Abstract
The exceptional high mortality of lung cancer can be instigated to a high degree by late diagnosis. Despite the plethora of studies on potential molecular biomarkers for lung cancer diagnosis, very few have reached clinical implementation. In this study we developed a panel of DNA methylation biomarkers and validated their diagnostic efficiency in bronchial washings from a large retrospective cohort. Candidate targets from previous high-throughput approaches were examined by Pyrosequencing in an independent set of 48 lung tumor/normal paired. Ten promoters were selected and quantitative methylation-specific PCR (qMSP) assays were developed and used to screen 655 bronchial washings (BWs) from the Liverpool Lung Project (LLP) subjects divided into training (194 cases and 214 Controls) and validation (139 cases and 109 controls) sets. Three statistical models were employed to select the optimal panel of markers and evaluate the performance of the discriminatory algorithms. The final logit regression model incorporated hypermethylation at p16, TERT, WT1 and RASSF1.The performance of this 4-gene methylation signature in the validation set demonstrated 82% sensitivity and 91% specificity. In comparison, cytology alone in this set provided 43% sensitivity at 100% specificity. The diagnostic efficiency of the panel did not show any biases with age, gender, smoking and the presence of a non-lung neoplasm. However, sensitivity was predictably higher in central (squamous and small cell) than peripheral (adenocarcinomas) tumors, as well as in stage 2 or greater tumors.These findings clearly demonstrate the impact of DNA methylation-based assays in the diagnosis of cytologically occult lung neoplasms. A prospective trial is currently imminent in the LLP study to provide data on the enhancement of diagnostic accuracy in a clinical setting, including by additional markers.
INTRODUCTION
Lung cancer causes more deaths than any other neoplasia in both the USA (1) and the UK (2); late detection is a major contributor to this high mortality rates (3). Bronchoscopic examination following suspicious imaging results can reveal the presence of a bronchial lesion, which is normally confirmed histologically by biopsy and/or bronchial washings (BWs - also referred to as bronchial lavage or bronchoalveolar lavage). However, a significant number of cases remain clinically occult after bronchoscopy as cytological examination tends to miss almost half of the cases (4, 5).
The implementation of molecular biomarkers in the early diagnosis of lung cancer has been a long standing goal. Particular focus was given in identifying such biomarkers in bronchial washings in individuals with a high risk of developing lung cancer. Previous attempts in bronchial washings to detect known molecular abnormalities in lung cancer, included genomic instability (6, 7), DNA mutations (8, 9) and more recently, DNA methylation (10, 11). The latter has certain advantages regarding its biomarker applicability; it is a covalent DNA modification, resistant to post-sampling processing and spans a significant nucleotide length, allowing for flexible assay design (12).
The feasibility of DNA methylation detection in the BW of lung cancer patients has been demonstrated in a number of studies (13-15) (reviewed in (12) and (16)). However, very few of the proposed biomarkers have been validated in large case control data sets. One such validated biomarker that has recently received CE IVD certification, under the commercial name of Epi proLung® BL Reflex Assay (Epigenomics, AG) is mSHOX2 (17)
In the current study, we describe the validation of a panel of DNA methylation biomarkers in a large retrospective case-control bronchial washings set (655 individuals) from the Liverpool Lung Project (18) and demonstrate a substantial gain in sensitivity of detection over standalone cytology.
MATERIALS & METHODS
Study design
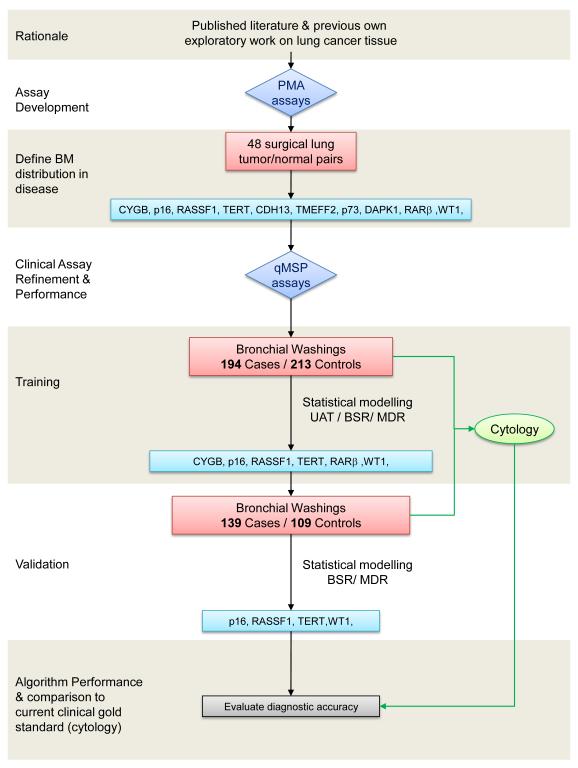
A brief outline of the study development is shown in Figure 1. The study extends over biomarker development phases 1 and 2, based on the EDRN guidelines (19). The promoter targets (p16, RASSF1, TMEFF2, TERT, CYGB, RARB, DAPK1, p73, WT1 and CDH13) were identified from previous work of our group (18, 20-23) and others (24-29) and validated by pyrosequencing in an independent set of 48 primary NSCLC surgical tissues (Supplementary Table 1). Quantitative Methylation PCR (qMSP) assays were developed for these ten markers in order to screen the bronchial washing specimens. For this phase, two nested case-control bronchial washing sets were selected from the Liverpool Lung Project (LLP) retrospective cohort. Inclusion criteria were, specimens with >2 years post-sampling follow-up information obtained through hospital records, the Merseyside & Cheshire Cancer Registry (MCCR) and the Office of National Statistics (ONS). Specimens were excluded if extracted DNA failed in quality control (see below in the qMSP description section). The case-control distributions of epidemiological and clinical characteristics for subjects in the training and test datasets are shown in Table 1 demonstrating overall similar patterns between the two classes, with the exception of smoking. Samples were randomized in 96-well plates and tested in a blinded fashion.
Figure 1.
