Abstract
Derivatives of pyridine-4-one are iron chelators with various biological activities including antifungal, anti-malarial, antiviral, anti-inflammatory and analgesic activities. This study was aimed to evaluate the analgesic effect of four new derivatives of 3-hydroxy pyridine-4-one in animal models. Acetic acid-induced writhing and formalin tests were used to assess the analgesic activity in male mice (18-22 g). Compound A (2.5-10 mg/kg), B (100-400 mg/kg), C and D (50-200 mg/kg) were administered intraperitoenally. Indomethacin and morphine were used as reference analgesic drugs. All tested compounds showed significant analgesia in acetic acid-induced writhing and the second phase of formalin test. All compounds except compound B also had analgesic activity in the first phase of formalin test. Among the investigated compounds, compound A which showed analgesic activity at doses of 2.5-10 mg/kg considered as the most potent analgesic compound. Structurally compound A lacks polar moieties such as −OH or −COOH and is less polar than the others. Therefore it seems that it has a better penetration into lipophilic sites of action.
Keywords: 3-Hydroxy-pyridine-4-one, Analgesic, Acetic acid-induced writhing, Formalin test
INTRODUCTION
Compounds with 4(1H) pyridineone structure have been synthesized for pharmacological evaluation of antifungal, anti-malarial, antiviral, anti-inflammatory and analgesic activities(1–4). The most important activity regarding their analgesic and anti-inflammatory actions belong to their iron chelating properties(5). Previous findings suggested that iron chelators are capable of inhibiting prostanoid synthesis in intact tissue through the removal or binding of Fe3+ linked to cyclo-oxygenase. These iron chelators may be of therapeutic value in the treatment of painful and other diseases via two mechanisms: 1- Inhibition of pro-inflammatory prostanoid synthesis and 2- Inhibition of toxic free radical generation by cyclo-oxygenase(6). Also it has been reported that desferrioxamine and 1,2-dimethyl-3-hydroxypyrid-4-one (L1) as important iron chelators inhibited human platelet aggregation in vitro as well as thromboxane A2 synthesis and conversion of arachidonate to lipoxygenase-derived products(7). Lipooxygenase is another key enzyme in inflammation pathway that produces leukotrienes(8). Lipooxygenase is a non-heme iron containing enzyme(9). Among the pharmacological properties of 4(1H)-pyridinone derivatives, the analgesic effects and the anti-inflammatory activity in the carrageenan-induced rat paw edema could suggest inhibition of prostaglandin E and arachidonic acid synthesis(10). Since compounds with 3-hydroxy-pyridine-4-one structure have iron chelating activity, this study was designed to evaluate the analgesic activity of four new derivatives of these compounds in animal models.
MATERIALS AND METHODS
Animals
Experiments were performed on male Swiss mice, weighing 18-22 g. All animals were maintained in standard laboratory conditions in the animal house of School of Pharmacy, Isfahan University of Medical Sciences (Isfahan, Iran). Animals were euthanized immediately after each experiment. All experiments were carried out in accordance with local guidelines for the care of laboratory animals of Isfahan University of Medical Sciences (Isfahan, Iran).
Chemicals
Four derivatives of 4(1H)-pyridinone (compounds A, B, C and D) as shown in Fig. 1 which had been synthesized in Department of Medicinal Chemistry, School of Pharmacy, Isfahan University of Medical Sciences (Isfahan, Iran) were used (11).
Fig. 1.
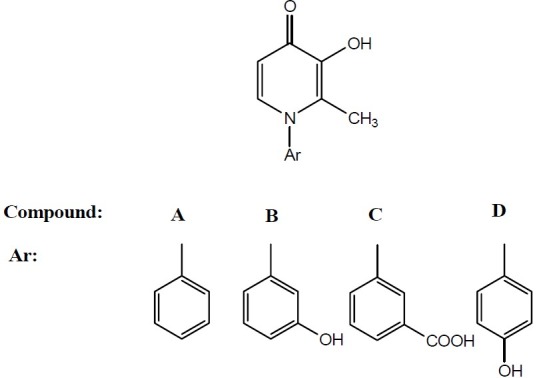
Chemical structures of four derivatives of 4(1H)-pyridinone (compounds A, B, C and D) which were used to test their analgesic activities.
