Abstract
Cancers may contain a small sub-population of uniquely tumorigenic cells that exhibit self-renewal and multipotency, i.e. cancer stem cells (CSC). These cells reside in invasive fronts in close proximity to blood vessels in many tumors, including head and neck squamous cell carcinomas (HNSCC). Recent evidence suggests that CSC resist chemotherapy and “drive” local recurrence and metastatic spread. Notably, endothelial cell-initiated signaling is critical for the survival and self-renewal of CSC and may play a role in resistance to therapy. Therefore, patients with head and neck cancer might benefit from therapies that target the CSC directly or their supportive perivascular niche.
Keywords: Tumorigenesis, Angiogenesis, Stemness, Oral cancer, Tumor initiation, Tumor microenvironment
1) Introduction
Head and neck squamous cell carcinoma (HNSCC) is a significant public health issue. HNSCC is the 8th most common cancer worldwide, and an estimated 263,900 new cases and 128,000 deaths from oral cavity cancer occurred in 2008 [1]. In 2012 in the US alone, it’s estimated that there will be 40,250 new cases diagnosed and 7,850 deaths attributed to HNSCC [2]. Approximately $3.1 billion will be spent for treatment for head and neck cancers [3], highlighting the public health burden. Treatment for patients with HNSCC typically involves surgery in combination with radiotherapy and platinum-based chemotherapy. Despite clinical advances and basic research efforts, the 5-year survival for patients with HNSCC has not improved significantly during the past 30 years, and remains at the unacceptably low 63% [2]. The development of a mechanism-based therapy that improves the survival and quality of life of patients will require better understanding of the pathogenesis of head and neck cancer.
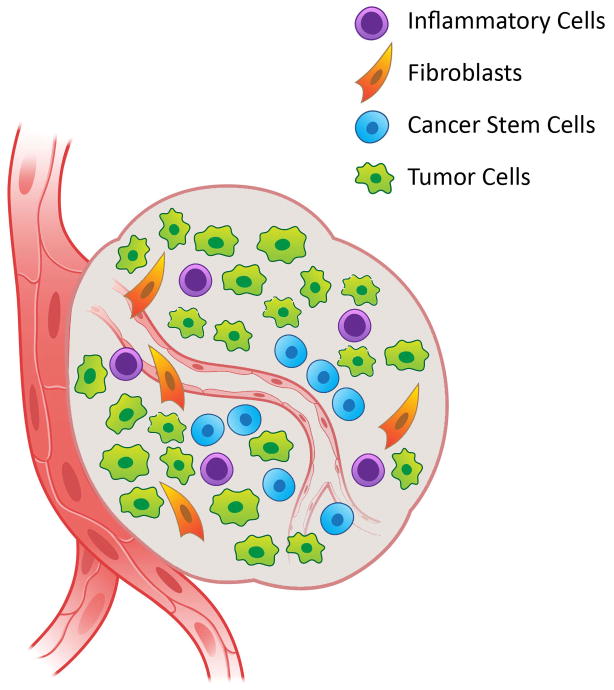
Recent evidence unveiled a small population of cancer cells that are highly tumorigenic, capable of self-renewal and that behave as tumor progenitor cells in HNSCC [4]. These features are consistent with the cancer stem cell (CSC) model. Cancer stem cells appear to play an important role in the initial stages of HNSCC tumorigenesis [5], as well as in tumor recurrence and metastatic spread. In HNSCC, cancer stem cells localize preferentially at the invasive fronts in close proximity to tumor blood vessels, in an area functionally defined as the perivascular niche (Fig. 1). Notably, the crosstalk with other cellular elements of the tumor microenvironment (e.g. endothelial cells) provides key signals for the survival and tumorigenic behavior of cancer stem cells [5]. It has been hypothesized that therapeutic targeting of the niche may disrupt the crosstalk between cancer stem cells and their partners, rendering the tumorigenic cells more susceptible to the effects of radiotherapy and conventional chemotherapy. This review discusses the proposed role of cancer stem cells in the pathobiology of head and neck cancer, and the potential impact of the cancer stem cell hypothesis to the clinical management of patients with this malignancy.