Outline of the study progress phases. The distribution of candidate biomarker (BM) targets is validated for by Pyrosequencing Methylation analysis (PMA) in an independent set of lung cancer tissues. Quantitative methylation-specific PCR (qMSP) assays are developed and evaluated for their robustness in clinical samples. These are used to screen the training bronchial washings (BW) set from lung cancer patients and age/sex matched controls. Samples were excluded if extracted DNA failed in quality control. Statistical modeling demonstrates six markers with higher discriminating efficiency and these are used to screen the validation BW set. Further statistical modeling is applied to test the derived algorithms in the validation set. The qualifying 4-marker panel incorporates cytological data in order to construct the final algorithm. UAT: Univariate Association Test, BSR: Best Subset Regression, MDR: Multifactor Dimensionality Reduction.
Table 1.
Frequency distribution of subject’s epidemiological and clinical characteristics by case-control status
| Subject characteristics | Training set (N=407) | Testing set (N=248) | ||
|---|---|---|---|---|
| Case (n=194) |
Control (n=213) |
Case (n=139) |
Control (n=109) |
|
| Age group↑ | ||||
| <60 | 33 (17.0) | 57 (26.8) | 18 (13.0) | 19 (17.4) |
| 60-79 | 150 (77.3) | 144 (67.6) | 110 (79.1) | 84 (77.1) |
| 80+ | 11 (5.7) | 12 (5.6) | 11 (7.9) | 6 (5.5) |
| Age summary statistic↑ | ||||
| mean ± sd | 68.7±7.56 | 66.4± 8.56 | 68.4±8.07 | 67.6±8.78 |
| Gender | ||||
| Male | 114 (58.8) | 115 (54.0) | 80 (57.6) | 63 (57.8) |
| Female | 80 (41.2) | 98 (46.0) | 59 (42.5) | 46 (42.2) |
| Smoking status* | ||||
| Non-smoker | 8 (4.1) | 40 (18.8) | 4 (2.9) | 25 (22.9) |
| Ex-smoker | 103 (53.1) | 91 (42.7) | 63 (45.3) | 65 (59.6) |
| Current smoker | 74 (38.1) | 42 (19.7) | 72 (51.8) | 18 (16.5) |
| Unknown | 9 (4.6) | 40 (18.8) | 0 (0.0) | 1 (0.9) |
| Summary of: | ||||
| Smoking durationb | ||||
| mean ±sd | 44.7±12.06 | 39.0±13.73 | 43.9±13.14 | 34.6±14.58 |
| median | 46 | 41 | 45 | 37 |
| Smoking pack years¶ | ||||
| mean ±sd | 45.0±26.93 | 42.4±29.66 | 50.7±34.54 | 32.0±19.82 |
| median | 42.1 | 39.4 | 45 | 28 |
| Cytology*‡ | ||||
| Negative | 113 (58.3) | 213 (100.0) | 76 (54.7) | 108 (99.1) |
| Positive | 67 (34.5) | 0 (0.0) | 46 (33.1) | 0 (0.0) |
| Suspicious | 14 (7.2) | 0 (0.0) | 17 (12.2) | 1 (0.9) |
| Histology Diagnosis | ||||
| Others a | 3 (1.6) | 20 (14.4) | ||
| Large cell carcinoma | 25 (12.9) | 16 (11.5) | ||
| Small cell carcinoma | 4 (2.0) | 39 (28.1) | ||
| Squamous cell | 91 (46.9) | 31 (22.3) | ||
| carcinoma | 68 (35.0) | 22 (15.8) | ||
| Adenocarcinoma | 3 (1.6) | 11 (7.9) | ||
| Unknown | ||||
| Sample duration (yrs) ‡ | ||||
| <5 | 75 (38.7) | 96 (45.1) | 10 (7.2) | 39 (35.8) |
| 5+ | 119 (61.3) | 117 (54.9) | 129 (92.8) | 70 (64.2) |
boarderline significant in training set
Statistically significant in training set (p<0.05)
Statistically significant in testing set (p<0.05)
Statistically significant in testing set with p-value from Mann-Whitney test
Others (adenocarcinoid, adenosquamous, Carcinoid, Carcinoma, NOS, Neoplasm, malignant, Tumor cells, malignant, Basal cell carcinoma)
Statistically significant in both dataset with p-value from Mann-Whitney test
Study size and power calculations
Power calculations were based on the target methylation frequencies found in the validation lung cancer tissue set (Supplementary Table 2). Assuming a minimum of 87% positives for at least two markers (null hypothesis, TPR0=0.87) and an anticipated sensitivity of 95% for the markers combination (alternative hypothesis, TPR1 =0.95) we deduce power associated with different sample sizes, case-control ratios and acceptable false positive rates in a simulation study (30) as shown in Supplementary Table 2. This indicated that a set of ≥200 cases is required in a 1:1 ratio with controls to achieve 86% power for a 5% false positive rate at the 95% confidence level.
Patients, samples and DNA
The two study sets comprised a total of 655 individuals (333 lung cancer cases/322 age/sex-matched controls) (Figure 1). Patients had been retrospectively recruited through the Liverpool Heart & Chest Hospital under the Liverpool Lung Project (LLP) umbrella. All patients were referred to the bronchoscopy clinics with a clinical suspicion of lung cancer. At the end of the clinical work up, the diagnoses for the majority of non lung cancer patients were, bronchitis, COPD, bronchiectasis and chest infections while at lower frequency heart conditions, sarcoidosis, asbestosis were diagnosed. It has to be noted that 36 individuals in the control group(s) had other (non-lung) cancers diagnosed such as colon, breast, prostate, skin, esophagus and oral as well as four mesotheliomas. The Liverpool Lung Project has received ethical approval and all the recruited patients provided informed consent.
DNA from frozen lung tumor and paired normal tissue was extracted as previously described (22). Bronchial washings were stored in Saccomanno’s fixative in an air-conditioned (18°C) room and the specimens’ cytological adequacy was judged by the presence of alveolar macrophages. DNA was extracted using the Blood and Tissue kit (Qiagen), quantified using Picogreen (Invitrogen) and up to 1μg DNA was bisulphite converted using the EZ-96 DNA Methylation-Gold Kit (ZymoResearch).
