for 10 min. The supernatants were transferred to new tubes and dried under a stream of nitrogen gas. The residues were suspended in 50% acetonitrile in water and injected onto the LC/MS for further analysis. HPLC separation of the GSH adducts of selegiline was carried out using a Phenomenex Luna C18 reverse phase column (4.6 × 100 mm; Phenomenex). The flow rate was 300 μl/min. For HPLC-ESI-MS/MS analysis, initial conditions were 95% of 0.1% (v/v) acetic acid in water (solvent A) and 5% of 0.1% acetic acid in acetonitrile (solvent B). The percentage of B was maintained for 5 min followed by linear gradient to 30% B from 5 to 15 min, to 80% B from 15 to 35 min, and to 90 from 35 to 40 min. The column was returned to initial conditions and equilibrated for 10 min before the next injection. ESI-MS/MS was carried out using a Thermoquest LCQ ion trap mass spectrometer (Thermo Fisher Scientific) interfaced with a Hewlett Packard 1100 series HPLC system (Hewlett Packard, Palo Alto, CA). The sheath gas was set at 80 arbitrary units, and the auxiliary gas was set at 15 arbitrary units. The spray voltage was 5 kV, and the capillary temperature was 180°C. Data were acquired in the positive ion mode using the Xcalibur software (Thermoquest Corporation, Manchester, UK), with two data dependent scans on the first two most intense ions.
Identification of the Selegiline Modified Site on the P4502B6 Apoprotein.
The protein mixture contained 1 nmol of P4502B6 and 2 nmol of reductase in a volume of 500 μl. The final concentration of selegiline was 12 μM. Control samples were incubated with selegiline in the absence of NADPH. The inactivation of P4502B6 was carried out as described above. After incubation for 30 min at 37°C, the control and the selegiline-inactivated samples were denatured by adding urea to give a final concentration of 8 M and then incubated at 55°C for 30 min followed by dilution with 50 mM ammonium bicarbonate to a final volume of 7 ml (to reduce the concentration of urea). The samples were then concentrated using Amicon Ultracentrifuge filter devices (Millipore Corporation, Billerica, MA) at 4°C. The sample volumes were concentrated to 75 μl. The samples were subsequently reduced with 5 mM dithiothreitol at 60°C for 30 min. Trypsin digestion was carried out using a 1:20 trypsin/protein ratio. The samples were incubated overnight in a shaking water bath at 37°C. The reactions were terminated by addition of 1 μl of 10% trifluoroacetic acid, and the digested samples were centrifuged at 13,000g in an Eppendorf centrifuge (Eppendorf North America, New York, NY) for 10 min. The supernatants were used for a LC-MS/MS analysis.
The LC/MS analysis was performed using a Shimadzu LCQ-Deca XP ion-trap mass spectrometer equipped with a Shimadzu LC-10AD HPLC system (Shimadzu). Chromatographic separation of the tryptic peptide samples was carried out using a C18 column (Jupiter 150 × 2.00 mm, 5 micron; Phenomenex). The mobile phase consisted of 0.025% trifluoroacetic acid, 0.025% formic acid in water (solvent A) and 0.025% trifluoroacetic acid, and 0.025% formic acid in acetonitrile (solvent B). The flow rate was 0.3 ml/min. Initial conditions were 10% B, and the proportion of solvent B was increased from 10% B to 35% B over 45 min and then to 90% B over 90 min. The column was washed with 90% B for 10 min before returning to the initial conditions and equilibrating the column for 15 min at the initial conditions. The instrument was operated in positive electrospray ionization mode with the following parameters: activation time of 30 ms, activation Q of 0.3, and the normalized collision set at 35%. The dynamic exclusion width was set at 1.5. The mass spectrometer was operated with the capillary temperature of 210°C and a capillary voltage of 2 V. The analysis was performed in the data-dependent acquisition mode in which a full scan was recorded over the mass range of 150 to 2000 followed by collision-induced dissociation of the six most abundant ions. Data acquisition and analysis were performed using both Xcalibur software (version 1.2; Thermoquest) and SEQUEST Bioworks (Thermo Electron).
The peptides were identified automatically using the SEQUEST BioWorks computer program, which correlated the experimental tandem mass spectra with the theoretical tandem mass spectra from the amino acid sequences for the 2B6 peptides obtained from the National Center for Biotechnology Information sequence database. The following criteria were applied for filtering the results: peptides were identified on the basis of XCorr values, which were set at 2.0, 2.2, and 3.75 for single-, double-, or triple-charged ions, respectively, and Sp values >400 and 600 for singly and doubly charged ions. XCorr is the cross correlation score between the actual and the predicted MS/MS spectrum. The Sp value indicates the preliminary score of the peptide and shows the number of fragment ions matched out of the total number of fragment masses for the peptide. Peptides that had a probability score <1.0 × 10−4 were not considered, and sequence assignments were based on selecting the peptide that displayed a ΔCn greater than 0.1 (ΔCn is the relative difference between the correlation score for the top matching peptide and the second best matching peptide). In addition, manual inspection of the peptide identifications from the MS/MS spectrum was performed to ensure that the major MS/MS fragmentation peaks matched the theoretical peaks.
Results
Inhibition of Bupropion Metabolism by Selegiline.
