Abstract
Quinazolinones are interesting materials because of their valuable biological effects. In this study some new 2,3-disubstituted-4(3H)quinqzolinone derivatives were synthesized from anthranilic acid in six steps by introducing a new chiral center to the aliphatic side chain of the quinazolinone. In the last step, a single acylation on the hydrazine moiety afforded final compounds. The structures of compounds were confirmed by IR, 1HNMR and Mass spectra.
Keywords: Anthranilic acid, 4(3H)-Quinazolinone, Synthesis
INTRODUCTION
Synthesis of different classes of heterocyclic molecules is one of the most important targets in the synthetic organic chemistry. Among the nitrogen-containing heterocyclic compounds, quinazolinones (Fig. 1) have attracted interest of many researchers because, introduction of various substituents to different positions of quinazolinones have produced compounds with valuable biological activities.
Fig. 1.

4(3H)-Quinazolinone chemical structure
Some of their most frequently reported biological properties include cytotoxic, antibacterial, antifungal, anticonvulsant, antitubercular, anti-HIV, antiviral, anti-inflammatory and antihistaminic activities(1–5). Other properties of quinazolinones which have been reported in the literature are antihelmentic, CNS depressant, antidiabetic, antiallergic, antihistaminic, analgesic and hypolipidemic effects(6–10).
Quinazolinones are also the main component of nearly 150 natural alkaloids existing in some families of plants, animals and microorganisms(11). Febrifugine and isofebrifugine which are known as antimalarial agents, are two wellknown natural alkaloids with quinazolinone structure (Fig. 2) (3).
Fig. 2.

Representative examples of natural quiazolinones
Several synthetic methods have been reported for preparing these pharmacologically active substances(12). The synthesis of quinazolinones may be performed by cyclization of benzene or pyrimidine substrates containing appropriate substituents, by using benzoxazin-4(one) (benzoxazinone) or by modification of appropriate derivatives of other heterocyclic systems(13). Among various methods for quinazolinone synthesis, application of benzoxazinone has been repeatedly reported.
In the present study, anthranilic acid as starting material was reacted with butyryl chloride to produce N-butyryl anthranilic acid followed by a ring closure in acetic anhydride to afford the corresponding benzoxazinone. Replacement of oxygen in benzoxazinone ring system with primary amines resulted in production of 2,3-disubstituted quinazolinones. Synthesized quinazolinones have either phenyl or benzyl moiety at position 3 (Fig. 1) due to the application of aniline or benzyl amine as primary amines. Substitution of phenyl hydrazine on alkyl side chain at position 2 (Fig. 1) of quinazolinone backbone resulted in production of novel hydrazid derivatives as the first class of final compounds. Subsequently, acylation of these derivatives with various acyl chlorides was performed successfully to obtain second group of novel quinazolinons as substituted hydrazides.
MATERIALS AND METHODS
Instrumentation
Melting points were determined in open capillaries using electrothermal 9200 melting point apparatus and are uncorrected. The IR spectra were determined by a WQF-510 FT-IR spectrophotometer using potassium bromide technique. 1HNMR spectra were recorded in CDCl3 solution on Bruker 400 or 500 MHz spectrometers. Mass spectra were measured on a Shimadzu Mass spectrometer using EI+ technique.
Preparation of compounds
In this study, we have prepared some new 4(3H)-quinazolinone derivatives from anthranilic acid 1 by a six-step procedure (Fig. 3). Anthranilic acid was reacted with butyryl chloride to obtain the corresponding amides. The amides were reacted with acetic anhydride to obtain benzoxazin-4(one) 3 as crystalline product.
Fig. 3.
