Abstract
Clostridium difficile is the causal agent of antibiotic-associated diarrhea and is a leading cause of hospital-acquired infections in the US. C. difficile has been known to cause severe diarrhea and colitis for more than 30 years, but the emergence of a newer, hypervirulent strain of C. difficile (BI/NAP1) has further compounded the problem, and recently both number of cases and mortality associated with C. difficile-associated diarrhea has been increasing. One of the major drivers of disease pathogenesis is believed to be an excessive host inflammatory response. A better understanding of the host inflammation and immune mechanisms that modulate the course of disease and control host susceptibility to C. difficile could lead to novel (host-targeted) strategies for combating the challenges posed by this deadly infection. This review summarizes our current knowledge of the host inflammatory response during C. difficile infection.
Keywords: Psuedomembranous colitis, antibiotic-associated diarrhea, toxic megacolon, chemokines, neutrophils, immune response
Clostridium difficile infection
Clostridium difficile is a Gram-positive, anaerobic, spore-forming, toxin-producing bacterium that was initially identified in 1935 as part of the normal gut flora of neonates [1]. It was initially named Bacillus difficilis due to its slow growth and the difficulty encountered in culturing the bacterium [1]. The role of C. difficile as a pathogen and as a causative agent for antibiotic-associated diarrhea and pseudomembranous colitis was first defined in 1978 [2, 3]. During the past 30 years, C. difficile infection has become one of the most common nosocomial, or hospital-acquired, infections in the U.S. and a major cause of antibiotic-associated diarrhea and pseudomembranous colitis in hospitalized patients and patients in long term care facilities [4, 5]. In fact, the incidence of C. difficile infections in some community hospitals is now greater than methicillin-resistant Staphylococcus aureus (MRSA) infections [5]. The epidemiology of the disease has also been changing with the emergence of newer, more virulent strains [6, 7] and an increase in the incidence of community-acquired C. difficile infections [8, 9].
C. difficile infection is almost always associated with disruption of endogenous gut flora, which is postulated to allow the bacterium to propagate and cause disease [10]. Although some studies had shown that prior treatment with ciprofloxacin, clindamycin, penicillins, and cephalosporins are most frequently associated with disease, the use of almost any antibiotic can lead to C. difficile infection [11, 12, 14]. The use of drugs to suppress gastric acid production (proton pump inhibitors and H2 blockers) has also been associated with an increased risk of C. difficile infection [13, 14]. Apart from treatment with certain drugs, other factors associated with an increased risk of C. difficile infection include old age, underlying chronic disease, recent hospitalization, gastrointestinal surgeries, and tube feeds [11, 13, 15, 16, 17]. In the past few years, a new, hyper-virulent strain of C. difficile (BI/NAP1/027) has emerged. This strain has been responsible for an epidemic of C. difficile that was originally identified in Quebec and has now spread to parts of the US [7, 18]. Although the exact mechanisms of increased virulence are still not clear, this strain is characterized by increased production of toxins A and B, the presence of a binary toxin (CDT), deletion of the gene tcdC (a negative regulator of toxin production), and increased resistance to fluoroquinolones [6, 7, 19, 20].
Clinically, patients with C. difficile colitis present with abdominal pain, cramps, diffuse watery diarrhea, and leukocytosis. Overall, the disease spectrum can range from asymptomatic colonization to mild diarrhea to severe complicated infections that include fulminant colitis, toxic megacolon, and shock. Many different scoring systems comprising a combination of clinical and laboratory parameters (e.g., altered mental status, fever, abdominal pain, leukocytosis, hypoalbuminemia, and elevated serum creatinine levels) have been studied [21, 22], and although there is no single best predictor of severe disease, the degree of leukocytosis is widely believed to correlate with more severe disease [21, 22].
The mainstay of treatment is discontinuation of the offending antibiotic and administration of metronidazole or vancomycin. Recently, fidaxomicin, a macrocyclic antibiotic, has been shown to slightly reduce the rate of recurrent infections, but the difference was seen in only non-NAP1 strains of C. difficile [23]. Other treatment modalities (including the antibiotics nitazoxanide, rifaximin, tigecycline, and teicoplanin), fecal transplant, probiotics, intravenous immune globulin, and the administration of humanized monoclonal antibody against toxin have been tried with limited success and need further evaluation before being broadly used [24]. Despite current therapeutic options, the incidence of C. difficile-associated diarrhea has been increasing during the past decade and is now the most common cause of hospital-acquired infection [5].