Pyrosequencing Methylation Analysis (PMA)
Pyrosequencing assays were designed for early validation of targets in the lung tumor solid tissue. Standard protocols that have been previously described (22, 23) were used. The primers for the pyrosequencing analysis are provided in Supplementary Table 3.
Quantitative Methylation Specific PCR (qMSP)
The qMSP assays were designed to specifically amplify bisulphite-converted methylated DNA target sequences in the presence of an excess of unmethylated counterpart sequences. The methylation-specific primer and probe sequences are listed in Supplementary Table 4. During assay development, it became apparent that probes bearing minor groove binding moiety (Taqman MGB probes) provided a significant improvement in the assay specificity and, due to their smaller size, allowed for a more flexible assay design. A lengthy optimization process identified primer concentrations and thermal profiles ensuring reproducible specificity. The qMSP reactions contained 1× TaqMan® Universal Master Mix II (Applied Biosystems) 250 nM probe, 300-900 nM primers (Supplementary Table 2) and 2 μl eluate from the bisulphate treated DNA sample. The reactions were performed on a 7500 FAST real time cycler (Applied Biosystems) under the following thermal profile: 95°C for 10 min, 50 cycles consisted of 95°C for 15 sec, 60°C-62°C for 1 min.
The sensitivity/specificity of the assays was tested on serial dilutions of artificially (SssI) methylated DNA in lymphocyte DNA. In addition, whole genome amplified (WGA) DNA was constructed unsing the RepliG screening kit (Qiagen) as an absolute unmethylated DNA standard. Following multiple repetitions the sensitivity threshold was selected to 0.5% (1:200) as it provided total reproducibility, while higher dilutions (0.1%) proved less reliable. A methylation-independent assay with non-CpG bearing primers/probe was designed for the ACTB gene in order to normalize for input DNA, but also to be used as an exclusion criterion. We experimentally established that a cycle threshold (Ct)=29 for ACTB assays corresponded 6.9 ng DNA (1000 diploid genomes). This cut-off was employed to ensure 5× genome coverage at the 1:200 sensitivity threshold.
The training set was screened with CYGB, p16, RASSF1, TERT, CDH13, TMEFF2, p73, DAPK1, RARβ and WT1. Following statistical analysis, CYGB, p16, RASSF1, TERT, RARβ and WT1, which demonstrated the highest independent sensitivity/specificity or selected by various multivariate models, were evaluated in the independent validation set.
Statistical analysis
Exploratory univariate analysis
The distribution of subjects’ epidemiological, clinical and methylation characteristics were described separately for training and testing datasets. Categorical characteristics were compared between cases and controls using Chi-square test and Fisher’s exact test when less than 5 individuals were observed. Student t-test was used to investigate statistical significant case-control difference in quantitative characteristics. The Mann-Whitney non-parametric test was employed where normality assumption failed.
Identification of optimum markers
Univariate exploratory analysis was used to provide insight into the marginal effect of each marker on subject status. The best generalized linear model (best GLM) was used to identify the best additive logit combination mostly predictive of subject status. The model was fitted using Akaike information criterion (AIC), Bayesian information criterion (BIC, BICq) and cross-validation (CV) as selection methods. Multifactor Dimensionality Reduction (MDR) was used to investigate non-additive combination of the markers, which provides an assessment of epistasis (non-linear interactions) among the markers (31). The significance of the association between subject’s disease status and each marker interaction was tested based on the Model-based Multifactor Dimensionality Reduction permutation test (32).
Model-based logit algorithms were derived in the training dataset for discrimination and prediction of subject status and validated in the testing dataset. These were done separately for (a) the top 6 markers from the univariate analysis, (b) markers selected from the ‘overall’ best subset GLM and (c) markers from the ‘overall’ best MDR combination. Cytology was added as an additional factor to the best discriminatory/predictive model.
The predictive performance of each algorithm was evaluated in the test data. The disease probability (ranging from 0 to 1) was used to classify (training subjects) or predict (test subjects) as cases for probability ≥0.5 or controls otherwise. The classification and predictive accuracies were assessed using diagnostic measures including accuracy, sensitivity and specificity. The area under ROC curve (AUC) was used to summarize performance over the range of predicted probabilities. The overall performance of the best discriminatory model and its extended version that incorporates cytology was evaluated in a combined training and testing data, stratified by epidemiological and clinical factors such as age, gender, smoking status, lung cancer histological subtype and time distance from specimen collection to diagnosis. The independent ROC-AUCs from the stratified analyses were compared using the DeLong test (33) extended for unpaired ROC curves.
RESULTS
Diagnostic efficiency of the DNA methylation panel
Pyrosequencing methylation analysis of the set of 48 surgical NSCLC specimens resulted in a set of 10 promoters (CYGB, p16, RASSF1, TERT, CDH13, TMEFF2, p73, DAPK1, RARβ and WT1) demonstrating high frequency of methylation in tumor tissue and absence of methylation in the normal adjacent counterpart (Supplementary Table 1). The training BW case-control set was subsequently screened with the developed qMSP assays. Three statistical models (Univariate association test, Marker combination by Best Subset Regression (BSR) and Markers combination by Multifactor Dimensionality Reduction (MDR)) were tested in order to identify the optimal marker panel(s) and algorithm(s) for improved diagnostic efficiency. Univariate analysis of the ten examined markers is presented in Table 2. All three models pointed to six markers (CYGB, p16, RASSF1, TERT, RARβ and WT1) which were subsequently used to screen the validation set (Supplementary Table 5).
Table 2.