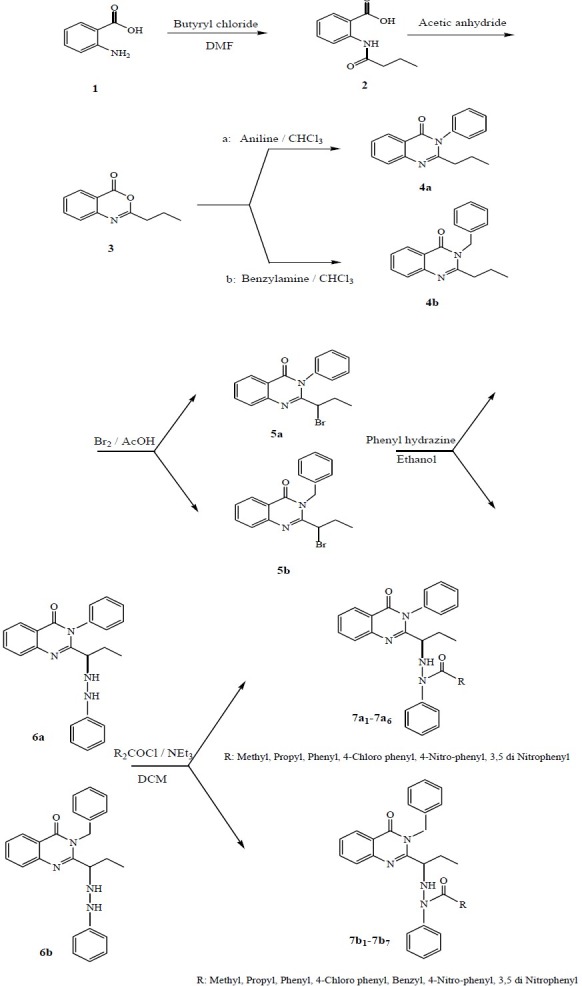
General reaction scheme for preparation of the final compounds
The benzoxazinone was subsequently refluxed with two different amines to give the corresponding quinazolinones 4a and 4b. The quinazolinones were brominated (5a, 5b) and subsequent treatment with phenyl hydrazine afforded 6a and 6b. Finally 6a and 6b were reacted with different acid chlorides to obtain compounds 7a1-7a6 and 7b1-7b7. These compounds were purified by column chromatography or preparative thin layer chromatography (PTLC) using several solvent systems. The structures of synthesized compounds were confirmed by IR, 1HNMR, and Mass spectra.
All atoms in the chemical structures of the final compounds have been numerically assigned for the ease of interpretation of the 1HNMR results (Fig 4, 5).
Fig. 4.
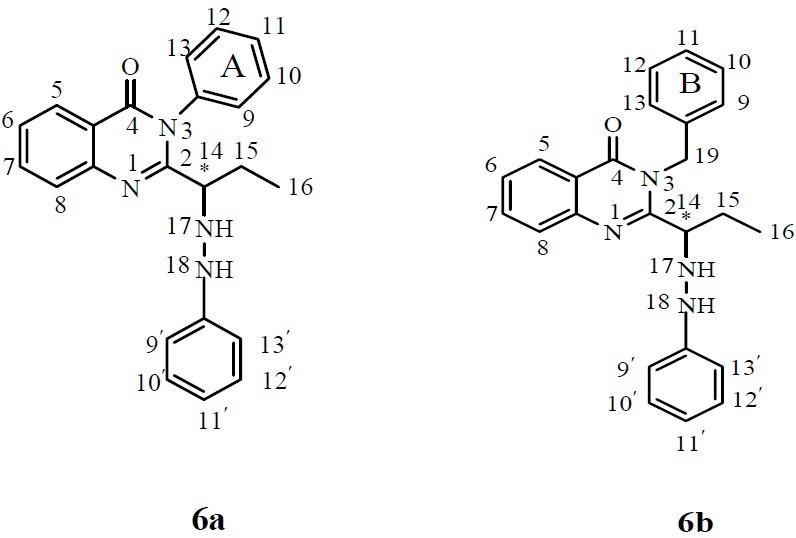
Chemical structures of compounds 6a and 6b
Fig. 5.
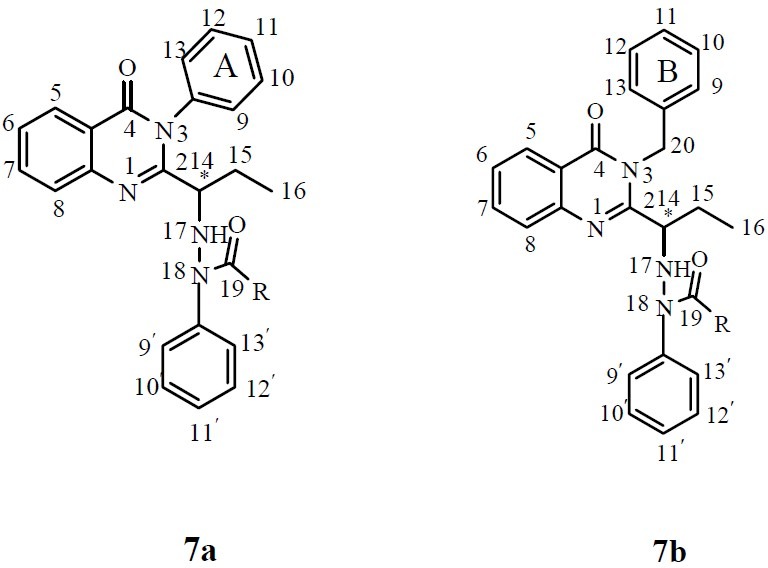
Chemical structures of compounds 7a and 7b
RESULTS
Details of preparation procedures and chemistry of synthesized compounds
Compounds 2 and 3 were prepared as described by Eissa and coworkers (14). Compound 4a was synthesized as reported by Kacker and coworkers(15) and compounds 4b, 5a and 5b were prepared based on the method used by Finer and coworkers(16).