Figure 1.
The perivascular niche for head and neck cancer stem cells. The perivascular niche is a specialized microenvironment composed of several cell types including tumor cells, inflammatory cells, cancer-associated fibroblasts, and cancer stem cells localized in close proximity to blood vessels. Molecular interactions among these cell types provide for a supportive microenvironment that maintains the stemness properties (i.e. self-renewal, multipotency, and tumorigenic potential) of cancer stem cells.
2) Cancer Stem Cells
Today, we understand the head and neck tumors as an “organ” composed of transformed cells that interact with stromal cells within the tumor microenvironment through a mutually relevant crosstalk. The cancer stem cells or “tumor-initiating cells” are capable of initiating tumor formation. However, the bulk of the tumor tissue is composed of rapidly proliferating cells called transit-amplifying cells and post-mitotic differentiated cells. These cells do not contribute to tumor initiation, but are derived from the cancer stem cells. According to the stochastic hypothesis, cancers are comprised of a functionally homogenous cell population, with each cancer cell having equal ability to initiate tumor formation and metastasis, and entry into the cell cycle is governed by low probability stochastic events [6]. In contrast, the cancer stem cell hypothesis purports that not all tumor cells have equal tumorigenic potential [6]. This subpopulation of ‘cancer stem cells’ exhibits properties of normal stem cells [7], although they are not necessarily derived from normal tissue stem cells [8]. The term ‘cancer stem cells’ (CSC) has been debated in the literature. This cell population has also been called ‘tumor initiating cells’ [9] or tumor progenitor cells [10]. The definition of cancer stem cells includes functional characteristics of these cells, including ability to generate tumors in immunodeficient mice, self-renewal, and multipotency or the ability to differentiate into other cells that compose the tumor microenvironment [6].
The concept of cancer stem cells first originated in 1855, when the pathologist Rudolf Virchow proposed that cancers arise from the activation of dormant, embryonic cell remnants [11]. Another early landmark study demonstrating cancer stem cell concepts by Furth and Kahn in 1937 showed that a single tumor cell could generate a tumor in a recipient mouse [12]. Fifty years later, with the emergence of methodologies such as Fluorescence Activated Cell Sorting (FACS), Lapidot and colleagues found the first evidence for the cancer stem cell model when they used the cell-surface markers CD34 and CD38 to isolate an unique sub-population of tumor-initiating cells in acute myeloid leukemia (AML) [13].
Although it was initially not clear whether this concept might apply to solid tumors, the Clarke laboratory provided the first evidence of cancer stem cells in breast tumors in 2003 [14]. Importantly, this study showed that the xenograft tumors generated did indeed reproduce the cellular heterogeneity of the original tumor; therefore, providing strong evidence for the multipotency of these cancer stem cells. Since then, evidence has accumulated for cancer stem cells in many solid tumors, including colon, pancreatic, and brain [15; 16; 17; 18]. A recent report revealed that tumor-initiating cells are very rare (<1 in 2500 cells) in many tumor types, including pancreatic, lung, and head and neck tumors [19].
It is important to point out, however, that some tumors follow the stochastic model. Most notably, the Morrison laboratory provided strong evidence that melanoma does not follow the cancer stem cell hypothesis and that melanomas are not hierarchically organized [20]. Such findings would suggest that in some cancers (e.g. melanoma) essentially every tumor cell would have to be eliminated for prevention of metastases/recurrence and long lasting patient cure. In contrast, in tumor types that follow the cancer stem cell hypothesis, targeted elimination of the most highly tumorigenic cells (i.e. CSC) would be sufficient to prevent metastases and recurrence. Of course, these are still highly controversial issues that warrant further investigation.
