Abstract
The retinoic-acid-receptor-related orphan receptors (RORs) are members of the nuclear receptor (NR) superfamily whose activity has been implicated in a number of physiological and pathological processes. The RORs, specifically RORα and RORγ, are considered master regulators of TH17 cells, a recently described subset of CD4+ T helper cells that have been demonstrated to have a pathological role in autoimmune disease. As with most members of the NR superfamily, RORs are ligand regulated, suggesting that their activity can be modulated by synthetic ligands. Recent advances in the field have established that selective inhibition of the RORs is a viable therapeutic approach for not only the treatment of autoimmune disorders, but ROR-mediated metabolic disorders as well.
Keywords: Nuclear receptor, steroid receptor, lipid, oxysterol, autoimmunity
Introduction
The human nuclear receptor (NR) superfamily is a highly conserved family of transcription factors comprised of 48 members. NRs function as ligand-dependent transcription factors and share considerable amino-acid sequence homology1. General structural characteristics of NRs are a variable amino-terminal A/B domain, a central, highly conserved DNA binding domain (DBD), also termed a C region, a hinge region (D), and a carboxy-terminal ligand binding domain (LBD, or E region). The LBD is responsible for the recognition and binding of the receptor’s ligand as well as ligand-dependent transcriptional activity. Some receptors contain an additional C-terminal region, or F domain, of which the function is poorly understood.
Approximately half of the NR superfamily have well characterized natural ligands whereas the remaining receptors are considered “orphan” receptors and remain the focus of intense research2. The majority of NRs with identified natural ligands are also validated targets for clinical purposes and are a rich source of therapeutics aimed at the treatment of a great number of diseases, including inflammation, cancer, and metabolic disorders. Orphan NRs are an active area of research due to the potential for identification of ligands that may be used to modulate these receptors with the goal of developing targeted therapeutics for various diseases3. Over the past few years, there have been significant breakthroughs in the identification of novel ligands, both natural and synthetic, for several orphan NRs. This review examines the progress made in the identification of ligands for the retinoic acid receptor-related orphan receptors (RORs) and their roles in immune and metabolic processes.
RORs: the basics
Comprised of three members [RORα (NR1F1), RORβ (NR1F2), and RORγ (NR1F3], the RORs are considered “orphan” receptors as their endogenous ligands have yet to be definitively agreed upon. Due to their known roles in metabolic and immune processes, there is significant interest in the identification of ligands that regulate the RORs due to their potential for clinical utilization. Unlike most family members, the RORs recognize and bind as monomers to specific sequences of DNA, termed ROR response elements (ROREs), typically consisting of an AGGTCA “half site” with a 5′ AT-rich extension, in the regulatory region of the target gene4–6. When bound to this element within the promoter of their target genes, the RORs constitutively recruit co-activators resulting in continual activation of transcription of their target genes7,8. Another group of NRs, the REV-ERBs, recognize the same response elements as the RORs and are co-expressed in many tissues9–11. The REV-ERBs are ligand-dependent transcriptional repressors, and in many cases, functionally antagonize the action of the RORs12–14.
The three RORs display significant sequence similarity and conservation between species. Each ROR generates multiple isoforms based on alternative promoter usage andexon splicing, with all of the isoforms varying only in the amino-terminal region of the receptor7. The RORs display distinct patterns of tissue expression and are involved in the regulation of various physiological processes. RORα is widely expressed and is found in liver, skeletal muscle, skin, lungs, adipose tissue, kidney, thymus, and brain15,16. The expression of RORβ is extremely restricted and is limited to the central nervous system17,18. RORγt has been the focus of considerable attention due to its role in T helper 17 cells (TH17) cell development and autoimmune disease pathology. RORγ, specifically RORγ2 (also called RORγt), is highly expressed in immune tissues, including the thymus, but there is significant expression of RORγ in the liver, skeletal muscle, adipose tissue, and kidney7. Due to significant sequence and functional similarities, ROR subtypes co-expressed in cells may exhibit functional overlap7. However, the physiological relevance and responsiveness of all of the different isoforms of each ROR has yet to be clarified.
ROR regulation in circadian rhythms
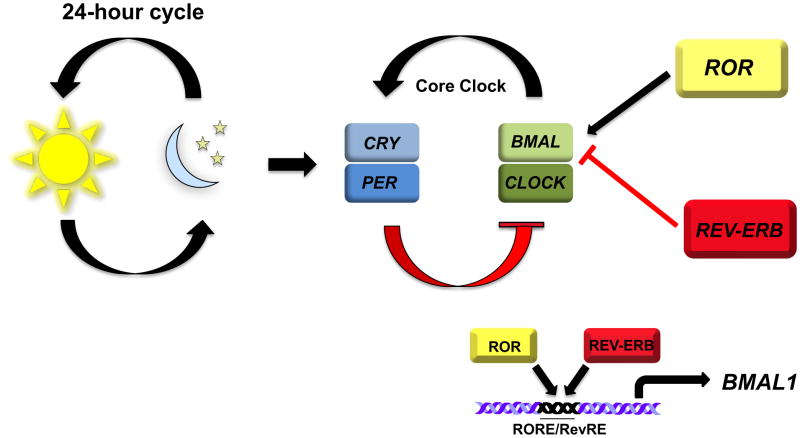
Circadian rhythms are daily cycles of biochemical, behavioral, and physiological processes controlled by endogenous “clocks” that play essential roles in the regulation of an organism’s physiology, including metabolism (Figure 1)19. In mammals, the master circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Aberrant circadian rhythms are associated with numerous disorders in humans, including sleep and mood disorders. The circadian rhythm is generated by a feedback loop where heterodimers of BMAL1 and CLOCK (the positive arm) activate the expression of the cryptochrome (Cry) and period (Per) genes (the negative arm). RORα is a core part of the clock machinery that positively regulates the expression of BMAL120,21. RORα competes with REV-ERBα for binding to their shared DNA response element in the BMAL1 promoter resulting in REV-ERBα mediated repression or RORα mediated activation of BMAL1 expression21–23. This oscillating expression of RORα and REV-ERBα in the SCN leads to the circadian pattern of BMAL1 expression thus interconnecting the positive and negative arms of the core circadian clock. (Figure 1). Therefore, RORα influences the period length and stability of the clock20.