Univariate association tests for the examined biomarkers in the training and validation bronchial washing sets.
| Markers | Training Set | Validation Set | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positives | χ2 p-value |
Model-based classification* | Positives | χ2 p-value |
Prediction using trained univariate logit model* |
|||||
| Case n=194 |
Control n=213 |
Accuracy (%) | AUC (95% CI) | Case (n=139) |
Control (n=109) |
Accuracy (%) | AUC (95% CI) | |||
| TERT | 130 | 35 | <10−4 | 75.7 | 0.75 (0.71, 0.79) | 75 | 2 | <10−3 | 73.4 | 0.76 (0.72, 0.80) |
| RASSF1 | 75 | 7 | <10−4 | 69.0 | 0.68 (0.64, 0.71) | 71 | 0 | <10−4 | 72.6 | 0.76 (0.71, 0.80) |
| WT1 | 70 | 10 | <10−4 | 67.1 | 0.66 (0.62, 0.69) | 73 | 8 | <10−3 | 70.2 | 0.73 (0.68, 0.77) |
| p16 | 36 | 1 | <10−4 | 60.9 | 0.59 (0.56, 0.62) | 18 | 0 | <10−4 | 51.2 | 0.57 (0.54, 0.59) |
| CYGB | 36 | 16 | <10−3 | 57.3 | 0.56 (0.52, 0.59) | 15 | 0 | <10−4 | 50.0 | 0.55 (0.53, 0.58) |
| RARb | 28 | 10 | 10−3 | 56.8 | 0.55 (0.52, 0.58) | 67 | 18 | <10−4 | 63.7 | 0.66 (0.60, 0.71) |
| p73 | 30 | 17 | 0.08 | 53.8 | 0.52 (0.49, 0.55) | |||||
| DAPK | 11 | 6 | 0.15 | 53.6 | 0.51 (0.50, 0.53) | |||||
| CDH13 | 30 | 43 | 0.22 | 52.3 | 0.52 (0.49, 0.56) | |||||
| TMEFF | 14 | 14 | 0.80 | 52.3 | 0.50 (0.48, 0.53) | |||||
Disease class prediction based predicted Pr(D) ≥ 0.5
The performance of the different discriminatory algorithms in training and validation data is shown in Table 3. All the logit discriminatory algorithms performed well in the training set. The performance of the top 6 univariate markers and the best subset with BICq or CV in the test data was similar, although the best subset algorithm was more sensitive but less specific in the training data. The MDR algorithm was slightly more specific but less sensitive than the best subset model with BICq or CV criteria in the training data, its performance in the test data was only similar to that of the best subset in terms of specificity (sp=0.98) and lower with regards to sensitivity (se=0.77). The addition of the top MB-MDR 2- and 3-way interactions into any of the best logit models did not alter their performances (data not shown). Overall, the best subset logit model with BICq or CV criteria including TERT, WT1, p16 and RASSF1, is the most parsimonious and best performed algorithm which was then applied in the validation set.
Table 3.
Evaluation of classification and predictive accuracies of discriminatory algorithms in training and testing dataset
| Performance measure | Discriminatory algorithms | |||
|---|---|---|---|---|
| Top 6 Univariate |
Best subset logit | MDR | ||
| (AIC or BIC) | (BICq or CV) | |||
| Classification performance in training dataset |
||||
| Se/Sp (%)* | 79.4/79.8 | 80.4/80.3 | 80.4/77.9 | 77.8/79.8 |
| DA (%)* | 79.6 | 80.3 | 79.1 | 78.9 |
| AUC (95% CI) | 0.85 (0.82, 0.89) |
0.86 (0.83, 0.90) |
0.85 (0.81, 0.88) |
0.82 (0.78, 0.86) |
| Predictive performance in test dataset |
||||
| SE/Sp/sp (%)* | 82.0/90.8 | - | 82.0/90.8 | 77.0/90.8 |
| DA (%)* | 85.9 | 85.9 | 83.1 | |
| AUC (95% CI) | 0.90 (0.87, 0.94) |
0.89 (0.85, 0.93) |
0.85 (0.81, 0.89) |
|
Evaluated at probability of disease =0.5, Se: sensitivity, Sp: specificty, DA: discriminatory accuracy
AIC: Akaike Information Criterion, BICq: Bayesian Information Criterion, CV: Cross-validation
The diagnostic efficiency of this algorithm in the validation set is depicted in Table 4. The sensitivity of the panel was higher (90%) in the cytology positive cases than the cytology negative ones (75%). Overall the sensitivity was 82% while the specificity is very high (91%). Therefore the panel classified correctly 213/248 individuals of the validation set (diagnostic accuracy = 85.9%). When including the cytology result into the model the sensitivity (82%) specificity (92%) and diagnostic accuracy (86.3%) remained similar. However, the diagnostic efficiency of the methylation panel is profoundly higher in comparison to the cytological evaluation alone, which demonstrates 45% sensitivity and 99% specificity.
Table 4.
Validation of the best subset logit model in the bronchial washings validation set. Comparative efficiency of the models including DNA methylation (p16, RASSF1, WT1, TERT) only and DNA methylation with incorporated cytology versus cytology only.