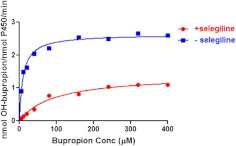
Figure 2 shows the rates of formation of hydroxybupropion by CYP2B6 in the presence and absence of selegiline as a function of increasing concentrations of bupropion. Hydroxybupropion formation was measured by integrating the area under the peak and comparing to a standard curve generated by injecting different amounts of authentic hydroxybupropion on the HPLC column. The Km of the wild-type enzyme for bupropion metabolism (10 μM) is identical to the previously reported value of 10 μM (Bumpus et al., 2005). In the presence of 12 μM selegiline, the Km value increased by 9-fold to 92 μM. Selegiline also decreased the kcat value for bupropion by 50% from 2.6 to 1.30 nmol hydroxybupropion/nmol P450/min. The change in the apparent Km value indicates that selegiline decreases the apparent affinity of CYP2B6 for bupropion, whereas the decrease in the kcat is probably the result of irreversible mechanism-based inactivation of bupropion hydroxylation activity (Fig. 3B) by selegiline that could not be overcome by high concentrations of bupropion. With respect to the catalytic efficiency, the presence of 12 μM selegiline decreased the kcat/km ratio by 19-fold.
Fig. 2.
Kinetics for the hydroxylation of bupropion by CYP2B6 in the presence and absence of selegiline. Samples were reconstituted with (12 μM) or without the addition of selegiline and incubated with bupropion ranging in concentration (Conc) from 40 to 400 μM. HPLC analysis and quantitation were performed as described under Materials and Methods. The data represent the means ± S.D. from two different experiments with duplicate samples.
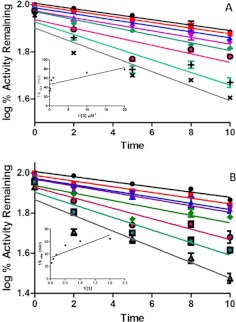
Fig. 3.
Time- and concentration-dependent inactivation of CYP2B6 by selegiline. A, purified recombinant CYP2B6 reconstituted with reductase was incubated with various concentrations of selegiline and NADPH (1.2 mM) at 30°C. Aliquots of the mixture were taken at the time intervals indicated and added to a secondary mixture for the determination of residual 7-EFC O-deethylation activity as described under Materials and Methods. The concentrations of selegiline used were 0 (●), 0.01 (■), 0.05 (▴) 0.1 (▾), 1 (♦), 2 (○), 5 (+), and 10 μM (×) done in duplicate. The data show the mean ± S.D. from three different experiments. The inset shows the rate for the time-dependent decrease in the 7-HFC formed at the various concentration of selegiline. B, the reaction mixtures were incubated without (●) or with 0.1 (■), 0.5 (▴), 1 (▾), 1 (♦), 5 (○), 10 (□), and 25 μM (▵) selegiline for the times indicated in the presence of NADPH, and then aliquots were removed and assayed for the formation hydroxybupropion as described under Materials and Methods. The inset shows the double-reciprocal plot for the initial rate of inactivation of hydroxybupropion formation as a function of the concentration of inactivator. The data shown represent the mean ± S.E. from three experiments.
Inactivation of P4502B6 by Selegiline.
In the presence of selegiline, a time-, concentration-, and NADPH-dependent inactivation of the 7-EFC O-deethylation activity of CYP2B6 was observed in the reconstituted system. Pseudo-first-order kinetics were observed for concentrations ranging from 0.1 to 10 μM as seen in Fig. 3A. The kinetic constants were determined from the double-reciprocal plot of the inverse of the initial rate of inactivation as a function of the reciprocal of the selegiline concentration (Fig. 3A, inset). Under our current experimental conditions, the kinetic constants describing the inactivation of CYP2B6 were characterized by a KI of 0.14 μM, a kinact of 0.022 min−1, and the t1/2 for inactivation of 31.5 min. A significant decrease in the 0 time activity was observed with increasing selegiline concentrations, suggesting that selegiline inhibits 7-EFC O-deethylase activity in a competitive manner. Selegiline was also a mechanism-based inactivator with respect to bupropion metabolism as shown in Fig. 3B. The kinetic constants describing this inactivation were characterized by a KI of 0.6 μM, kinact of 0.029, and t1/2 of 24 min.
Spectrophotometric and HPLC Analysis of Heme Remaining.
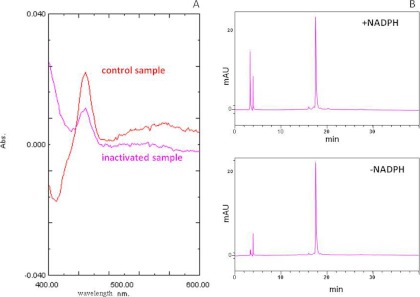
The 7-EFC O-deethylation activity of CYP2B6 in the reconstituted system containing purified CYP2B6 and NADPH-reductase decreased by 70% after incubation with 10 μM selegiline for 20 min in the presence of NADPH. As shown in Fig. 4A, the loss in activity was accompanied by a 30% loss in the reduced CO spectrum of the enzyme. The red line is for the control sample following reduction by sodium dithionite in the presence of CO, which is higher than the pink line, which represents the inactivated sample in the presence of CO. Evidence that the heme moiety itself was neither modified nor destroyed was obtained from reverse-phase HPLC analysis where the heme was monitored at 405 nm (Fig. 4B). Virtually no differences in the retention times or areas of the heme peaks were observed between the control and selegiline-inactivated CYP2B6. The loss in the reduced CO spectrum, with no loss in total heme as measured by HPLC, suggests that binding of the selegiline or its metabolite causes a change in the conformation of the protein and thus may affect the binding of CO to the reduced P450. Similar changes in reduced CO binding, with no loss in heme, have been seen before with other mechanism-based inactivators.
