Abstract
Quantum dots (QDs) have not been used to label cytoskeleton structure of live cells owing to the limitations of the delivery strategies and QDs conjugation methods as well as the non-specific binding issues. We conjugated tubulin to QDs and applied the emerging method of nanoblade to deliver QD-tubulin conjugates into live Hela cells. This method will open new opportunities for QDs cytosolic targeting live cells.
Keywords: nanoblade delivery, QD-tubulin, cytosolic targeting
Since their introduction as biological imaging probes1,2, quantum dots (QDs) have gained prominence in various imaging applications due to their unique attributes such as high brightness, broad excitation spectrum, narrow emission spectrum and excellent photostability3–5. However, QDs also suffer from several shortcomings such as relatively large size6, non-specific binding (due to the charge of surface coatings) 7,8, large avidity9, blinking10,11, and cytotoxicity12. These shortcomings have restricted the general utility for quantum dots to only a subset of imaging applications.
An important, but elusive goal has been the delivery and targeting of QDs to cytosolic targets. Such a capability would enable multiplexed detection and single particle tracking over long periods of times of transiently interacting proteins and dynamic cellular machines. Successful cytosolic targeting requires, however, an efficient delivery that escapes the endocytotic pathway, supplying QDs to the cytosol that is not engulfed in membranous organelles (endosome-free QDs). In addition, once delivered, QDs should have minimal steric hindrance (i.e. small size) and minimal nonspecific binding so that they can freely diffuse and sample the full cytosolic volume in order to find and specifically bind to their target(s).
Although extensive efforts have been vested in developing cytosolic targeting methods and many reports claim to have achieved successful targeting13, a general, widely applicable protocol and convincing demonstrations of specific cytosolic targeting of fine structures such as the cytoskeleton are still lacking. These methods can be classified into two main categories: (i) Facilitated delivery strategies, using cell penetrating peptides (CPP)14,15, proton sponge polymer carriers16,17, pinocytosis18,19, and transfection reagents18. Methods belonging to this category provide high throughput delivery, but suffer from low efficiency release of endosome-free QDs (i.e. most QDs still end up trapped in endosomes). (ii) Active delivery methods include electroporation20,21 and microinjection22. Electroporation offers an efficient way of delivery by temporally destabilizing the plasma membrane to create transient pores using high voltage electrical pulses. However, this method suffers from low cell viability, aggregation of the payload and low uptake of large objects18. The other active method, microinjection, is the most efficient and direct way to deliver QDs into the cytoplasm. The delivery is done via a sharp glass microcapillary tip (with a diameter < 0.5 μm) that mechanically penetrates the cell membrane while maintaining reasonable cell viability23,24. However, the injection of large cargo (>0.5 μm) or aggregation-prone objects such as QDs and QDs-protein conjugates is difficult due to repeated tip clogging.
Here we applied the photothermal nanoblade technique25–27 to deliver tubulin-QDs conjugates into the cytoplasm of HeLa cells. As shown in Figure 1a, a laser pulse is used to excite surface plasmons in the thin titanium coating of the tip of a glass capillary pipette; the plasmon absorption conducts heat into the liquid medium in close proximity to the metal which in turn produces nanosecond-short explosive vapor bubbles right next to the cell membrane. These bubbles produce large transient cuts or pores in the cell membrane. Concurrently, by pressurizing the capillary, a transient liquid flow is generated, enabling the delivery of the payload into the cytosol. In contrast to traditional microinjection, the photothermal nanoblade is brought in gentle contact with the cell membrane, eliminating the need for damaging, mechanical puncturing. It also allows, for the same reason, use of a relatively large tip orifice (up to ~2μm) and injection of relatively large objects such as bacteria15.
Figure 1.
(a) photothermal nanoblade delivery of tubulin-QD conjugates into the cytosol; (b) three-step tubulin-QDs conjugation strategy; (c) single-step tubulin-QD conjugation strategy.