Identifying whether tumors follow the cancer stem cell hypothesis has important therapeutic implications as recent evidence from several types of cancer suggests that the CSC population contributes to chemotherapy and radiation-therapy resistance. It is known that cancer stem cells possess innate resistance mechanisms against radiation therapy and chemotherapy, preventing therapy-induced cell death and allowing them to survive and promote tumor recurrence. For example, radiation resistant glioblastomas have been shown to have increased expression of the cancer stem cell marker CD133+ [21]. These findings suggest that the tumorigenic CD133+ cells are uniquely resistant to radiation therapy, which may help to explain the lack of response observed in this sub-set of glioblastomas.
Several resistance mechanisms may play a role in cancer stem cell resistance to therapy. It is possible that mechanisms of resistance involve slow proliferative rates of cancer stem cells in situ [22], amplified checkpoint activation [21], and deregulation of DNA damage repair processes [23]. The Wnt/B-catenin and Notch signaling have also been shown to play a role in resistance to radiotherapy of mammary progenitor cells [24]. Notably, the demonstration that the most tumorigenic sub-set of cells within a tumor is also uniquely resistant to therapy provides strong rationale for studies focused on the understanding of mechanisms underpinning this resistance and subsequent therapeutic targeting of these pathways as a means to sensitize such tumors to chemotherapeutic drugs and/or radiation.
3) Head and neck cancer stem cells
Highly tumorigenic, stem-like cells were first reported in HNSCC by the Prince laboratory in 2007 [4]. Tumor cells were sorted using FACS for expression of the cell-surface marker CD44, and tumor initiation in immunodeficient mice was observed with as few as 5×103 CD44+ cells, although higher numbers of CD44− cells did not induce tumor formation. Importantly, CD44+ cells generated complex tumors composed of a heterogeneous population of tumor cells mixed with an abundant stromal component [4]. This provided the first evidence that head and neck squamous cell carcinoma might follow the cancer stem cell model. Following this landmark work, other laboratories confirmed that in HNSCC, CD44+ cells fit the functional definition of a cancer stem cells [25], [26], [27]. Further work from the Prince laboratory was able to isolate cancer stem cells using a single marker Aldehyde Dehydrogenase (ALDH) [28]. In addition to isolating tumor initiating cells using the marker ALDH from primary human tumors, these cells have been isolated from HNSCC cell lines, e.g. UM-SCC-104 and UM-SCC-22B [29] [30]. Notably, combination of both, ALDH and CD44, further refines one’s ability to identify a small sub-population of uniquely tumorigenic head and neck cancer stem cells [5].
Distant metastases in head and neck squamous cell carcinoma (HNSCC) contribute to the high mortality associated with the disease [31]. Cancer stem cells may mediate the processes involved in evasive resistance to therapy and distant metastases in HNSCC.
4) Putative cancer stem cell markers in HNSCC
The ability to identify and isolate sub-population of tumor cells and characterize them for tumorigenic behavior has revealed that not all head and neck tumor cells are equal. This process typically involves a sorting step (e.g. flow cytometry, columns/microbeads) and the use of putative markers of cancer stemness. Although many putative markers have been identified, to date no consensus exists on what marker might provide the best specificity for head and neck cancer stem cells (Table 1). Indeed, most likely there will not be a single marker that can unequivocally distinguish cancer stem cells from non-stem cells. Nevertheless, the following is a short description of markers that have been frequently used for head and neck cancer stem cell identification.
Table 1.
Cancer stem cell markers in head and neck squamous cell carcinomas
4.1. CD44
CD44 is a cell-surface glycoprotein that functions as a receptor for hyaluronic acid and is involved in cell-cell crosstalk, cell adhesion and migration [32]. CD44 has been implicated in head and neck tumor progression and chemoresistance [33]. Recent evidence suggests that CD44 expression correlates with HNSCC tumor aggressiveness [34], [35]. CD44+ cells have been shown to express high levels of Bmi-1 [4], an important self-renewal protein associated with embryonic and post-natal stem cells [36]. Notably, CD44 was the cancer stem cell marker used in the work that first described the function of cancer stem cells in HNSCC [4]
4.2. CD133
Another cell-surface antigen, CD133 (also known as Prominin 1; PROM1) is a transmembrane glycoprotein that has been characterized as a potential marker for cancer stem cells. In some HNSCC cell lines, CD133+ cells were found to have increased clonality when compared to CD133− cells [37]. Oral cancer stem-like cells from cell lines and primary tumors were found to have an increased expression of CD133, and displayed increased migration and tumorigenicity when compared to controls. Furthermore, CD133 status has been associated with poor cancer prognosis for HNSCC patients [38]. Recent evidence suggests that CD133+ cells were found to present increased clonogenicity, invasiveness, and tumorigenicity compared to CD133− [39]. Emerging data clearly support the use of CD133 as a putative cancer stem cell marker in head and neck tumor models.