Fig. 4.
Effect of selegiline on the reduced CO spectrum and the heme remaining as measured by HPLC. A, portions of the control and selegiline inactivated samples were bubbled with CO in the presence of dithionite, and the reduced CO spectra were monitored between 400 and 500 nm on a spectrophotometer. Abs, absorbance. B, the control and selegiline inactivated CYP2B6 samples were injected onto a C4 column, and the areas under the heme peaks at 405 nm were integrated to calculate the heme loss. The inactivations and analyses were performed as described under Materials and Methods.
Metabolite Identification by LC-ESI/MS.
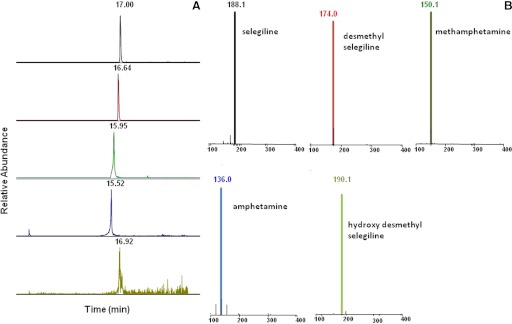
Metabolism of selegiline by CYP2B6 was assessed in a reconstituted system. LC-ESI was used to determine the metabolites formed by CYP2B6 when selegiline was incubated with the P450 in the presence of NADPH. The LC-ESI mass spectra of the individual [MH+] ions are shown in Fig. 5. Figure 5A shows the extracted ion chromatograms for the parent and the various metabolites, and Fig. 5B shows the MS spectrum of the associated [MH+] m/z spectra of the respective metabolites generated by the metabolism of selegiline when incubated with CYP2B6. Unchanged selegiline eluted at 17 min, with a protonated molecular ion [MH+] at m/z 188. The mass spectrum of the MH+ ion at m/z 188 gave fragment ions at m/z 119 and 91 (data not shown). The peaks at 16.64 and 15.95 min gave molecular ions at m/z 174 and 150, indicating that selegiline undergoes N-demethylation to yield N-desmethylselegiline (m/z 174) and N-depropargylation to methamphetamine (m/z 150). Both metabolites then undergo further demethylation to yield amphetamine with an m/z of 136 and with a retention time of 15.52 min. Small amounts of some secondary metabolite were also detected, eluting at 16.92 min, with an m/z of 190, which is p-hydroxydesmethylselegiline (data not shown).
Fig. 5.
LC-MS results for the metabolism of selegiline incubated with CYP2B6 in the reconstituted system. Details of the incubation and analytical conditions are described under Materials and Methods. The metabolites formed by CYP2B6 were separated by LC-ESI. A, the extracted ion chromatograms for the peaks eluting at different time points for the parent and the products that include methamphetamine, amphetamine, N-desmethylamphetamine, and hydroxyl-desmethylselegiline. B, the MS spectra for the parent and the product peaks.
In Vitro Trapping of the Reactive Metabolite with GSH.
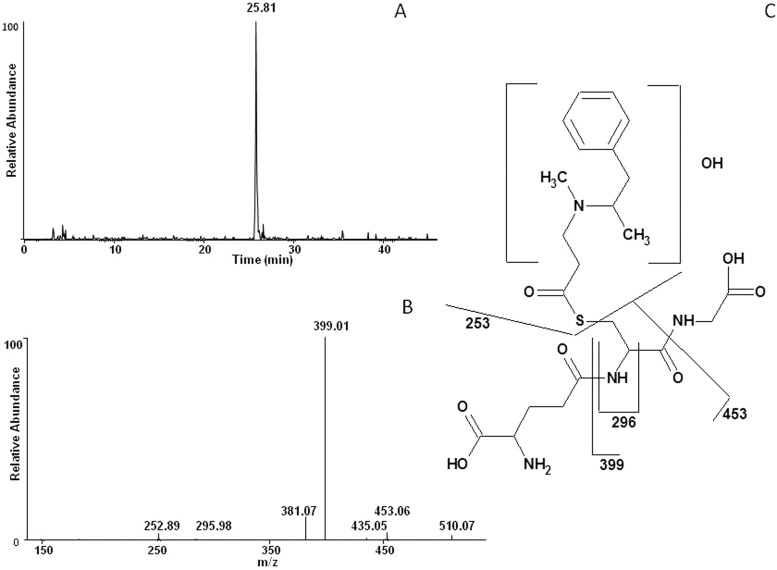
The electrophilic reactive metabolite generated by incubating selegiline with CYP2B6 in the reconstituted system in the presence of NADPH was trapped using the nucleophilic trapping reagent GSH and analyzed by LC/MS. The GSH adduct exhibited a molecular ion at m/z 528, which corresponds with the addition of the glutathionyl moiety (307 amu) and two oxygen atoms to the parent compound (221 + 307). Figure 6A shows the extracted ion chromatogram from a reaction mixture in which reconstituted CYP2B6 was incubated with selegiline in the presence of NADPH and GSH. The peak eluting at approximately 25.81 min with an m/z of 528 was seen only in samples with NADPH. The peak with m/z 528 corresponds to a GSH adduct generated by trapping an electrophilic intermediate of selegiline formed by CYP2B6. Figure 6B shows the MS/MS fragmentation for the ion with m/z 528. Loss of glycine (−75 amu) and pyroglutamate (−129 amu) residues from the parent ion led to fragment ions at m/z 453 and 399, respectively. This characteristic loss of glycine and pyroglutamate residues is diagnostic in confirming the formation of GSH adducts. The fragment ion with m/z 435 is from the loss of the glycyl moiety + water. The product ions at m/z 510 and 381 are from the loss of water from the adducted GSH adduct and the 399 fragment ion. The fragment at m/z 296 is from the combined loss of glycine + glutamate + CO. The ion at m/z 253 is derived from cleavage of the carbon-sulfur bond between the GSH moiety and the hydroxylated selegiline leading to the retention of sulfur on the hydroxylated selegiline. The exact position where the hydroxylation occurs could not be determined from the MS/MS spectrum. Furthermore, whether the oxygen added to the triple bond is transferred to the terminal or the internal carbon atom could not be determined from the fragmentation pattern. It has been reported that insertion of an oxygen atom at the internal or terminal acetylenic carbon results in heme or protein modification, respectively (Ortiz de Montellano and Komives, 1985). Because we do not see heme loss, the insertion of oxygen possibly occurs at the terminal carbon. The fragmentation pattern for the major ions observed in the MS/MS spectrum of the GSH-selegiline adduct is shown in Fig. 6C. Control incubations using only the inhibitor in the absence of NADPH did not show the formation of this adduct.
