Abstract
To study the impact of new, expensive, and unproven therapies to treat prostate cancer, we investigated the dissemination of intensity-modulated radiotherapy (IMRT). IMRT is an innovative treatment for prostate cancer that delivers higher doses of radiation with improved precision compared to alternative radiotherapies. We observed rapid adoption of this new treatment among men diagnosed with prostate cancer from 2001 through 2007, despite uncertainty about its relative effectiveness. We compared patient and disease characteristics of those receiving IMRT and the previous radiation standard of care, three-dimensional conformal therapy; assessed intermediate-term outcomes; and examined potential factors associated with the increased use of IMRT. We found that in the early period of IMRT adoption (2001–03) men with high-risk disease were more likely to receive IMRT, whereas after IMRT’s initial dissemination (2004–07) men with low-risk disease had fairly similar likelihoods of receiving IMRT as men with high-risk disease. This raises concerns about overtreatment, as well as considerable health care costs, because treatment with IMRT costs $15,000–$20,000 more than other standard therapies. As health care delivery reforms gain traction, policy makers must balance the promotion of new, yet unproven, technology with the risk of overuse.
More than 200,000 men are diagnosed with prostate cancer in the United States each year. Given the condition’s protracted clinical course, this is among the most costly malignancies, accounting for more than $7 billion in spending annually. During the past decade, growth of spending for prostate cancer has averaged 11 percent a year, outpacing rates for other common conditions such as cardiovascular and pulmonary diseases.1 This is due, at least in part, to the introduction of novel, expensive technologies.
Chief among these is intensity-modulated radiotherapy (IMRT), which costs an additional $15,000-$20,000 more per treatment course than surgical or other radiation options.2 IMRT uses complex equipment and computer algorithms to deliver radiation precisely to the prostate.3
Relative to the prior radiation standard of three-dimensional conformal therapy, IMRT yields two potential benefits resulting from its targeting capabilities. First, IMRT delivers higher doses of radiation to cancer sites, which may result in improved cancer control and lower recurrence rates.4 Second, by reducing radiation exposure to surrounding tissue, IMRT may limit acute and chronic toxicity, including declines in bowel, urinary, and sexual function.5,6
Ultimately, if these benefits prove to hold true, then IMRT would represent a major therapeutic advance for men with prostate cancer.
However, evidence supporting IMRT as the new standard is limited. In contrast, three-dimensional conformal therapy, IMRT’s immediate predecessor, has high-level evidence for both reduced morbidity compared to the prior standard7 and improved cancer control with higher doses.8,9 Unfortunately, observational studies involving IMRT are inconsistent. A systematic review noted that only 20 percent of comparative studies demonstrated any difference in cancer control between the approaches.10 Furthermore, only a third of the studies reported any improvements in patient-reported quality of life, and only half showed any benefit in terms of avoiding bowel toxicity.
Finally, because of its dose distribution and longer delivery times, IMRT may undertreat areas, a result known as the geographical miss, and expose large volumes of normal tissue to low doses of radiation,10,11 which raises concerns about secondary malignancies.12
For these reasons, we undertook a study to better understand the trends in adoption of IMRT. In addition, we compared patient and disease characteristics between the two radiation approaches, assessed intermediate-term outcomes, and examined potential factors associated with the increased use of IMRT.
Study Data And Methods
Data Source And Study Population
We used the most current Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data available to identify men age sixty-six and older diagnosed with prostate cancer between 2001 and 2007. The SEER database comprises a nationally representative collection of population-based registries of all incident cancers and captures about 26 percent of the US population.13
Sixty-five-year-olds were excluded to ensure accurate comorbidity estimation using Medicare claims for the year prior to diagnosis.14 In addition, we included only Medicare beneficiaries who were enrolled in both Parts A and B from one year prior to diagnosis until one year after diagnosis.
Using these exclusion criteria, we identified 125,299 men diagnosed with prostate cancer. Patients undergoing IMRT and three-dimensional conformal therapy within one year of diagnosis were identified using the Healthcare Common Procedure Coding System codes from the carrier (that is, the physician) and outpatient files (see online Appendix A).15 With these methods, we identified 19,846 men treated with IMRT and 16,644 men treated with three-dimensional conformal therapy.
