Abstract
Salmonella spp. are gram-negative flagellated bacteria that can cause food and water-borne gastroenteritis and typhoid fever in humans. We now report that flagellin from Salmonella spp. is recognized in mouse intestine by Toll-like receptor 11 (TLR11). Absence of TLR11 renders mice more susceptible to infection by S. typhimurium, with increased dissemination of the bacteria and enhanced lethality. Unlike S. typhimurium, S. typhi, a human obligatory pathogen that causes typhoid fever, is normally unable to infect mice. TLR11 is expressed in mice but not in humans, and remarkably, we find that tlr11−/− mice are efficiently infected with orally-administered S. typhi. We also find that tlr11−/− mice can be immunized against S. typhi. Therefore, tlr11−/− mice represent the first small animal model for the study of the immune response to S. typhi, and for the development of vaccines against this important human pathogen.
Introduction
Innate immune responses provide the first-line of host defense against microbial pathogens and, together with adaptive immunity, ensure effective clearance of invading pathogens. Toll like receptors (TLRs), which are expressed in sentinel cells such as macrophages and dendritic cells, recognize conserved components of microbes known as pathogen-associated molecular patterns (PAMPs) (Kawai and Akira, 2010). Mice express twelve TLRs including TLRs 11, 12 and 13, which are not expressed in humans (Roach et al., 2005). We have previously characterized the role of the mouse-specific TLR11 in responses to uropathogenic E. coli and the apicomplexan parasite Toxoplasma gondii (Yarovinsky et al., 2005; Zhang et al., 2004). The T. gondii PAMP recognized by TLR11 is the apicomplexan profilin protein (Yarovinsky et al., 2005). The finding that TLR11 recognized a protein ligand from T. gondii was consistent with phylogenetic analyses demonstrating that TLR11 is most closely related to TLR5 (Roach et al., 2005; Zhang et al., 2004) which recognizes the bacterial protein PAMP, flagellin (Hayashi et al., 2001).
In contrast, to T. gondii, the ligand from uropathogenic E.coli (UPECs) that triggers innate responses through TLR11 in the urogenital tract has remained uncharacterized (Zhang et al., 2004). Our initial efforts in purifying this ligand suggested that it was a protein component of UPEC (5), although we were not able to identify the specific molecule recognized by TLR11(Zhang et al., 2004). Given that PAMPs are rarely restricted to specific pathogens (Kawai and Akira, 2010), we chose to investigate the potential role of TLR11 in recognizing other pathogens in order to identify another source from which we could potentially purify the bacterial ligand for TLR11. Consistent with TLR11 mediating the innate response to UPECs at the urogenital epithelium, we noted that TLR11 is also highly expressed in the intestinal epithelium. This led us to speculate that TLR11 might also function in the recognition of enteropathogenic bacteria.
Gram-negative Salmonella sp. are a common bacterial enteropathogen and are widely used in laboratory studies aimed towards understanding the basis of mucosal immune responses, and diseases such as gastroenteritis and typhoid. Most laboratory studies are carried out using S. typhimurium in mice, where a disseminated infection with some similarities to human typhoid is observed. Typhoid fever affects more than twenty million individuals and causes more than 220,000 deaths annually (Crump et al., 2004; Woc-Colburn and Bobak, 2009). However typhoid disease in humans is caused by the specific Salmonella enterica serovars Typhi and, to a lesser extent Paratyphi (Crump et al., 2004), which do not infect mice. There are marked genetic differences between S. typhimurium and S. typhi, which presumably account for the divergent disease states caused by these organisms in people (Sabbagh et al., 2010). As a result, animal models of S. typhi infection are of significant interest for understanding the biology of this important pathogen. Typhoid is endemic in third-world countries, where it has been difficult to achieve adequate improvements in sanitation and water supply to have a timely impact on the disease. Clearly what are required are effective vaccination strategies. Unfortunately, the two approved vaccines have only modest efficacy (50–80% protection) (Guzman et al., 2006). The development of better vaccination would require a more complete understanding of the immune response to bacteria as well as the availability of experimentally tractable small-animal models that can be infected by S. typhi.
In this study we describe the contribution of TLR11 in Salmonella spp. infection of the mouse. We have found that TLR11 deficient mice are severely compromised in innate epithelial responses to S. typhimurium and exhibit poorly controlled and widely disseminated infection resulting in enhanced lethality. We have purified the ligand from S. typhimurium and found that like TLR5, TLR11 recognizes flagellin. Flagellin is also the TLR11 ligand from UPECs. Flagellin binds TLR11 and induces innate responses in mice independent of TLR5. Remarkably, loss of TLR5 results in upregulation of TLR11 in lamina propria macrophages, suggesting enhanced TLR11 responses may contribute to the resistance of TLR5 deficient mice to S. typhimurium infection. Because TLR11 contributes significantly to protection of mice from S. typhimurium and the presence of TLR11 correlates with the host restriction of typhoidal Salmonella infection, we tested whether TLR11 might be responsible for mouse resistance to S. typhi infection. As for S. typhimurium, we found that TLR11 recognized S. typhi flagellin and that S. typhi could lethally infect mice lacking TLR11 upon oral infection. Low dose oral infection with S. typhi produced a febrile illness with features of human typhoid. Existing small animal models of S. typhi infection rely on use of severely immunocompromised animals (Mian et al., 2011) and, therefore, cannot serve as systems in which to study host responses, or for vaccine development. In contrast, TLR11 deficient mice can be protected from infection by vaccination with heat killed S. typhi or by passive transfer of serum from immunized mice. Therefore, these results provide the first genetic evidence of host restriction enforced by species-specific TLR expression, and the generation of an immunologically intact model for assessing S. typhi host-pathogen interactions and testing new vaccine approaches.
Results
Lack of TLR11 leads to enhanced susceptibility to infection by S. typhimurium
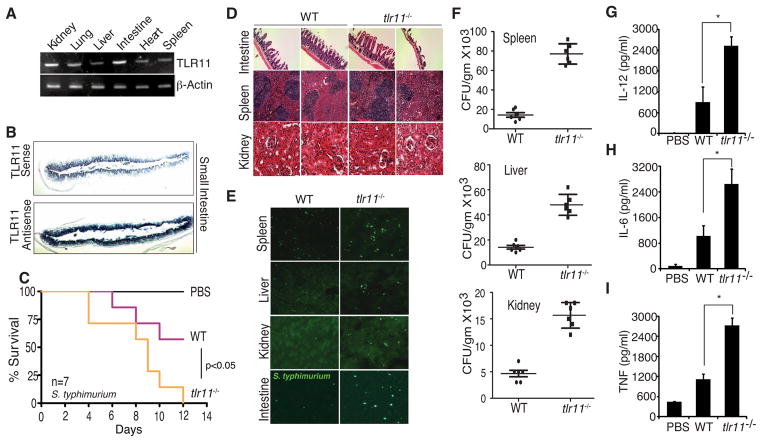
Although we had previously suggested that the PAMP from UPECs recognized by TLR11 was a protein (Zhang et al., 2004), our initial efforts to identify the ligand were unfruitful. We suspected that TLR11 was likely to recognize similar ligands in other pathogenic bacteria and to help identify additional pathogens that might trigger TLR11 dependent responses, we examined the tissue distribution of TLR11. We carried out RT-PCR to examine TLR11 expression in liver, spleen, kidney, heart, and intestine and observed high relative expression of TLR11 in the small intestine (Fig 1A, Supplementary Fig 1A). We examined the expression of TLR11 in sections from the small intestine using in situ hybridization. Similar to the pattern of expression seen in the urinary bladder, TLR11 mRNA was strongly enriched in the epithelial layer of the small intestine (Fig 1B, Supplementary Fig 1B).