Figure 1. ROR Regulation of the Circadian Rhythm.
Circadian rhythms are biological processes that display endogenous oscillations of approximately 24 hours and are regulated by a core circadian clock. The master circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. There are several interconnected transcriptional auto-regulatory feedback loops controlling the circadian cycle. Heterodimers of BMAL1 and CLOCK activate the expression of CRY and PER genes. Once the CRY/PER heterodimers reaches a critical threshold, they enter the nucleus and repress BMAL/CLOCK transactivation. RORα and REV-ERBα have been demonstrated to positively or negatively regulate the expression of BMAL1, respectively. RORα competes with REV-ERBa for binding of their shared DNA response element in the BMAL1 promoter. The oscillating pattern of RORα and REVERBα in the SCN dictates the circadian pattern of of BMAL1 expression. This RORα/REV-ERBα feedback loops interconnects the positive and negative arms of the core circadian clock.
Genetic models in which the RORs are either modified or have been deleted, have been instrumental in identifying their roles in the circadian rhythm. The staggerer mouse (RORαsg/sg) is a natural mouse mutant that carries an intragenic insertion within the RORα gene, which results in a frameshift and premature stop codon, rendering RORα inactive15. Staggerer mice exhibit severe cerebellar ataxia as well as a shortened period length when placed under constant dark conditions20. RORβ−/− mice also exhibit aberrant circadian rhythm, such that under constant dark conditions, RORβ−/− mice have a longer period length than wild type (wt) mice17. While no overt circadian abnormalities were apparent in RORγ−/− mice, recent work has demonstrated that RORγ directly regulates neuronal PAS domain protein 2 (Npas2) in vivo suggesting a regulatory role for this receptor in Npas2-dependent physiological processs7,24. Likewise, several lines of evidence suggest a link between cardiovascular disease, metabolic disturbances and mood disorders with disrupted circadian rhythms25. Given its extensive role in the regulation of the circadian rhythm, targeted modulation of RORα appears a feasible means by which to regulate these disorders.
RORs in metabolism and metabolic disease
The aforementioned genetic models have also been invaluable in identifying the roles of the RORs in physiological processes. On a normal diet, Staggerer mice display hypo-α-lipoproteinemia, have lower total plasma cholesterol levels, lower high density lipoprotein (HDL), apolipoprotein AI (Apoa1, the major constituent of HDL), lower apolipoprotein CIII levels (Apoc3), Apoa2, and triglycerides, compared to wild type mice26–28. Staggerer mice have decreased expression of the reverse cholesterol transporters Abca1 and Abca8/g1 in their liver and intestine, and are much less susceptible to hepatic steatosis and weight gain, compared to wt mice29. Sterol regulatory element-binding protein 1, isoform c (Srebp-1c) is reduced in the liver and muscle of staggerer mice as is the enzyme fatty acid synthase (Fas)29,30. Expression of the co-activators Peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and β, proteins involved in the regulation of oxidative metabolism and gluconeogenesis are increased in staggerer mice31. Furthermore, expression of the p450 enzyme Cyp7b1 is reduced in staggerer mice. RORα directly regulates Cyp7b1 expression by binding to a functional RORE in the promoter regulatory region of the Cyp7b1 gene30,32. These observations suggest that RORα functions as a positive regulator of Cyp7b1 function30,32. Staggerer mice also have smaller brown and white adipose cells than wt mice and when fed a high fat diet, staggerer mice are resistant to weight gain and hepatic steatosis29.
Evidence supporting RORα’s role in glucose metabolism is derived from studies in steroid receptor coactivator-2 (SRC-2) knockout mice. These mice display symptoms similar to von Gierke’s disease, which is associated with severe hypoglycemia and abnormal accumulation of glucose in the liver. SRC-2 controls the expression of hepatic Glucose-6-Phosphatase (G6Pase), an enzyme that is critical for maintaining fasting blood sugar levels by increasing hepatic glucose production and co-activates RORα bound to the RORE on theG6Pase promoter 33. Finally, it was recently demonstrated that RORα controls the expression and secretion of Fibroblast Growth Factor 21 (FGF21), a hepatic hormone that regulates peripheral glucose tolerance and hepatic lipid metabolism34. Since RORα is critical in regulating the expression of key enzymes in the gluconeogenic pathway, suppression of RORα activity may lead to a decrease in the elevated hepatic glucose output levels observed in type 2 diabetes.
Initial characterization of RORγ−/− mice revealed that they display normal cholesterol and triglyceride levels, with slightly lower blood glucose levels than their wt counterparts30. However, recent evidence suggests that RORγ may indeed have a role in metabolism through regulation of adipogenesis and insulin sensitivity. Meissburger et al. demonstrate that RORγ is a negative regulator of adipocyte differentiation in vitro. When overexpressed during adipocyte differentiation, RORγ decreases the amount of differentiated adipocytes. However, in vivo differentiation of adipocyte precursors in RORγ−/− mice was enhanced but showed decreased size. The smaller adipocytes were insulin sensitive and protected the mice from obesity induced hyperglycemia and insulin resistance 35. Moreover, analysis of adipose stromal-vascular fractions from obese human subjects demonstrated a positive correlation between RORγ expression and adipocyte size that was negatively correlated with adipogenesis and insulin sensitivity. These findings suggest that RORγ may be a novel target for the treatment of obesity-associated insulin resistance35.