Statistical Analysis
We obtained patient and tumor characteristics from the SEER registry data. This included information on patients’ age, race or ethnicity, socioeconomic status evaluated at the patient ZIP code level,16 and tumor characteristics (tumor grade and stage). Tumor grade classifies the degree of abnormality of cancer cells, and tumor stage reflects the extent of cancer. Together, these tumor characteristics help predict a patient’s prognosis.
We used Medicare data to measure patients’ comorbidities. Methods used to evaluate comorbidity were based on Medicare claims for the twelve-month period prior to diagnosis.14 As described previously,17 we categorized patients as having “low risk” prostate cancer if they had well-differentiated tumors, regardless of their age, or if they had moderately differentiated tumors and were age sixty-five or older at the time of diagnosis. All other patients were categorized as having “high risk” prostate cancer. Well- and moderately differentiated tumors are less aggressive than poorly differentiated tumors. Tumor differentiation was based solely on grade and not stage.
A sensitivity analysis was performed using SEER data beginning with 2004, when information on prostate-specific antigen levels and Gleason scores became available. The analysis was used to define low-risk disease using the D’Amico classification,18 which includes a prostate-specific antigen of 10 ng/mL or less, a Gleason score of 6 or lower, and a clinical stage designation of no more than T2a. Gleason scores grade the architecture of prostate cancer cells with scores ranging from 2 to 10; cancers with higher Gleason scores are more aggressive. We calculated a kappa statistic to measure the concordance of these two definitions of low-risk disease. A kappa statistic is a standard way to assess the agreement between two categorical measurements. Our kappa value of 0.54 indicated that there was good agreement between these two definitions of low-risk disease (0.40 ≤ κ < 0.75).
We analyzed rates of use of IMRT, three-dimensional conformal therapy, and overall use of either IMRT or three-dimensional conformal therapy. Patient demographics and disease characteristics were compared between the two approaches using chi-square tests.
For a subset of patients with at least three years of follow-up, we assessed the use of treatment for cancer recurrence among men who received IMRT or three-dimensional conformal therapy without additional treatments around the time of radiation. In addition, we evaluated the risk of needing an intervention for a bowel or urinary complication. For both these outcomes, we used a survival analysis, which is a standard way of modeling the event rate over time.
In these models, we adjusted for age, race or ethnicity, comorbidity, socioeconomic status, and tumor grade and stage.
To examine potential factors associated with IMRT use, we stratified our data based on race (white, African American), socioeconomic status (low, medium, high), and disease classification (low risk, high risk), and we fitted a logistic regression model for each stratum. We then obtained annual predicted probabilities of treatment with IMRT compared to other forms of radiation, such as three-dimensional conformal therapy, two-dimensional external beam radiotherapy, and brachytherapy.
We adjusted for a variety of patient and clinical characteristics, which are specified in the exhibits. The Institutional Review Board of the University of Michigan approved the study protocol.
Limitations
Our findings should be interpreted in the context of some limitations. First, our findings can be generalized only to Medicare beneficiaries undergoing radiation therapy for prostate cancer. Although approximately one-third of patients with prostate cancer are younger than sixty-five,19 those treated with radiation tend to be older than those undergoing alternative therapies (median age: sixty-nine years),20 which makes our findings generalizable to the vast majority of men undergoing radiation for prostate cancer.
Second, because we used observational data, our inference may be biased by unmeasured differences between the two populations. To help combat this, we adjusted for a variety of patient characteristics and used clinical registry data, allowing us to assess tumor stage and grade— arguably two of the most important determinants of treatment.
Third, our examination of the drivers of IMRT use would be more complete if we had data on provider characteristics, including physicians’ ownership of treatment facilities. Our ability to characterize providers was somewhat limited by our data set.
Physician surveys have revealed that providers who adopt IMRT tend to be fewer years removed from training and in larger academic or private practices, compared to those who do not adopt IMRT.21 However, more empirical data investigating the association between provider factors and use of IMRT are needed.
Despite these limitations, our findings highlight some of the patient factors, tumor characteristics, and financial considerations pertaining to the adoption of IMRT, as well as some of the intermediate-term outcomes demonstrating IMRT’s mixed results, which have both clinical and policy implications.