Fig. 6.
LC-MS/MS analysis of the GSH adduct obtained when selegiline was incubated with CYP2B6 in the presence of NADPH. CYP2B6 was reconstituted with reductase and incubated with selegiline and GSH. The separation and analysis of the adduct were as described under Materials and Methods. A, the extracted ion chromatogram of the primary peak ion eluting at 25.81 min with an m/z 528. B, the MS/MS spectrum of the peak eluting at 25.81 with an m/z of 528. C, the proposed structure of the selegiline-GSH adduct with the primary sites of fragmentation indicated.
Identification of the Selegiline Modified Site on the P4502B6 Apoprotein.
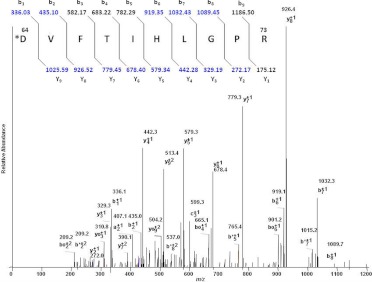
CYP2B6 that had been inactivated by incubation with selegiline was digested with trypsin and analyzed by LC/MS. Sequest Bioworks 3.2 software was used to determine the peptide fragment and the amino acid modified by the inactivator. The Sequest search showed sequence coverage of 81% for the modified 2B6. Analysis of the tryptic digests revealed only one peptide having the sequence DVFTVHLGPR as the peptide that had been modified during the inactivation of 2B6 by selegiline. The Sequest search resulted in a doubly charged peptide with an m/z 681.12 as the precursor ion and the singly charged peptide with an m/z 1361.24. The mass of the unadducted peptide in the control sample was 1140.6 (data not shown), indicating that the adducted peptide exhibited a mass increase of 220 Da. The site of adduction was localized to aspartic acid 64 as demonstrated by the 220 shift between the observed b1 ion in the inactivated sample and the calculated b1 ion based on the sequence and the observed b1 ion in the −NADPH sample (data not shown). The mass of the parent peptide, [MH+] is 1140.67, and the adducted peptide is 1361.246. Figure 7 shows the spectrum of the doubly charged [M+H]2+ ion at m/z 681.12 from the selegiline-modified peptide, indicating the expected b and y ions due to fragmentation and an increase of 220 for the aspartic acid residue at position 64. Several of the singly charged fragment ions of both y and b ions were observed in the MS/MS analysis. The fragment ions included the following: m/z 272.17 (y2+1), 329.19 (y3+1), 442.28 (y4+1), 579.34 (y5+1), 678.40 (y6+1), 779.45 (y7+1), 926.52 (y8+1), 1025.59 (y9+1), 435.10 (b2+1), 582.17 (b3+1), 919.35 (b6+1), 1032.43 (b7+1), and 1089.45 (b8+1). In addition, several B* (B-NH3), Bo (B-H2O), Y* (Y-NH3), and Yo (Y-H2O) ions were identified. These data suggest that the modification of the peptide occurred at the N terminus on the Asp64 residue. In the Sequest search, the modified peptide was identified with a probability of 9.8 × 10−6, and the XCorr value was 3.145 for a doubly charged ion. The Sp value, which indicates the preliminary score of the peptide, was 742. The RSp value, which indicates the ranking of the match during Sp scoring, was 1. Manual interpretation of the MS/MS spectrum to verify the identification of labeled peptide was done using Xcalibur software. A peptide with a mass of 1361 was not seen in control samples of P4502B6 that were incubated with selegiline in the absence of NADPH and digested with trypsin (data not shown). Although our studies only detected and identified a single amino acid residue, this does not preclude the possibility that one or more other residues in CYP2B6 may have been modified, which may have eluded detection by our techniques.
Fig. 7.
LC-MS/MS spectrum of the CYP2B6 tryptic peptide modified by selegiline. P4502B6 was reconstituted with reductase and selegiline in the presence or absence of NADPH and subsequently analyzed by LC-MS/MS and Sequest software as described under Materials and Methods. This figure shows the profile of the doubly charged ion (m/z 681.12) for the modified peptide 64DVFTVHLGPR73. All of the y ions and several b ions were identified. In addition, several B* (B-NH3), Bo (B-H2O), Y* (Y-NH3), and Yo (Y-H2O) ions were identified. The inset shows the b ions and y ions calculated for the modified peptide, and the b and y ions identified in the observed MS/MS spectrum are shown in the blue.