Indomethacin (Sigma, USA) and morphine hydrochloride (Darou Pakhsh, Iran) were used as reference analgesic drugs. At first a trial dose (100 mg/kg) of each compound was assessed for analgesic activity and the other doses were selected based on the results of the trial dose. Compound A showed toxicity at the trial dose and a one-tenth dose (10 mg/kg) was considered as the maximum dose for this compound.
Acetic acid-induced writhing test
Modified acetic acid writhing test for screening analgesic activity was used(12,13). Compounds A, B, C and D were suspended in 1% aqueous solution of tween 80 (v/v) and administered intraperitoenally to mice. Control animals received vehicle (10 ml/kg) and the reference group received indomethacin (10 mg/kg). Thirty min later, all animals received acetic acid (10 ml/kg of 1% acetic acid in saline solution, i.p.). Immediately after the injection of acetic acid, each animal was isolated in an individual box and abdominal constrictions counted during a 10 min period starting 10 min after acetic acid injection(11). Percent inhibition was calculated using the following formula:
Inhibition (%)=((Number of twitches in control group – Number of twitches in test group) / Number of twitches in control group) * 100
Formalin test
Twenty microliter of 2.5% formalin (v/v in 0.9% saline) was injected into the subplantar space of the right hind paw of mice 30 min after i.p. injection of above-mentioned doses of the test compounds, vehicle or reference drug (morphine, 10 mg/kg). The time that animals spent for licking the injected paw was recorded and compared. Two distinct periods of intensive licking activity were identified. The initial phase (acute phase) was 0-5 min and the second phase (chronic phase) was 20-30 after formalin injection(14,15).
Statistical analysis
The results were expressed as mean ± SEM. The data obtained in experimental groups were analyzed by one way analysis of variance (ANOVA) followed by Scheffe post hoc test. P values less than 0.05 were considered significant.
RESULTS
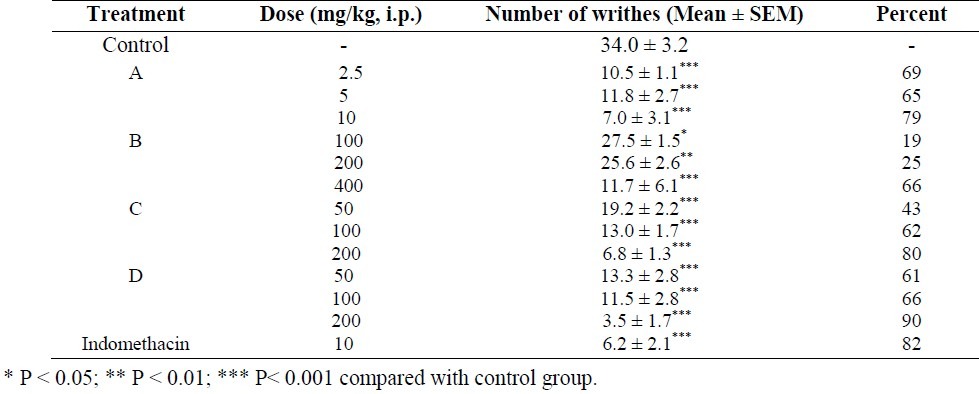
Four new derivatives of hydroxyl pyridinone (compounds A, B, C and D) were investigated for their analgesic effect. As it is seen in Table 1, indomethacin as a standard analgesic drug inhibited acetic acid-induced abdominal constrictions by 82%. The tested compounds also showed significant analgesia in this test. The maximum applied dose of compounds A (10 mg/kg), B (400 mg/kg), C and D (200 mg/kg) produced 79%, 66%, 80% and 90% inhibition of acetic acid-induced twitches respectively.