| Cytology | Negative | Positive | Sensitivit y |
Specificit y |
||
|---|---|---|---|---|---|---|
| Methylation Panel Model |
Lung Cancer | Negative | 19 | 57 | 75% | |
| Positive | 6 | 57 | 90% | |||
| Overall | 25 | 114 | 82% | |||
|
| ||||||
| Controls | Negative | 98 | 10 | 91% | ||
| Positive | 1 | 0 | 100% | |||
| Overall | 99 | 10 | 91% | |||
|
| ||||||
| Methylation Panel +Cytology model |
Lung Cancer | 25 | 114 | 82% | ||
| Controls | 100 | 9 | 92% | |||
|
| ||||||
| Cytology only | Lung Cancer | 76 | 63 | 45% | ||
| Controls | 108 | 1 | 99% | |||
Overall performance of the panel in clinical subgroups
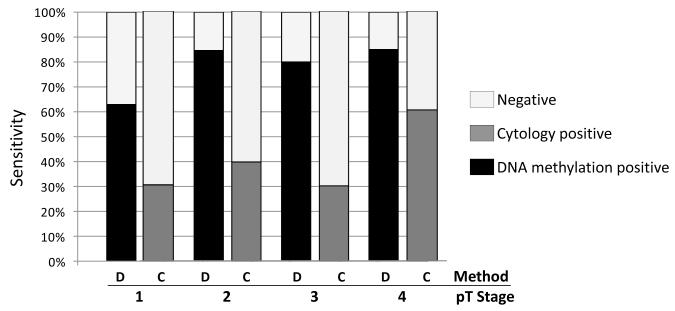
Following the validation of the 4-gene panel signature in the test set, an overall performance analysis of this panel was undertaken including both sets. The purpose of this was to identify possible biases in diverse epidemiological and clinical subgroups. Table 5 demonstrates the details of this analysis. The model performed equally among different age and gender groups. In addition, no differences in the sensitivity and specificity of detection were observed in relation to the age of the specimen in storage. Most importantly no significant sensitivity/specificity differences were observed among different smoking groups. Interestingly, the specificity of the panel was similar to the control sub-group bearing no malignant tumors at all (82.1%) and the control sub-group bearing tumors in other organs of the body except lung (83.3%). As expected, the sensitivity of the methylation panel was higher in cytology positive bronchial washings. Methylation positives were found in 140/189 (74.1%) cytology negative samples, 27/31 (87.1%) samples with suspicious cytology and 103/113 (91.1%) samples with a lung cancer cytological diagnosis (chi-square test, p=0.001). It was also evident that stage T1 tumors were less detectable (63%) than T2, T3 and T4 (over 80%) while no such difference was seen for nodal metastasis. When comparing sensitivities of cytology and DNA methylation in diverse pT groups (Figure 2), two points become obvious; (1) DNA methylation sensitivity is consistently higher in all groups than that of cytology and (2) that cytology demonstrates higher sensitivity in T4s as opposed to T1, T2, T3 (chi-square test, p=0.002) while DNA methylation has equally high sensitivity in T2, T3, T4 compared to T1 (chi-square test, p=0.004). Concerning histology, the panel demonstrated a higher efficiency in detecting small cell (100%) and squamous cell (83%) lung tumors in comparison to adenocarcinomas (75%). Our cohort also included a few inoperable cases with unconfirmed pathology thus such analysis was not applicable to these specimens.
Table 5.
Overall performance of best discriminatory algorithm by epidemiologic and clinical characteristics in both training and validation sets.
| Clinical characteristics | Number of specimens |
% | Chi-square test p- value |
|
|---|---|---|---|---|
| se | (sp) | |||
| Diagnosis | ||||
| Lung Cancer | 333 | 81.1 | ||
| Other (non-lung) cancer | 36 | (83.3) | ||
| No malignancy | 286 | (82.1) | ||
| Age | ||||
| <60 | 117 | 78.1 | (89.5) | |
| 60-79 | 498 | 81.1 | (80.3) | 0.81 |
| 80+ | 40 | 86.4 | (77.8) | |
| Gender | ||||
| Male | 372 | 81.3 | (82.6) | |
| Female | 283 | 80.9 | (82.0) | 0.77 |
| Smoking status | ||||
| None | 77 | 83.3 | (86.2) | |
| Former | 222 | 78.9 | (83.3) | 0.78 |
| Current | 206 | 82.9 | (81.7) | |
| Specimen in storage (yrs) | ||||
| <5 | 220 | 84.7 | (81.5) | |
| >5 | 435 | 79.8 | (82.9) | 0.34 |
|
| ||||
| Lung cancer cases only | ||||
|
| ||||
| Cytology | ||||
| Negative | 189 | 74.1 | ||
| Positive | 144 | 90.3 | <0.001 | |
| Stage (pT) | ||||
| 1 | 46 | 63.0 | ||
| 2 | 91 | 84.6 | 0.018 | |
| 3 | 20 | 80.0 | ||
| 4 | 53 | 84.9 | ||
| Nodal status (pN) | ||||
| 0 | 94 | 74.5 | ||
| 1 | 35 | 85.7 | 0.34 | |
| 2 | 63 | 84.1 | ||
| 3 | 13 | 84.6 | ||
| Histology diagnosis | ||||
| Adenocarcinoma | 92 | 75.0 | ||
| Squamous cell carcinoma | 118 | 83.1 | 0.003 | |
| Small cell carcinoma | 41 | 100.0 | ||
| Others* | 82 | 75.6 | ||
Includes adenosquamous carcinomas (n=3), large cell carcinomas (n=2) carcinoids (n=5), lung carcinomas of non confirmed pathology (n=72)
Figure 2.
Sensitivities of cytology and DNA methylation in different pathological stages of lung cancer. DNA methylation demonstrates superior sensitivity across all stages. D: DNA methylation panel, C: cytology.
Two hundred and thirty six samples included in this study (116 lung cancer cases, 120 controls) were also included in our previous collaborative studies for mSHOX2 methylation validation (34, 35). mSHOX2 positivity in the LLP methylation panel subgroups is provided in supplementary table 7. In this specific set of overlapping samples, mSHOX2 demonstrated 65.5% sensitivity and 96.5% specificity while the LLP panel including cytology demonstrated 90% sensitivity and 86.2% specificity (Supplementary Table 8). The combination of the two (score positive samples with at least one of two) did not result in improving detection parameters.
DISCUSSION
The late diagnosis of lung cancer remains the major reason for the large number of deaths due to this disease. Earlier diagnosis with successful surgical intervention is currently the best way forward. The advent of early detection through CT screening holds future promise but still has to be implemented (36, 37). Cytological diagnosis of the disease remains one of the major investigative tools but unfortunately it can miss up to half of the lung cancer cases. Thus the diagnostic efficiency in cytologically occult bronchoscopic material is essential. Despite the number of articles suggesting potential biomarkers, very few have progressed to the next level towards clinical evaluation. The main reasons include low study size and thus statistical power, extensive diversity of methods and lack of assay optimization to reach clinical standards (12).