Figure 1. TLR11 is expressed in intestine and protects against S. typhimurium.
(A) RT PCR analysis of TLR11 in various organs. (B) In situ hybridization staining (antisense) for TLR11 in small-intestine tissue section. (C) Survival of wt and tlr11−/− mice (n=7) orally infected with S. typhimurium (108 CFU). (D) Tissue damage during S. typhimurium infection shown by H&E staining in small intestine, spleen and kidney on day 5 post infection. (E) Intestinal invasion of S. typhimurium expressing GFP. (F) Dissemination of GFP S. typhimurium in orally infected wt or tlr11−/− mice was detected on day 5 post infection by measuring CFU per gram of spleen, liver and kidney. (G-I) Day 5 serum cytokines from wt or tlr11−/− mice orally infected with S. typhimurium measured by ELISA. * p<0.001
The intestinal localization of TLR11 suggested that TLR11 might recognize enteropathogens. As we had established recognition of E. coli spp. by TLR11 (Zhang et al., 2004), we wished to determine whether other well-known intestinal pathogens might be recognized by TLR11. Salmonella spp. are medically relevant enteropathogenic bacteria and S. typhimurium is widely used in mouse studies. Given that TLR11 was expressed at the small intestinal epithelium through which S. typhimurium gains entry to the host, and in the macrophage populations in which S. typhimurium is found, we tested whether TLR11 was important for immune responses to orally-administered S. typhimurium, to which mice are susceptible. As reported previously, oral administration of high doses of S. typhimurium led to infection and lethality in wild-type mice, however the lethality was significantly enhanced in tlr11−/− mice (Fig 1C). Histological studies demonstrated enhanced destruction of the intestinal wall in mice lacking TLR11, consistent with an important role in epithelial immunity (Fig 1D). Using S. typhimurium expressing GFP we were able to observe increased bacterial dissemination that spread beyond the severely damaged intestinal wall in tlr11−/− mice (Fig 1E). Significantly increased recovery of viable S. typhimurium from the liver, spleen, and kidney of tlr11−/− mice confirmed the enhanced dissemination in the absence of TLR11 (Fig 1F). Identical results, including intestinal damage and dissemination were seen with non-GFP expressing S. typhimurium (Supplementary Fig 1C). Analyses of serum cytokine levels revealed elevated IL-12 (Fig 1G), IL-6 (fig 1H) and TNF (Fig 1I) in tlr11−/− mice, suggesting that bacterial dissemination causes death of the animals by triggering a cytokine storm.
Flagellin is a ligand for TLR11
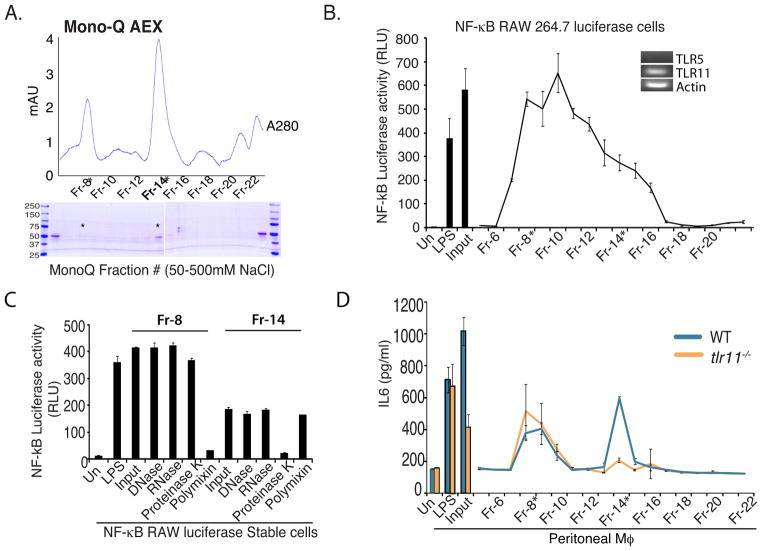
The TLR11 stimulatory activity found in lysates of the UPEC strain 8NU has a protein component as this activity is abolished by treatment with proteinase K, but not RNase, DNase or concanavalin A (ConA) (Zhang et al., 2004). Based on this finding, we speculated that the TLR11 PAMP in Salmonella also had a protein component that could be isolated by column chromatography. We fractionated heat-killed lysates of S. typhimurium on a DEAE-Sephacel anion-exchange column, followed by CM-sepharose cation exchange chromatography, and finally Mono Q FPLC chromatography using linear NaCl gradients (Fig 2A). The stimulatory activity of dialyzed fractions from each chromatographic step was tracked by testing induction of luciferase in RAW cells, which express TLR11, stably transfected with an NF-κB-luciferase reporter. The stimulatory activity eluted broadly and corresponded to the two major protein peaks (Fraction 8 and Fraction 14) following Mono Q fractionation (Fig 2B). To confirm that the stimulatory activity in these fractions was of a similar nature to the TLR11 PAMP, we treated Fraction 8 and Fraction 14 with DNAse, RNAse, and proteinase K. As LPS is a major PAMP in gram-negative bacteria, we also depleted LPS using polymyxin B sepharose. As shown in Figure 2C, the NF-κB stimulatory activity in Fraction 8 was unaffected by DNAse, RNAse and proteinase K, but was severely diminished by polymyxin B treatment, and hence the activity in Fraction 8 is likely due to LPS. In contrast the stimulatory activity in Fraction 14, was unaffected by DNAse, RNAse, and polymyxin B, but abolished by proteinase K treatment, strongly suggesting a protein PAMP.
Figure 2. TLR11 recognizes S. typhimurium flagellin.
(A) The TLR11 ligand was purified by mechanical disruption of S. typhimurium followed by sequential DEAE-sephacel and CMSephacel chromatography. Fractions with stimulatory activity in Raw-κB-luc cells were further subjected to MonoQ FPLC with a linear NaCl gradient. Shown is absorbance at 280nm over the course of the elution. The fractions indicated were resolved by SDS PAGE and proteins were visualized by Coomassie staining. (B) Stimulatory activity of MonoQ fractions was assessed in Raw-κB-luc cells. (C) Fractions #8 and #14 were subjected to DNase, RNase, Proteinase K and Polymixin B treatment and assessed for stimulatory activity in Raw-κB-luc cells. (D) Peritoneal macrophages from WT or tlr11−/− mice were stimulated with Mono-Q fractions and IL-6 production was analyzed by ELISA.
To test the specificity of these fractions for TLR11, we measured the induction of IL6 following stimulation of peritoneal macrophages from either WT or tlr11−/− mice. The stimulatory activity in Fraction 8 was unaffected by the absence of TLR11, however the ability of Fraction 14 to stimulate IL6 production was completely abolished in the tlr11−/− cells (Fig 2D) or upon treatment with proteinase K (Supplementary Fig 2A). Therefore, Fraction 14 contains a Salmonella-derived protein-with TLR11-dependent stimulatory activity. SDS-PAGE analysis of Fraction 14 revealed few protein bands, but included a prominent band around 50 kDa with an elution pattern that matched the TLR11-dependent activity (Fig 2A,B). We excised that band and analyzed it by mass-spectrometry and surprisingly, the only protein detected was S. typhimurium flagellin (Supplementary Fig 2B).
Although the stimulatory activity observed in Fraction 14 was dependent upon the expression of TLR11 (Figure 2D and Supplementary Fig 2A), we were concerned that stimulation might occur through the known flagellin receptor, TLR5. However, consistent with previous reports (West et al., 2005), we found that the mouse macrophage cell line RAW 264.7 does not express TLR5 (Fig 2B, inset), suggesting that the stimulation by Fraction 14 (and hence flagellin) was not due to TLR5. The recognition of Salmonella flagellin by TLR5 is believed to be important for mounting of innate responses to this bacteria (Fournier et al., 2009; Uematsu et al., 2006) although the role of TLR5 is not straightforward: mice lacking TLR5 show enhanced resistance to S. typhimurium (Uematsu et al., 2006). Our results suggested that TLR11 might play a more significant role in murine resistance to S. typhimurium.