3-phenyl -2- (1- (2-phenylhydrazinyl) propyl) quinazolin-4(3H)-one (6a)
Phenylhydrazin (3.62 ml, 0.04 mol) was added to a solution of compound 5a (3.43 g, 0.01 mol) in ethanol (15 ml). The reaction mixture was refluxed for 6 h. After cooling the mixture, the precipitated product was filtered off and crystallized from ethanol to obtain compound 6a, as white crystals, yield: 30%. m.p 216-217°C, (Found : M 370, C23H22N4O requires 370) νmax, 3309, 3265 (NH), 3051 (Ar-H), 2931, 2870 (R-H), 1660 (C=O) cm-1; 1HNMR δH (400 MHz; CDCl3) 8.33 (1H, d, J=8.0 Hz, H-C5 Ar), 7.83 (1H, t, J=8.0 Hz, H-C7 Ar), 7.78 (1H, d, J=8.0 Hz, H-C8 Ar), 7.55 (2H, t, J=6.8 Hz, H-C10, H-C12 Ar), 7.46 (1H, t, J=7.2 Hz, H-C6 Ar), 7.36 (1H, t, J=7.2 Hz, H-C11 Ar), 7.28(1H, d, J=7.6 Hz, H-C9 Ar), 7.18 (2H, t, J=7.6 Hz, H-C10’, H-C12’ Ar), 7.00(1H ,d, J=7.6 Hz, H-C13 Ar), 6.88 (2H, d, J=8.0 Hz, H-C9’, H-C13′ Ar), 6.79 (1H, t, J=7.2 Hz, H-C11’ Ar), 5.53 (1H, s, H-N18), 4.52 (1H, d, J=9.6 Hz, H-N17), 3.65-3.75 (1H, m, H-C14), 1.59-1.70(1H, m, H -C15-H’), 1.40-1.53 (1H, m, H-C15 - H’), 0.87 (3H, t, J=7.2 Hz, H-C16).
3-benzyl -2- (1- (2-phenylhydrazinyl) propyl) quinazolin-4(3H)-one (6b)
Phenylhydrazin (3.62 ml, 0.04 mol) and a solution of compound 5b (3.57 g, 0.01 mol) in ethanol (15 ml) were reacted as described for 6a to give 6b as white crystal, yield: 50%. m.p188-189°C, (Found: M 384, C24H24N4O requires 384) νmax, 3350, 3284 (NH), 3051 (Ar-H), 2933, 2871 (R-H), 1660 (C=O) cm-1; 1HNMR δH (400 MHz ; CDCl3 ) 8.34 (1H, d, J=8.0 Hz, H-C5 Ar), 7.80 (1H, t, J=8.0 Hz, H-C7 Ar), 7.72 (1H, d, J=8.0 Hz, H-C8 Ar), 7.52 (1H, t, J=7.2 Hz, H-C6 Ar), 7.08-7.23 (7H, m, C19 -C6H5, H-C10’, H-C12’ Ar), 6.83 (2H, d, J=8.0 Hz, H-C9’, H-C13’ Ar), 6.77(1H, t, J=7.2 Hz, H-C11’ Ar), 5.48(1H, d, J=16 Hz, H -C19 -H’) , 5.34 (1H, s, H-N18), 5.17(1H, d, J=15.6 Hz, H-C19 - H’), 4.62 (1H, d, J=9.2 Hz, H-N17), 4.07-4.17 (1H, m, H-C14), 1.48-1.70 (2H, m, H-C15), 1.09 (3H, t, J=7.2 Hz, H-C16).
General procedure for the preparation of final compounds 7a1-6
Various acid chlorides (1.1 mmol) were added drop wise to a solution of compound 6a (0.37 g, 1 mmol) in dichloromethane (40 ml) and triethylamine (0.21 ml, 1.5 mmol). The reaction mixture was stirred at 0°C for 4-5 h and purified by column or thin layer chromato-graphy to give the final compounds 7a1-6 as white or yellow powders, yields: 25-30%.
N’-(1-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)-N-phenylacetohydrazide (7a1)
The compound was purified by PTLC (chloroform: methanol; 100:1), m.p 123-124°C (Found: M 412, C25H24N4O2 requires 412) νmax, 3257(NH), 3064 (Ar-H), 2964, 2929 (R-H), 1689 (C=O) cm-1; 1HNMR δH (500 MHz ; CDCl3) 8.29 (1H, d, J=5.0 Hz, H-C5 Ar), 7.75-7.85 (2H, m, H-C7, H-C8 Ar), 7.44-7.55 (3H, m, H-C10, H-C12, H-C6, Ar), 7.25-7.40 (4H, m, H-C11, H-C9, H-C10’, H-C12’ Ar), 7.10-7.25 (4H, m, H-C13, H-C11’, H-C9’, H-C13’ Ar), 3.4-3.6 (1H, brs, H-C14), 1.8-2.1 (4H, m, C19 -CH3, H-C15-H’), 1.5-1.8 (1H, m, H-C15-H ’), 0.6-0.8 (3H, brs, H-C16).