To demonstrate successful QD delivery and cytosolic targeting, we incorporated QDs-tubulin conjugates into growing microtubules in live cells. The large size of QDs could possibly hinder the polymerization of the conjugates in growing filaments. To circumvent this, a multi-step scheme was therefore devised (Figure 1b): (i) amine-derivatized, PEG-coated QDs were reacted with bis[sulfosuccinimidyl] suberate (BS3) cross-linker followed by a gel filtration step (to remove excess BS3); (ii) tubulin monomers were polymerized at 37°C in the presence of GTP and DMSO; (iii) when the tubulin solution became turbid (due to microtubule polymerization), pre-activated QDs were added to react with amine groups on polymerized tubulin molecules; and (iv) as a last step, the reaction solution was quenched with hydroxylamine and centrifugation at 15000xg at 4°C for 15 min. The pellet was then de-polymerized at 4°C in a DMSO-free buffer and purified by a 100K molecular weight cut-off (MWCO) centrifuge filter. As a control, a random conjugation method was also implemented (Figure 1c). The pre-activated QDs were mixed with tubulin dimmers, resulting in non-specific conjugation via amine groups.
Following photothermal nanoblade delivery of de-polymerized tubulin-QD conjugates (scheme of Figure 1b) to HeLa cells, filamentous structures were observed by wide-field fluorescence microscopy for long periods of times (Figure 2a and 2b), indicating successful delivery and targeting. Non-specific background and incomplete filamentous structures were observed after nanoblade delivery of conjugates prepared according to the scheme described in Figure 1c, possibly due to conjugation-induced blocking of the tubulin binding site (Figure 2c). Also, nanoblade-based delivery of bare amine-derivatized PEG-coated QDs into HeLa cells resulted in interspersed punctuated spots over a uniform staining of the cytosol (Figure 2e), possibly indicating aggregation of probes in the cytosol. In the above, the representative images (more data in supporting information) showed the successful cytocol staining on microtubules. However, the effect of QDs size on cyto-staining still remains further study. Although it has been reported that big size of QDs probes may result in multi-valent conjugation, crosslinking, steric hindrance, reduced diffusion and potential alternation of function of bio-molecules28–30, a thorough and systemic study is still needed in the future.
Figure 2.

Images of live HeLa cells after photothermal nanoblade delivery of (a) tubulin-QD conjugates prepared with the three-step conjugation strategy (scheme in Figure 1b); (b) zoom-in of boxed area in (a); (c) tubulin-QD conjugates prepared with the single-step conjugation strategy (Figure 1c); (d) zoom-in of boxed area in (c); (e) bare amine-derivatized PEG-coated QDs. Scale bar: 8 μm.
In order to confirm specific targeting and QD incorporation into growing filaments, cells with QD-stained filamentous structures were fixed and sequentially labeled with anti-tubulin IgG (mouse) followed by alexa-647 anti-mouse IgG (goat). Cells injected with tubulin-QDs conjugates (Figure 1b) displayed good co-localization between the QDs and the alexa-647 channels (Figures 3a – 3c), whereas cells injected with bare amine-derivatized PEG-coated QDs displayed filamentous structures only on the alexa-647 channel and, therefore, poor co-localization (Figures 3d – 3f).
Figure 3.
Images of fixed HeLa cells after photothermal nanoblade delivery of tubulin-QD conjugates (green) and immunocytochemistry labeling of tubulin (red): (a) tubulin-QD conjugates (scheme in Figure 1b); (b) immunocytochemistry image of same cell as in (a); (c) overlay of (a) and (b); (d) bare amine-derivatized PEG-coated QDs; (e) immunocytochemistry image of same cell as in (c); (f) overlay of (d) and (e). Scale bar: 8 μm.