Study Results
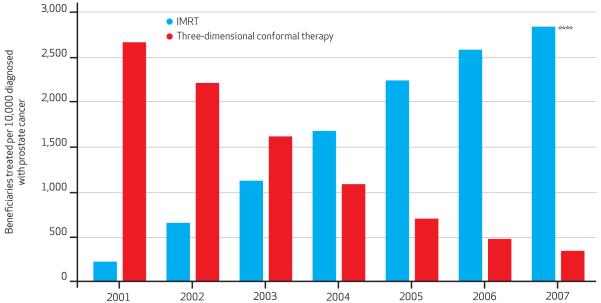
From 2001 to 2007 the rate of three-dimensional conformal therapy use decreased nearly 90 percent, while the rate of IMRT use increased more than tenfold, from roughly 220 cases per 10,000 Medicare beneficiaries diagnosed with prostate cancer to more than 2,800 cases per 10,000 such beneficiaries (Exhibit 1). The overall rate of radiation treatment with either IMRT or three-dimensional conformal therapy increased 10percent, from roughly 2,880 to nearly 3,200 cases per 10,000 Medicare beneficiaries diagnosed with prostate cancer.
Exhibit 1. Trends In Use Of IMRT Or Three-Dimensional Conformal Therapy For The Treatment Of Medicare Beneficiaries Diagnosed With Prostate Cancer, 2001–07.
SOURCE Authors’ analysis of linked Surveillance, Epidemiology, and End Results (SEER)-Medicare data. NOTES Among beneficiaries diagnosed with prostate cancer, there was an 87 percent decrease in the use of three-dimensional conformal therapy along with amore than 1,000 percent increase in the use of intensity-modulated radiotherapy (IMRT) from 2001 through 2007. Statistical significance denotes test for trend. ****p < 0:001
Patient And Disease Characteristics
During the study period, whites transitioned from receiving more IMRT to receiving more three-dimensional conformal therapy (Exhibit 2). Compared to patients of medium and high socioeconomic status, the majority of those receiving either treatment were of low socioeconomic status. Initially, 11 percent more men with low socioeconomic status received IMRT compared to three-dimensional conformal therapy, although this difference diminished by 2007.
EXHIBIT 2. Patient And Disease Characteristics Of The Study Population, Study Of Medicare Beneficiaries Treated For Prostate Cancer, Selected Years 2001-07.
| 2001 (%) |
2004 (%) |
2007 (%) |
||||
|---|---|---|---|---|---|---|
| Characteristic | 3D-CRT (n = 4,874) |
IMRT (n = 401) |
3D-CRT (n = 1,973) |
IMRT (n = 3,052) |
3D-CRT (n = 579) |
IMRT (n = 4,842) |
| AGE (YEARS) | ||||||
| 65-69 | 21 | 19 | 25 | 23** | 26 | 23**** |
| 70-74 | 38 | 41 | 34 | 37 | 33 | 36 |
| 75-79 | 31 | 30 | 27 | 29 | 26 | 28 |
| 80+ | 10 | 9 | 13 | 11 | 15 | 13 |
| RACE/ETHNLCITY | ||||||
| White | 85 | 89**** | 83 | 83** | 86 | 82** |
| African American | 9 | 3 | 11 | 10 | 9 | 10 |
| Other | 5 | 8 | 6 | 8 | 5 | 8 |
| SOCIOECONOMIC STATUS | ||||||
| Low | 54 | 65**** | 53 | 56** | 53 | 57 |
| Medium | 24 | 13 | 24 | 21 | 27 | 23 |
| High | 22 | 22 | 23 | 23 | 19 | 19 |
| NUMBER OF COMORBIDITIES | ||||||
| 0 | 66 | 68 | 64 | 66 | 59 | 61 |
| 1 | 23 | 21 | 23 | 23 | 25 | 25 |
| 2 or more | 11 | 10 | 13 | 12 | 16 | 14 |
| DISEASE CLASSIFICATION | ||||||
| Low risk | 57 | 55 | 25 | 32**** | 18 | 27**** |
| High risk | 43 | 45 | 75 | 68 | 82 | 73 |
SOURCE Authors’ analysis of linked Surveillance, Epidemiology, and End Results (SEER)-Medicare data. NOTES IMRT is intensity-modulated radiotherapy. 3D-CRT is three-dimensional conformal therapy. Chi-square tests were performed for all categorical variables. Percentages might not sum to 100 because of rounding.
p < 0.05
p < 0.001
Overall, there was a trend toward decreasing radiation treatment—either IMRT or three-dimensional conformal therapy—for men with low-risk disease. However, by 2007, 9 percent more men with low-risk disease received IMRT than three-dimensional conformal therapy. Similarly, more men treated with IMRT had lowergrade and lower-stage tumors (see online Appendix B).15
For the subset of patients with at least three years of follow-up, the predicted use of treatment for cancer recurrence among men receiving IMRT or three-dimensional conformal therapy without additional treatments around the time of radiation was 6 percent and 9 percent, respectively, at three years (p < 0.001). Compared to three-dimensional conformal therapy, men treated with IMRT had a higher likelihood of receiving an intervention for a bowel (22 percent versus 18 percent, p < 0.001) or urinary complication (8 percent versus 6 percent, p < 0.001).