4.3. Aldehyde dehydrogenase (ALDH)
In addition to expression of cell-surface antigens, assessing enzymatic function has been used to identify the sub-population of cancer stem cells in HNSCC. ALDH is an intracellular enzyme that is involved in converting retinol to retinoic acid [40]. ALDH+ cells were shown to be highly tumorigenic in cancers of the breast and brain [41], [42]. In our own lab, we demonstrated that using the marker ALDH+ in addition to CD44+ to sort HNSCC cells, tumors can be consistently generated from as few as 1,000 cells (13 tumors out of 15 implantations), whereas 10,000 ALDH-CD44− cells were significantly less tumorigenic (2 tumors out of 15 implantations) [5]. Further, xenografts generated from ALDH+CD44+ implantations were successfully serially passaged, indicating self-renewal properties, whereas ALDH−/CD44− did not exhibit this capacity. In other labs, ALDH was found to enrich for cancer stem cells and is involved in epithelial-to-mesenchymal transition [43]. More recent evidence supports the use of ALDH+ as a single marker to identify cancer stem cells in HNSCC [28].
4.4. Side population (SP)
Side population is defined as a sub-group of cells that behave differently than the main population when analyzed with a specific marker. The efflux of vital dyes by cell multidrug transporters has been investigated as a potential marker for cancer stem cells. The cells’ ability to actively pump the dye Hoechst 33342 by the ATP-binding cassette transporter (ABC) can be used to identify cells with increased longevity, and this concept has been applied to cancer stem cells which are thought to remain in a quiescent state [44]. Zhang and colleagues demonstrated that SP cells retrieved from oral squamous cell carcinomas exhibit features consistent with those of cancer stem cells [45]. Several other research groups confirmed these observations and demonstrated that SP cells are uniquely tumorigenic in head and neck cancer models [46].
5) Perivascular niche in head and neck cancer
It is generally believed that stem cells reside in ‘niches’ or specialized local microenvironments that are conducive to their function [47]. Niches form a complex environment where complex interactions among cells and matrix components define stem cell survival and stemness [48]. In glioblastoma, vascular endothelial cells are able to maintain a supportive niche for cancer stem cells [49]. This work suggests that the perivascular niche prevents the apoptosis of brain cancer stem cells and maintains an adequate balance between the self-renewal and differentiation. Brain cancer stem cells had accelerated growth when co-implanted with endothelial cells in immunodeficient mice, suggesting endothelial cells secrete important factors that may promote the growth and progression of tumors. Interestingly, recent data suggest that pre-cancerous stem cells can serve as tumor vasculogenic progenitors [50].
Evidence exists for a supportive perivascular niche in head and neck cancer. We observed that the majority of the cancer stem cells are found within a 100 m-radius of blood vessels in primary human HNSCC [5]. Using the SCID mouse model of human tumor angiogenesis that allows for the engineering of xenograft HNSCC with humanized blood vessels [51], we observed that selective ablation of tumor-associated endothelial cells with an artificial death switch (iCaspase-9) results in the decrease of the fraction of head and neck cancer stem cells [5]. These data demonstrated that disruption of the tumor vasculature is sufficient to reduce the proportion of tumor-initiating cells in pre-clinical models of HNSCC, and provide evidence for the existence of a supportive niche for stem cells in these tumors.