Table 1.
Effect of 3-hydroxy pyridine-4-one derivatives on acetic acid-induced writhing in mice (n=6)
In formalin test, compound A at doses of 5 and 10 mg/kg significantly (p<0.01) reduced licking behavior of the acute phase of formalin test. It also significantly (p<0.001) inhibited the pain response of the second phase of formalin at all applied doses. Morphine as the reference drug inhibited the pain behavior of both phases by 92% and 97% respectively (Fig. 2). The analgesic activity of compound B has been shown in Fig. 3. This compound at doses of 100, 200 and 400 mg/kg could not exert any significant pain relieving effect in the first phase of formalin test, but its analgesic effect was significant (p<0.01) in the second phase. As it is seen in Fig. 4, compound C only at the maximum dose (200 mg/kg) could suppress the licking behavior of both phases of formalin test by 39% and 83%, respectively. The results of the analgesic activity of compound D is illustrated in Fig. 5. This compound at doses of 100 and 200 mg/kg significantly (p<0.001) inhibited the pain response of both phases.
Fig. 2.
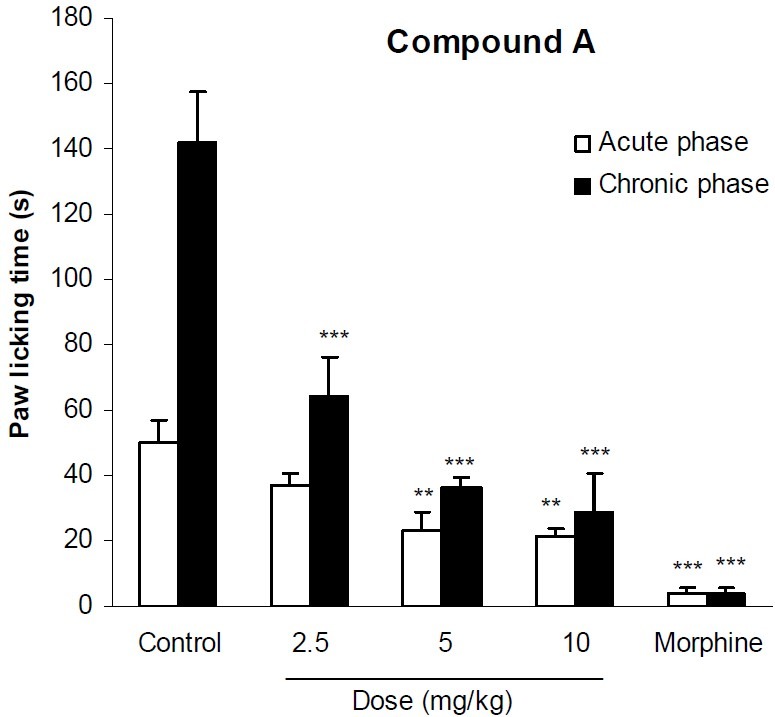
The analgesic activity of compound A in formalin test. Vehicle and different doses of the compound A were administered 30 min prior to subplantar injection of formalin and the time spent for licking was measured during a 0-5 min (acute phase) and 20-30 min (chronic phase) period starting after formalin injection. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM of 6 animals in each group. ** P <0.01; *** P < 0.001 significantly different from control group (ANOVA with Scheffe post hoc test).
Fig. 3.