N’-(1-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)-N-phenylbutyrohydrazide (7a2)
The compound was purified by PTLC (chloroform: methanol; 100:1), m.p 118-119°C (Found: M 440, C27H28N4O2 requires 440) νmax , 3280 (NH), 3064 (Ar-H), 2929, 2871 (R-H), 1687 (C=O) cm-1 ; 1HNMR δH (500 MHz ; CDCl3) 8.27 (1H, brs, H-C 5 Ar), 7.70-7.85 (2H, m, H-C7, H-C8 Ar), 7.40-7.55 (4H, m, H-C10, H-C12, H-C6 , H-C11 Ar), 7.22-7.40 (3H, m, H-C9, H-C10’, H-C12’ Ar),7.10-7.22 (4H, m, H-C13, H-C11’, H-C9’, H-C13’ Ar), 6.55 (1H, brs, H-N17), 3.4-3.5 (1H, brs, H-C14), 2.0-2.15 (2H, brs, C19-CH2), 1.85-1.95 (1H, m, H-C15-H’), 1.6-1.7 (3H, m, C19-CH2-CH2, H-C15 - H’), 0.75-0.85 (3H, brs, C19-CH2-CH2-C H3), 0.65-0.75 (3H, brs, H-C16)
N’-(1-(4-oxo-3-phenyl-3,4-dihydroquinazolin-2-yl)propyl)-N-phenylbenzohydrazide (7a3)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 110-111°C (Found: M 474, C30H26N4O2 requires 474) νmax, 3270 (NH), 3064 (Ar-H), 2966, 2927 (R-H), 1685(C=O) cm-1; 1HNMR δH (400 MHz ; CDCl3) 8.31(1H, d, J=8.0 Hz, H-C5 Ar), 7.75-7.85 (2H, brs, H-C7, H-C8 Ar), 7.35-7.55 (4H, m, H-C10, H-C12, H-C6, H-C11 Ar), 7.29 (1H, d, J=7.6 Hz , H-C9 Ar), 7.04-7.27 (9H, m, C19-C6H5, H-C10′, H-C12’, H-C13, H-C11’ Ar), 6.99 (2H, d, J=7.2 Hz, H-C9’, H-C13’ Ar), 6.50-6.65 (1H, brs, H-N17), 3.56 (1H, t, J=6.4 Hz, H-C14), 1.80-1.95 (1H, m, H -C15-H’), 1.60-1.75 (1H, m, H-C15- H’), 0.68 (3H, t, J=7.2 Hz, H-C16).
4- chloro-N’- (1- (4-oxo-3- phenyl-3, 4-dihydro quinazolin -2 -yl) propyl) -N- phenylbenzo hydraide (7a4)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 127-128°C (Found: M 509, C30H25ClN4O2 requires 509) νmax, 3251 (NH), 3070 (Ar-H), 2962,2873 (R-H), 1680 (C=O) cm-1; 1HNMR δH (400 MHz ; CDCl3) 8.31 (1H, d, J=8.0 Hz, H-C5 Ar), 7.77-7.90 (2H, m, H-C7, H-C8 Ar), 7.51 (2H, t, J=8.0 Hz, H-C10 , H-C12 Ar), 7.47(1H, t, J=7.2 Hz, H-C6 Ar), 7.4(1H, t, J=7.6 Hz, H-C11 Ar), 7.05-7.33(9H, m, H-C9, C19-C6 H4Cl, H-C10’ , H-C12’, H-C13, H-C 11’ Ar), 7.0 (2H, d, J=7.6 Hz, H-C9’, H-C13’ Ar), 3.55(1H, t, J=6.4 Hz, H-C14), 1.80-1.92 (1H, m, H-C15-H’), 1.65-1.80 (1H, m, H-C15-H’), 0.69 (3H, t, J=7.2 Hz, H-C16).