It has been known for several years that endothelial cell-secreted factors (e.g. IL-6, EGF, CXCL8) enhance the migration of tumor cells and protect these cells against anoikis by activating key signaling pathways, i.e. STAT3, ERK and Akt [52]. More recently, we reported that endothelial cell-secreted factors induce Bmi-1 expression and promote the self-renewal of head and neck cancer stem cells [5]. This emerging evidence is beginning to characterize drugable signaling pathways that play a critical role in the biology of cancer stem cells and that can be exploited therapeutically in HNSCC.
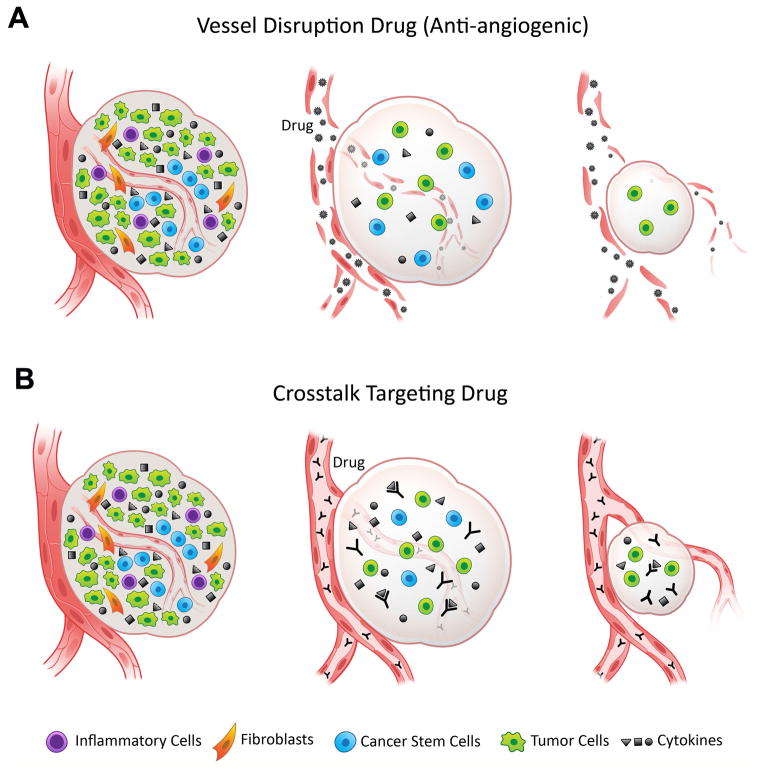
Targeted disruption of the crosstalk between endothelial cells and cancer stem cells might be beneficial for the treatment of head and neck cancer patients. Two general strategies are emerging from these pre-clinical studies (Fig. 2), as follows: A) Anti-angiogenic therapies that eliminate tumor blood vessels and therefore disrupt the perivascular niche. Such strategy was used by Kerbel’s laboratory that showed that anti-angiogenic therapy with the mouse VEGFR2 targeting antibody DC101 sensitizes brain tumor stem-like cells to therapy with a cytotoxic drug in pre-clinical models of glioma [53]. And B) Specific therapies targeting key signaling events that mediate the crosstalk between tumor-associated endothelial cells and cancer stem cells. Indeed, the ongoing Phase II clinical trial evaluating the effect of combination therapy with Cetuximab and the Hedgehog signaling inhibitor IPI-926 in recurrent head and neck cancer might be considered one of the first attempts to interfere with cancer stem cell signaling in HNSCC patients [54].
Figure 2.