In this study, we undertook a retrospective case-control design to evaluate DNA methylation biomarkers utilizing a training and a validation sample set (overall 655 individuals) from the Liverpool Lung Project. The study was designed to maximize compliance to the EDRN guidelines (19, 38), the Cancer Research UK Diagnostic Biomarker Roadmap (39) and STARD (40) recommendations for reporting in this manuscript. We developed very robust qMSP assays and established sensitivity and specificity through thousands of repetitions. qMSP is currently considered the gold standard method for reliably detecting DNA methylation in high dilution (41, 42). It must be noted that white blood cells, which are frequently present in bronchial washings, are not de facto methylation-free for all genes. Thus we determined a positive control based cut-off (0.5% methylation dilution), which was always at least 4-fold higher (>2 ΔCt) from the lymphocyte methylation signal. We have also used a methylation-independent assay for the ACTB gene to quantify the DNA input and thus (a) be used as an exclusion criterion, indicative of inadequate amount of DNA and (b) provide normalization for the target gene signal.
Our biomarker qualification process through training and validation sets demonstrated that a panel of TERT, WT1, p16 and RASSF1 methylation markers provides a parsimonious and efficient algorithm for correctly predicting lung cancer status in 85.9% of tested bronchial washing specimens. We utilized three different models to identify a useful marker panel and develop the discriminatory/predictive algorithm utilizing them. The consistency of various analyses conducted supports the usefulness of the markers, providing further support to previous suggestions on the use of marker panels than single markers in order to improve sensitivity and specificity (43, 44).
RASSF1 methylation in bronchial washings has been recently shown to increase diagnostic sensitivity (42) Our study is also in agreement with a previous report on p16 and RASSF1 and RARβ methylation specificity in cancer cases (although RARβ was not eventually included in the final panel) (45). However, CDH13 appears as a cancer-specific marker in the latter while in our study had clearly no discrimination efficiency. The methodological approach (endpoint MSP vs qMSP) may be a source of this difference.
It is apparent that the DNA methylation panel reported in this manuscript has superior sensitivity (82%) compared to cytology alone (45%), while its specificity is marginally lower (92% compared to 99% of cytology only). Cytology is currently the clinical gold standard for BW evaluation but it is known to have a low sensitivity of detection (4, 5). Therefore the use of DNA methylation biomarkers can be used in a clinical setting to improve the diagnostic efficiency for lung cancer. The incorporation of cytology into the model did not alter the diagnostic efficiency in our validation set. A larger cohort of specimens is currently being recruited in the LLP in an effort to confirm whether this DNA methylation marker panel can substitute or complement the cytological report in bronchial washings for lung cancer diagnosis. In any case, the diagnostic benefit of this panel in cytologically occult specimens is profound. However, the cost/gain ratio of the clinical benefit is yet to be evaluated prospectively. It must be determined if the significant increase in detection sensitivity, and thus expected improvement of patient survival can outweigh the drop in specificity as the latter may have significant ethical and health economics implications.The major clinical need at this time in the arena of lung cancer CT screening programmes, is the issue of indeterminate nodules which require repeat CT scans (i.e. which nodules require surgical resection). The implementation of CT screening in the USA and Europe will be funded by differing health care systems, however, initial data indicates that the cost could be in the order of $18,000 per per life-year saved (2012 dollars) (46). The real advancement in the implementation planning of future CT screening programmes on an international level will be the reduction in the number of repeat CT scans for such indeterminate CT identified nodules, which was one of the major recommendations from the IASLC CT Screening Workshop Report 2011 (37). Thus the use of a validated biomarker assay in this clinical setting it urgently needed. The methylation study reported in this paper has validated a set of methylation biomarkers with a good AUC value, however, the individuals in this study did not have CT scan data and bronchial washings are not routinely used within lung cancer clinical work-up, across all clinical settings internationally. The validation of methylation biomarkers in sputum or in plasma samples in a CT screened population is urgently required.
There are a number of studies demonstrating the feasibility of DNA methylation detection in plasma and serum of lung cancer patients (reviewed in (16)) These involve a variety of genes examined and report diverse sensitivities and specificities. Two of the genes included in our 4-gene panel (p16 and RASSF1) are also used in some of the above studies while WT1 and TERT have not been examined. The diagnostic efficiency of the LLP methylation panel in this set of bronchial washings is generally superior to those reported in plasma and serum studies. The main reason for this is certainly the sample origin, as bronchial washings have the advantage directly representing the lung epithelium. Plasma and serum contain the seeded cancer DNA in high dilution but also represent the whole body. Thus, neoplastic or preneoplastic foci in other than lung organs may contribute to lower specificity of lung cancer detection. The diagnostic efficiency of our panel in plasma/serum is yet to be investigated in our prospective trials involving individuals with CT information from the UKLS study (47)
It is important that this panel demonstrated no biases related to age and gender. Most importantly, its diagnostic efficiency is independent of the smoking status, suggesting that it detects cancer-specific alterations rather than tobacco-related field cancerization. It is also of note that correct classification was not influenced by the presence of other (non-lung) cancers in the control population. As RASSF1, TERT, WT1 and p16 are common epigenetic players in cancer development, this has to do with the origin of the specimen (i.e. the lung) rather than the specificity of these four markers to lung cancer.
The better performance in central (small cell and squamous carcinomas) rather than peripheral adenocarcinomas was not surprising as bronchoscopy is expected to sample the latter at lower efficiency. It is of note that while our initial marker selection was based on adenocarcinoma and squamous carcinoma tissue only, their performance in the BW from patients with other histological subtypes (e.g small cell, carcinoids etc) was equally efficient. It is also of no surprise that lower sensitivity was achieved in smaller (T1) tumors, as these presumable seed less cells in the lung cavity. It is still important though that DNA methylation detected more than half of T1 tumors, group in which cytology has particularly low sensitivity.
The combination of mSHOX2 and LLP panel results, scoring positives on the basis of either LLP or mSHOX2 positive) did not result in improving detection parameters in the common set of 236 samples. Of course, the limited data of the overlapping samples did not allow for appropriate data modeling and recalibration of the LLP algorithm. Such modeling requires synchronous screening of a larger number of individuals, as is planned for the prospective trial which is currently being set up.