The deletion of both RORα and RORγ exhibit similar changes in cholesterol, triglyceride, and blood glucose levels as the single knock out mice. Gene expression analysis from livers of double knock out (DKO) mice suggests a degree of functional redundancy between RORα and RORγ which is most likely due to the similarities in RORE binding affinities30. However, the recent evidence regarding obesity and insulin resistance in the RORγ−/− mice highlights the differences between the two NRs in metabolic processes.
RORs and (auto)immunity
Host defense against invading pathogens is largely dependent upon distinct adaptive immune responses facilitated by the differentiation of CD4+ T cells into specific lineages of effector T helper cells (TH1, TH2, and TH17 cells)36. Both RORα and RORγ, specifically RORγt, have generated a significant amount of attention over the past few years due to their essential role in the development of TH17 cells. Until recently, it was generally thought that there were only two T helper subsets within the CD4+ T cell repertoire, TH1 and TH2. TH1 cells mediated cellular immunity against intracellular bacteria and viruses whereas TH2 cells were thought to be involved in the humoral response to parasitic pathogens36. However, TH1 cells that responded to self-antigen can lead to autoimmune diseases whereas dysregulation of TH2 responses to allergens and parasites can cause specific allergic and parasitic pathology. TH1 cells had long been thought to be the mediators of tissue damage in autoimmune disease36. Key experiments using two established mouse models of autoimmunity, experimental autoimmune encephalomyelitis (EAE) and type II collagen-induced arthritis (CIA), largely led to the discovery of another T helper subset known as TH17 cells (Figure 2). In this setting, TH17 cells were critical mediators of much of the pathology associated with EAE and CIA. TH17 cells are defined by a specific cytokine profile and secrete IL-17, IL-9, IL-21, IL-22, IL-26, and CCL2037. These mediators are responsible for a number of different effector functions in host defense as well as autoimmune diseases.
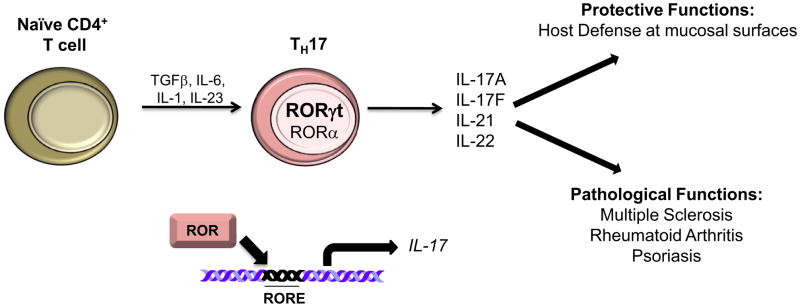
Figure 2. RORα and RORγ in TH17 Cell Differentiation.
In the presence of several exogenous factors, including TGFβ, IL-6, and IL-1, naïve CD4+ T cells differentiate into TH17 cells. Exogenous IL-23 is necessary for the propagation of pathogenic TH17 cells. The expression of RORα and particularly RORγt is necessary for TH17 cell differentiation and for the expression of IL-17A and IL-17F, among other cytokines. TH17 cells play a significant role in host defense against extracellular pathogens at mucosal surfaces. However, aberrant TH17 cell activity has been associated with the pathology of several autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, and psoriasis.
Despite the negative implications for TH17 cells, this cell type plays a significant role in host defense against extracellular pathogens, specifically gram-negative bacteria at mucosal surfaces, as well as obligates intracellular pathogens, including intracellular bacteria and fungi38. In addition, TH17 cells have been shown to exhibit general tissue protective functions37.
Key factors in the development of TH17 cells involve the RORs, specifically RORα and RORγt, one isoform of RORγ that is exclusively detected in a few distinct types of cells in the immune system39. Overexpression of RORγt in naïve CD4+ T cells was demonstrated to drive the induction and development of TH17 cells40. Furthermore, RORγt−/− mice display impaired TH17 cell development40. Mice deficient in both RORα and RORγ completely lack TH17 cells and are resistant to the development of several autoimmune diseases, including EAE41,42. Collectively, these data suggested that targeted inhibition of RORα and RORγ with specific, synthetic ligands could be one mechanism to potentially reduce autoimmune pathology.
Regulation of RORs by endogenous ligands
The ligand binding domains of NRs are multifunctional. Typically, ligand binding induces a conformational change in the receptor resulting in dissociation of corepressors and recruitment of co-activators1. However, RORs are constitutively active meaning that they are in an active conformation in the absence of ligand and that ligand binding might actually repress receptor activity (inverse agonist, see box 1). While identification of the endogenous ligands for RORs has been controversial, recent evidence suggests that, similar to the liver X receptor’s (LXRs), oxygenated sterols may function as high affinity ligands. Indeed, 7-oxygenated sterols (7α-OHC, 7β-OHC, and 7-ketocholesterol) function as inverse agonists to both RORs. The 7-oxygenated sterols bind to both RORα and RORγ isoforms with an affinity significantly greater than the affinity for cholesterol and cholesterol sulfate, and suppress their transactivation properties. It was also shown that both RORα and RORγ are constitutively active in the absence of ligand, able to bind co-activator peptides, and activate transcription. Furthermore, the 7-oxygenated sterols modulated the expression of RORα/γ-dependent target genes in a receptor dependent manner8 and possess the ability to induce the conformational change necessary to alter cofactor binding and transcriptional activity, a core requirement for a bona fide ligand (Figure 3).
Box 1. Definition of a NR ligand.