Structural Analysis of the Effect of Modification of Asp64 in CYP2B6 by Selegiline.
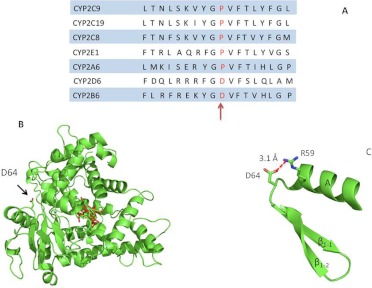
The recent high-resolution crystal structure of CYP2B6 at 2.0 Å (PBD code 3IBD) allowed us to determine the potential structural effects of formation of an adduct of Asp64 by a reactive intermediate of selegiline on the three-dimensional structure of CYP2B6. Specifically, Asp64 is located in the periphery of CYP2B6 (Fig. 8B), approximately 24 Å away from and coplanar with the heme iron. It is surface-exposed and present in a loop between helix A and the β1-1 sheet (Gay et al., 2010). According to the CYP2B6 crystal structure, the carboxyl group of Asp64 is within hydrogen bonding distance of Arg59 (Fig. 8C), and chemical modification of Asp64 by selegiline may impair this intermolecular interaction and compromise the secondary or tertiary structures of CYP2B6, thus leading to a loss in the catalytic activity of CYP2B6.
Fig. 8.
A, alignment of the amino acid sequences of members of the CYP2 family in the region corresponding to amino acid residues 54 to 72. The sequence alignment was obtained from the database at http://bioinformatics.charite.de/supercyp/. B, PyMol-generated cartoon representation of the CYP2B6 crystal structure (Protein Data Bank code 3IBD) showing the location of Asp64, which is the site identified for the covalent modification by selegiline. Asp64 is located in the periphery of the protein, approximately 24 Å away from the heme (shown in red). C, Asp64 is located in a loop between helix A and the β1-1 sheet. The carboxyl group of Asp64 forms a hydrogen bond with the primary amine of Arg59. Covalent modification of the carboxyl group of Asp64 by a reactive metabolite of selegiline may impair formation of this hydrogen bond and lead to a loss in CYP2B6 structural integrity.
Discussion
Selegiline, used in the treatment of Parkinson's disease, belongs to the class of drugs called phenethylamines and has a propargyl group (Knoll and Magyar, 1972; Fowler et al., 1981). It is estimated that up to 47% of patients with Parkinson's disease develops depression and requires antidepressant drugs (Dooneief et al., 1992) such as bupropion. One of the objectives of this study was to assess the potential for drug-drug interactions associated with the use of the combination of selegiline and the antidepressant bupropion that is also metabolized primarily by CYP2B6. Our findings here show that CYP2B6 produced the formation of the hydroxylated product of bupropion at a rate that significantly changed in the presence 12 μM selegiline (1.39 min−1) or absence of selegiline (2.6 min−1). In addition, metabolism of bupropion by purified CYP2B6 shows that, in the presence of selegiline, the Km for bupropion increases approximately 9-fold compared with that in the absence of selegiline. Thus, our results demonstrate that the kcat/Km value for bupropion in the presence of selegiline was 0.014, which was 19 times lower than in the absence of selegiline (0.26), indicating that apparent Km values make a significant difference in the catalytic efficiency of the enzyme to metabolize bupropion (Fig. 2). Selegiline also inactivated the 7-EFC O-deethylation activity of CYP2B6 and acted as a mechanism-based inactivation of bupropion metabolism as measured by the decreasing rates of formation of hydroxybupropion with time of incubation as shown in Fig. 3, A and B, respectively.
Bupropion has been shown to be an indirect dopaminergic agonist and is also used in the treatment of patients with Parkinson's disease. It is also used to treat nicotine dependence and act as an antidepressant (Cooper et al., 1980; Lerman et al., 2003). Selegiline, a drug to treat Parkinson's disease, also acts by inhibiting the uptake of dopamine and has also been used as an antidepressant and a smoking cessation drug (Fowler et al., 1981; George et al., 2003). Although bupropion is contraindicated in conjunction with monoamine oxidase inhibitors (Foley et al., 2006), studies by Ritter and Alexander (1997) have assessed the safety of combining selegiline with various antidepressants, including bupropion. On the basis of adverse reactions reported with the various antidepressants, bupropion was the first choice in combination with selegiline. However, a report by Richard and Kurlan (1997) indicates that 51% of the time physicians use selective serotonin reuptake inhibitors as the first line of therapy for depression followed by tricyclic antidepressants (41%) and other drugs such as bupropion (8%). This confirms that there is uncertainty regarding the efficacy of antidepressant therapy in patients with Parkinson's disease. Although the mechanism of action of selegiline is not fully understood, it is presumed to be linked to potentiation of monoamine neurotransmitter activity in the central nervous system resulting from its inhibition of MAO-B activity. Human brains obtained at autopsy from selegiline-treated patients showed approximately 80% inhibition of MAO-B activity (Riederer et al., 1978). MAO-B is involved in the oxidative deamination of dopamine in the brain and selegiline binds to MAO-B within the nigrostriatal pathway, thus preventing the metabolism of dopamine and enhancing the dopaminergic activity in the patients with Parkinson's disease. The evidence that selegiline and its metabolites can cross the blood-brain barrier comes from postmortem analysis of human brain tissues in patients with Parkinson's disease who have been treated with selegiline (Gerlach et al., 1996). Reynolds et al. (1978) also showed higher concentrations of amphetamine and phenylethylamine in Parkinson brains following administration of deprenyl. In the brain, CYP2B6 is expressed in neurons and glial cells in the hippocampus, cerebellum, caudate nucleus, and putamen (Miksys and Tyndale, 2002), and the highest levels of MAO (both A and B) activity are found in caudate nucleus and putamen. This report establishes that metabolites of selegiline can be formed by CYP2B6 in a reconstituted system. Selegiline undergoes demethylation to form N-desmethylselegiline, which further undergoes hydroxylation to form hydroxydesmethylselegiline. It also undergoes N-depropionylation to form methamphetamine. Both these primary metabolites may undergo further metabolism to form amphetamine as shown in Fig. 1. A small amount of phenylethylamine was also detected during metabolism by 2B6 (data not shown). It has previously been shown that MAO-B has an affinity for phenylethylamine and is selectively inhibited by low concentrations of selegiline (Riederer and Youdim, 1986). Phenylethylamine has been shown to induce the release of dopamine in both in vitro and in vivo experiments (Paterson et al., 1990), and thus, the increase in phenylethylamine concentration may inhibit the reuptake of dopamine, thereby enhancing the effect of selegiline (Paterson et al., 1990; Gerlach et al., 1996).