Although the current sensitivity can be improved by expanding the existing panel, it is still almost double of the current gold standard, which is cytology. Thus clinical implementation could proceed provided that the diagnostic efficiency reported here is further validated in an independent cohort; preferably a multi-site case control study should be undertaken. One of the main problems appears to be the potential shortage of DNA from bronchial washings if higher numbers of markers need to be included. This can be overcome by the use of microfluidic PCR arrays that significantly reduce reaction volumes and thus required input DNA.
In this study, we utilized a training and a validation cohort to identify a panel of DNA methylation based biomarkers with potential diagnostic utility for lung cancer detection in bronchial washings specimens. This 4-marker panel significantly improves the diagnosis rate compared to cytological evaluation only clearly demonstrating that DNA methylation biomarkers can become a useful clinical tool for the diagnosis of lung cancer, especially in cytologically occult bronchoscopic material. However, the timely delivery of such molecular diagnostic tools can only be accomplished through consortia which share samples and information and utilize common methodologies throughout the diagnostic process from sampling to reporting.
Supplementary Material
Acknowledgements
This research was supported by Cancer Research UK (Grant ref: C1340/A12091) and the Roy Castle Lung Cancer Foundation, UK. Authors would like to thank Mrs Jenny Goggin for assisting with specimen processing.
Footnotes
Conflict of interest statement: No conflict of interest to disclose
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jack RH, Davies EA, Moller H. Lung cancer incidence and survival in different ethnic groups in South East England. Br J Cancer. 2011;105:1049–53. doi: 10.1038/bjc.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulshine JL, van Klaveren RJ. Lung cancer screening: what is the benefit and what do we do about it? Lung Cancer. 2011;71:247–8. doi: 10.1016/j.lungcan.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Van’t Westeinde SC, van Klaveren RJ. Screening and early detection of lung cancer. Cancer J. 2011;17:3–10. doi: 10.1097/PPO.0b013e3182099319. [DOI] [PubMed] [Google Scholar]
- 5.Dobler CC, Crawford AB. Bronchoscopic diagnosis of endoscopically visible lung malignancies: should cytologic examinations be performed routinely? Intern Med J. 2009 doi: 10.1111/j.1445-5994.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- 6.Liloglou T, Maloney P, Xinarianos G, Hulbert M, Walshaw MJ, Gosney JR, et al. Cancer-specific genomic instability in bronchial lavage: a molecular tool for lung cancer detection. Cancer Res. 2001;61:1624–8. [PubMed] [Google Scholar]
- 7.Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–30. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Somers VA, van Henten AM, ten Velde GP, Arends JW, Thunnissen FB. Additional value of K-ras point mutations in bronchial wash fluids for diagnosis of peripheral lung tumours. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 1999;13:1120–4. doi: 10.1034/j.1399-3003.1999.13e30.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahrendt SA, Chow JT, Xu LH, Yang SC, Eisenberger CF, Esteller M, et al. Molecular detection of tumor cells in bronchoalveolar lavage fluid from patients with early stage lung cancer. J Natl Cancer Inst. 1999;91:332–9. doi: 10.1093/jnci/91.4.332. [DOI] [PubMed] [Google Scholar]
- 10.Schneider KU, Dietrich D, Fleischhacker M, Leschber G, Merk J, Schaper F, et al. Correlation of SHOX2 gene amplification and DNA methylation in lung cancer tumors. BMC Cancer. 2011;11:102. doi: 10.1186/1471-2407-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Fraipont F, Moro-Sibilot D, Michelland S, Brambilla E, Brambilla C, Favrot MC. Promoter methylation of genes in bronchial lavages: a marker for early diagnosis of primary and relapsing non-small cell lung cancer? Lung Cancer. 2005;50:199–209. doi: 10.1016/j.lungcan.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Liloglou T, Field JK. Detection of DNA methylation changes in body fluids. Adv Genet. 2010;71:177–207. doi: 10.1016/B978-0-12-380864-6.00006-7. [DOI] [PubMed] [Google Scholar]
- 13.Baryshnikova E, Destro A, Infante MV, Cavuto S, Cariboni U, Alloisio M, et al. Molecular alterations in spontaneous sputum of cancer-free heavy smokers: results from a large screening program. Clin Cancer Res. 2008;14:1913–9. doi: 10.1158/1078-0432.CCR-07-1741. [DOI] [PubMed] [Google Scholar]
- 14.Hsu HS, Chen TP, Wen CK, Hung CH, Chen CY, Chen JT, et al. Multiple genetic and epigenetic biomarkers for lung cancer detection in cytologically negative sputum and a nested case-control study for risk assessment. J Pathol. 2007;213:412–9. doi: 10.1002/path.2246. [DOI] [PubMed] [Google Scholar]
- 15.Grote HJ. Aberrant promoter methylation as biomarker for molecular cytological diagnosis of lung cancer. Verh Dtsch Ges Pathol. 2006;90:216–26. [PubMed] [Google Scholar]
- 16.Liloglou T, Bediaga NG, Brown BR, Field JK, Davies MP. Epigenetic biomarkers in lung cancer. Cancer letters. 2012 doi: 10.1016/j.canlet.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich D, Kneip C, Raji O, Liloglou T, Seegebarth A, Schlegel T, et al. Performance evaluation of the DNA methylation biomarker SHOX2 for the aid in diagnosis of lung cancer based on the analysis of bronchial aspirates. International journal of oncology. 2012;40:825–32. doi: 10.3892/ijo.2011.1264. [DOI] [PubMed] [Google Scholar]
- 18.Field JK, Smith DL, Duffy S, Cassidy A. The Liverpool Lung Project research protocol. Int J Oncol. 2005;27:1633–45. [PubMed] [Google Scholar]