4- nitro-N’- (1- (4-oxo-3- phenyl-3, 4-dihydro quinazolin -2- yl) propyl) -N- phenylbenzo hydrazide(7a5)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 191-192°C (Found: M 519, C30H25N5O4 requires 519) νmax, 3288 (NH), 3064 (Ar-H), 2966, 2925 (R-H), 1685 (C=O), 1522, 1344 (NO2) cm-1; 1HNMR δH (500 MHz ; CDCl3) 8.34 (1H, d, J=7.9 Hz, H-C5 Ar), 8.02 (2H, d, J=8.3 Hz, C19 -C-CH-C H Ar), 7.8-7.9 (2H, m, H-C7, H-C8 Ar), 7.5-7.6 (2H, m, H-C10, H-C12 Ar), 7.48 (1H, t, J=7.2 Hz, H-C6 Ar), 7.43 (1H, t, J=7.5 Hz, H-C11 Ar), 7.15-7.35 (7H, m, C19 -C-C H ,H-C9, H-C10’, H-C12’ H-C13, H-C11’ Ar), 7.05 (2H, d, J=5.5 Hz, H-C9’, H-C13’ Ar), 3.55-3.62 (1H, m, H-C14), 1.85-1.97 (1H, m, H-C15-H’), 1.65-1.75 (1H, m, H-C15-H’), 0.69 (3H,t, J=5.0 Hz, H-C16).
3,5-dinitro-N’- (1-(4-oxo-3-phenyl-3,4-dihydro quinazolin -2 -yl) propyl) -N- phenylbenzo hydrazide (7a6)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 208-209°C (Found: M 564, C30H24N6O6 requires 564) νmax, 3240 (NH), 3095 (Ar-H), 2968, 2927 (R-H), 1684 (C=O), 1541, 1344 (NO2) cm-1; 1HNMR δH (500 MHz; CDCl3) 8.94 (1H, s, C19-C-CH-CNO2-CH-CNO2 Ar), 8.2-8.4 (3H, m, H-C5, C19-C-C H Ar), 7.8-7.9 (2H, m, H-C7, H-C8 Ar), 7.50-7.64 (3H, m, H-C10, H-C12, H-C6, Ar), 7.47(1H, t, J=7.5 Hz, H-C11 Ar), 7.05-7.40 (7H, m, H-C9 , H-C10’, H-C12’, H-C13, H-C11’, H-C9’, H-C13’ Ar), 3.5-3.64 (1H,m, H-C14), 1.8-2.0 (1H, m, H-C15-H), 1.60-1.75 (1H, m, H-C15-H’), 0.6-0.8 (3H, brs, H-C16).
General procedure for the preparation of final compounds 7b1-7
To a solution of compound 6b (0.384 g, 1 mmol) in dichloromethane (40 ml) was added triethylamine (0.21 ml, 1.5 mmol) and different acid chlorides (1.1 mmol). The reaction mixture was stirred at 0°C for 4-5 h and was purified by column or thin layer chro-matography to give the final compounds 7b1-7 as white or yellow powders, yields: 25-30%.
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)propyl)-N-phenylacetohydrazide (7b1)
The compound was purified by PTLC (chloroform: methanol; 100:1) m.p 149-150°C (Found: M 426, C26H26N4O2 requires 426) νmax, 3244 (NH), 3064 (Ar-H), 2968, 2870 (R-H), 1674 (C=O) cm-1; 1HNMR δH (400 MHz ; CDCl3 ) 8.29 (1H, d, J=7.6 Hz, H-C5 Ar), 7.65-7.78 (2H, m, H-C7, H-C8 Ar), 7.48(1H, t, J=7.6 Hz, H-C6 Ar), 7.17-7.40 (6H, m, H-C10’, H-C11’, H-C12’, H-C10, H-C11, H-C12 Ar), 7.12 (2H, d, J=5.6 Hz, H-C9’, H-C13’ Ar), 6.75-6.85 (2H, m, H-C9, H-C13 Ar), 6.57 (1H, d, J=5.6 Hz, H-N17), 5.57 (1H, d, J=16 Hz, H-C20-H’), 4.92 (1H, d, J=15.2 Hz, H-C20-H’), 3.85 (1H, dd, J=7.6 Hz,J=6.8 Hz, H-C14), 1.90-2.05 (1H, m, H-C15-H’), 1.8-1.9 (3H, m, C19-C H3) 1.6-1.8 (1H, m, H-C15-H’), 0.5-0.6 (3H, brs, H-C16).
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)propyl)-N-phenylbutyrohydrazide (7b2)
The compound was purified by PTLC (chloroform: methanol; 100:1), m.p 168-169°C (Found: M 454, C28H30N4O2 requires 454) νmax, 3246 (NH), 3055 (Ar-H), 2964, 2927 (R-H), 1668 (C=O) cm-1; 1HNMR δH (500 MHz ; CDCl3) 8.29 (1H, d, J=7.5 Hz, H-C5 Ar), 7.6-7.8 (2H, m, H-C7, H-C8 Ar), 7.48 (1H, t, J=7.5 Hz, H-C6 Ar ),7.2-7.4 (6H, m, H-C10’, H-C11’, H-C12’, H-C10, H-C11, H-C12 Ar), 7.05-7.2 (2H, brs, H-C9’, H-C13’ Ar), 6.75-6.90 (2H, brs, H-C9, H-C13 Ar),6.5-6.7(1H. brs, H-N17), 5.62 (1H, d, J=17.0 Hz, H-C20-H’), 4.88 (1H, d, J=14.0 Hz, H-C20-H’), 3.80-3.95 (1H, m, H-C14), 1.9-2.2 (3H, m, C19-CH2, H-C15-H’), 1.5-1.8 (3H, m, H-C15-H’, C19-CH2-CH2), 0.75-0.90 (3H, brs, C19-CH2-CH2-C H3), 0.5-0.7 (3H, brs, H-C16).