Use Of Therapies
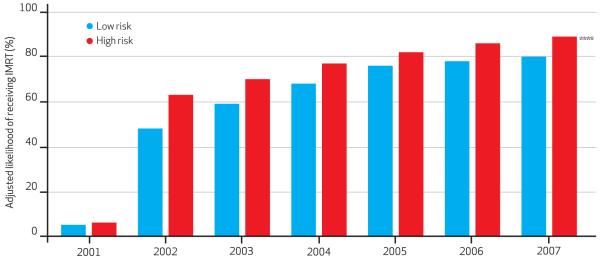
The adjusted percentage of IMRT use is shown according to disease classification (Exhibit 3), race (Exhibit 4), and socio-economic status (online Appendix C).15 These models were adjusted for several patient and tumor characteristics. The rate of IMRT adoption was very rapid for patients with both low-risk and high-risk disease. In the early period of IMRT adoption—2001–03—men with high-risk disease were more likely to receive IMRT compared to three-dimensional conformal therapy, two-dimensional external beam radiotherapy, or brachytherapy.
Exhibit 3. Adjusted Likelihood Of Receiving IMRT Compared To Other Radiation Treatments For Prostate Cancer, According To Disease Risk Classification, 2001–07.
SOURCE Authors’ analysis of linked Surveillance, Epidemiology, and End Results (SEER)-Medicare data. NOTES IMRT is intensity-modulated radiotherapy. Model was adjusted for age, race, socioeconomic status, and comorbidity. Statistical significance was determined using the omnibus Wald test. See Liao TF. Comparing social groups:Wald statistics for testing equality among multiple logit models. Int J Comp Sociol. 2004;45(3):3-16. ****p < 0:001
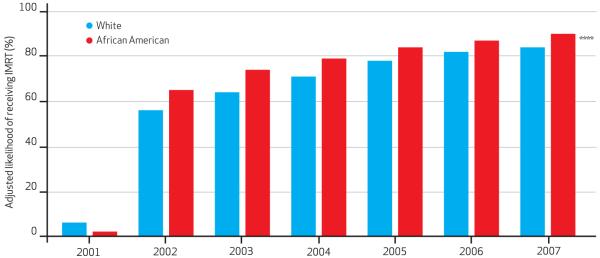
Exhibit 4. Adjusted Likelihood Of Receiving IMRT Compared To Other Radiation Treatments For Prostate Cancer, According To Race, 2001–07.
SOURCE Authors’ analysis of linked Surveillance, Epidemiology, and End Results (SEER)-Medicare data. Notes IMRT is intensity-modulated radiotherapy. Model was adjusted for age, socioeconomic status, comorbidity, and tumor grade and stage. Statistical significance was determined using the omnibus Wald test. See Liao TF. Comparing social groups:Wald statistics for testing equality among multiple logit models. Int J Comp Sociol. 2004;45(3):3-16. ****p < 0:001
Conversely, after IMRT’s initial dissemination—2004–07—men with low-risk disease and men with high-risk disease had fairly similar likelihoods of receiving IMRT. Except in when IMRT was rare, African Americans whites had similar likelihoods of receiving compared to the three alternative therapies. discrepancy in likelihoods of IMRT use for can Americans and whites was always less 10 percent, making this difference marginal.
Similarly, IMRT diffused fairly evenly across all socioeconomic classes. Here, the difference in likelihoods of IMRT use was always less than 5 percent, which suggests no clinical differences in use across socioeconomic classes.
Discussion
Despite its unclear clinical superiority over its predecessor, three-dimensional conformal therapy, IMRT for prostate cancer has disseminated rapidly. Furthermore, this dissemination has been widespread, with fairly uniform use among men of different races and socioeconomic classes.