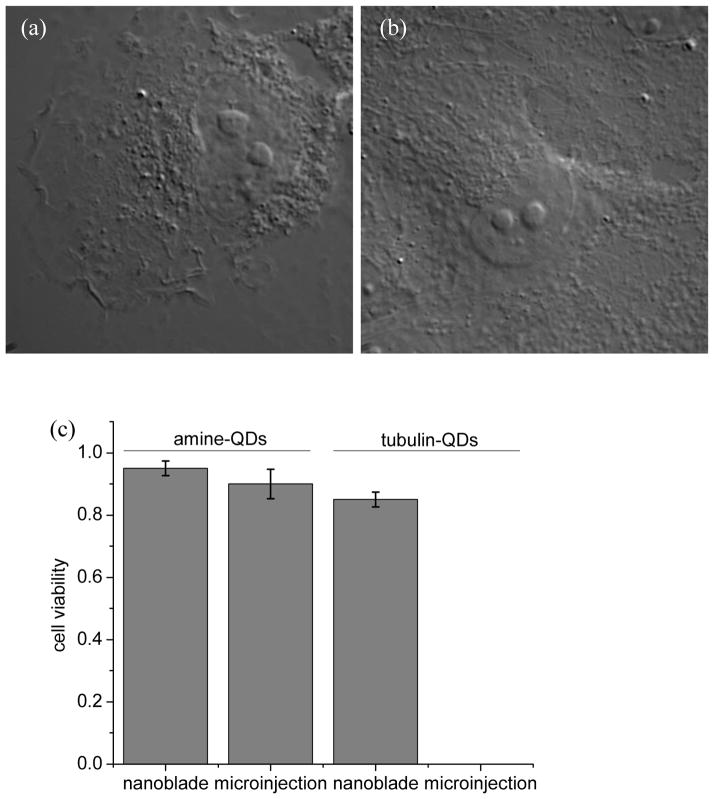
Figure 3 provides evidence for successful delivery and targeting of QDs to a cytosolic target, i.e. to the polymerizing microtubule network. We also tested the delivery of the same conjugates using the conventional microinjection method. The two injection methods were compared with regard to successful targeting and cell viability post injection (Figure 4). Cell viability was estimated by surface morphology 1 hour after injection. Conventional microinjection of amine-derivatized PEG-coated QDs was difficult (due to aggregation and clogging), but still possible. Cell viabilities post deliveries of these QDs were comparable for both methods (Figure 4). Conventional microinjection of tubulin-QDs conjugates was not at all possible for ~0.5 μm microcapillary tips due to severe aggregation and clogging. When tip diameters were increased to ~1 μm, some successful injections could be carried out, but with greater difficulty in penetrating the cell membrane (higher mechanic force was needed to puncture the cell membrane) and with significant reduction in cell viability post-injection. In contrast, photothermal nanoblade delivery was as efficient for tubulin-QDs conjugates as for amine-derivatized PEG-coated QDs, while maintaining good cell viability post-injection (Figure 4). Furthermore, photothermal nanoblade delivery is significantly easier as it requires only a gentle approach to the cell membrane, and therefore is devoid of attempts that end up with broken pipette tips.
Figure 4.
Examples of cell morphology after (a) microinjection and (b) photothermal nanoblade (tip ~1 μm) delivery of tubulin-QD conjugates. (c) Cell viability post-photothemal nanoblade and conventional microinjections of bare amine-derivatized PEG-coated QDs and tubulin-QD conjugates (scheme in Figure 1b).
To summarize, we conjugated tubulin to QDs and successfully delivered and targeted them into growing microtubule structures inside live cells using the photothermal nanoblade delivery method. This method achieved efficient delivery of endosome-free QD conjugates with high cell viabilities albeit at a low throughput. Nanotechnology-based solutions that are inspired by the photothermal nanoblade could potentially provide higher throughput delivery to cells in the future.
Supplementary Material
Acknowledgments
This work was supported by NIH grant #5R01EB000312, NIH grant #1R01GM086197, NSF (CBET 0853500 and ECCS 0901154), NIH (R21EB014456), a UC Discovery/Abraxis BioScience Biotechnology Award (#178517), and the Prostate Cancer Foundation Challenge Award. Conventional microinjection was done at the CNSI Advanced Light Microscopy/Spectroscopy Shared Facility at UCLA.