Two major problems are the largest obstacles to successful treatment of C. difficile infections: (i) the emergence of a newer, hypervirulent strain of C. difficile (BI/NAP1) that has been associated with more severe disease, including 30 day mortality in approximately 15% of cases [25, 26]; and (ii) higher rates of recurrence [27, 28]. Although the use of fidaxomicin decreases the incidence of recurrence in non-NAP1 strains, the rate is still very high (between 13–15%) [23]. It is important to note that the current standard of practice for treating C. difficile infection is the use of antibiotics that further disrupt the microbial flora, which in turn may create a niche for additional C. difficile infections (and recurrences). A close look at disease pathogenesis suggests that although targeting the bacterium is important, excessive inflammation also plays an important role in disease pathogenesis. Interestingly, asymptomatic colonization with C. difficile is seen in a large number of infants [86, 87] and in about 4% of the adult population [14]. The fact that asymptomatic colonization persists without any overt disease suggests that host-associated factors (immune response and inflammation) may play a critical role in determining the disease outcomes. These intriguing observations suggest that targeting the host inflammation may be a novel adjunctive strategy for combating C. difficile infection.
Pathogenesis
Changes to the endogenous microbiome by broad-spectrum antibiotics provide an ideal niche for C. difficile infection [12], and infection with C. difficile leads to toxin-mediated intestinal inflammation and diarrheal disease [29, 30]. Typically, bacterial spores are transmitted by the fecal-oral route; spores survive the gastric acid barrier and germinate when they reach the anaerobic environment of the colon. The resulting vegetative cells then penetrate the mucus layer and adhere to intestinal epithelial cells. This step is followed by secretion of the large clostridial toxins toxin A and toxin B that are believed to be the major virulence factors. In vitro studies have shown that the toxins lead to a characteristic inflammatory response, which includes damage to the intestinal epithelial cells, neutrophilic infiltration, and local chemokine and cytokine secretion [31, 32]. Because it is at least in part a toxin-mediated disease, one of the key determinants of disease severity after infection is the host inflammatory response [33] and blocking the inflammatory response can ameliorate the disease in animal models [31, 34].
C. difficile virulence factors
Clostridial Toxins
Following colonization and replication of the vegetative forms, the bacteria secrete toxin A (TcdA) and toxin B (TcdB). These toxins were identified in the early 1980s [29, 30], and, at least in animal models, administration of the toxins has been shown to replicate all the histopathologic features of C. difficile infection [29]. The toxins share 63% amino acid sequence similarity [35] and are members of the clostridial glucosylating toxin family whose activity is due to their monoglucosylation of a threonine residue onto the Rho protein family of GTPases, leading to Rho inactivation [32]. Because Rho proteins are important molecular switches that control actin cytoskeleton re-organization, the inactivation of Rho leads to disruption of the intracellular actin cytoskeleton and, ultimately, cell death [32]. In addition, the toxins induce the release of inflammatory mediators from intestinal epithelial, neuronal, and immune cells via activation of different molecular pathways including TLR4, TLR5, and NOD1 signaling [36, 37, 38, 39].
The relative contributions of toxin A and B in the pathogenesis of C. difficile disease remain controversial. Earlier studies had shown that purified toxin A alone, but not B, replicated symptoms of C. difficile infection [40]; toxin B was believed to be dispensable, but it was then shown that, in fact, toxin B is essential for virulence and toxin A is dispensable [41]. Using isogenic tcdA and tcdB mutants in a hamster model, it was shown that toxin B is a key virulence determinant [41]. Most recently, another study [42] used similar methods to show that, in fact, both toxin A and toxin B were important mediators of in vitro cytotoxicity and in vivo virulence [42]. The differing findings in these two studies could be due to genetic differences in the strains of C. difficile used for mutagenesis. Although both groups used derivatives of strain 630, these strains had been independently passaged for many years, and, in fact, the strain used in the latter study [42] produced 3-fold more toxin than the equivalent strain used in the earlier study [41, 43]. The end points used to determine the in vivo pathogenicity of the strains were also different in the two studies; death was an end-point in the earlier study [41], and a clinical scoring system comprising weight loss, behavioral changes, and wet tail, followed by sacrifice of moribund and sick animals, was used in the later study [42]. In any case, toxins are essential for disease because toxin A−B− strains are avirulent [42, 44]. Interestingly, in human C. difficile infection, naturally occurring toxin A+B− isolates have not been reported while toxin A−B+ variants are being isolated with increasing frequency and have similar disease severity as toxin A+B+ strains [44, 45]. More studies in mouse models of C. difficile infection would help identify the relative role of each toxin in the pathogenesis of C. difficile infection.
