Abstract
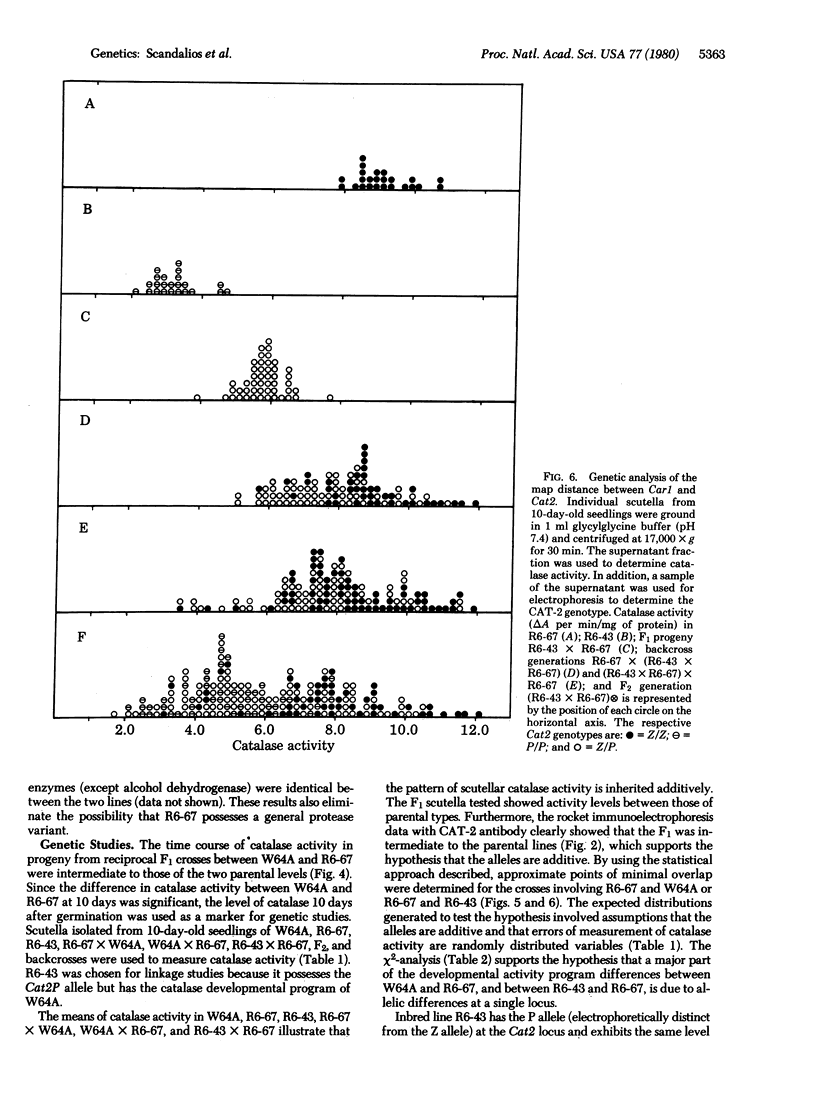
Genetic and biochemical analyses suggest that the developmental program of catalase (H2O2:H2O2 oxidoreductase, EC 1.11.1.6) activity in maize scutella is controlled by a temporal regulatory gene (Car1) that is distinct from the structural genes thus far identified. Recombination data show that Car1 is located about 37 map units from the Cat2 structural gene on the chromosome 1S. Turnover studies indicate that Car1 may act by regulating the rate of catalase synthesis.
Keywords: gene expression, enzyme turnover, additive trans-acting gene
Full text
PDF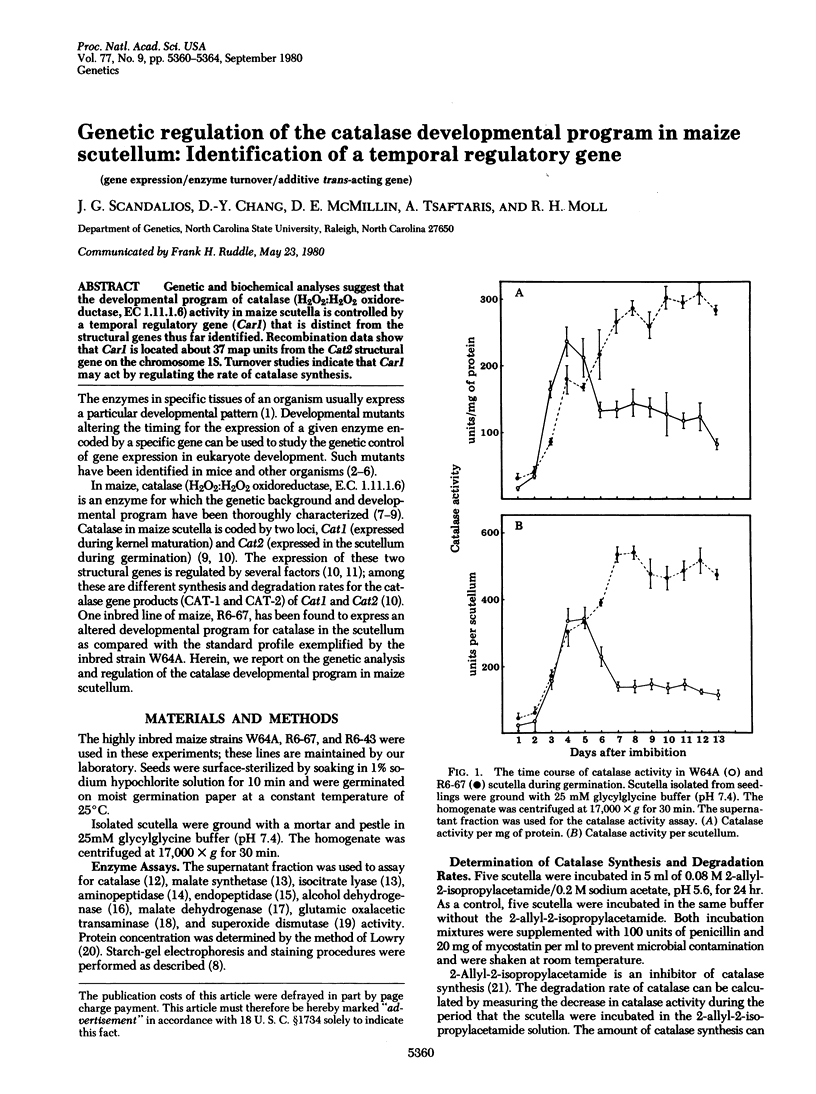



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham I., Doane W. W. Genetic regulation of tissue-specific expression of amylase structural genes in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4446–4450. doi: 10.1073/pnas.75.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Dickinson W. J. A genetic locus affecting the developmental expression of an enzyme in Drosophilia melanogaster. Dev Biol. 1975 Jan;42(1):131–140. doi: 10.1016/0012-1606(75)90319-x. [DOI] [PubMed] [Google Scholar]
- Felder M. R., Scandalios J. G., Liu E. H. Purification and partial characterization of two genetically defined alcohol dehydrogenase isozymes in maize. Biochim Biophys Acta. 1973 Jul 12;317(1):149–159. doi: 10.1016/0005-2795(73)90207-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., Paigen K. Genetic determination of the alpha-galactosidase developmental program in mice. Cell. 1975 Nov;6(3):371–378. doi: 10.1016/0092-8674(75)90186-5. [DOI] [PubMed] [Google Scholar]
- Melville J. C., Scandalios J. G. Maize endopeptidase: genetic control, chemical characterization, and relationship to an endogenous trypsin inhibitor. Biochem Genet. 1972 Aug;7(1):15–31. doi: 10.1007/BF00487006. [DOI] [PubMed] [Google Scholar]
- Ott L., Scandalios J. G. Genetic Control and Linkage Relationships among Aminopeptidases in Maize. Genetics. 1978 May;89(1):137–146. doi: 10.1093/genetics/89.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIGEN K. The genetic control of enzyme activity during differentiation. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1641–1649. doi: 10.1073/pnas.47.10.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE V. E., STERLING W. R., TARANTOLA V. A., HARTLEY R. W., Jr, RECHCIGL M., Jr The kinetics of catalase synthesis and destruction in vivo. J Biol Chem. 1962 Nov;237:3468–3475. [PubMed] [Google Scholar]