NRs are generally characterized as ligand-dependent transcription factors. Typically, NR ligands are small, hydrophobic molecules including steroid hormones, fatty acids, and lipophilic vitamin derivatives. True ligands bind in the LBD of NRs inducing a conformational change within the receptor thus providing an interface for cofactor binding. Co-factors can be either co-activators or co-repressors. Ligands are classified according to their ability to regulate a specific NRs transcriptional activity.
Agonist
An agonist binds to the LBD and induces a conformational change resulting in increased recruitment of co-activator proteins. This results in maximal alterations in target gene transcription.
Antagonist
An antagonist does not provoke a response from the receptor. Rather, an antagonist binds the LBD and blocks the ability of an agonist to bind and activate the receptor.
Inverse agonist
An inverse agonist binds within the LBD of a given receptor, but inhibits the basal constitutive activity of the receptor. This generally describes a ligand to a particular NR that in its basal conformation is not bound by any ligand but allows for interaction with a cofactor protein (either coactivator or corepressor) leading to transcriptional activity. An inverse agonist induces a conformational change within the receptor that decreases the affinity of the receptor for a cofactor protein.
Partial agonists
Partial agonists bind and activate a receptor, but only with partial efficacy relative to a ligand that elicits a maximal response.
The RORs have intrinsic transcriptional activity, meaning that they are constitutively active since it has been demonstrated that they bind co-activator proteins in the absence of ligand. When ligand binding occurs, it represses the transcriptional activity of the receptor.
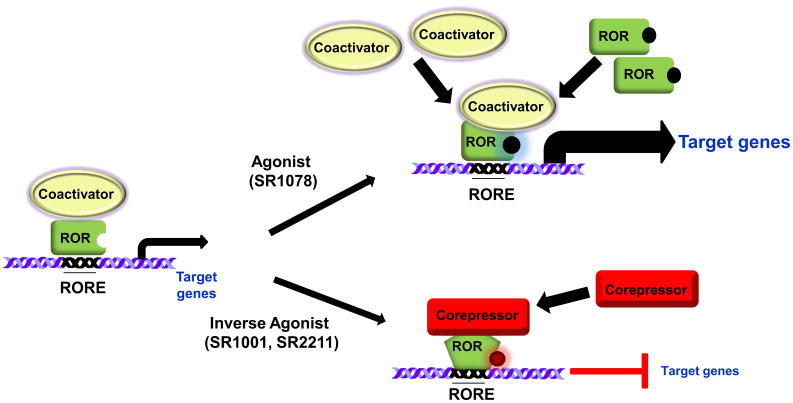
Figure 3. Regulation of ROR activity with synthetic ligands.
The RORs are considered to have intrinsic transcriptional activity, meaning that they are constitutively active and bind coactivators in the absence of ligand. However, due to the ubiquitous expression of putative ROR ligands, it remains to be determined whether the RORs are ever in an unbound state or require ligand for receptor stability. Treatment with an agonist(SR1078) would result in the recruitment of more coactivator proteins, thereby enhancing transcriptional activity. Inverse agonists, when bound to the RORs LBD, induce a conformational change in the receptor resulting in dissociation of coactivator proteins and recruitment of corepressor proteins. Inverse agonists repress the activity of the receptors.
Several other endogenous RORα and RORγ ligands have been described recently. 24S-hydroxycholesterol (24S-OHC) is a high affinity ligand for RORα and RORγ, and similar to the 7-oxygentated sterols, 24S-OHC acts as an inverse agonist and dose dependently reduces the RORα and RORγ constitutive activity43. As a consequence, expression of BMAL1 and REV-ERBα mRNAs are also reduced. In a similar manner, 24S,25-epoxycholesterol (24,25-epoC) and 24R-cholesterol (24R-OHC) also selectively bind to and regulate the activity of RORγ43.
20α-hydroxycholesterol (20α-OHC), 22R-hydroxycholesterol (22R-OHC), and 25-hydroxycholesterol (25-OHC) were also shown to be putative endogenous ligands for RORγ44 as all three ligands dose dependently increased the recruitment of coactivator peptides to RORγ, in vitro44. In addition, elucidation of the RORγ crystal structure revealed that these three ligands bind to RORγ in a similar manner. The RORγ crystal structures also demonstrated that the AF-2 domain at the C- terminus of the receptor, together with helices H3, H4, and H5 form a charge clamp pocket, the area that facilitates binding of co-activator proteins to NRs. Mutational studies of this region revealed that an intact charge clamp pocket was required for these hydroxycholesterols to affect RORγ activity44 (Table 1).
Table 1. Structure of ROR Ligands.
Both natural and synthetic ligands are displayed.