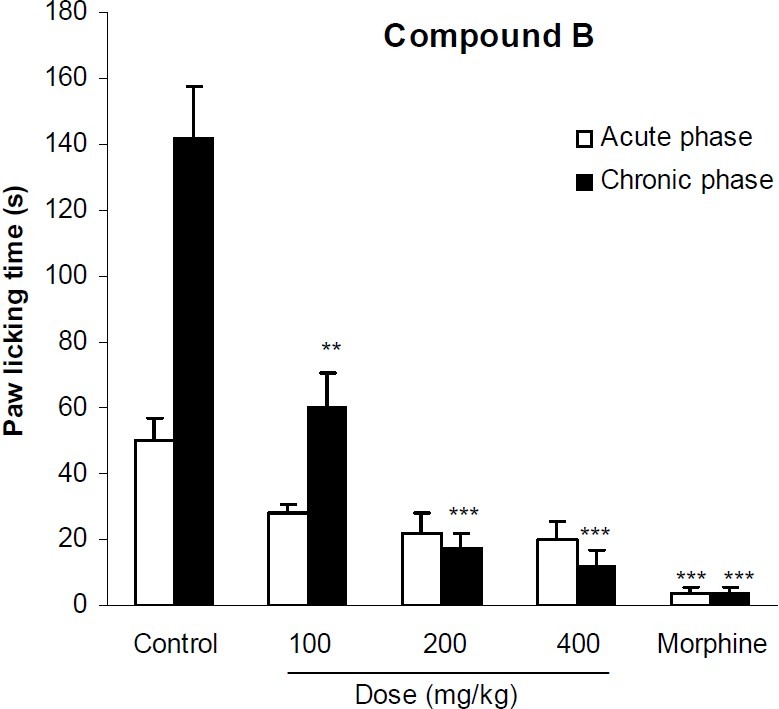
The analgesic activity of compound B in formalin test. Vehicle and different doses of the compound B were administered 30 min prior to subplantar injection of formalin and the time spent for licking was measured during a 0-5 min (acute phase) and 20-30 min (chronic phase) period starting after formalin injection. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM of 6 animals in each group. ** P <0.01; *** P < 0.001 significantly different from control group (ANOVA with Scheffe post hoc test).
Fig. 4.
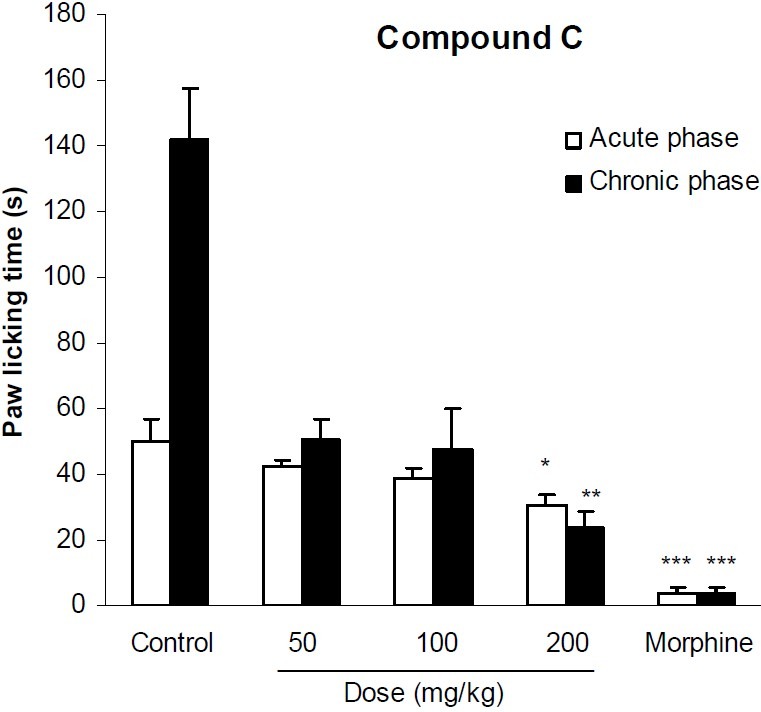
The analgesic activity of compound C in formalin test. Vehicle and different doses of the compound C were administered 30 min prior to subplantar injection of formalin and the time spent for licking was measured during a 0-5 min (acute phase) and 20-30 min (chronic phase) period starting after formalin injection. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM of 6 animals in each group. * P <0.05; ** P < 0.01; *** P < 0.001 significantly different from control group (ANOVA with Scheffe post hoc test).
Fig. 5.