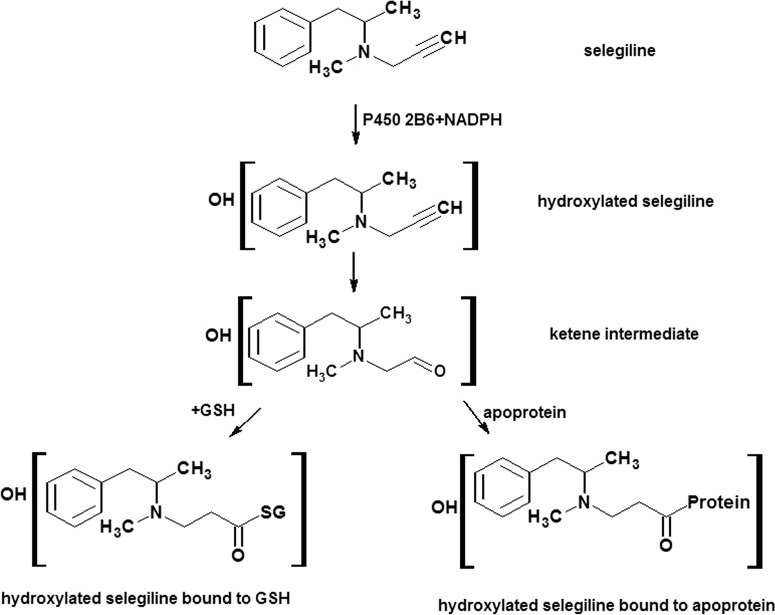
The lack of heme loss during inactivation by selegiline in our studies indicates that selegiline forms a covalent adduct with the apoprotein of the CYP2B6. The NADPH-dependent GSH adduct was observed using LC-MS and exhibited an m/z 528. We propose that the mechanism for the formation of the 2B6-selegiline adduct involves initial hydroxylation of the selegiline followed by oxidation of the acetylenic group to give a ketene intermediate with subsequent nucleophilic attack by GSH at the ketene as shown in Scheme 1. Although LC-MS has been a useful tool in characterizing GSH adducts, there are limitations in obtaining complete structural information on the breakdown of the adduct, and the exact position of the hydroxylation on the aromatic ring of selegiline could not be determined from the MS/MS spectra. CYP2B6 was incubated with selegiline in either the presence or absence of NADPH and analyzed by LC-ESI/MS to determine the change in mass of the adducted protein. Even though the unmodified enzyme could be readily observed by LC-ESI/MS and its mass was determined from the deconvoluted spectrum (data not shown), we have not been able to detect the adducted protein formed by the reaction of selegiline with CYP2B6 in the presence of NADPH, presumably because the modified CYP2B6 aggregates under LC-MS conditions, making it difficult to deconvolute the resulting multiple ionization envelopes.
Scheme. 1.
Pathway for the bioactivation of selegiline to the reactive intermediate that was trapped with glutathione. The species considered to be the reactive intermediate is the monohydroxylated selegiline that reacts with GSH following the formation of the ketene moiety.
However, LC-MS and Sequest analysis of the tryptic digest of the modified protein revealed that the peptide DVFTVHLGPR (positions 64–73 in the sequence) was the tryptic peptide modified by the reactive metabolite derived from selegiline and that the site of adduct formation was Asp64. The position of the Asp64 residue is on the periphery of the 2B6 structure in the loop between helix A and the β1-1 sheet as shown in Fig. 8B. The distance between the Asp64 and the prosthetic heme is approximately 24 Å in the 2B6 structure. Recent reports indicate that substrates may bind to the enzyme in multiple orientations and that ligand binding produces conformational changes in P450s that may involve changes in the hydrogen bond interactions between the heme and the protein (Chen et al., 2004). A report on the crystal structure of 2C9 with warfarin bound reversibly in the active site indicated that an aspartic acid residue (Asp293) is involved in hydrogen bonding with Arg59 (Williams et al., 2003). In addition, the volume of the active site may change significantly upon binding of a substrate to the P450, thus changing the number of residues within 5 Å of the bound ligand (Scott et al., 2004). Thus, the Asp64 residue may not be involved directly in substrate binding, but binding to the substrate may propagate a change in the heme or the active site residues. The alignment of amino acid sequences shown in Fig. 8A indicates that the Asp64 residue is located in a conserved region of the P450 2 family. The amino acid sequence GPVFT is shared among many members of the 2 family. Alignment studies show that Pro64 is highly conserved in all members of the 2 family, with the exception of CYP2B6 and 2D6, where Pro is replaced by Asp. Molecular dynamics simulations from our laboratory on CYP2B1 using the program Caver (Zhang et al., 2009) have shown that a substrate access channel referred to as the “white” channel seems to go past the helix A in CYP2B1. Alignment of CYP2B1 and 2B6 shows that Asp64 is conserved between these two isoforms and that in CYP2B6, similar to CYP2B1, Asp64 is located just after helix A. Therefore, it is probable that Asp64 is close to substrate access channel but may not be part of the channel itself. Because Asp64 forms a hydrogen bond with Arg59, chemical modification of Asp64 by selegiline may also perturb this potentially structurally important intramolecular interaction and lead to loss of catalytic activity.