Our previous results had demonstrated that UPEC also possess a proteinase K sensitive ligand recognized by TLR11. We therefore carried out an independent purification similar to that used for S.typhimurium. The peak, proteinase K-sensitive, TLR11-stimulatory fraction from Mono Q column chromatography of 8NU lysates was sequenced by mass spectrometry, and yielded E.coli flagellin as the primary component (Supplementary Fig 2C-E). Therefore, TLR11, which is highly enriched at epithelial surfaces, including bladder (Zhang et al., 2004), kidney and small intestine (Supplementary Fig 1A) is capable of recognizing flagellin from enteropathogenic and uropathogenic bacteria. Taken with the enhanced susceptibility of TLR11-deficient mice to S.typhimurium and UPEC infection, these data suggest non-redundant roles for TLR5 and TLR11 in the recognition of flagellated bacteria.
Recognition of flagellin by TLR5 and TLR11
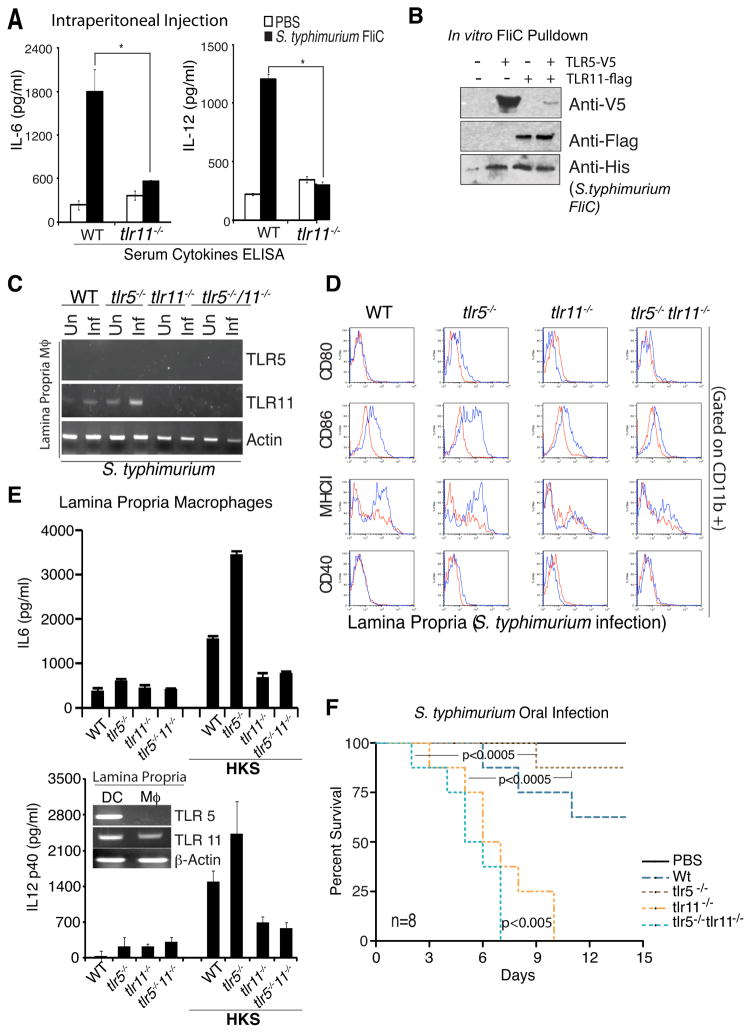
To directly examine whether flagellin responses depend on TLR11, we purified recombinant S. typhimurium flagellin to homogeneity, followed by passage through polymyxin B sepharose resin to remove contaminating LPS. We injected this purified flagellin into WT and tlr11−/− mice and measured serum levels of IL-6 and IL-12. TLR11 knock-out mice produced significantly less IL-6 and IL-12 following injection of recombinant flagellin (Fig 3A). Consistent with previous reports (Feuillet et al., 2006; Uematsu et al., 2006), production of serum IL-6 was also partially reduced when flagellin was injected into TLR5 knockout animals (not shown), suggesting both can independently recognize flagellin.
Figure 3. Recognition of S. typhimurium flagellin by TLR5 and TLR11.
(A) WT and tlr11−/− mice (n=5) were injected intraperitoneally with recombinant S. typhimurium flagellin and 2 hours later serum IL-6 and IL-12 were measured by ELSIA. * p<0.01. (B) TLR5 and TLR11 were expressed in HEK293 cells for 48 hours and TLR/flagellin complexes were formed by incubating cell lysates with His-tagged recombinant S. typhimurium flagellin (1μg/ml) overnight at 40C. Binding was assessed by Ni-sepharose pulldown followed by Western blotting with Anti-Flag, Anti-V5, and Anti-His antibodies. (C) Wild type, tlr11−/− and tlr5−/−/ tlr11−/− mice were challenged with S. typhimurium and 5-days later mice were sacrificed to obtained lamina propria macrophages by sorting (CD11C−CD11b+ MHC-II+). RNA was prepared and analyzed for TLR5 and TLR11 expression by RT-PCR. (D) Lamina propria cells from above were analyzed for macrophage activation markers (CD80, CD86, MHC-II, CD40) by FACS. The blue traces are from infected mice. (E) Lamina propria macrophages and DCs were sorted from wild type mice and analyzed for TLR5 and TLR11 expression by RT-PCR (inset). Cells from wild type, tlr11−/− and tlr5− /−/ tlr11−/− mice were stimulated in vitro with heat killed S. typhimurium and IL-6 (upper graph) and IL-12 (lower graph) production was measured by ELISA. (F) Wild type, tlr11−/− and tlr5−/−/ tlr11−/− mice (n=8) were challenged with S. typhimurium.
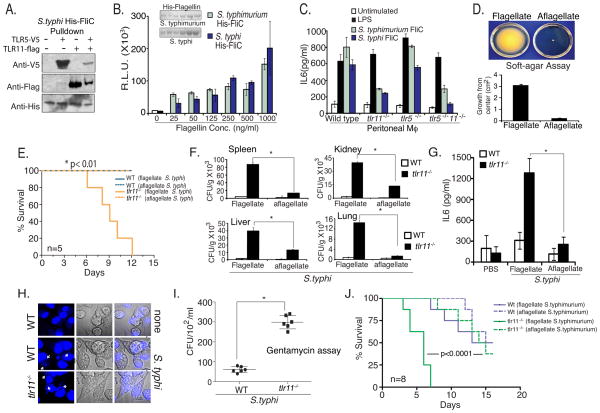
To further explore the role of TLR5 and TLR11 in the recognition of S. typhimurium flagellin, we generated C-terminally epitope tagged versions of TLR5 (TLR5-V5) and TLR11 (TLR11-Flag). Using lysates from HEK293 T cells expressing TLR5, TLR11, or both, we performed pull-downs with recombinant polyhistidine-tagged S. typhimurium flagellin. Flagellin was captured using nickel-beads, and binding of TLR5 and TLR11 was assessed by immunoblotting. Consistent with the ability of TLR5 and TLR11 to activate cytokine expression in response to flagellin, both TLRs were capable of interacting with flagellin (Fig 3B). To examine whether TLR5 and TLR11 might interact with each other, and hence act as a heterodimer in cells where they are co-expressed, we mixed Flag-tagged TLR11 with V5-tagged TLR5, and HA-tagged TLR11. Interestingly TLR11 can form a heterodimer with TLR5, but can also form homodimers (Supplementary Fig 3A).