From a quality-of-care standpoint, it is reassuring that African Americans who receive radiation are at least as likely to get IMRT as whites, given prior evidence that African Americans less frequently received adequate doses of conformal radiation—a prostate cancer quality-of-care indicator.22
Reasons For Rapid Adoption
Reasons underlying IMRT’s rapid adoption are likely to be complex and multifactorial. First, because of the potential advantages afforded by a more conformal and higher-dose delivery, IMRTmay truly provide better cancer control and lower morbidity. In this scenario, proponents are correct in arguing that withholding IMRT until the data become better developed would be unjust. This may be particularly true insofar as more men are being diagnosed with aggressive cancers, which may benefit from higher doses of treatment. However, over time, men with low-risk disease had fairly similar likelihoods of receiving IMRT as those with high-risk disease, which seems to dampen this argument.
Furthermore, evidence supporting IMRT’s improved cancer control and toxicity profile is mixed.10 In a subset of patients with at least three years of follow-up, we found that men receiving IMRT without additional treatments around the time of radiation were less likely to require treatments for cancer recurrence than men receiving three-dimensional conformal therapy. However, those treated with IMRT also had a higher chance of receiving an intervention for a bowel or urinary complication.
Second, with increasing patient demands—fueled by growing accessibility of information— physicians may feel compelled to position themselves at the vanguard of therapeutic options to maintain their prostate cancer treatment market share. For example, many physicians considered economic competition a factor in their decision to adopt IMRT.21
A third reason may relate to financial incentives inherent in the fee-for-service delivery system. Medicare started reimbursing for IMRT in 2002, setting rates high to account for the cost of IMRT equipment and personnel.23 Because of intense physics planning, quality assurance time, treatment delivery time, and equipment costs, IMRTis more expensive than three-dimensional conformal therapy.24
On one hand, high Medicare reimbursement rates may be appropriate because lower reimbursement rates might have precluded the diffusion of a promising, yet expensive, new technology. On the other hand, reimbursements for IMRT are more than $14,000 more per patient than are reimbursements for three-dimensional conformal therapy.25 Especially for treatments with small or questionable benefits, these financial incentives may influence physicians’ behavior.26
Thus, the rapid adoption of IMRT may have occurred before sufficient evidence surfaced in support of its clinical benefits and cost-effectiveness.2 As with three-dimensional conformal therapy, high reimbursement rates for IMRT should be temporary and decrease after its introduction.
Indeed, reimbursement for the technical component—that is, the cost of equipment and technical support for administering therapy, not of physicians’ involvement in care—has decreased over time for IMRT.25 However, throughout our study period, the reimbursements for treatment delivery remained roughly five times higher for IMRT than for three-dimensional conformal therapy.27
Concern Regarding Overuse
The concern about overuse of IMRT as a result of high reimbursement rates is compounded by the prospect of physicians’ ownership of treatment facilities and self-referral. Within radiation oncology, these issues have persisted for quite some time.
Twenty years ago, radiation therapy facilities with ownership interests by nonradiation oncologists performed 58 percent more procedures than did facilities without ownership conflicts.28 Around that time, the Stark Law (section 1877 of the Social Security Act)29 was passed, prohibiting a physician from referring patients to facilities in which the physician, or an immediate family member, had a financial relationship through ownership or compensation.30 However, under the in-office ancillary exception to the Stark Law, physicians could refer patients for self-owned services if they had a supervisory or managerial role, and the services were provided in a building used by the referring physician.30,31
Because IMRT is often provided on site, it is not in violation of the Stark Law.29 As a result, some physicians may view IMRT as an investment opportunity. The shift of radiation delivery from oncologists to urologists has raised such speculation in the lay press.23 In fact, some companies have aggressively marketed IMRT to urologists, claiming that treating 1.5 new patients monthly with IMRT could generate more than $425,000 in additional revenue per physician each year.32
In this context, financial pressures induced by considerable start-up costs may encourage IMRT use in the marginal patient. Indeed, overuse, such as treatment among those who do not need it, may be greatest among patients receiving radiation therapy.17
Encouragingly, the use of radiation has decreased for both IMRT and three-dimensional conformal therapy among low-risk patients. This suggests that perhaps more patients with indolent disease are undergoing active surveillance.