| Name | Structure | Origin | Receptor preference | Ligand type | Affinity (Ki) | REFS |
|---|---|---|---|---|---|---|
| T0901317 |
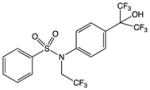
|
Human NR specificity screen | RORα, RORγ, LXRα LXRβ PXR FXR other | RORs: Inverse agonist LXRs, PXR, FXR: Agonist |
RORα: 132nM RORγ: 51nM |
67, 68, 49, 50, |
| SR1001 |
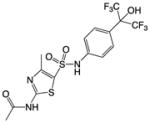
|
Synthetic small molecule – analog of T0901317 | RORα RORγ | Inverse agonist | RORα: 172nM RORγ: 111nM |
56 |
| SR1078 |
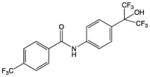
|
Synthetic small molecule – analog of T0901317 | RORα RORγ | Agonist | IC50: 1–3μM | 51 |
| SR3335 |
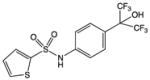
|
Synthetic small molecule – analog of T0901317 | RORα | Inverse agonist | 220nM | 55 |
| SR2211 |
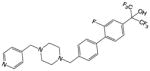
|
Synthetic small molecule – analog of T0901317 | RORγ | Inverse agonist | 105nM | 62 |
| Cholesterol |
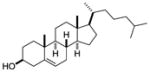
|
Sf-9 insect cells ubiquitously expressed in all mammalian cells |
RORα | agonist | EC50: 200nM | 45, 44 |
| Cholesterol Sulfate |
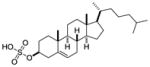
|
Sf-9 insect cells ubiquitously expressed in all mammalian cells |
RORα | agonist | 46 | |
| Ursolic Acid |
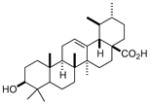
|
Small chemical library screen, carboxylic acid expressed in plants | RORγ | Inverse agonist | IC50: 680nM | 59 |
| Digoxin |
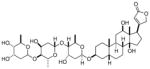
|
chemical screen, isolated from Foxglove plant | RORγ | Inverse agonist | Kd: 109nM | 57 |
| 7α-hydroxy- cholesterol |
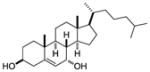
|
Screen of oxysterols for ROR activity | RORα RORγ | Inverse agonist | RORα: 12–18nM RORγ: 17–31nM |
8 |
| 7β-hydroxy- cholesterol |
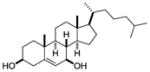
|
Screen of oxysterols for ROR activity | RORα RORγ | Inverse agonist | RORα: 12–18nM RORγ: 17–31nM |
8 |
| 7-keto- cholesterol |
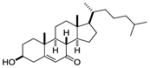
|
Screen of oxysterols for ROR activity | RORα RORγ | Inverse agonist | RORα: 12–18nM RORγ: 17–31nM |
8 |
| 20α-hydroxy- cholsterol |
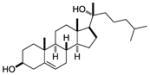
|
Alpha screen of cholesterol and hydroxycholesterols | RORγ | agonist | EC50: 20–40nM | 44 |
| 22R hydroxy- cholsterol |
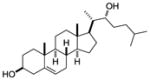
|
Alpha screen of cholesterol and hydroxycholesterols | RORγ | agonist | EC50: 20–40nM | 44 |
| 25-hydroxy- cholsterol |
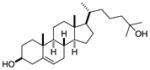
|
Alpha screen of cholesterol and hydroxycholesterols | RORγ | agonist | EC50: 20–40nM | 44 |
| 24S-hydroxy- cholsterol |
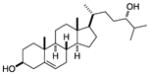
|
Screen of oxysterols for ROR activity | RORα RORγ | inverse agonist | 25nM | 43 |
| 24,25 -epoxy- cholsterol |
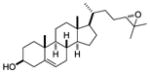
|
Screen of oxysterols for ROR activity | RORγ | inverse agonist | 20nM | 43 |
| 24R-hydroxy- cholsterol |
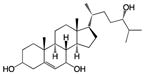
|
Screen of oxysterols for ROR activity | RORγ | inverse agonist | 102nM | 43 |
Despite the identification of these putative ligands for the RORs, their physiological significance and whether they are regulatory or structural is not clear. For instance, several studies using RORα LBD purified from insect cells identified and characterized cholesterol, cholesterol sulfate, and several other cholesterol derivatives as endogenous putative ligands45,46. While subsequent studies have since established that several of these ligands are fortuitous, they suggest a requirement for ligand-bound LBD for receptor stability47. Mutations in several key amino acids known to be involved in ligand binding abolishes the constitutive activity of the receptor, which could be attributed to the instability of the receptor in the absence of ligand48. Furthermore, much of the work describing ROR ligands has occurred using artificial systems in vitro with luciferase reporter systems. To date, only the 7-oxygenated sterols, 24S-OHC, 24,25-epoC, and 24R-OHC were shown to affect ROR target gene expression in vitro8,43. It is also difficult to envisage how many of these putative ligands could function as regulatory ligands due to their circulating levels within tissues. Whether these ligand associate with the RORs in vivo has yet to be determined. These questions need to be answered in order to determine whether the described ROR ligands are deemed “endogenous” and regulatory. With this in mind, using these ligands as “tools” to understand the biology of the RORs should be met with caution. Despite the relative discrepancy, these data suggests that the RORs may function as lipid sensors and thus play a major role in the regulation of lipid metabolism.
Modulation of ROR activity with synthetic ligands
The identification of endogenous ligands to RORα and RORγ intensified the search for synthetic ligands that could modulate ROR activity (Table 1). The synthetic LXR agonist T0901317 was the first synthetic inverse agonist identified for both RORα and RORγ. Despite its potency at activating RORα and RORγ, T0901317 displays promiscuity and binds to several NRs including LXR, farnesoid X receptor (FXR), and pregnane X receptor (PXR), thus limiting its use as a chemical tool to explore the activity of the RORs in physiological settings49,50.
A focused medicinal chemistry approach to develop analogs of T0901317 that activated RORs but not other NRs led to the development of several ROR-selective modulators. The first RORα/γ-specific synthetic ligand characterized was the amide SR1078. (Figure 3). SR1078 was initially identified as an inverse agonist as it repressed the constitutive activity of RORα and RORγ and inhibited the recruitment of co-activators to RORγ in a dose dependent manner51. However, further examination revealed that SR1078 acts as an agonist and stimulated expression of two ROR target genes, G6Pase and FGF21, in the liver. Pharmacokinetic studies revealed that SR1078 displays reasonable plasma exposure, thus enabling its use as a chemical tool to probe the function of RORα and RORγ both in vitro and in vivo52.