We had been intrigued by the increased resistance of mice lacking TLR5 to infection by S. typhimurium (Uematsu et al., 2006), and wondered whether TLR11, given that it could signal in macrophages lacking TLR5, might have a role in this resistance. Different explanations have been advanced to explain this phenomenon, including the possible influence of altered commensal bacterial flora and enhanced IL-1β activity in the TLR5 knock-outs (Carvalho et al., 2011; Vijay-Kumar et al., 2008). In addition to epithelial cells, sentinel cells of the intestine include lamina propria (LP) macrophages (MΦ’s) and dendritic cells (DC’s). We therefore analyzed TLR5 and TLR11 mRNA expression in these two cell types. While LP-DCs express both TLR5 and TLR11, we found that LP-MΦ’s express TLR11 but not TLR5, which is consistent with previous reports (Means et al., 2003; Uematsu et al., 2006) (Fig 3E, inset). Therefore it is likely that in LP-DCs, flagellin-recognition is mediated by both TLR5 and TLR11, possibly as a heterodimer, whereas in LP-MΦ, TLR11 homodimers recognize flagellin. Therefore, although TLR11 and TLR5 both recognize flagellin and can form a heterodimer, consistent with the lack of TLR11 in humans, heterodimer formation is clearly not a prerequisite for flagellin recognition.
We had noted that TLR11 is upregualted in macrophages upon exposure to S. typhimurium or flagellin (Supplementary Fig 3B). Therefore, we decided to examine the expression of TLR5 and TLR11 in LP Mφ before and after infection with S. typhimurium. Consistent with the in vitro data, we observed an increase in TLR11 mRNA upon infection. Surprisingly, TLR11 mRNA was upregulated in tlr5−/− macrophages, both before, and to a greater extent, after infection (Fig 3C and Supplementary Fig 3C). This suggests that in tlr5−/− mice, the LP Mφ are activated, most likely indirectly, and as a result the level of TLR11 is increased in these cells. Sorted CD11b+ LP Mφ from tlr5−/− mice express increased levels of the activation markers CD86 and MHC class II after infection, similar to the WT mice, consistent with the activated status of these macrophages (Fig 3D). Consistent with enhanced TLR11 expression in tlr5−/− LP-MΦ, these cells were hyper-responsive to heat killed S. typhimurium (HKS) (Fig 3E), and infection. Although there was a modest increase in basal cytokine production observed in the unstimulated LP-MΦ, only the HKS induced response was abrogated upon deletion of TLR11 (Fig 3E). Therefore, hyper-responsiveness of TLR5-/- LP-MΦ to S. typhimurium PAMPs required TLR11. These results suggest that increased TLR11 expression in TLR5 KO macrophages, leads to their enhanced activation, and increased recruitment of CD4 T-cells to the lamina propria, thereby facilitating clearance of Salmonella from these mice. This model suggests that the TLR11 knock-out phenotype would be dominant during S. typhimurium infection. Indeed, tlr5−/−/tlr11−/− mice exhibit enhanced lethality like tlr11−/− mice (Fig 3F). Arguably, the lethality of the double knockout mice and S. typhimurium dissemination is further enhanced (Supplementary Fig 3D), suggesting that loss of TLR11 may reveal a protective role for TLR5.
TLR11 knock-out mice are susceptible to infection by S.typhi
The vast majority of animal studies on Salmonella have been carried out using S. typhimurium, a Salmonella enterica serovar that causes a typhoid like-disease only in mice. In contrast, S. typhi causes typhoid fever in humans, but does not infect mice (Tsolis et al., 1999). This lack of a convenient small animal model, has prevented careful study of the immune response to S.typhi (Raffatellu et al., 2008). As a result, there are significant outstanding questions regarding S.typhi biology and, especially, the host immune response to this important pathogen. Consequently development of effective vaccines against S.typhi has been significantly hampered. Although oral S. typhi vaccines exist, efficacy has been limited to approximately 70% (Levine et al., 1999; Levine et al., 2007), while more effective conjugate vaccines are available, these are ineffective in young children (Lin et al., 2001). Furthermore, the development of Salmonella spp. as potential vaccine vectors is also hindered by the inability to infect rodent models (Mestecky et al., 2008).
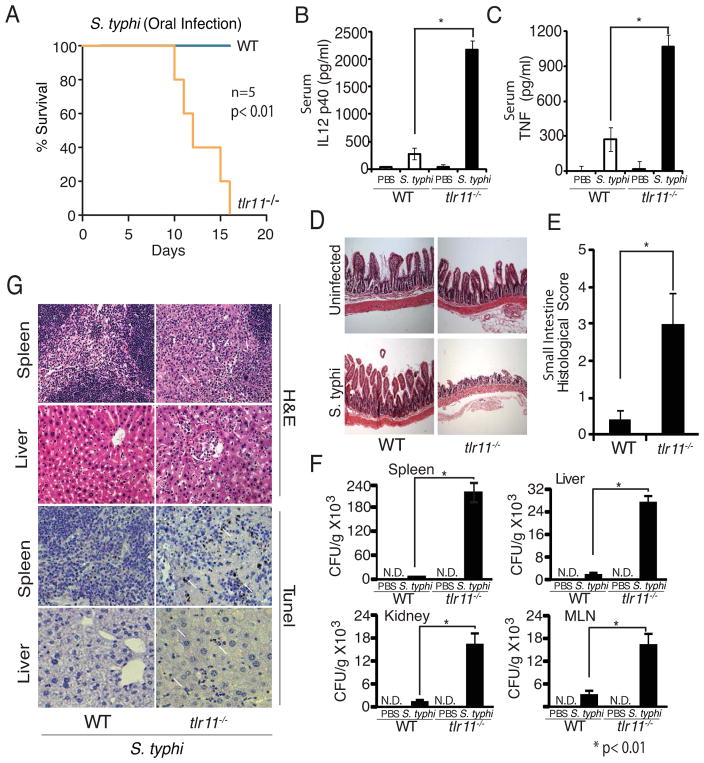
TLR11 is expressed in mice, but not humans, and we hypothesized that expression of TLR11 might be responsible for preventing infection by S. typhi. Although S. typhi is thought to modulate expression of flagellin in order to evade recognition by the host, this occurs after engaging the epithelial barrier (Arricau et al., 1998; Winter et al., 2008). Thus expression of TLR11 and recognition of S. typhi at the epithelium in mice may be a crucial determinant of murine resistance to S. typhi. To test this hypothesis we orally-administered S.typhi to WT and tlr11−/− mice. As expected, WT mice were unaffected by S. typhi. In contrast, all of the tlr11−/− mice were infected, and displayed significant mortality (Fig 4A). Infection of tlr11−/−, but not WT, mice was accompanied by significant upregulation of serum IL-12 (Fig 4B) and TNF (Fig 4C), suggesting disseminated disease. Indeed, severe intestinal tissue damage with areas of complete epithelial breakdown was observed in tlr11−/− mice (Fig 4D, E). Furthermore, bacterial dissemination to various organs, along with high CFU load was observed in tlr11−/− mice (Fig 4F). We also examined the presence of cell death in spleen and liver sections in infected mice using tunel staining, and observed increased tunel-positive cells in sections from tlr11−/− mice (Fig 4G). While previous studies have shown mild differences in TLR5 responses to Salmonella sp. flagellin, these results suggest that the lack of TLR11 in humans may be a more crucial determinant of Salmonella susceptibility. Thus, the host tropism of S. typhi is restricted by a host-specific TLR.
Figure 4. Tlr11−/− mice are susceptible to oral infection with Salmonella typhi.