Furthermore, in the earlier years of IMRT adoption, men being treated with IMRT were more likely to have high-risk than low-risk disease, which seems appropriate given IMRT’s ability to deliver higher doses of radiation. However, after IMRT’s initial dissemination, men with low-risk disease had fairly similar likelihoods of receiving IMRT as men with high-risk disease, which raises concern regarding overtreatment.
Cost Implications
IMRT’s widespread adoption comes at a high cost. The provision of IMRT by hospitals or physician groups requires a substantial capital investment of approximately $2 million and the hiring of advanced support staff. These considerable outlays translate into higher per patient costs.2,25 In fact, it is estimated that replacing conformal therapy with IMRT for treating prostate cancer alone would increase spending by $1.4 billion per year.25
At issue, then, is what price society is willing to pay for small incremental benefits in outcome from a more costly technology. In the context of limited resources, does everybody deserve a “Cadillac” when a “Buick” is almost as good?
Although IMRT has largely supplanted conformal therapy as the standard method for prostate cancer, this example of technology diffusion is just the tip of the iceberg. Proton beam therapy is touted as an improved form of radiation and thought to deliver doses more precisely than IMRT does. These hypothetical improvements come with a hefty price tag, with start-up costs exceeding $100 million per facility.33 Price not-withstanding, several centers in the United States either have built or have plans to build facilities for proton beam therapy, although the ultimate bearer of the added costs is most assuredly the taxpayer.
Policy Implications
Given the current economic climate, spending growth for health care is unsustainable, particularly when it occurs in such dramatic increments for relatively little gain. Moving forward, several policy initiatives may help bend the cost curve associated with new technology dissemination.
Delivery System Changes
First, proposed changes to the delivery system, in the form of accountable care organizations, may mitigate some of the incentives for physicians to do more, which are intrinsic to the fee-for-service system. By sharing the responsibility of health care for a defined patient population, providers participating in these organizations may be encouraged to provide more-efficient care, perhaps discouraging the unregulated use of “Cadillac” treatments.
Payment Policy Changes
Second, changes in payment policies could limit the use of new, as yet unproven technology. For instance, the use of bundled payments may deter less-efficient use of resources. Under bundled payments, Medicare will pay a fixed amount for a full range of services provided during an episode of care.
Another payment policy that may gain footing involves prior authorization initiatives, which have been shown to reduce the inappropriate use of imaging procedures.34 Implementing prior authorization for prostate cancer treatments, however, would require defining appropriate treatment, a controversial term.
Medicare could also modify its payment policies to reduce the professional or technical fees associated with IMRT. As previously shown, the administration of hormone therapy for prostate cancer declined rapidly after reimbursement levels were cut.35
Assessing Effectiveness
Third, efforts to better understand treatment effectiveness are blossoming, although they are still in nascent stages.36 The American Recovery and Reinvestment Act of 2009, also known as the stimulus bill, earmarked $1.1 billion for comparative effectiveness research, and the Affordable Care Act of 2010 created the research. Patient-Centered Outcomes Research Institute to identify and address research priorities for comparative effectiveness research.
Coverage With Evidence Development
Fourth, and perhaps most intriguing, is the idea of “coverage with evidence development.” This policy avoids the “yes or no” dilemma of whether to support therapies and instead grants Medicare coverage for designated new treatments provided that patients participate in research, such as a clinical trial or disease registry.37 The research component generates clinical evidence to help determine whether a treatment has a health benefit.38
An early application of coverage with evidence development involved Medicare’s coverage novel colorectal cancer drugs for subjects willing to participate in clinical trials.39 In terms of evaluating new radiation treatments, coverage with evidence development is an attractive policy for prostate cancer treatment because it allows all participants to receive new treatments, in the case of a registry as opposed to a randomized trial—in which some participants do not receive the innovative treatment—and collects data evaluate their relative effectiveness.
Conclusion
Collectively, these policy reforms may strike better balance between technology dissemination and establishing relative clinical effectiveness while, at the same time, not deterring search and development. Paying for what works, even if it does not incorporate the newest, most expensive treatments with all the “bells and whistles,” will serve us best as we attempt expand health care coverage and limit overall spending. ■
Supplementary Material
Acknowledgments
Bruce Jacobs is supported in part by an American Cancer Society Postdoctoral Fellowship Grant (121805-PF-12-008-01-CPHPS) and by National Institutes of Health Training Grant No. 5 T32 DK007782-12. Brent Hollenbeck is supported in part by an American Cancer Society Pennsylvania Division-Dr. William and Rita Conrady Mentored Research Scholar Grant (MSRG-07-006-01-CPHPS). The authors thank David C. Miller, from the Department of Urology at the University of Michigan, for his review of the manuscript. The views expressed in this article do not reflect the views of the federal government.