- Paigen K., Meisler M., Felton J., Chapman V. Genetic determination of the beta-galactosidase developmental program in mouse liver. Cell. 1976 Dec;9(4 Pt 1):533–539. doi: 10.1016/0092-8674(76)90035-0. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Scandalios J. G. Turnover of genetically defined catalase isozymes in maize. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1402–1406. doi: 10.1073/pnas.68.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindranath S. D., Fridovich I. Isolation and characterization of a manganese-containing superoxide dismutase from yeast. J Biol Chem. 1975 Aug 10;250(15):6107–6112. [PubMed] [Google Scholar]
- Scandalios J. G., Espiritu L. G. Mutant aminopeptidases of Pisum sativum. I. Developmental genetics and chemical characteristics. Mol Gen Genet. 1969 Oct 13;105(2):101–112. doi: 10.1007/BF00445679. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. Genetic control of multiple molecular forms of catalase in maize. Ann N Y Acad Sci. 1968 Jun 14;151(1):274–293. doi: 10.1111/j.1749-6632.1968.tb11896.x. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G., Liu E. H., Campeau M. A. The effects of intragenic and intergenic complementation on catalase structure and function in maize: a molecular approach to heterosis. Arch Biochem Biophys. 1972 Dec;153(2):695–705. doi: 10.1016/0003-9861(72)90388-8. [DOI] [PubMed] [Google Scholar]
- Scandalios J. G. Subunit dissociation and recombination of catalase isozymes. Proc Natl Acad Sci U S A. 1965 May;53(5):1035–1040. doi: 10.1073/pnas.53.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson J. C., Ganapathy P. S., Scandalios J. G. Regulation of maize catalase by changing rates of synthesis and degradation. Biochem J. 1977 Apr 15;164(1):113–117. doi: 10.1042/bj1640113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Ning-Sun, Scandalios J. G. De novo synthesis and developmental control of the multiple gene-controlled malate dehydrogenase isozymes in maize scutella. Biochim Biophys Acta. 1975 Apr 19;384(2):293–306. doi: 10.1016/0005-2744(75)90031-5. [DOI] [PubMed] [Google Scholar]