In conclusion, we have demonstrated that the metabolism of bupropion to hydroxybupropion by reconstituted CYP2B6 is inhibited in the presence of selegiline. Selegiline also inactivated CYP2B6 7-EFC O-deethylation activity in a mechanism-based manner, and its kinetic parameters were determined. The potential for drug-drug interactions identified here for treatment regimens involving bupropion thus indicate that it is important that the investigation of drugs other than selegiline for the treatment of depression in patients with Parkinson's disease be pursued. CYP2B6 metabolizes selegiline metabolites, including desmethylselegiline, methamphetamine, and amphetamine, and the inactivation of CYP2B6 by selegiline is attributed to generation of a reactive intermediate that is consistent with the mass of selegiline plus two oxygen atoms. Additional studies using site-directed mutagenesis will be performed to investigate the possible mechanism for the inactivation.
Acknowledgments
We thank Hsia-lien Lin for the purification of reductase and Drs. Haoming Zhang and Jaime D'Agostino for helpful discussions.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA16954].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- P450
- cytochrome P450
- LC
- liquid chromatography
- MAO
- monoamine oxidase
- ESI
- electrospray ionization
- MS/MS
- tandem mass spectrometry
- 7-EFC
- 7-ethoxy-4-(trifluoromethyl)coumarin
- HPLC
- high-performance liquid chromatography
- CYP2B6
- cytochrome P4502B6
- amu
- atomic mass unit.
Authorship Contributions
Participated in research design: Sridar, Kenaan, and Hollenberg.
Conducted experiments: Sridar.
Contributed new reagents or analytic tools: Kenaan.
Performed data analysis: Sridar, Kenaan, and Hollenberg.
Wrote or contributed to the writing of the manuscript: Sridar, Kenaan, and Hollenberg.
References
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E. (1995) Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 56:395–401 [PubMed] [Google Scholar]
- Benetton SA, Fang C, Yang YO, Alok R, Year M, Lin CC, Yeh LT. (2007) P450 phenotyping of the metabolism of selegiline to desmethylselegiline and methamphetamine. Drug Metab Pharmacokinet 22:78–87 [DOI] [PubMed] [Google Scholar]
- Bumpus NN, Sridar C, Kent UM, Hollenberg PF. (2005) The naturally occurring cytochrome P450 (P450) 2B6 K262R mutant of P450 2B6 exhibits alterations in substrate metabolism and inactivation. Drug Metab Dispos 33:795–802 [DOI] [PubMed] [Google Scholar]
- Buters JT, Schiller CD, Chou RC. (1993) A highly sensitive tool for the assay of cytochrome P450 enzyme activity in rat, dog and man. Direct fluorescence monitoring of the deethylation of 7-ethoxy-4-trifluoromethylcoumarin. Biochem Pharmacol 46:1577–1584 [DOI] [PubMed] [Google Scholar]
- Chang TK, Weber GF, Crespi CL, Waxman DJ. (1993) Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637 [PubMed] [Google Scholar]
- Chen Z, Ost TW, Schelvis JP. (2004) Phe393 mutants of cytochrome P450 BM3 with modified heme redox potentials have altered heme vinyl and propionate conformations. Biochemistry 43:1798–1808 [DOI] [PubMed] [Google Scholar]
- Cooper BR, Hester TJ, Maxwell RA. (1980) Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther 215:127–134 [PubMed] [Google Scholar]
- Dooneief G, Mirabello E, Bell K, Marder K, Stern Y, Mayeux R. (1992) An estimate of the incidence of depression in idiopathic Parkinson's disease. Arch Neurol 49:305–307 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230 [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE. (2006) Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother 6:1249–1265 [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Oreland L, Callingham BA. (1981) The acetylenic monoamine oxidase inhibitors clorgyline, deprenyl, pargyline and J-508: their properties and applications. J Pharm Pharmacol 33:341–347 [DOI] [PubMed] [Google Scholar]
- Gay SC, Shah MB, Talakad JC, Maekawa K, Roberts AG, Wilderman PR, Sun L, Yang JY, Huelga SC, Hong WX, et al. (2010) Crystal structure of a cytochrome P450 2B6 genetic variant in complex with the inhibitor 4-(4-chlorophenyl)imidazole at 2.0-A resolution. Mol Pharmacol 77:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Jatlow PI, Kosten TR, O'Malley SS. (2003) A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biol Psychiatry 53:136–143 [DOI] [PubMed] [Google Scholar]
- Gerlach M, Youdim MB, Riederer P. (1996) Pharmacology of selegiline. Neurology 47:S137–S145 [DOI] [PubMed] [Google Scholar]
- Hanna IH, Reed JR, Guengerich FP, Hollenberg PF. (2000) Expression of human cytochrome P450 2B6 in Escherichia coli: characterization of catalytic activity and expression levels in human liver. Arch Biochem Biophys 376:206–216 [DOI] [PubMed] [Google Scholar]