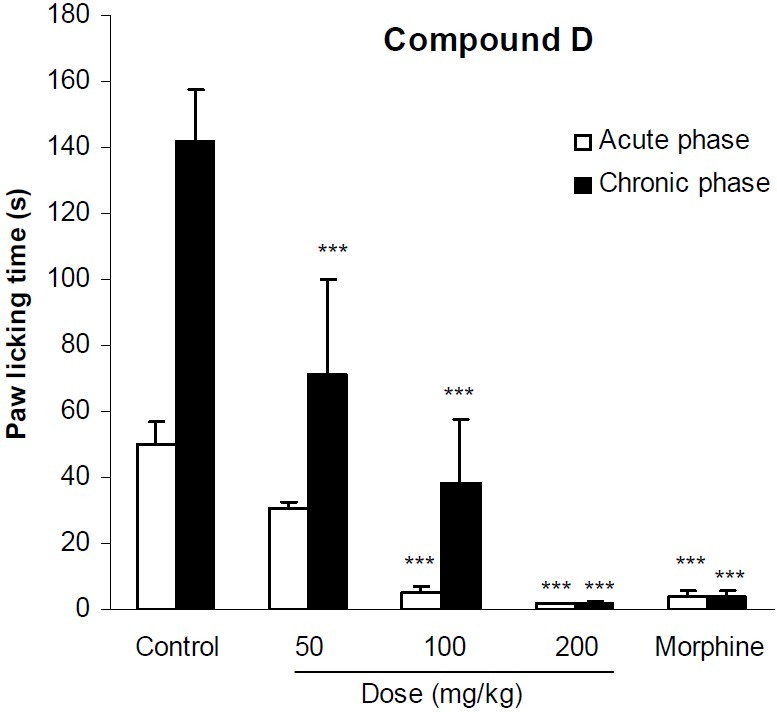
The analgesic activity of compound D in formalin test. Vehicle and different doses of the compound D were administered 30 min prior to subplantar injection of formalin and the time spent for licking was measured during a 0-5 min (acute phase) and 20-30 min (chronic phase) period starting after formalin injection. Morphine (10 mg/kg, i.p.) was used as reference drug. Data are mean ± SEM of 6 animals in each group. *** P < 0.001 significantly different from control group (ANOVA with Scheffe post hoc test).
DISCUSSION
In this study, four new derivatives of 3-hydroxy-pyridine-4-one were evaluated for their possible analgesic activity. As it is seen in result section, all compounds showed significant analgesia in both acetic acid and formalin tests. Acetic acid-induced writhing is a non-specific pain model and many compounds belonging to diverse pharmacological categories including opioids, non-steroidal anti-inflammatory drugs, calcium channel blockers, anticholinergics, antihistamines, and cortico-steroids show analgesic activity in this test(15). Acetic acid test which is a visceral pain model produces a painful reaction and acute inflammation in the peritoneal area. Release of arachidonic acid, and biosynthesis of prostaglandin via cyclooxygenase pathway plays a role in the nociceptive mechanism of this test(16). The analgesic effect of the tested compounds may be mediated through inhibition of cyclooxygenase and/or lipooxygenase (and other inflammatory mediators)(15,17). However, further studies are needed to elucidate the exact mechanism of analgesic activity of these compounds in this test.
The formalin test is considered as a more specific model which evaluates both acute and chronic pain(18). In this test, animals present two distinct nociceptive behavioral phases, which probably involves different stimulating signals. The early phase initiates immediately after formalin injection and lasts about 5 min, resulting from chemical stimulation of nociceptors. The late phase initiates 15-20 min after formalin injection and lasts for about 20-40 min. It has been reported that bradykinin is involved in the early phase, while histamine, 5-hydroxytryptamine (5-HT), prostaglandins and bradykinin are the main mediators of the late phase(14). Also it has been reported that the late phase is dependent on the combination of an inflammatory reaction in the peripheral tissue and functional changes in the dorsal horn of the spinal cord. In this experiment, the analgesic effect of experimental compounds was more significant in the late phase, which indicates an action related to the inflammatory process. The results of the present study are in agreement with previous studies(3,5,10) and indicate that 3-hydroxy-pyridine-4-one have analgesic and anti-inflammatory effects.