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)propyl) -N-phenylbenzohydrazide (7b3)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 166-167°C (Found: M 488, C31H28N4O2 requires 488) νmax, 3242 (NH), 3057 (Ar-H), 2931, 2873 (R-H) cm-1; 1HNMR δH (400MHz; CDCl3) 8.31 (1H, d, J=8.0 Hz, H-C5 Ar), 7.70-7.80 (2H, m, H-C7, H-C8 Ar), 7.48 (1H, t, J=7.6 Hz, H-C6 Ar), 7.1-7.3 (11H, m, C19-C6H5, H-C10’, H-C11’, H-C12’, H-C10, H-C11, H-C12 Ar), 7.01 (2H, d, J=7.6 Hz, H-C9’, H-C 13’ Ar), 6.9 (2H, d, J=6.4 Hz, H-C9, H-C13 Ar), 6.5-6.7 (1H, brs, H-N17), 5.67 (1H, d, J=16.4 Hz, H-C20-H’), 4.96 (1H, d, J=16 Hz, H-C20-H’), 4.05 (1H, t, J=6.4 Hz, H-C14), 1.9-2.2 (1H, m, H-C15-H’), 1.70-1.82 (1H, m, H-C15-H’), 0.53 (3H, t, J=7.2 Hz, H-C16).
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)propyl)-4-chloro-N-phenylbenzohydrazide (7b4)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 130-131°C (Found: M 523, C31H27ClN4O2 requires 523) νmax, 3249 (NH), 3068 (Ar-H), 2962, 2873 (R-H), 1680 (C=O) cm-1 ; 1HNMR δH (400 MHz ; CDCl3) 8.32 (1H, d, J=8.0 Hz, H-C5 Ar), 7.67-7.80 (2H, m, H-C7, H-C8 Ar), 7.5 (1H, t, J=6.8 Hz, H-C6 Ar), 7.08-7.30 (10H, m, C19-C6H4Cl, H-C10’, H-C11’, H-C12’, H-C10, H-C11, H-C12 Ar), 6.97 (2H, d, J=7.2 Hz, H-C9’, H-C13’ Ar), 6.91 (2H, d, J=6.0 Hz, H-C9, H-C13 Ar), 6.59 (1H, d, J=7.2 Hz, H-N17), 5.58 (1H, d, J=16.4 Hz, H-C20-H’), 5.0 (1H,d, J=15.6 Hz, H-C20- H′), 3.97-4.1 (1H, m, H-C14), 1.85-2.0 (1H, m, H -C15-H’) ,1.65-1.80 (1H, m, H-C15-H’), 0.55 (3H, t, J=7.2 Hz, H-C16).
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)propyl)-N,2-diphenylacetohydrazide (7b5)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 165-166°C (Found: M 502, C32H30N4O2 requires 502) νmax, 3290 (NH), 3055 (Ar-H), 2925, 2852 (R-H), 1676 (C=O) cm-1; 1HNMR δH (500 MHz; CDCl3) 8.2-8.4 (1H, brs, H-C5 Ar), 7.6-7.8 (2H, m, H-C7, H-C8 Ar), 7.48 (1H, t, J=7.5 Hz, H-C6 Ar), 7.10-7.45 (9H, m, H-C10’, H-C11’, H-C12’, H-C10, H-C11, H-C12, C19-CH2-C-CH-CH-CH Ar), 6.95-7.1 (4H, m, H-C9’, H-C13’, C19-CH2-C-C H Ar), 6.75-6.9 (2H, brs, H-C9 , H-C13 Ar), 5.61 (1H, d,J=17 Hz, H -C20-H’) , 4.8 (1H, d, J=12.5 Hz, H-C20- H’), 3.85-3.95 (1H, m, H-C14), 3.4-3.6 (2H, brs, C19-CH2), 1.85-2.0 (1H, m, H-C15-H’), 1.6-1.8 (1H, m, H-C15-H’), 0.5-0.6 (3H, brs, H-C16).