Putative clinical implications of therapeutic targeting of the perivascular niche. A, Anti-angiogenic drugs eliminate tumor-associated blood vessels and disrupt the supporting perivascular niche for cancer stem cells. Examples of such drugs are the humanized anti-VEGF antibody bevacizumab (Avastin, Genentech/Roche) or the receptor tyrosine kinase inhibitor sunitinib (Sutent, Pfizer). B, Targeted therapies (e.g. antibodies) that specifically inhibit molecules responsible for the survival and self-renewal of cancer stem cells. Putative signaling molecules mediating the crosstalk between endothelial cells and cancer stem cells are IL-6 and CXCL8 (IL-8). Both interleukins have been shown to regulate stemness of cancer stem cells [59,60] and are secreted by endothelial cells. Examples of drugs targeting these pathways are Tocizilumab, a humanized monoclonal antibody against interleukin-6 receptor-IL-6R (Chugai/Roche) and Repertaxin, a non-competitive allosteric blocker of CXCR1/CXCR2 (Dompé). In both cases, elimination of cancer stem cells could potentially limit the generation of differentiated tumor cells and potentially mediate tumor regression.
However, it is important to point out that anti-angiogenic therapies may have unintended consequences to patient outcomes, as pointed out in a recent work from the Wicha laboratory. In pre-clinical models of breast cancer, the use of anti-angiogenic therapy with bevacizumab or sunitinib enhanced hypoxia and increased the fraction of cancer stem cells [55]. Further, they showed that Akt/β-catenin signaling, an important pathway in the biology of breast cancer stem cells, is activated under hypoxic conditions caused by the use of anti-angiogenic agents. This work suggests that anti-angiogenic agents might need to be combined with anti-cancer stem cell drugs, at least in breast cancer.
These findings may help to explain an intriguing dilemma in the field, i.e. that cancer stem cells are found primarily in close proximity to blood vessels [5], yet hypoxia enhances the stem cell phenotype [55]. We postulate that within the unchallenged tumor growth, cancer stem cells find a conducive microenvironment in close proximity to blood vessels and that endothelial cells secrete factors that maintain their self-renewal and multipotency features [5]. However, when a tumor is challenged with anti-angiogenic agents, the resulting hypoxia induces Akt/β-catenin signaling [55], which in turn enhances the survival and proliferation of the cancer stem cells. As a consequence, the overall fraction of cancer stem cells is increased.
Hypoxic states generated by anti-angiogenic therapy correlate with enhanced tumor cell motility and the development of evasive resistance to therapy [56]. Notably, seminal studies from the Weinberg laboratory provided evidence that epithelial-mesenchymal transition (EMT) is involved in the acquisition of stem cell properties [57]. Further, EMT appears to play a role in the biology of stem cells in head and neck tumors [43]. Together, the fact that tumor cells become more invasive under hypoxia states, and that EMT generates cancer stem cells, generate what can be called a “perfect storm” in which invasive cells are endowed with the highly tumorigenic features associated with the cancer stem cell phenotype. This might help to explain the recent observation suggesting that tumors that escape anti-angiogenic therapy tend to assume a more aggressive behavior leading to metastases [58].
A potential advantage of targeted disruption of the signaling events initiated by endothelial cells as a means to eliminate cancer stem cells, instead of killing the endothelial cell and disrupting the blood vessel, is that the former may not lead to the development of hypoxia. As discussed above, the development of hypoxia might result in an enhancement of the invasive phenotype of highly tumorigenic cells. On the other hand, targeted disruption of key signaling pathways that support the survival and behavior of cancer stem cells (i.e. stemness, pluripotency, and tumor-initiating ability) without causing hypoxia might be a more effective strategy to manage advanced cancer. Notably, such strategy would likely require combination with conventional cancer therapies that debulk the tumors by eliminating more differentiated tumor cells.
6) Future directions and final thoughts
Although accumulating evidence suggests an important role for stem cells in many types of cancer including HNSCC, many questions remain. If cancer stem cells prove to truly resist conventional therapy and “drive” local recurrence and metastatic spread, then targeting these cells and/or the perivascular niche may result in an effective treatment modality for patients with HNSCC. However, if research eventually demonstrates that HNSCC do not follow the cancer stem cell hypothesis, such therapeutic strategy will have little or no effect. It is unquestionable that more research is warranted to better understand the role of cancer stem cells in the pathobiology of head and neck cancer. Notably, understanding the impact of key signaling events within the perivascular niche to the function of cancer stem cells will be critical for the development of mechanism-based therapies that aim at the improvement of the survival and quality of life of patients with head and neck cancer.