(A) Survival of wild type and tlr11−/− mice (n=5 mice) orally infected with 109 CFU S. typhi (S. typhi-Ty2, ATCC 700931). (B) Day 10 serum IL-12 p40 from mice orally infected with 109 CFU S. typhi per mouse. (C) ELISA analysis of serum TNF from mice orally infected with S. typhi as in (B). * p<0.01 (D) Histology of orally infected wild type and tlr11−/− mice was assessed on day 10 post infection using small intestine sections and (E) histological score plotted. (F) S. typhi dissemination detected by Colony forming units (CFU) per gram of tissue on LB plates from spleen, MLN, liver and kidney. (G) H&E staining and tunel staining of tissues from day 10 post infection with S. typhi. The arrows indicate tunel positive cells.
Non-typhoidal Salmonella serovars (NTS) cause a very different disease compared to typhoidal Salmonella serovars such as S. typhi and S. paratyphi. In humans, where NTS cause local gastroenteritis and diarrhea, these bacteria do not breach the epithelial barriers and are cleared by the mucosal immune system. In contrast S. typhi overcomes the mucosal barrier even in healthy adults, and is disseminated to internal organs. A feature of typhoid infection in humans is its persistence, where the patient develops high fever for extended periods. We therefore wanted to test whether tlr11−/− mice infected with S. typhi also developed fever. The pathogenic S. typhi strain used in these studies is well studied and characterized [S. typhi-Ty2 pathogenic strain from ATCC, #700931; (11-12)], and expresses the Vi capsular antigen (Supplementary Fig 4A), which is a major virulence factor for S. typhi. Tlr11−/− mice infected with a relatively low dose (CFU 106) of orally administered S. typhi developed fever, whereas, as expected, the WT mice were uninfected (Supplementary Fig 4B). Fever persisted for approximately three weeks before dissipating. In contrast when the mice were infected with the normal, higher dose (CFU 5×108) of S. typhi, the TLR11 KOs developed fever but then developed sepsis around d10 with hypothermia and death (Supplementary Fig 4C). Stool samples from the infected mice revealed significantly increased levels of gross blood and bloody diarrhea in the tlr11−/− mice (Supplementary Fig 4D). Careful examination of the ileum, colon and caecum of the infected tlr11−/− mice showed breaches in the epithelial layer that were likely responsible for the bleeding that was observed (Supplementary Fig 5A,B). Histology from tlr11−/− demonstrated evidence of disseminated disease in spleen, kidney and lung (Supplementary Fig 5C,D).
During typhoid infection, the gall bladder is often colonized by S.typhi and can serve as a reservoir in chronic carriers, the most notorious example of which was the case of Typhoid Mary. Mice infected with S. typhimurium can also show infection of the gall-bladder (Menendez et al., 2009). We examined the gall-bladders from tlr11−/− mice infected with S.typhi. Seven days post infection we observed significant numbers of bacteria in the gall-bladders both microscopically and upon culturing the bacteria from the tissue (Supplementary Fig 5E,F). Therefore tlr11−/− mice infected with S.typhi show many of the hallmarks of human typhoid.
Infection of TLR11 knockout mice by S. typhi is dependent on flagellin
To test whether the flagellin from S. typhi was also recognized by TLR11, we cloned and expressed a His-tagged form of flagellin from S. typhi. In in vitro pulldown experiments, performed in parallel with the S. typhimurium flagellin pulldown shown in Figure 3B, we find that S. typhi flagellin is capable of binding both TLR5 and TLR11 similarly (Fig 5A). We purified flagellin from S.typhimurium and S.typhi using nickel-affinity columns followed by passage through a polymyxin B column to remove LPS (Fig 5B, inset), and tested their ability to stimulate NF-κB activity in the RAW 264.7-luciferase reporter cell line that does not express TLR5 (Fig 2B). Flagellin from both spp. were equivalent in the ability to stimulate NF-κB in these cells. To test the TLR-dependence of flagellin-recognition, we stimulated peritoneal macrophages from WT, tlr11−/−, tlr5−/−, and tlr11−/−/tlr5−/− mice, and measured IL-6 production by ELISA. The level of IL-6 produced was dramatically reduced in cells from tlr11−/− mice, but not from tlr5−/− mice (Fig 5C), consistent with the lack of significant expression of TLR5 in macrophages(Means et al., 2003; Uematsu et al., 2006).
Figure 5. S. typhi infection of tlr11−/− mice requires flagellin.
(A) TLR/flagellin complexes were formed by incubating cells expressing TLR11 or TLR5 with His-tagged recombinant S. typhi flagellin. Binding was assessed by Ni-sepharose pulldown followed by Western blotting with Anti-Flag, Anti-V5, and Anti-His antibodies. (B) S. typhimurium and S. typhi flagellin were cloned in pET-15b vector and flagellin was purified by affinity chromatography (inset). RAW NF-kB luciferase cells were stimulated with increasing doses of flagellin and assayed for luciferase activity. (C) Wild type, tlr11−/− and tlr5−/−/ tlr11−/− peritoneal macrophages were stimulated with Flagellin (500ng/ml) and supernatant IL6 was measured by ELISA. (D) Aflagellate S. typhi was obtained from the Salmonella Genome Stock center (SGSC; Calgary, Canada) and examined for motility on soft agar plate, grown in a humid chamber overnight at 370C, bacterial growth were measured from center and plotted. (E) WT and tlr11−/− mice (n=5) were orally infected with flagellate or aflagellate Salmonella typhi (5×108 CFU). (F) Mice were sacrificed at day 10 and tissue CFU following dissemination into spleen, kidney, liver and lung were measured using LBagar plates. * p<0.01 (G) On day-10 serum cytokines were analyzed by ELISA. (H) Wild type and tlr11−/− mouse peritoneal macrophages were infected with S. typhi at an MOI of 10 for 6 hours and stained with DAPI (indicated by arrows). (I) Macrophages infected for 2 hours were washed three times, placed in media with 100 μg/ml gentamycin and incubated for four hours. Macrophages lysates prepared with Triton-X100 containing media were plated on the LB agar plates to determine CFU. (J) Wild type and tlr11−/− mice (n=8) were orally inoculated with flagellate and aflagellate Salmonella typhimurium (CFU-108/ mice).
As flagellin from both S. typhi and S. typhimurium are recognized by TLR11, we wondered why mice would be resistant to only S. typhi. TLR11 is absent in humans, and interestingly similar to the situation in mice, S. typhi does not infect a variety of other species, which coincidentally also express TLR11. Sequencing efforts have revealed that S. typhi has a significantly degraded genome that expresses far fewer virulence factors compared to S. typhimurium (Parkhill et al. 2001). Hence a possible explanation for the species-specific resistance to S. typhi by TLR11 would be the unique dependence of S.typhi on flagellin as a virulence factor. Consistent with such a hypothesis, a previous study found minimal effects of the loss of flagella on S. typhimurium infection in mice suggesting that other virulence factors were substituting for the absence of flagellin (Lockman and Curtiss, 1990; Schmitt et al., 2001). In contrast, aflagellate S. typhi exhibited defects in epithelial cell invasion in vitro that were not solely due to lack of motility (Liu et al., 1988). To directly test the role of flagellin in the infection of mice by S .typhi and S. typhimurium, we obtained aflagellate versions of both strains. We first ensured that the aflagellate S. typhi also expressed Vi antigen (Supplementary Fig 4A). As expected the motility of aflagellate S. typhi on a soft agar assay was dramatically reduced (Fig 5D). We then compared the ability of flagellated and aflagellated S. typhi to infect WT and tlr11−/− mice. Only tlr11−/− mice developed lethal infection with flagellated S. typhi, however, these mice were unaffected by aflagellated S. typhi (Fig. 5E). We also characterized the mice infected with the aflagellated S. typhi and observed reduced dissemination (Fig 5F) and cytokine production (Fig 5G). These data, with previous in vitro experiments (Liu et al., 1988), suggest a particular dependence of S. typhi on flagellin as a key virulence factor in vivo.