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)propyl)-4-nitro-N-phenylbenzohydrazide (7b6)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 131-132°C (Found: M 533, C31H27N5O4 requires 533) νmax , 3275 (NH), 3064 (Ar-H), 2928, 2871(R-H), 1674 (C=O), 1522, 1344 (NO2) cm-1; 1HNMR δH (500 MHz ; CDCl3) 8.35 (1H, d, J=7.5 Hz, H-C5 Ar), 8.04 (2H, d, J=8.0 Hz, C19-C-CH-C H-CNO2 Ar), 7.75-7.85 (2H, m, H-C7, H-C8 Ar), 7.54 (1H, t, J=8.0 Hz H-C6 Ar), 7.15-7.40 (8H, m, C19 -C-CH, H-C10’, H-C11’, H-C12’ , H-C10, H-C11, H-C12 Ar), 6.92-7.05(4H, m, H-C9’, H-C13’, H-C9, H-C13 Ar), 5.5 (1H, d, J=15 Hz, H -C20 -H’), 5.12 (1H, d, J=15 Hz, H-C20 - H’), 4.0-4.1 (1H, brs, H-C14), 1. 9-2.0 (1H, m, H -C15-H’), 1.75-1.9 (1H, m, H-C15-H’), 0.55-0.65 (3H, brs, H-C16).
N’- (1- (3-benzyl-4-oxo-3,4-dihydroquinazolin-2- yl) propyl) -3, 5- dinitro -N- phenylbenzo hydrazide (7b7)
The compound was purified by column chromatography (petroleum ether: ethyl acetate; gradient), m.p 120-121°C (Found: M 578, C31H26N6O6 requires 578) νmax, 3267 (NH), 3095 (Ar-H), 2927, 2873 (R-H), 1666 (C=O), 1539, 1344 (NO2) cm-1; 1HNMR δH (500 MHz ; CDCl3) 8.94 (1H, s, C19-C-CH-CNO2-CH-CNO2 Ar), 8.2-8.6 (3H, m, C19 -C-C H -NO2, H-C 5 Ar), 7.80-7.92 (2H, m, H-C7, H-C8 Ar), 7.58 (1H, t, J=7.4 Hz, H-C6 Ar), 7.27-7.45(6H, m, H-C10’, H-C 11’, H-C12’, H-C10, H-C11, H-C12 Ar), 7.2 (2H, d, J=6.0 Hz, H-C9’, H-C13’ Ar), 7.1 (2H, d, J=6.0 Hz, H-C9, H-C13 Ar), 5.4-5.6 (1H, brs, H-C20-H’), 5.1-5.3 (1H, brs, H-C20-H’), 4.0-4.2 (1H, brs, H-C14), 1.75-2.0 (1H, m, H-C15-H’), 1.5-1.7 (1H, m, H-C15-H’), 0.65-0.80 (3H, brs, H-C16).
DISCUSSION
To prepare N-butyryl anthranilic acid 2, anthranilic acid 1 was treated with butyryl chloride in a nucleophilic substitution reaction.
In the second step, the amide 2 was reacted with acetic anhydride to accelerate ring closure and water removal to get 1,3 benzoxazine-4(one) 3 as a crystalline product.
In the third step 1,3 benzoxazine-4(one) 3 was refluxed with two different amines to give the corresponding quinazolinone 4a and 4b as a result of a nucleophilic substitution and subsequently dehydration of compound.
Brominated quinazolinones 5a and 5b (Fig. 3) were prepared by treating the quinazolinones with bromine in glacial acetic acid. After bromination, a chiral centre was introduced at the position 14 of the propyl side chain. This chiral centre also presents in all compounds synthesized from 5a and 5b as illustrated in Fig. 4, 5.
According to 1HNMR data, aromatic hydrogens at positions 9 and 13 of the phenyl ring (ring A) are seen as two separate doublets in 1 HNMR spectra due to the neighboring effects of this chiral center (Fig. 4, 5). This neighboring effect is also observed for two hydrogens of the benzylic CH2 next to the ring B and the aliphatic CH2 at position 15 of the propyl side chain (Fig. 4, 5).
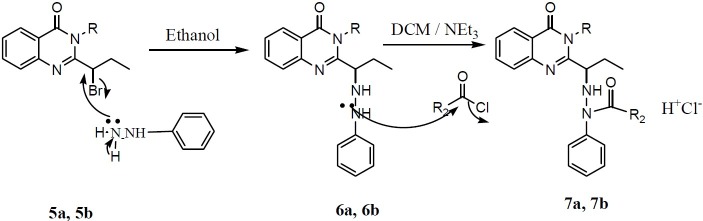
In the fifth step, treatment of brominated quinazolinone 5a and 5b with phenyl hydrazine afforded compounds 6a and 6b. In this step, the Br atom has been displaced as a leaving group with NH group of phenyl hydrazine as a result of a nucleophilic substitution (Fig. 6).
Fig. 6.
The proposed mechanism for the production of final compounds
To obtain the final compounds, compounds 6a and 6b were reacted with different acid chlorides via a nucleophilic substitution (Fig. 6).