Acknowledgments
We thank Chris Jung for his help with the illustrations. Support for this work was provided by Weathermax foundation, University of Michigan Comprehensive Cancer Center; grant P50-CA-97248 (University of Michigan Head and Neck SPORE) from the NIH/NCI, and R01-DE15948, R21-DE19279, R01-DE21139 and Training Grant T32-DE007057 from the NIH/NIDCR.
Footnotes
Conflict of Interest Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer. Journal for Clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.NCI. Cancer Trends Progress Report 2009/2010 update. [Google Scholar]
- 4.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a Subpopulation of Cells with Cancer Stem Cell Properties in Head and Neck Squamous Cell Carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nör JE. Endothelial Cell-Initiated Signaling Promotes the Survival and Self-Renewal of Cancer Stem Cells. Cancer Research. 2010;70:9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Clarke MFDJ, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer research (Baltimore) 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Fan T, Zhao Q, Zeng W, Zaslavsky E, Chen JJ, Frohman MA, Golightly MG, Madajewicz S, Chen W-T. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. International Journal of Cancer. 2010;126:669–683. doi: 10.1002/ijc.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virchow R. Die Cellularpathologie in ihrer Begrundung auf physiologische und pathologische Gewebelehre. Berlin: August Hirschwald; 1858. [Google Scholar]
- 12.Furth J, Kahn MC. The transmission of leukemia in mice with a single cell. Am J Cancer. 1937 [Google Scholar]
- 13.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer research (Baltimore) 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 16.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic Characterization of Human Colorectal Cancer Stem Cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer research (Baltimore) 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 18.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer research (Baltimore) 2003;63:5821–5828. [PubMed] [Google Scholar]
- 19.Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, Hidalgo M, Tsao M, Ailles L, Waddell TK, Maitra A, Neel BG, Matsui W. Tumor-Initiating Cells Are Rare in Many Human Tumors. Cell Stem Cell. 2010;7:279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, Morrison SJ. Phenotypic Heterogeneity among Tumorigenic Melanoma Cells from Patients that Is Reversible and Not Hierarchically Organized. Cancer Cell. 2010;18:510–523. doi: 10.1016/j.ccr.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 22.Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H, Olson S, Gabrielli B, Osborne G, Vescovi A, Reynolds BA. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain. 2011;134:1331–1343. doi: 10.1093/brain/awr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCord AM, Jamal M, Williams ES, Camphausen K, Tofilon PJ. CD133+ Glioblastoma Stem-like Cells are Radiosensitive with a Defective DNA Damage Response Compared with Established Cell Lines. Clinical Cancer Research. 2009;15:5145–5153. doi: 10.1158/1078-0432.CCR-09-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/Beta-Catenin Mediates Radiation Resistance of Mouse Mammary Progenitor Cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumann M, Krause M. CD44: A Cancer Stem Cell Related Biomarker with Predictive Potential for Radiotherapy. Clinical Cancer Research. 2010;16:5091–5093. doi: 10.1158/1078-0432.CCR-10-2244. [DOI] [PubMed] [Google Scholar]
- 26.Chikamatsu K, Ishii H, Takahashi G, Okamoto A, Moriyama M, Sakakura K, Masuyama K. Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head & Neck. 2011:336–343. doi: 10.1002/hed.21732. [DOI] [PubMed] [Google Scholar]
- 27.Faber A, Barth C, Hörmann K, Kassner S, Schultz JD, Sommer U, Stern-Straeter J, Thorn C, Goessler UR. CD44 as a stem cell marker in head and neck squamous cell carcinoma. Oncology reports. 2011;26:321–326. doi: 10.3892/or.2011.1322. [DOI] [PubMed] [Google Scholar]