Compounds with 3-hydroxy-pyridine-4-one structure have iron chelating properties(19). Since cyclooxygenase and lipooxygenase enzymes, as key enzymes of inflammation and pain are depended on iron(6,9) perhaps the tested compounds inhibit these enzymes by chelating iron and result in analgesic and anti-inflammatory effects. Also It has been reported that iron chelators can strongly induce transcription of NO synthase (NOS) and increase the release of interleukin-1β (IL-1β) in human alveolar macrophages(20,21). These observations suggest that iron chelators may modulate certain inflammatory mediators and regulate inflammatory processes. Iron chelators have potent antioxidant activity and since free radicals are involved in the inflammatory process(22), therefore the antioxidant properties of the tested compounds might have some role in their anti-inflammatory effect. Treatment of beta-thalassemia patients with iron chelators, deferasirox or desferrioxamine resulted in lower concentrations of malondi-aldehyde and high-sensitivity C-reactive protein which are biomarkers of oxidative-stress and inflammation respectively((23). These results clearly indicate that iron is involved in both processes. Although further investigations are needed to present a definitive mechanism of action for the analgesic and anti-inflammatory effects of the applied compounds, it seems that iron chelating and consequent antioxidant activity of the compounds have important roles in their observed pharmacological effects.
Among the investigated compounds, compound A at the doses of 2.5-10 mg/kg inhibited acetic acid-induced writhing and formalin-induced nociceptive behavior and considered as the most potent analgesic compound. When compound A is structurally compared with other compounds, it lacks polar moieties such as −OH or −COOH. Therefore compound A is less polar than the others and it seems that it has a better penetration into lipophilic sites of action. Consistent with our results, Abeysinghe and coworkers have shown that more lipophilic iron chelators reveal more potent inhibition of lipooxygenase(9).
CONCLUSION
All of four compounds which had been designed and synthesized as iron chelators showed analgesic activity in acetic acid and formalin tests and compound A which was less polar, was more potent than the others. Further studies are needed to find out the exact mechanism(s) of these compounds.
ACKNOWLEDGMENT
This work was supported by the Research Council of Isfahan University of Medical Sciences, Isfahan, I.R.Iran.
REFERENCES
- 1.Hershko C, Theanacho EN, Spira DT, Peter HH, Dobbin P, Hider RC. The effect of N-alkyl modification on the antimalarial activity of 3-hydroxypyridin-4-one oral iron chelators. Blood. 1991;77:637–643. [PubMed] [Google Scholar]
- 2.Le Van K, Cauvin C, De Walque S, Georges B, Boland S, Martinelli V, et al. New pyridinone derivatives as potent HIV-1 nonnucleoside reverse transcriptase inhibitors. J Med Chem. 2009;52:3636–3643. doi: 10.1021/jm801438e. [DOI] [PubMed] [Google Scholar]
- 3.Aytemir MD, Uzbay T, Erol DD. New 4(1H)-pyridinone derivatives as analgesic agents. Arzneimittelforschung. 1999;49:250–254. doi: 10.1055/s-0031-1300409. [DOI] [PubMed] [Google Scholar]
- 4.Saelens JK, Bernard PS, Wilson DE. Baclofen as an analgesic. Brain Res Bull. 1980;5:553–557. [Google Scholar]
- 5.Ozturk G, Erol DD, Aytemir MD, Uzbay T. New analgesic and antiinflammatory agents 4(H)-pyridinone derivatives. Eur J Med Chem. 2002;37:829–834. doi: 10.1016/s0223-5234(02)01390-9. [DOI] [PubMed] [Google Scholar]