Footnotes
Supporting Information Available: Additional information about materials and methods, sample preparation and more imagines are available as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 2.Chan WCW, Nie S. Science. 1998;281:2016. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 3.Murray CB, Kagan CR, Bawendi MG. Ann Rev Mater Sci. 2000;30:545–610. [Google Scholar]
- 4.Alivisatos AP. Nature Biotech. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 5.Pinaud F, Michalet X, Bentolila LA, Tsay JM, Doose S, Li JJ, Iyer G, Weiss S. Biomaterials. 2005;27:1679–1687. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith A, Nie S. Nature Biotech. 2009;27:732–733. doi: 10.1038/nbt0809-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susumu K, Uyeda HT, Medintz IL, Pons T, Delehanty JB, Mattoussi H. J Am Chem Soc. 2007;129:13987–13996. doi: 10.1021/ja0749744. [DOI] [PubMed] [Google Scholar]
- 8.Breus W, Heyes CD, Tron K, Nienhaus GU. ACS Nano. 2009;3:2573–2580. doi: 10.1021/nn900600w. [DOI] [PubMed] [Google Scholar]
- 9.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Ren X, Kahen K, Hahn M, Rajeswaran M, Maccagnano-Zacher S, Silcox J, Cragg G, Efros A, Krauss T. Nature. 2009;459:686–689. doi: 10.1038/nature08072. [DOI] [PubMed] [Google Scholar]
- 11.Mahler B, Spinicelli P, Buil S, Quelin X, Hermier J, Dubertret B. Nature Mater. 2008;7:659–664. doi: 10.1038/nmat2222. [DOI] [PubMed] [Google Scholar]
- 12.Chang E, Thekkek N, Yu W, Colvin V, Drezek R. Small. 2006;2:1412–1417. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 13.Nelson S, Ali M, Trybus K, Warshaw D. Biophys J. 2009;97:509–518. doi: 10.1016/j.bpj.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan G, Agrawal A, Marcus AI, Nie S. J Am Chem Soc. 2007;129:14759–14766. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 15.Medintz IL, Pons T, Delehanty JB, Susumu K, Brunel FM, Dawson PE, Mattoussi H. Bioconjug Chem. 2008;19:1785–1795. doi: 10.1021/bc800089r. [DOI] [PubMed] [Google Scholar]
- 16.Bayles A, Chahal H, Chahal D, Goldbeck C, Cohen B, Helms B. Nano Lett. 2010;10:4086–4092. doi: 10.1021/nl102172j. [DOI] [PubMed] [Google Scholar]
- 17.Yezhelyev M, Qi L, O’Regan R, Nie S, Gao X. J Am Chem Soc. 2008;130:9006–9012. doi: 10.1021/ja800086u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delehanty JB, Bradburne CE, Boeneman K, Susumu K, Farrell D, Mei B, Blanco-Canosa JB, Dawson G, Dawson PE, Mattoussi H, Medintz IL. Integr Biol. 2010;2:265–277. doi: 10.1039/c0ib00002g. [DOI] [PubMed] [Google Scholar]
- 19.Buono C, Anzinger J, Amar M, Kruth H. J Clin Invest. 2009;119:1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derfus AM, Chan WCW, Bhatia SN. Adv Mater. 2004;16:961–966. [Google Scholar]
- 21.Chen FQ, Gerion D. Nano Lett. 2004;4:1827–1832. [Google Scholar]
- 22.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 23.Han S-W, Nakamura C, Kotobuki N, Obataya I, Ohgushi H, Nagamune T, Miyake J. Nanomed Nanotechnol Biol Med. 2008;4:215–225. doi: 10.1016/j.nano.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y. Nat Protoc. http://www.natureprotocols.com/2007/11/02/microinjection_technique_and_p.php, published online November 2, 2007.
- 25.Wu TH, Teslaa T, Kalim S, French CT, Moghadam S, Wall R, Miller JF, Witte ON, Teitell MA, Chiou PY. Anal Chem. 2011;83:1321–1327. doi: 10.1021/ac102532w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu TH, Teslaa T, Teitell MA, Chiou PY. Opt Express. 2010;18:23153–23160. doi: 10.1364/OE.18.023153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Shimada E, Zhang J, Hong JS, Smith G, Teitell MA, Koehler CM. Proc Natl Acad Sci USA. 2012;109:4840–4845. doi: 10.1073/pnas.1116792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howarth M, Liu W, Puthenveetil S, Zheng Y, Marshall L, Schmidt M, Wittrup K, Bawendi M, Ting A. Nature Methods. 2008;5:397–399. doi: 10.1038/nmeth.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith A, Wen M, Nie S. Biochem (Lond) 2010;32:12. [PMC free article] [PubMed] [Google Scholar]
- 30.Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian A. Biomaterials. 2007;28:4717–4732. doi: 10.1016/j.biomaterials.2007.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.