RORα expression is induced in response to some types of cellular stress and is downregulated in several breast, prostate, and ovarian cancer cell lines 53. Interestingly, activation of RORα by SR1078 in this setting results in an increase in p53 levels and apoptosis suggesting that RORα represents a novel target for the development of cancer therapeutics54.
The inverse agonist, SR3335 (Table 1) was initially identified based on its ability to inhibit the constitutive activity of RORα. Furthermore, SR3335 directly bound to the LBD of RORα, with little effect at RORγ, and suppressed expression of RORα target genes involved in hepatic gluconeogenesis including G6Pase and Phosphoenolpyruvate Carboxykinase (PEPCK). Pharmacokinetic studies revealed that SR3335 had reasonable plasma exposure and administration of this ligand to diet induced obese mice (DIO) led to reduced plasma glucose levels following a pyruvate tolerance test (PTT), an indicator of gluconeogenesis55. Given that elevated glucose output is observed in type 2 diabetes (T2D), suppression of RORα activity with novel ligands like SR3335 may hold utility in the treatment of metabolic disorders, including T2D.
With the accumulating evidence surrounding RORα and RORγt’s role in TH17 cell development and autoimmune pathology, identification of a dual and highly selective RORα/γ inverse agonist that inhibits TH17-mediated pathology is extremely enticing and such efforts led to the identification and characterization of SR1001 (Table 1), a first-in-class RORα/γ-specific inverse agonist. (Figure 3). SR1001 directly binds to the LBD of both RORα and RORγ resulting in a conformational change that decreases affinity for coactivators and increased affinity for corepressors56. When screened against all 48 human nuclear receptors, SR1001 displayed activity only at RORα and RORγ. In vitro, SR1001 inhibited IL-17 expression and TH17 cell development without affecting the differentiation and function of any of the other T helper cell lineages56. More importantly, in vivo administration of SR1001 delayed the onset and severity of EAE, through inhibition of TH17 cell development and function. These data demonstrate that small molecule inhibitors of ROR activity are effective at suppressing TH17-mediated autoimmune diseases56.
Huh et al. identified the well-known cardiac glycoside digoxin (Table 1), a small molecule inhibitor of RORγ activity. Currently, digoxin is used clinically in the treatment for various heart conditions. Digoxin normally competes with K+ ions for the same binding site on the Na+/K+ ATPase pump, thereby altering electrical conduction in the heart. Digoxin suppressed RORγ-mediated activity only, and displayed no activity for RORα, Drosophila hormone receptor 3 (DHR3), the C. elegans nuclear hormone receptor Dauer formation-12 (DAF-12), or the androgen receptor57. Digoxin inhibited TH17 cell differentiation and function and delayed the onset and severity of EAE57. Despite its efficacy in this model, major drawbacks with digoxin are its toxicity, occurrence of adverse drug reactions associated with use of this drug, and a narrow therapeutic window. Given these issues, less toxic digoxin analogs have been derived that are able to inhibit RORγ activity and TH17 cell differentiation and function, in vitro57. The crystal structure of the RORγ LBD bound to digoxin was recently resolved demonstrating the mechanism by which digoxin inhibited RORγ activity. Similar to SR1001, when bound to RORγ, digoxin inhibited co-activator binding58. These findings demonstrate the feasibility of targeting RORγ or both RORα and RORγ with small molecules for the treatment of TH17-mediated autoimmune disorders.
Ursolic acid has also been demonstrated to target RORγ and thus inhibit TH17 cell differentiation. When administered in vivo, mice treated with ursolic acid exhibited a delay of onset with decreased severity of symptoms of EAE. Biochemical assays indicate that ursolic acid effectively binds the LBD of RORγ leading to displacement of co-activator binding, whereas it had little effect at RORα59. Ursolic acid, which is present in many plants, including apples, was originally described asa potential anti- cancer therapeutic able to inhibit various types of cancer cells by inhibiting STAT3 activation60. Further examination suggested that ursolic acid reduced the expression of matrix metalloproteinase-9 (MMP-9) by potentially acting through the glucocorticoid receptor (GR)61. Given the steroidal-like structures of both ursolic acid and digoxin, the possibility that both compounds exhibit activity at GR complicates the in vivo interpretations. Glucocorticoids are very effective at inhibiting symptoms of EAE and are in fact routinely prescribed by neurologists to reduce the severity and duration of relapses in MS patients.
While SR1001 was effective at delaying the onset and reducing the severity of EAE, there was some concern that this compound, which modified the activity of RORα, would induce a phenotype similar to that of the staggerer mouse, including ataxia and a disrupted circadian rhythm15,56. Furthermore, while several RORγ selective modulators had already been described, their utility as candidates for further drug development was limited. Therefore, further development of RORγ modulator was warranted. SR2211 is a selective RORγ modulator that binds to the LBD of RORγ and functions as an inverse agonist suppressing receptor activity 62. Therefore, SR2211 is a potent and efficacious RORγ modulator with potential utility in the treatment of TH17-mediated autoimmune disorders.
Despite their high profile roles in TH17-mediated autoimmunity, the expression of RORγt and RORα are not restricted to this cell type, nor are all TH17 cells pathogenic. Recent evidence links RORα to the maintenance of IgA+ memory B cells63. Certain types of innate lymphoid cells, including lymphoid tissue inducer cells (LTi), γδ T cells, and intestinal epithelial cells (IEPs), express RORγt 64,65. Innate lympoid cells play important roles in tissue surveillance and can be the first lines of defense against a number of invading pathogens65. Similarly, TH17 cells have proven to be essential for host defense against some gram-negative bacteria and fungal infections at mucosal surfaces66. Given the increasing number of immune cells expressing the RORs, inhibiting their activity during particular immune system assaults may be detrimental. Therefore, careful assessment of the infection and invading pathogen(s) may be warranted prior to administration. Alternatively, some infections due to specific gram-negative bacteria or fungi may require the use of ROR agonists to amplify the immune response from these ROR-restricted cell types.