Mice are ordinarily resistant to S. typhi even when introduced by intravenous or intraperitoneal inoculation. The transplantation of mice with human immune cells can render mice susceptible to S. typhi infection by intraperitoneal inoculation, suggesting that the host-specificity of S. typhi also results from a requirement for human mononuclear cells (Firoz Mian et al., 2011; Libby et al., 2010; Song et al., 2010). Our studies suggest that TLR11 is critical for preventing infection by Salmonella typhi following the normal, oral, transmission route. However to test directly whether TLR11 also affects the ability of the bacteria to infect macrophages, we isolated peritoneal macrophages, which lack TLR5, from wild-type and TLR11 knock-out mice and infected them with S. typhi. The TLR11 knock-out macrophages were infected with significantly greater numbers of bacteria as determined by direct microscopic analysis (Fig. 5H). S. typhi in infected tlr11−/− macrophages was viable and could be recovered from infected macrophages. Infected macrophages were incubated in gentamycin containing media after infection, to kill non-internalized bacteria, followed by separating and washing the cells, and performing CFU assays (Fig 5I). We also compared the ability of flagellated and aflagellated S.typhi to infect TLR11 knock-out peritoneal macrophages in culture, using the same microscopic and gentamycin culture assays, and observed a clear dependence on flagellin for S.typhi infection of mouse macrophages (Supplementary Fig 6A). Finally, we wanted to test the role of TLR11 when mice were infected by intraperitoneal injection of S. typhi. It is well known that the restriction against S. typhi in mice is not due to a barrier effect as mice are even resistant to intraperitoneal infection (Collins and Carter, 1978). Recent chimeric humanized mouse models demonstrate that the host restriction in bone marrow derived cells applies to systemically administered S. typhi (Firoz Mian et al., 2011; Libby et al., 2010; Song et al., 2010). Consistent with the infection experiments in culture, S. typhi did not cause infection of wild-type mice whereas the absence of TLR11 led to lethal systemic infection, even with a low dose infection (104 CFU per animal), with dissemination of the bacteria to different organs (Supplementary Fig 6C,D). In contrast to S. typhi, when oral infections were performed with flagellated and aflagellated S. typhimurium, we observed significant and similar lethality in WT and tlr11−/− mice with the aflagellated S. typhimurium (Fig 5J). These results suggest that other virulence factors present in S. typhimurium, but not S. typhi, mitigate the requirement for flagellin, and offer a plausible explanation for why S. typhi, but not S. typhimurium, exhibits strict human host restriction.
TLR11 KO mice can be immunized against infection by S. typhi
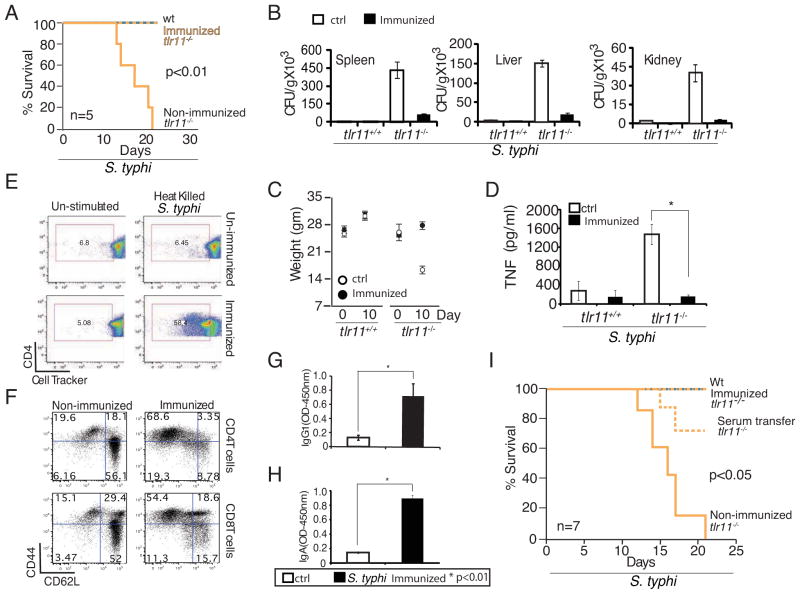
Analysis of the tlr11−/− mice infected with S.typhi suggests that TLR11 provides effective immune responses at the intestinal epithelial barrier, preventing entry and subsequent dissemination of the bacteria. As S. typhi are gram-negative bacteria that express numerous other PAMPs, and the immune system of these mice is not otherwise compromised, we assumed that adaptive immune responses to injected S. typhi would be maintained. The more delayed disease course in tlr11−/− mice compared with recent humanized mouse models (Libby et al., 2010) is consistent with tlr11−/− mice having otherwise normal immune systems. The recovery of mice that are given low dose oral infection and exhibit a typhoid-like disease course, further distinguishes the tlr11−/− mice as a S. typhi disease model. These findings led us to speculate that tlr11−/− mice would be a useful model for vaccine development. We therefore immunized wild-type and tlr11−/− mice by injecting heat killed S. typhi intraperitoneally, with the first injection followed by four booster injections at 4 day intervals. As a control the unimmunized mice were injected with PBS. Fourteen days after the final booster injection (i.e. 30 days after the first injection), the mice were given live S.typhi (CFU 5×108) orally and monitored for three weeks. While all unimmunized tlr11−/− mice died by day 20 of infection, immunization provided complete protection against S.typhi infection in tlr11−/− mice (Fig 6A). There was very little dissemination of S.typhi and immunized tlr11−/− mice gained weight similar to WT mice (Fig 6B,C). Immunization of tlr11−/− mice also reduced cytokine responses to levels that were comparable to those of WT mice (Fig 6D). We examined the antibodies in the immunized animals and detected elevated IgG1 and IgA (Fig 6G,H). Proliferation of CD4+ T cells was augmented (Fig 6E), while the number of CD62L low/ CD44 high cells was significantly increased in immunized animals (Fig 6F), suggesting development of strong memory responses that are critical for successful vaccination.
Figure 6. Generation of protective immunity in tlr11−/− mice by immunization.
(A) WT and tlr11−/− mice immunized with or without 108 CFU equivalents of heat killed S. typhi (HKS) at day - 30, -26, -22, -18, and -14 were challenged by oral administration of 5X108 CFU live S. typhi on day 0 (n=5 per group). (B) Day 10 post infection CFU from spleen, liver, and kidney homogenates measured by plating on LB agar plates. (C) Weight of WT and tlr11−/− mice injected with or without heat killed S. typhi, and subsequently orally challenged with live S. typhi as in (A) (n=5). (D) TNF ELISA performed from serum samples collected at day 10 post infections of HKS immunized versus control, non-immunized mice (as in A). * p<0.01 (E) Proliferation of Cell Tracker labeled CD4 T cells cultured in vitro for 48 hours with antigen presenting cells simulated with HKS was analyzed by FACS. (F) Memory phenotype of splenic T cells from tlr11−/− mice, either non-immunized or HKS immunized, was assessed by analysis of CD44 and CD62L by FACS. (G,H). Serum IgG1 and IgA measured by ELISA from uninfected animals immunized as in (A). (I) Survival of WT and tlr11−/− mice infected with S. typhi. The tlr11−/− mice were either nonimmunized or HKS immunized or were injected with serum (100μl) from immunized WT mice, before challenge with live, orally administered S. typhi (CFU 5×108).