- 28.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head & Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang AL, Hauff SJ, Owen JH, Graham MP, Czerwinski MJ, Park JJ, Walline H, Papagerakis S, Stoerker J, McHugh JB, Chepeha DB, Bradford CR, Carey TE, Prince ME. UM-SCC-104: A New human papillomavirus-16–positive cancer stem cell–containing head and neck squamous cell carcinoma cell line. Head & Neck. 2011 doi: 10.1002/hed.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos MS, Neiva KG, Meyers KA, Krishnamurthy S, Nör JE. Endothelial derived factors inhibit anoikis of head and neck cancer stem cells. Oral Oncology. 2012;48:26–32. doi: 10.1016/j.oraloncology.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano D, Myers J. Metastasis of squamous cell carcinoma of the oral tongue. Cancer and Metastasis Reviews. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 32.Gao L, Yan L, Lin B, Gao J, Liang X, Wang Y, Liu J, Zhang S, Masao I. Enhancive effects of Lewis y antigen on CD44-mediated adhesion and spreading of human ovarian cancer cell line RMG-I. Journal of Experimental & Clinical Cancer Research. 2011;30:15. doi: 10.1186/1756-9966-30-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang SJ, Bourguignon LYW. Role of Hyaluronan-Mediated CD44 Signaling in Head and Neck Squamous Cell Carcinoma Progression and Chemoresistance. The American Journal of Pathology. 2011;178:956–963. doi: 10.1016/j.ajpath.2010.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshua B, Kaplan MJ, Doweck I, Pai R, Weissman IL, Prince ME, Ailles LE. Frequency of cells expressing CD44, a Head and Neck cancer stem cell marker: Correlation with tumor aggressiveness. Head & Neck. 2012;34:42–49. doi: 10.1002/hed.21699. [DOI] [PubMed] [Google Scholar]
- 35.Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS, Jr, Dunn GP, Bui JD, Sunwoo JB, Uppaluri R. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer research (Baltimore) 2012;72:365–374. doi: 10.1158/0008-5472.CAN-11-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molofsky AV, Pardal R, Iwashita T, Park I-K, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, MacCallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head & Neck. 2009;31:94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 38.Chiou S-H, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clinical Cancer Research. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133+ cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Letters. 2010;289:151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Ma I, Allan A. The Role of Human Aldehyde Dehydrogenase in Normal and Cancer Stem Cells. Stem Cell Reviews and Reports. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 41.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasper M, Schäfer A, Piontek G, Teufel J, Brockhoff G, Ringel F, Heindl S, Zimmer C, Jr, Schlegel Aldehyde dehydrogenase 1 positive glioblastoma cells show brain tumor stem cell capacity. Neuro-Oncology. 2010;12:1024–1033. doi: 10.1093/neuonc/noq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for Epithelial-Mesenchymal Transition in Cancer Stem Cells of Head and Neck Squamous Cell Carcinoma. PLoS ONE. 2011;6:e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. The Journal of Experimental Medicine. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Letters. 2009;277:227–234. doi: 10.1016/j.canlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Yanamoto S, Kawasaki G, Yamada S-i, Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T, Umeda M. Isolation and characterization of cancer stem-like side population cells in human oral cancer cells. Oral Oncology. 2011;47:855–860. doi: 10.1016/j.oraloncology.2011.06.501. [DOI] [PubMed] [Google Scholar]
- 47.Morrison SJ, Spradling AC. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borovski T, De Sousa E, Melo F, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer research (Baltimore) 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 49.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Shen R, Ye Y, Chen L, Yan Q, Barsky SH, Gao J-X. Precancerous Stem Cells Can Serve As Tumor Vasculogenic Progenitors. PLoS ONE. 2008;3:e1652. doi: 10.1371/journal.pone.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and Characterization of Functional Human Microvessels in Immunodeficient Mice. Lab Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 52.Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nör JE. Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia (New York, NY) 2009;11:583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 54.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Pilot Study of Cetuximab and the Hedgehog Inhibitor IPI-926 in Recurrent Head and Neck Cancer. [cited 2012 Mar 30]. Available from: http://clinicaltrials.gov/ct2/show/NCT01255800. [Google Scholar]
- 55.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]