Biography

Bruce L. Jacobs is a fellow in urologic oncology, endourology, and health services research at the University of Michigan.
In this month’s Health Affairs, Bruce Jacobs and coauthors report on their examination of intensity-modulated radiotherapy (IMRT), a high-cost treatment for prostate cancer that has been widely adopted despite uncertainty about its effectiveness compared to alternative therapies. The authors found that as the use of the therapy spread from 2004 to 2007, men whose prostate cancer was considered low risk—that is, less likely to be a clinically significant cancer—were nonetheless as likely to receive IMRT as men at high risk, which raises serious concerns about overtreatment.
“With limited financial resources, our health care system must establish that ‘Cadillac’ therapies, such as IMRT, are truly worth the investment before they become the standard,” says Jacobs. At the same time, he adds, “we must ensure that incentives used to encourage the utilization of new technology do not unintentionally create incentives for overuse.”
Jacobs is a fellow in urologic oncology, endourology, and health services research at the University of Michigan. His primary research focus is on the adoption and diffusion of new technologies for the treatment of prostate cancer and the implications of those technologies for health policy. He received his medical degree from Vanderbilt University.

Yun Zhang is a health economist at the University of Michigan.
Yun Zhang is a health economist at the University of Michigan. His research concentrates on the adoption and cost-effectiveness of health technologies and on medical decision making. Zhang received his doctorate in economics from the University of North Carolina.

Ted A. Skolarus is an assistant professor of urology in the University of Michigan Health System.
Ted Skolarus is an assistant professor of urology in the University of Michigan Health System and an investigator in the Veterans Affairs HSR&D Center for Clinical Management Research, in Ann Arbor, Michigan. His research aims to increase knowedge about the delivery of prostate cancer survivorship care, support the ongoing needs of prostate cancer survivors, and identify opportunities to improve care coordination among cancer specialists and primary care providers. Skolarus earned his medical degree from Wayne State University.

Brent K. Hollenbeck is director of the Herbert H. and Grace A. Dow Division of Health Services Research, University of Michigan.
Brent Hollenbeck is an associate professor and director of the Herbert H. and Grace A. Dow Division of Health Services Research at the University of Michigan. His longitudinal research focuses on understanding the upstream influences and downstream consequences of variation in physician practice. Hollenbeck received his medical degree from Indiana University.
Contributor Information
Bruce L. Jacobs, fellow in urologic oncology, endourology, and health services research at the University of Michigan, in Ann Arbor
Yun Zhang, health economist at the University of Michigan.
Ted A. Skolarus, assistant professor of urology in the University of Michigan Health System and an investigator in the Veterans Affairs HSR&D Center for Clinical Management Research
Brent K. Hollenbeck, associate professor and director of the Herbert H. and Grace A. Dow Division of Health Services Research, University of Michigan
NOTES
- 1.Roehrig C, Miller G, Lake C, Bryant J. National health spending by medical condition, 1996-2005. Health Aff (Millwood) 2009;28(2):w358–67. doi: 10.1377/hlthaff.28.2.w358. DOI: 10.1377/hlthaff.28.2.w358. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517–24. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling CC, Burman C, Chui CS, Kutcher GJ, Leibel SA, LoSasso T, et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35(4):721–30. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 4.Vora SA, Wong WW, Schild SE, Ezzell GA, Halyard MY. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68(4):1053–8. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–9. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Jani AB, Su A, Milano MT. Intensity-modulated versus conventional pelvic radiotherapy for prostate cancer: analysis of acute toxicity. Urology. 2006;67(1):147–51. doi: 10.1016/j.urology.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Koper PC, Stroom JC, van Putten WL, Korevaar GA, Heijmen BJ, Wijnmaalen A, et al. Acute morbidity reduction using 3DCRT for prostate carcinoma: a randomized study. Int J Radiat Oncol Biol Phys. 1999;43(4):727–34. doi: 10.1016/s0360-3016(98)00406-4. [DOI] [PubMed] [Google Scholar]