Concluding remarks
To date, several groups have developed or described numerous small molecule ligands to RORα and RORγ. Collectively, these data demonstrate that these orphan NRs are not only valid drug targets, but have efficacy at suppressing TH17 cell development and function both in vitro and in vivo. While further optimization of the small molecules is still needed, it is obvious that targeting the RORs for the treatment of TH17-mediated autoimmune disorders represents a promising endeavor as novel treatments of autoimmune disorders. Current treatments for known TH17-mediated autoimmune diseases, including multiple sclerosis, use agents that are general immunosuppressants and thus the side-effect profile is significant. The targeting of the RORs present a significant advantage over the current therapies as they specifically target the one arm of the immune system mediating disease, rather that the immune system as a whole. Finally, extensive analysis of the use of these ligands in vivo has yet to occur. While genetic studies are valuable tools for elucidating the roles these receptors play in physiology, the roles of the RORs during metabolic and autoimmune disease progression can be extensively studies through in vivo use of specific synthetic ligands.
Glossary
- Co-activator
a nuclear receptor co-activator is a transcriptional coregulatory protein that contains nuclear receptor interacting domains. The co-activator is unable to bind DNA by itself but assists nuclear receptors to bind to HREs on target gene promoter site and increase transcription
- Corepressor
a nuclear receptor co-repressor, similar to a co-activator, contains nuclear receptor interacting domains. The co- repressor assists nuclear receptors in the downregulation of target gene expression
- Diet induced obesity (DIO)
a mouse model of pre-diabetic type 2 diabetes and obesity with elevated blood glucose and impaired glucose tolerance
- Experimental autoimmune encephalomyelitis (EAE)
a mouse model of autoimmunity. Symptoms and disease progression in EAE are similar to those experienced by those of multiple sclerosis patients
- Hormone response element (HRE)
a short DNA sequence in the promoter of a gene that binds a specific NR complex and regulates transcription. An HRE is most commonly composed of two inverted repeats separated by three nucleotides, which allows the receptor to bind as a dimer
- Ligand binding domain (LBD)
a domain found in NRs that is highly conserved between the various NR where ligands bind and modulate gene transcription. The LBD contributes to the dimerization interface of the receptor and in addition binds coactivator and corepressor proteins
- Nuclear receptors (NRs)
highly conserved transcription factors that generally regulate gene transcription in a ligand-dependent manner. Steroid hormones are perhaps the most recognized members of the NR superfamily
- Orphan receptors
A NR is considered an orphan receptor when it has no known, or generally agreed upon, endogenous ligand(s) identified
- Retinoic-acid-receptor-related orphan receptor (ROR)
a member of the NR superfamily. Three isoforms of ROR exist, ROR-α, -β, and –γ, each encoded by a different gene. RORs bind as monomers to hormone response elements as opposed to the majority of other nuclear receptors which bind as dimmers
- T helper 17 cells (Th17)
a subset of T helper cells that are developmentally distinct from Th1 and Th2 cells and that produce interleukin 17 (IL-17). Th17 cells are thought to play a key role in autoimmune disease such as multiple sclerosis, psoriasis, juvenile diabetes, rheumatoid arthritis, and Crohn’s disease
- Type II collagen-induced arthritis(CIA)
an animal model of polyarthritis that is induced by immunization with type II collagen of susceptible mice and rats
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hummasti S, Tontonoz P. Adopting new orphans into the family of metabolic regulators. Mol Endocrinol. 2008;22:1743–53. doi: 10.1210/me.2007-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohan R, Heyman RA. Orphan nuclear receptor modulators. Curr Top Med Chem. 2003;3:1637–47. doi: 10.2174/1568026033451709. [DOI] [PubMed] [Google Scholar]
- 4.Giguere V, et al. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–53. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- 5.Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLamarter JF, Becker-Andre M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol. 1994;8:757–70. doi: 10.1210/mend.8.6.7935491. [DOI] [PubMed] [Google Scholar]
- 6.Hirose T, Smith RJ, Jetten AM. ROR gamma: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun. 1994;205:1976–83. doi: 10.1006/bbrc.1994.2902. [DOI] [PubMed] [Google Scholar]
- 7.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, et al. Modulation of ROR{alpha} and ROR{gamma} activity by 7-oxygenated sterol ligands. J Biol Chem. 2009;285:5013–25. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duez H, Staels B. Rev-erb-alpha: an integrator of circadian rhythms and metabolism. J Appl Physiol. 2009;107:1972–80. doi: 10.1152/japplphysiol.00570.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Med Chem. 3:623–38. doi: 10.4155/fmc.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin L, Wu N, Lazar MA. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl Recept Signal. 8:e001. doi: 10.1621/nrs.08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burris TP. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol Endocrinol. 2008;22:1509–20. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghuram S, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–13. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin L, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–9. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton BA, et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature. 1996;379:736–9. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- 16.Steinmayr M, et al. staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc Natl Acad Sci U S A. 1998;95:3960–5. doi: 10.1073/pnas.95.7.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andre E, et al. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17:3867–77. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andre E, Gawlas K, Becker-Andre M. A novel isoform of the orphan nuclear receptor RORbeta is specifically expressed in pineal gland and retina. Gene. 1998;216:277–83. doi: 10.1016/s0378-1119(98)00348-5. [DOI] [PubMed] [Google Scholar]