From two available positions on hydrazine for acylation, substitution of acyl group on nitrogen atom next to the phenyl ring, position 18 (Fig. 5), was confirmed by 1HNMR. The singlet hydrogen's signal of phenyl-bonded NH has been disappeared after substitution and doublet hydrogen's signal of CH-bonded NH, position 17, has been shifted to down field region due to the deshielding effect of carbonyl functional group
CONCLUSION
In the current work, quinazolinones as biologically active substances were conjugated with another well known moiety (phenyl hydrazine) in a multi step reaction procedure to produce interesting novel hydrazide derivatives of quinazolinone. These compounds will be subjected to various biological evaluations to investigate their possible activities.
ACKNOWLEDGMENT
This work has been performed in the Department of Medicinal Chemistry and financially supported by the Research Council of Isfahan University of Medical Sciences.
REFERENCES
- 1.Khosropour RA, Mohammadpoor-Baltork I, Ghorbankhani H. Bi (TFA) 3-[nbp] FeCl4: a new, efficient and reusable promoter system for the synthesis of 4 (3H)-quinazolinone derivatives. Tetrahedron Lett. 2006;47:3561–3564. [Google Scholar]
- 2.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis and antimicrobial activity of some novel substituted 2-imidazolyl-N-(4-oxo-quinazolin-3(4H)-yl) acetamides. Chem Pharm Bull. 2007;55:1615–1619. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 3.Philipova I, Dobrikov G, Krumova K, Kaneti J. Convenient synthesis of some 2 substituted 4 (3H) quinazolinone derivatives. J Heterocyclic Chem. 2006;43:1057–1063. [Google Scholar]
- 4.Laddha SS, Wadodkar SG, Meghal SK. Studies on some biologically active substituted 4 (3H)-quinazolinones. Part 1. Synthesis, characterization and anti-inflammatory-antimicrobial activity $ of 6, 8-disubstituted 2-phenyl-3-[substituted-benzothiazol-2-yl]-4 (3H)-quinazolinones. Arkivoc. 2006;11:1–20. [Google Scholar]
- 5.Zhou Y, Murphy DE, Sun Z, Gregor VE. Novel parallel synthesis of N- (4-oxo-2-substituted -4- H-quinazolin -3- yl) -substituted sulfonamides. Tetrahedron Lett. 2004;45:8049–8051. [Google Scholar]
- 6.Dinakaran M, Selvam P, DeClercq E, Sridhar SK. Synthesis, antiviral and cytotoxic activity of 6-bromo-2, 3-disubstituted-4 (3H)-quinazolinones. Biol Pharm Bull. 2003;26:1278–1282. doi: 10.1248/bpb.26.1278. [DOI] [PubMed] [Google Scholar]
- 7.Nanda AK, Ganguli S, Chakraborty R. Antibacterial activity of some 3-(Arylideneamino)-2-phenyl quinazoline-4 (3H)-ones: synthesis and preliminary QSAR studies. Molecules. 2007;12:2413–2426. doi: 10.3390/12102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghabrial SS, Gaber HM. Dipolar cycloaddition reactions with quinazolinones: A new route for the synthesis of several annelated pyrrolo and pyridazinoquinazoline derivatives. Molecules. 2003;8:401–410. [Google Scholar]
- 9.Refaie FM, Esmat AY, Gawad SMA, Ibrahim AM, Mohamed MA. The antihyperlipidemic activities of 4 (3H) quinazolinone and two halogenated derivatives in rats. Lipids Health Dis. 2005;4:22. doi: 10.1186/1476-511X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mourad AE, Aly AA, Farag HH, Beshr EA. Microwave assited synthesis of triazoloquinazolinones and benzimidazoqinazolinones. Beilstein J Org Chem. 2007;3:1–5. doi: 10.1186/1860-5397-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mhaske SB, Argade NP. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron. 2006;62:9787–9826. [Google Scholar]
- 12.Connolly DJ, Cusack D, O’Sullivan TP, Guiry PJ. Synthesis of quinazolinones and quinazolines. Tetrahedron. 2005;61:10153–10202. [Google Scholar]
- 13.Brown DJ. Quinazolines (Supplement I) New York: John Wiley & Sons; 1997. pp. 1–5. [Google Scholar]
- 14.Eissa A, El-Mebvally A, El-Hashash M, El-Gohary A. Synthesis and biological evaluation of some new 2-propyl-4 (3H)-quinazolinone derivatives as antibacteria. J Korean Chem Soc. 2008;52:328–337. [Google Scholar]
- 15.Kacker IK, Zaheer SH. Potential analgesics. I. Synthesis of substituted 4-quinazolones. J Indian Chem Soc. 1951;28:344–346. [Google Scholar]
- 16.Finer JT, Bergnes G, Smith WW, Chabala JC, Feng B. Methods and Compositions utilizing quinazolinones. US Patents. 2003 6545004. [Google Scholar]