- 19.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 20.Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes. 2006;20:60–3. doi: 10.1016/j.mcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Xinarianos G, McRonald FE, Risk JM, Bowers NL, Nikolaidis G, Field JK, et al. Frequent genetic and epigenetic abnormalities contribute to the deregulation of cytoglobin in non-small cell lung cancer. Hum Mol Genet. 2006;15:2038–44. doi: 10.1093/hmg/ddl128. [DOI] [PubMed] [Google Scholar]
- 22.Daskalos A, Nikolaidis G, Xinarianos G, Savvari P, Cassidy A, Zakopoulou R, et al. Hypomethylation of retrotransposable elements correlates with genomic instability in non-small cell lung cancer. Int J Cancer. 2009;124:81–7. doi: 10.1002/ijc.23849. [DOI] [PubMed] [Google Scholar]
- 23.Daskalos A, Logotheti S, Markopoulou S, Xinarianos G, Gosney JR, Kastania AN, et al. Global DNA hypomethylation-induced DeltaNp73 transcriptional activation in non-small cell lung cancer. Cancer Lett. 2011;300:79–86. doi: 10.1016/j.canlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Palmisano WA, Divine KK, Saccomanno G, Gilliland FD, Baylin SB, Herman JG, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–8. [PubMed] [Google Scholar]
- 25.Virmani AK, Rathi A, Zochbauer-Muller S, Sacchi N, Fukuyama Y, Bryant D, et al. Promoter methylation and silencing of the retinoic acid receptor-beta gene in lung carcinomas. J Natl Cancer Inst. 2000;92:1303–7. doi: 10.1093/jnci/92.16.1303. [DOI] [PubMed] [Google Scholar]
- 26.Toyooka S, Toyooka KO, Maruyama R, Virmani AK, Girard L, Miyajima K, et al. DNA methylation profiles of lung tumors. Molecular cancer therapeutics. 2001;1:61–7. [PubMed] [Google Scholar]
- 27.Belinsky SA, Palmisano WA, Gilliland FD, Crooks LA, Divine KK, Winters SA, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62:2370–7. [PubMed] [Google Scholar]
- 28.Zochbauer-Muller S, Lam S, Toyooka S, Virmani AK, Toyooka KO, Seidl S, et al. Aberrant methylation of multiple genes in the upper aerodigestive tract epithelium of heavy smokers. Int J Cancer. 2003;107:612–6. doi: 10.1002/ijc.11458. [DOI] [PubMed] [Google Scholar]
- 29.Shivapurkar N, Stastny V, Suzuki M, Wistuba II, Li L, Zheng Y, et al. Application of a methylation gene panel by quantitative PCR for lung cancers. Cancer Lett. 2007;247:56–71. doi: 10.1016/j.canlet.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janes H, Pepe M. The optimal ratio of cases to controls for estimating the classification accuracy of a biomarker. Biostatistics. 2006;7:456–68. doi: 10.1093/biostatistics/kxj018. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69:138–47. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calle ML, Urrea V, Vellalta G, Malats N, Steen KV. Improving strategies for detecting genetic patterns of disease susceptibility in association studies. Stat Med. 2008;27:6532–46. doi: 10.1002/sim.3431. [DOI] [PubMed] [Google Scholar]
- 33.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 34.Schmidt B, Liebenberg V, Dietrich D, Schlegel T, Kneip C, Seegebarth A, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer based on bronchial aspirates. BMC Cancer. 2010;10:600. doi: 10.1186/1471-2407-10-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietrich D, Kneip C, Raji O, Liloglou T, Seegebarth A, Schlegel T, et al. Performance evaluation of the DNA methylation biomarker SHOX2 for the aid in diagnosis of lung cancer based on the analysis of bronchial aspirates. Int J Oncol. 2012;40:825–32. doi: 10.3892/ijo.2011.1264. [DOI] [PubMed] [Google Scholar]
- 36.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011 doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Field JK, Smith RA, Aberle DR, Oudkerk M, Baldwin DR, Yankelevitz D, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 Report. J Thorac Oncol. 2012;7:10–9. doi: 10.1097/JTO.0b013e31823c58ab. [DOI] [PubMed] [Google Scholar]
- 38.Baker SG, Kramer BS, Srivastava S. Markers for early detection of cancer: statistical guidelines for nested case-control studies. BMC Med Res Methodol. 2002;2:4. doi: 10.1186/1471-2288-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CRUK 2009 http://science.cancerresearchuk.org/funding/find-grant/all-funding-schemes/biomarkers-imaging-discovery-development-project-grants/ssLINK/CR_027484.
- 40.Bossuyt PM, Reitsma JB. The STARD initiative. Lancet. 2003;361:71. doi: 10.1016/S0140-6736(03)12122-8. [DOI] [PubMed] [Google Scholar]
- 41.Hoque MO, Topaloglu O, Begum S, Henrique R, Rosenbaum E, Van Criekinge W, et al. Quantitative methylation-specific polymerase chain reaction gene patterns in urine sediment distinguish prostate cancer patients from control subjects. J Clin Oncol. 2005;23:6569–75. doi: 10.1200/JCO.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 42.van der Drift M, Prinsen C, Knuiman J, Janssen J, Dekhuijzen PN, Thunnissen E. Diagnosing peripheral lung cancer: the additional value of RASSF1A methylation and KRAS mutation analyses in washings in non-diagnostic bronchoscopy. Chest. 2011 doi: 10.1378/chest.10-2579. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Mohan A, Guleria R. Biomarkers in cancer screening, research and detection: present and future: a review. Biomarkers. 2006;11:385–405. doi: 10.1080/13547500600775011. [DOI] [PubMed] [Google Scholar]
- 44.Manne U, Srivastava RG, Srivastava S. Recent advances in biomarkers for cancer diagnosis and treatment. Drug Discov Today. 2005;10:965–76. doi: 10.1016/S1359-6446(05)03487-2. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Kwon YM, Kim JS, Lee H, Park JH, Shim YM, et al. Tumor-specific methylation in bronchial lavage for the early detection of non-small-cell lung cancer. J Clin Oncol. 2004;22:2363–70. doi: 10.1200/JCO.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 46.Pyenson BS, Sander MS, Jiang Y, Kahn H, Mulshine JL. An Actuarial Analysis Shows That Offering Lung Cancer Screening As An Insurance Benefit Would Save Lives At Relatively Low Cost. Health Affairs. 2012;31:770–9. doi: 10.1377/hlthaff.2011.0814. [DOI] [PubMed] [Google Scholar]
- 47.Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66:308–13. doi: 10.1136/thx.2010.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.