Finally, to see if immunity to S. typhi generated could be transferred to an unimmunized TLR11 KO, we isolated serum from the WT mice immunized with heat-killed S. typhi, and transferred it to uninfected TLR11 KO mice. The recipient mice were then infected with live S. typhi and we observed significant protection against lethality in these mice (Fig 6I). This result strongly suggests that the TLR11 KO mice are likely to be an excellent model to study protective immune responses to S. typhi, and hence facilitate efforts to develop more effective human vaccines.
Discussion
In this study we report that the non-human TLR, TLR11, recognizes flagellin, and helps prevent oral Salmonella spp. Infection and dissemination in mice. Interestingly, S. typhi, an obligate human pathogen which does not infect wild-type mice, can efficiently infect tlr11−/− mice. To our knowledge this represents the first demonstration of pathogen host restriction determined by species restricted TLR expression. As such, tlr11−/− mice resemble mice with a humanized innate immune system, at least with respect to recognition of Salmonella flagellin. Thus, absence of TLR11 abolishes the innate immunity that normally prevents S. typhi infection. This restriction likely occurs at the level of tissue resident macrophages, which express high levels of TLR11 and are non-permissive for S. typhi infection. Given that TLR11 is also expressed in small intestinal epithelial cells as well as in dendritic cells, it is possible that TLR11 acts to both promote barrier responses against Salmonella spp. and to augment systemic adaptive and innate responses after the epithelial barrier is breached. The relative contribution of various TLR11 expressing cellular compartments remains to be fully explored. Nevertheless, in the absence of TLR11, Salmonella spp. disseminate more readily to other organs including gall bladder, spleen, liver, and kidney. Upon infection with a high inoculum, there is significant epithelial damage and a robust disseminated infection is established. S. typhi PAMPs detected by TLRs such as TLR2, TLR4 and TLR5 are likely to lead to systemic cytokine production and septic shock.
In the context of a low-dose infection of otherwise immunocompetent tlr11−/− mice, a more classical resolving typhoidal disease is observed. In this context other aspects of the innate immune system are likely to be protective. Hence the tlr11−/− mice should allow the contribution of TLR5 and other components of the innate and adaptive immune response to be addressed in a small animal model that more closely mimics the complement of innate Salmonella sensors present in humans. It is possible that as for S. typhimurium infection, in which loss of TLR5 further sensitizes TLR11 knockout animals, tlr11−/− mice may reveal that TLR5 is a relevant sensor of S. typhi. The ability of tlr11−/− mice to respond normally to internalized bacteria, means that they can be immunized by injection of heat killed S. typhi. The protective immunity generated in immunized tlr11−/− mice is highly effective and completely blocks infection upon subsequent oral challenge with live bacteria. The ability to infect and successfully vaccinate the TLR11 knock-outs means that these mice represent the first mouse model for S. typhi infection, and should significantly facilitate future research into both the pathobiology of this important pathogen, and the development of effective vaccination strategies against S. typhi in humans.
Toll-like receptors have emerged as critical sensory mechanisms for recognizing and responding to infectious microbes. While most TLRs are shared between humans and other species such as mice, a subclass including TLR11, 12 and 13, are not expressed in humans. TLR11 is expressed in many species such as birds, dogs, cows but is absent in humans. Previous studies have shown that TLR11 recognizes profilin, a protein ligand from the apicomplexan parasite Toxoplasma gondii, and plays a critical role in the mounting of innate immune responses to this organism(Yarovinsky et al., 2005). In addition TLR11 was also shown to specifically respond to uropthogenic E.coli strains that typically infect the urogenital tract causing urinary tract infections(Zhang et al., 2004). The expression of TLR11 in epithelial cells, as well as macrophages and dendritic cells, likely explains its involvement in mediating responses to these different pathogens. The studies presented in this report show that TLR11 recognizes flagellin from both the UPEC strain 8NU and Salmonella spp. In this regard, it is particularly interesting to note that there are several important flagellated enteropathogenic bacteria, for example enteropathogenic E. coli (Mohawk and O'Brien, 2011) and Campylobacter jujuni (Young et al., 2007), for which mouse models do not recapitulate human disease. Instead, many studies must be carried out using immunocompromised neonatal mice, colonization of germ free and streptomycin treated mice, massive bacterial doses or non-physiological routes of administration, or more limited models such as the rabbit ileal loop or in vitro models. While these and other approaches have proven extremely useful, they also have significant limitations, most notably for the study of host immunity and for vaccine development. Unlike other animal models in which conserved aspects of the innate immune response have been removed, such as MyD88 or TLR4 deficient mice, the tlr11−/− mice maintain expression of all conserved components of the innate and adaptive immune system. As such, we speculate that tlr11−/− mice will be a useful animal model to study the microbiology and immunology of a wide variety of flagellated human pathogens, and will also prove useful in vaccine development.
Materials and Methods
Experimental Animals
Tlr11−/− mice (Zhang et al., 2004) were backcrossed to the C57/BL6 background (Jackson Laboratories; Bar Harbor, ME) for more than 10 generations. Mice used in experiments were generated by crossing tlr11−/+ mice. Many experiments utilized WT and KO littermates, while in others mice produced by crossing TLR11 KO males and females, and WT males and females, obtained from the first breeding, were used. Animals used for experiments were age and sex matched and were bred and housed in specific pathogen-free conditions (Yale University and Columbia University). All mouse protocols were approved by IACUC, Yale University and Columbia University.
Reagents and antibodies
LPS 0111:B4 was from Sigma (St. Louis, MO). Recombinant Flagellin produced in BL21 was purified by His-affinity chromatography and polymyxin B (Detoxi Gel; Thermo). Purified flagellin from mammalian cells was kindly provided by Dr. Andrew Gewirtz, [Emory University; (McSorley et al., 2002)]. Bacterial strains were S. typhi (ATCC-700931), Salmonella typhimurium (SL1344) and GFP-Salmonella strain (SL1344). Aflagellate S. typhimurium (SL-1344---Aflagellate fliC fljBfliC::Tn10> fljB5001::MudJ) was from Dr. Louise Teel (Uniformed Services University of the Health Sciences). Aflagellate S. typhi Ty2 [SA4717 (fliC::Tn10--- Fli(-)] was from the Salmonella Genetic Stock Center (Calgary, Canada). Uropathogenic E.coli 8NU was from Dr. D. Klumpp (Northwestern University, IL).
Purification of the S. typhimurium TLR11 ligand
S. typhimurium (SL1344) was grown overnight at 370C without shaking, collected by centrifugation, washed once in 10mM Tris, pH 8 and homogenized in a Warring Blender for 5×10 sec followed by heating at 600C for 30 minutes. The resulting product was centrifuged at 10k xg for 30 min, and the supernatant was ultracentrifuged at 100k × g for 60 min. The resulting supernatant was treated with polymyxin B (Thermo/Pierce) and applied to a DEAE Sephacel column and eluted by step elution with 250 and 500mM NaCl. Dialyzed fractions were assessed by NF- κB luiferase reported assay, and fractions of interest were pooled, and subjected to CM Sepharose chromatography. Fractions containing peak activity (flow through) was applied to Mono Q column and eluted with a linear 50-500 mM NaCl gradient (AKTA, GE biosciences). Fractions containing stimulatory activity were resolved by SDS-PAGE and visualized by Coomassie. A single band co-eluting with the the TLR11 stimulatory activity was excised, subjected to in gel trpytic digest and MS analysis (Columbia University Protein Core Facility).
mRNA expression
Total RNA was isolated using Trizol (Invitrogen) and 1 μg of RNA was used for RT. One-tenth of this reaction was used as a template for PCR amplification with Taq polymerase for 30 cycles at 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min. Primer sequences are in the Supplementary Online Materials.