- 19.Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121:2133–41. doi: 10.1172/JCI46043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–8. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 21.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 22.Forman BM, et al. Cross-talk among ROR alpha 1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol. 1994;8:1253–61. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- 23.Retnakaran R, Flock G, Giguere V. Identification of RVR, a novel orphan nuclear receptor that acts as a negative transcriptional regulator. Mol Endocrinol. 1994;8:1234–44. doi: 10.1210/mend.8.9.7838156. [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y, Kang HS, Angers M, Jetten AM. Retinoic acid-related orphan receptor gamma directly regulates neuronal PAS domain protein 2 transcription in vivo. Nucleic Acids Res. 2011;39:4769–82. doi: 10.1093/nar/gkq1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutsson A, Hallquist J, Reuterwall C, Theorell T, Akerstedt T. Shiftwork and myocardial infarction: a case-control study. Occup Environ Med. 1999;56:46–50. doi: 10.1136/oem.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raspe E, et al. Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha. J Biol Chem. 2001;276:2865–71. doi: 10.1074/jbc.M004982200. [DOI] [PubMed] [Google Scholar]
- 27.Mamontova A, et al. Severe atherosclerosis and hypoalphalipoproteinemia in the staggerer mouse, a mutant of the nuclear receptor RORalpha. Circulation. 1998;98:2738–43. doi: 10.1161/01.cir.98.24.2738. [DOI] [PubMed] [Google Scholar]
- 28.Vu-Dac N, et al. Transcriptional regulation of apolipoprotein A-I gene expression by the nuclear receptor RORalpha. J Biol Chem. 1997;272:22401–4. doi: 10.1074/jbc.272.36.22401. [DOI] [PubMed] [Google Scholar]
- 29.Lau P, et al. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem. 2008;283:18411–21. doi: 10.1074/jbc.M710526200. [DOI] [PubMed] [Google Scholar]
- 30.Kang HS, et al. Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics. 2007;31:281–94. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–81. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 32.Wada T, et al. Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3) Mol Pharmacol. 2008;73:891–9. doi: 10.1124/mol.107.040741. [DOI] [PubMed] [Google Scholar]
- 33.Chopra AR, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke’s disease. Science. 2008;322:1395–9. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem. 285:15668–73. doi: 10.1074/jbc.M110.102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meissburger B, et al. Adipogenesis and insulin sensitivity in obesity are regulated by retinoid-related orphan receptor gamma. EMBO Mol Med. 3:637–51. doi: 10.1002/emmm.201100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Graeber KE, Olsen NJ. Th17 cell cytokine secretion profile in host defense and autoimmunity. Inflamm Res. 61:87–96. doi: 10.1007/s00011-011-0419-1. [DOI] [PubMed] [Google Scholar]
- 38.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect Immun. 78:32–8. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korn T, Petermann F. Development and function of interleukin 17-producing gammadelta T cells. Ann N Y Acad Sci. 2012;1247:34–45. doi: 10.1111/j.1749-6632.2011.06355.x. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 146:772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Kumar N, Crumbley C, Griffin PR, Burris TP. A second class of nuclear receptors for oxysterols: Regulation of RORalpha and RORgamma activity by 24S-hydroxycholesterol (cerebrosterol) Biochim Biophys Acta. 2010;1801:917–23. doi: 10.1016/j.bbalip.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin L, et al. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol Endocrinol. 2010;24:923–9. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kallen JA, et al. X-ray structure of the hRORalpha LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORalpha. Structure. 2002;10:1697–707. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 46.Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem. 2004;279:14033–8. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- 47.Volkman JK. Sterols in microorganisms. Appl Microbiol Biotechnol. 2003;60:495–506. doi: 10.1007/s00253-002-1172-8. [DOI] [PubMed] [Google Scholar]
- 48.Moraitis AN, Giguere V, Thompson CC. Novel mechanism of nuclear receptor corepressor interaction dictated by activation function 2 helix determinants. Mol Cell Biol. 2002;22:6831–41. doi: 10.1128/MCB.22.19.6831-6841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houck KA, et al. T0901317 is a dual LXR/FXR agonist. Mol Genet Metab. 2004;83:184–7. doi: 10.1016/j.ymgme.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Mitro N, Vargas L, Romeo R, Koder A, Saez E. T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett. 2007;581:1721–6. doi: 10.1016/j.febslet.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, et al. Identification of SR1078, a Synthetic Agonist for the Orphan Nuclear Receptors RORalpha and RORgamma. ACS Chem Biol. 2010;5:1029–34. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, et al. Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORalpha and RORgamma. ACS Chem Biol. 5:1029–34. doi: 10.1021/cb100223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, McAvoy S, Kuhn R, Smith DI. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene. 2006;25:2901–8. doi: 10.1038/sj.onc.1209314. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Solt LA, Kojetin DJ, Burris TP. Regulation of p53 Stability and Apoptosis by a ROR Agonist. PLoS One. 2012;7:e34921. doi: 10.1371/journal.pone.0034921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar N, et al. Identification of SR3335 (ML-176): A Synthetic RORalpha Selective Inverse Agonist. ACS Chem Biol. 6:218–22. doi: 10.1021/cb1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 472:491–4. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 472:486–90. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujita-Sato S, et al. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 286:31409–17. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu T, et al. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem. 286:22707–10. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pathak AK, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, et al. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J Biomed Biotechnol. 2011:419343. doi: 10.1155/2011/419343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar N, et al. Identification of SR2211: A Potent Synthetic RORgamma-Selective Modulator. ACS Chem Biol. doi: 10.1021/cb200496y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang NS, et al. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORalpha. Nat Immunol. 2012 doi: 10.1038/ni.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vonarbourg C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–51. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 66.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–9. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 67.Kumar N, et al. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluorometh yl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-(alpha)/(gamma) inverse agonist. Mol Pharmacol. 2010;77:228–36. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz JR, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–8. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]