- Hanna IH, Teiber JF, Kokones KL, Hollenberg PF. (1998) Role of the alanine at position 363 of cytochrome P450 2B2 in influencing the NADPH- and hydroperoxide-supported activities. Arch Biochem Biophys 350:324–332 [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183 [PubMed] [Google Scholar]
- Hidestrand M, Oscarson M, Salonen JS, Nyman L, Pelkonen O, Turpeinen M, Ingelman-Sundberg M. (2001) CYP2B6 and CYP2C19 as the major enzymes responsible for the metabolism of selegiline, a drug used in the treatment of Parkinson's disease, as revealed from experiments with recombinant enzymes. Drug Metab Dispos 29:1480–1484 [PubMed] [Google Scholar]
- Hollenberg PF, Kent UM, Bumpus NN. (2008) Mechanism-based inactivation of human cytochromes P450s: experimental characterization, reactive intermediates, and clinical implications. Chem Res Toxicol 21:189–205 [DOI] [PubMed] [Google Scholar]
- Knoll J, Magyar K. (1972) Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol 5:393–408 [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Jr., Pinto A, Kucharski S, Krishnan S, Niaura R, Epstein LH. (2003) Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol 22:541–548 [DOI] [PubMed] [Google Scholar]
- Magyar K, Szende B, Lengyel J, Tekes K. (1996) The pharmacology of B-type selective monoamine oxidase inhibitors; milestones in (−)-deprenyl research. J Neural Transm Suppl 48:29–43 [DOI] [PubMed] [Google Scholar]
- Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. (2003) Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology 45:122–132 [DOI] [PubMed] [Google Scholar]
- Miksys SL, Tyndale RF. (2002) Drug-metabolizing cytochrome P450s in the brain. J Psychiatry Neurosci 27:406–415 [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Montellano PR, Komives EA. (1985) Branchpoint for heme alkylation and metabolite formation in the oxidation of arylacetylenes by cytochrome P-450. J Biol Chem 260:3330–3336 [PubMed] [Google Scholar]
- Paterson IA, Juorio AV, Boulton AA. (1990) 2-Phenylethylamine: a modulator of catecholamine transmission in the mammalian central nervous system? J Neurochem 55:1827–1837 [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Riederer P, Sandler M, Jellinger K, Seemann D. (1978) Amphetamine and 2-phenylethylamine in post-mortem Parkinsonian brain after (−)deprenyl administration. J Neural Transm 43:271–277 [DOI] [PubMed] [Google Scholar]
- Richard IH, Kurlan R. (1997) A survey of antidepressant drug use in Parkinson's disease. Parkinson Study Group. Neurology 49:1168–1170 [DOI] [PubMed] [Google Scholar]
- Riederer P, Youdim MB. (1986) Monoamine oxidase activity and monoamine metabolism in brains of parkinsonian patients treated with l-deprenyl. J Neurochem 46:1359–1365 [DOI] [PubMed] [Google Scholar]
- Riederer P, Youdim MB, Rausch WD, Birkmayer W, Jellinger K, Seemann D. (1978) On the mode of action of L-deprenyl in the human central nervous system. J Neural Transm 43:217–226 [DOI] [PubMed] [Google Scholar]
- Ritter JL, Alexander B. (1997) Retrospective study of selegiline-antidepressant drug interactions and a review of the literature. Ann Clin Psychiatry 9:7–13 [DOI] [PubMed] [Google Scholar]
- Scott EE, White MA, He YA, Johnson EF, Stout CD, Halpert JR. (2004) Structure of mammalian cytochrome P450 2B4 complexed with 4-(4-chlorophenyl)imidazole at 1.9-A resolution: insight into the range of P450 conformations and the coordination of redox partner binding. J Biol Chem 279:27294–27301 [DOI] [PubMed] [Google Scholar]
- Sharma U, Roberts ES, Hollenberg PF. (1996) Inactivation of cytochrome P4502B1 by the monoamine oxidase inhibitors R-(-)-deprenyl and clorgyline. Drug Metab Dispos 24:669–675 [PubMed] [Google Scholar]
- Siu EC, Tyndale RF. (2008) Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice. J Pharmacol Exp Ther 324:992–999 [DOI] [PubMed] [Google Scholar]
- Stresser DM, Kupfer D. (1999) Monospecific antipeptide antibody to cytochrome P-450 2B6. Drug Metab Dispos 27:517–525 [PubMed] [Google Scholar]
- Taavitsainen P, Anttila M, Nyman L, Karnani H, Salonen JS, Pelkonen O. (2000) Selegiline metabolism and cytochrome P450 enzymes: in vitro study in human liver microsomes. Pharmacol Toxicol 86:215–221 [DOI] [PubMed] [Google Scholar]
- Williams PA, Cosme J, Ward A, Angove HC, Matak Vinković D, Jhoti H. (2003) Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature 424:464–468 [DOI] [PubMed] [Google Scholar]
- Zhang H, Kenaan C, Hamdane D, Hoa GH, Hollenberg PF. (2009) Effect of conformational dynamics on substrate recognition and specificity as probed by the introduction of a de novo disulfide bond into cytochrome P450 2B1. J Biol Chem 284:25678–25686 [DOI] [PMC free article] [PubMed] [Google Scholar]