In Situ Hybridization
TLR11 cDNA fragments used as probe were amplified using specific primers (Supplementary Online Materials) to create a 450 bp fragment and sub cloned into the pcDNA3 vector. Sense probes were prepared after linearization with EcoRI and transcription with SP6 polymerase; antisense probes were prepared after linearization with XbaI and transcription with T7 polymerase. In situ hybridization was performed as described previously (Zhang et al. 2004).
NF-κB Luciferase Assays
RAW264.7 cells stably transected with a NF-κB Luciferase reporter construct pBIIX-luc (Raw-κB-luc) were plated and, after 24 hours, were stimulated with 20 ng/ml of LPS, or 1 μg /ml of Flagellin for 6 h. Luciferase activity was measured according to the manufacturer’s instructions (Promega).
Flow-cytometry and Cell Isolation
Cells were stained using the antibodies described (e-Biosciences or BD) and analyzed on a BD LSR-II. Analysis was performed with FlowJo software. For cell proliferation, splenocytes were plated in 96 well plates, stimulated with heat-killed Salmonella for 72 hours and were analyzed for cell proliferation using Cell Tracker Red (Invitrogen) according to the manufacturer’s instructions. Thioglycollate elicited macrophages (Rao et al., 2010) and lamina propria cells (Denning et al., 2007) were isolated from mice under sterile conditions, using established protocols (see Supplementary online materials). Lamina propria cells were sorted using a FACS ARIA at the Microbiology & Immunology core facility, Columbia University.
ELISA experiments
For in vitro ELISAs, peritoneal macrophages were cultured in RPMI1640 10% FBS and TNF, IL-12, and IL-6 were measured. For in vivo ELISAs, WT and tlr11−/− mice (n=5) were injected i.p. with 30 μg of recombinant Flagellin and bled at 2 hrs and serum IL-6 and IL-12p40 levels were measured. To assess cytokine production during infection, the same strains of mice were orally infected with S. typhimurium or S.typhi, surviving animals were bled and serum IL-12p40 and TNF, IL6 or IgG1, IgA levels were assessed by ELISA on d5 post-infection for S. typhimurium and day 8-10 post infection for S.typhi. ELISA kits were from BD Bioscience (San Jose, CA).
In vivo infection and Immunization
Wild type and tlr11−/− mice were orally infected with S. typhimurium (108) or Salmonella typhi (5×108 or 5×109) using a gavage needle unless otherwise indicated. For immunization WT and tlr11−/− mice were immunized by i.p. injection of heat killed S.typhi (108 CFU equivalents per animal) 5 times at 4 day intervals and challenged orally with live S. typhi at 14 days after the last immunization. Survival was monitored by daily observation and Kaplan Meier survival graphs were generated (Prism, GraphPad). N=5 mice were taken for each condition, unless otherwise indicated, significance is as given and was calculated in Prism.
Histology
Tissues were harvested and then sectioned and H&E stained at Yale or Columbia histology core facilities. Images were captured using a Zeiss Imager-M2. Tissue histology sections were examined and histological grading was performed by a GI pathologist at Yale University. Sections were scored as follows: 0, Normal; 1 Normal with focal epithelial loss; 2—Normal with mild surface epithelial loss; 3— Partial necrosis and diffuse epithelial loss; 4—Total Necrosis.
CFU
Bacterial burden was measured as follows: tissues were harvested and homogenized at 1gm per ml equivalent in LB, serially diluted 1:10 in LB and 100ul was plated on LB plates. For S. typhimurium CFU determination LB plates were supplemented with streptomycin. After 16 hours colonies were counted, data is presented as mean ± standard deviation of triplicate samples and is representative of at least three independent experiments.
Supplementary Material
Acknowledgments
The work in this manuscript was supported by grants from NIH (RO1-AI59440 and R37-AI33443) and institutional support from Columbia University. MSH is supported by ARRA P30 AR058886- 01. We would like to thank Ms. Crystal Bussey for help managing our animal colony and Dr. Suneeta Krishnareddy for help evaluating histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey PS, Popoff MY. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 2011;4:102–111. doi: 10.1038/mi.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FM, Carter PB. Growth of salmonellae in orally infected germfree mice. Infection and immunity. 1978;21:41–47. doi: 10.1128/iai.21.1.41-47.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firoz Mian M, Pek EA, Chenoweth MJ, Ashkar AA. Humanized mice are susceptible to Salmonella typhi infection. Cell Mol Immunol. 2011;8:83–87. doi: 10.1038/cmi.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier B, Williams IR, Gewirtz AT, Neish AS. Toll-like receptor 5- dependent regulation of inflammation in systemic Salmonella enterica Serovar typhimurium infection. Infect Immun. 2009;77:4121–4129. doi: 10.1128/IAI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, Favre D, Dietrich G. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17(Suppl 2):S22–27. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin Infect Dis. 2007;45(Suppl 1):S24–28. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, Smith KD, Cookson BT, Karlinsey JE, Kinkel TL, Porwollik S, et al. Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella Typhi infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15589–15594. doi: 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–1269. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- Liu SL, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infection and immunity. 1988;56:1967–1973. doi: 10.1128/iai.56.8.1967-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman HA, Curtiss R., 3rd Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infection and immunity. 1990;58:137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- Menendez A, Arena ET, Guttman JA, Thorson L, Vallance BA, Vogl W, Finlay BB. Salmonella infection of gallbladder epithelial cells drives local inflammation and injury in a model of acute typhoid fever. J Infect Dis. 2009;200:1703–1713. doi: 10.1086/646608. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Nguyen H, Czerkinsky C, Kiyono H. Oral immunization: an update. Curr Opin Gastroenterol. 2008;24:713–719. doi: 10.1097/MOG.0b013e32830d58be. [DOI] [PubMed] [Google Scholar]
- Mian MF, Pek EA, Chenoweth MJ, Coombes BK, Ashkar AA. Humanized mice for Salmonella typhi infection: new tools for an old problem. Virulence. 2011;2:248–252. doi: 10.4161/viru.2.3.16133. [DOI] [PubMed] [Google Scholar]
- Mohawk KL, O'Brien AD. Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J Biomed Biotechnol. 2011;2011:258185. doi: 10.1155/2011/258185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Wilson RP, Winter SE, Baumler AJ. Clinical pathogenesis of typhoid fever. J Infect Dev Ctries. 2008;2:260–266. doi: 10.3855/jidc.219. [DOI] [PubMed] [Google Scholar]
- Rao P, Hayden MS, Long M, Scott ML, West AP, Zhang D, Oeckinghaus A, Lynch C, Hoffmann A, Baltimore D, et al. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature. 2010;466:1115–1119. doi: 10.1038/nature09283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh SC, Forest CG, Lepage C, Leclerc JM, Daigle F. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett. 2010;305:1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O'Brien AD. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infection and immunity. 2001;69:5619–5625. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, Galan JE. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis RM, Kingsley RA, Townsend SM, Ficht TA, Adams LG, Baumler AJ. Of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv Exp Med Biol. 1999;473:261–274. [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Kumar A, Neish AS, Uematsu S, Akira S, Gewirtz AT. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infection and immunity. 2008;76:1276–1281. doi: 10.1128/IAI.01491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Dancho BA, Mizel SB. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to toll-like receptor 5. J Biol Chem. 2005;280:9482–9488. doi: 10.1074/jbc.M411875200. [DOI] [PubMed] [Google Scholar]
- Winter SE, Raffatellu M, Wilson RP, Russmann H, Baumler AJ. The Salmonella enterica serotype Typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell Microbiol. 2008;10:247–261. doi: 10.1111/j.1462-5822.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- Woc-Colburn L, Bobak DA. The expanding spectrum of disease due to salmonella: an international perspective. Curr Infect Dis Rep. 2009;11:120–124. doi: 10.1007/s11908-009-0018-2. [DOI] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007;5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.