- 8.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr., Miller DW, Adams JA, et al. Comparison of conventionaldose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 9.Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 10.Staffurth J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22(8):643–57. doi: 10.1016/j.clon.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70(3):660–5. doi: 10.1016/j.ijrobp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83–8. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 13.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER cancer statistics review, 1975-2007. National Cancer Institute; Bethesda (MD): 2010. [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 16.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 17.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134–41. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 18.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 19.Ries LAG, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, et al., editors. SEER cancer statistics review, 1975-2005. National Cancer Institute; Bethesda (MD): 2008. [Google Scholar]
- 20.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostatecancer survivors. N Engl J Med. 2008;358(12):1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 21.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005;104(6):1296–303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 22.Spencer BA, Miller DC, Litwin MS, Ritchey JD, Stewart AK, Dunn RL, et al. Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol. 2008;26(22):3735–42. doi: 10.1200/JCO.2007.13.2555. [DOI] [PubMed] [Google Scholar]
- 23.Carreyrou J, Tamman M. A device to kill cancer, lift revenue. Wall Street Journal. 2010 Dec 7; Health Industry section. [Google Scholar]
- 24.Cahlon O, Hunt M, Zelefsky MJ. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin Radiat Oncol. 2008;18(1):48–57. doi: 10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Konski A, Watkins-Bruner D, Feigenberg S, Hanlon A, Kulkarni S, Beck JR, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):408–15. doi: 10.1016/j.ijrobp.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Andersen R, Brook R, Kominski G, Albert PS, Wenger N. The effects of payment method on clinical decision-making: physician responses to clinical scenarios. Med Care. 2004;42(3):297–302. doi: 10.1097/01.mlr.0000114918.50088.1c. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Medicare and Medicaid Services . Physician fee schedule search [Internet] CMS; Baltimore (MD): [last modified 2012 Jan 8; cited 2012 Mar 6]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=7&T=0&HT=0&CT=0& H1=77418&M=5. [Google Scholar]
- 28.Mitchell JM, Sunshine JH. Consequences of physicians’ ownership of health care facilities-joint ventures in radiation therapy. N Engl J Med. 1992;327(21):1497–501. doi: 10.1056/NEJM199211193272106. [DOI] [PubMed] [Google Scholar]
- 29.42 US Code, sec. 1395nn.
- 30.Falit BP, Gross CP, Roberts KB. Integrated prostate cancer centers and over-utilization of IMRT: a close look at fee-for-service medicine in radiation oncology. Int J Radiat Oncol Biol Phys. 2010;76(5):1285–8. doi: 10.1016/j.ijrobp.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 31.42 US Code, sec. 1395nn(b)(2).
- 32.Urorad Healthcare . FAQ’s [Internet] Urorad Healthcare; Humble (TX): [cited 2012 Jan 27]. Available from: http://online.wsj.com/public/resources/documents/urorad-whois12082010.pdf. [Google Scholar]
- 33.Pollack A. Hospitals look to nuclear tool to fight cancer. New York Times; Dec 26, 2007. Business section. [Google Scholar]
- 34.Mitchell JM, Lagalia RR. Controlling the escalating use of advanced imaging: the role of radiology benefit management programs. Med Care Res Rev. 2009;66(3):339–51. doi: 10.1177/1077558709332055. [DOI] [PubMed] [Google Scholar]
- 35.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363(19):1822–32. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 36.Wu AW, Snyder C, Clancy CM, Steinwachs DM. Adding the patient perspective to comparative effectiveness research. Health Aff (Millwood) 2010;29(10):1863–71. doi: 10.1377/hlthaff.2010.0660. [DOI] [PubMed] [Google Scholar]
- 37.Miller FG, Pearson SD. Coverage with evidence development: ethical issues and policy implications. Med Care. 2008;46(7):746–51. doi: 10.1097/MLR.0b013e3181789453. [DOI] [PubMed] [Google Scholar]
- 38.Keenan PS, Neumann PJ, Phillips KA. Biotechnology and Medicare’s new technology policy: lessons from three case studies. Health Aff (Millwood) 2006;25(5):1260–9. doi: 10.1377/hlthaff.25.5.1260. [DOI] [PubMed] [Google Scholar]
- 39.Carino T, Williams RD, 2nd, Colbert AM, Bridger P. Medicare’s coverage of colorectal cancer drugs: a case study in evidence development and policy. Health Aff (Millwood) 2006;25(5):1231–9. doi: 10.1377/hlthaff.25.5.1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.