- 6.Jeremy JY, Kontoghiorghes GJ, Hoffbrand AV, Dandona P. The iron chelators desferrioxamine and 1-alkyl-2-methyl-3-hydroxypyrid-4-ones inhibit vascular prostacyclin synthesis in vitro. Biochem J. 1988;254:239–244. doi: 10.1042/bj2540239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barradas MA, Jeremy JY, Kontoghiorghes GJ, Mikhailidis DP, Hoffbrand AV, Dandona P. Iron chelators inhibit human platelet aggregation, thromboxane A2 synthesis and lipoxygenase activity. FEBS Lett. 1989;245:105–109. doi: 10.1016/0014-5793(89)80201-7. [DOI] [PubMed] [Google Scholar]
- 8.Brunton LL, Lazo JS, Parker KL. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 655–657. [Google Scholar]
- 9.Abeysinghe RD, Roberts PJ, Cooper CE, MacLean KH, Hider RC, Porter JB. The environment of the lipoxygenase iron binding site explored with novel hydroxypyridinone iron chelators. J Biol Chem. 1996;271:7965–7972. doi: 10.1074/jbc.271.14.7965. [DOI] [PubMed] [Google Scholar]
- 10.Ozturk G, Erol DD, Uzbay T, Aytemir MD. Synthesis of 4(1H)-pyridinone derivatives and investigation of analgesic and anti-inflammatory activities. Farmaco. 2001;56:251–256. doi: 10.1016/s0014-827x(01)01083-7. [DOI] [PubMed] [Google Scholar]
- 11.Nikazma A. Synthesis of 1-aryl-2-methyl-3- hydroxypyridine-4-one derivatives as iron chelators. School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, Isfahan, A PharmD Thesis. 2001:33–36. [Google Scholar]
- 12.Erol DD, Sunal R, Duru S. Synthesis and screening of analgesic and anti-inflammatory activity of 6- acyl -3- (4-substituted benzoylmethyl) -2 (3H)-benzoxazolones. Arzneimittelforschung. 1990;40:478–480. [PubMed] [Google Scholar]
- 13.Hayashi G, Takemori AE. The type of analgesic-receptor interaction involved in certain analgesic assays. Eur J Pharmacol. 1971;16:63–66. doi: 10.1016/0014-2999(71)90057-4. [DOI] [PubMed] [Google Scholar]
- 14.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 15.Vogel HG, Vogel WH. Drug Discovery and Evaluation. 1st ed. Berlin: Springer; 1997. pp. 376–377. [Google Scholar]
- 16.Franzotti EM, Santos CV, Rodrigues HM, Mourao RH, Andrade MR, Antoniolli AR. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca) J Ethnopharmacol. 2000;72:273–277. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 17.Koster R, anderson M, De Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:417. [Google Scholar]
- 18.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 19.Porter JB, Hoyes KP, Abeysinghe RD, Brooks PN, Huehns ER, Hider RC. Comparison of the subacute toxicity and efficacy of 3-hydroxypyridin-4-one iron chelators in overloaded and nonoverloaded mice. Blood. 1991;78:2727–2734. [PubMed] [Google Scholar]
- 20.Dlaska M, Weiss G. Central role of transcription factor NF-IL6 for cytokine and iron-mediated regulation of murine inducible nitric oxide synthase expression. J. Immunol. 1999;162:6171–6177. [PubMed] [Google Scholar]
- 21.O’Brien-Ladner AR, Blumer BM, Wesselius LJ. Differential regulation of human alveolar macrophage-derived interleukin-1β and tumor necrosis factor-α by iron. J Lab Clin Med. 1998;132:497–506. doi: 10.1016/s0022-2143(98)90128-7. [DOI] [PubMed] [Google Scholar]
- 22.Aruoma OI, Grootveld M, Bahorun T. Free radicals in biology and medicine: From inflammation to biotechnology. Biofactors. 2006;27:1–3. doi: 10.1002/biof.5520270101. [DOI] [PubMed] [Google Scholar]
- 23.Walter PB, Macklin EA, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the Novartis CICL670A0107 trial. Haematologica. 2008;93:817–825. doi: 10.3324/haematol.11755. [DOI] [PubMed] [Google Scholar]