The genes encoding the C. difficile toxins are located within a 19.6 kb region called the pathogenicity locus (PaLoc). Three other genes that regulate the expression and secretion of toxin genes are also present on the PaLoc: tcdC, which encodes a regulatory factor; tcdR, which encodes a sigma factor for the RNA polymerase; and tcdE, which encodes a holin-like protein. TcdC is believed to be a putative negative regulator of the toxin genes, TcdR is an alternative sigma factor important for the expression of toxin genes, and TcdE is believed to be important for secreting toxins from the bacterium [44].
Studies of the hypervirulent NAP1/027 strain of C. difficile have shown that it produces 16- to 20-fold higher amounts of toxins A and B [6], as well as a third toxin, binary toxin (CDT) [7]. The binary toxin gene is located on the CdT locus in the bacterial genome. In addition to the binary toxin, the hypervirulent strain BI/NAP1/027 has a nonsense mutation in the gene encoding the negative regulatory protein TcdC [6] that has been shown to increase expression of toxin A and toxin B genes as well as Vero cell cytotoxicity [6, 46]. The binary toxin induces formation of microtubule-based protrusions that increase bacterial adherence [47]. However, it may play only an adjunct role in C. difficile-associated disease; in a rabbit model of C. difficile-associated disease, CDT+ToxA−ToxB− strains led to fluid accumulation and colonization but did not cause death or disease [48].
Other C. difficile virulence factors
Beyond the large clostridial toxins, there is a second class of virulence determinants for C. difficile, the surface layer proteins (SLPs). Whereas the large clostridial toxins are the major virulence factors, some recent studies suggest that non-toxin C. difficile molecules, specifically the surface layer proteins (SLPs), have immunoregulatory roles [49, 36]. C. difficile non-toxin proteins include cell surface proteins, adhesins (cwp66), flagellar proteins (FLiC and FLiD), and fibronectin-binding proteins [50]. In vitro experiments show that SLPs bind to Vero cells, human epithelial cell lines, and gastrointestinal tissue obtained from biopsy samples [51], and additional in vitro studies using mouse bone marrow-derived dendritic cells and human monocyte-derived dendritic cells have shown that purified SLPs induce the production of both proinflammatory [TNF-α, interleukin (IL)-12, IL-23, and IL-1β] and anti-inflammatory (IL-10) cytokines [49, 36]. In mouse studies, this effect was mediated via TLR4 receptors in an NFκB-dependent manner [36]. Analysis of serum samples from patients, asymptomatic carriers, and healthy controls has shown specific antibody responses to these SLPs and flagellar proteins [52, 53]; in particular, patient samples had a significantly higher anti-SLP IgG levels as compared to carriers or controls, showing that SLPs are targeted by the host immune responses [53]. The exact role of these antibodies in controlling pathogenesis, however, is still unclear. Flagellar proteins bind to the mucus layer of mouse intestine in vitro and play a role in adherence to cecal epithelium in vivo [54]. All of these studies suggest that non-toxin C. difficile molecules may modify the disease process. However, it is important to note that most of the above referenced studies have been performed in vitro using cell lines or biopsy specimens. A more detailed analysis in a mouse model of C. difficile-associated diseases (CDAD) would help to define the relative contribution and exact roles of the non-toxin molecules in the pathogenesis of CDAD.
Host immune response during Clostridium difficile infection
The spectrum of disease following C. difficile infection ranges from asymptomatic colonization to mild diarrhea to fulminant colitis and death. Such variability in disease outcome is believed to be at least partly dependent on host factors including age, co-morbidities, and the immune responses generated by exposure to the bacterium. Following C. difficile infection, both the adaptive and innate arms of the immune system are activated.
Because toxins are a major C. difficile virulence factor, and administration of toxin A was shown to replicate all the histopathologic features of C. difficile infection in animal models, rabbit and hamster ileal loop models of C. difficile toxin A challenge have been employed to study the host response. The toxins disrupt the actin cytoskeleton and activate the inflammasome and NFκB-mediated pathways that lead to proinflammatory cytokine and chemokine production. The actions of C. difficile toxins can be broadly divided into cytopathic effects characterized by disruption of the cellular barrier and enterotoxic effects with the initiation and propagation of an inflammatory response.
Innate immune responses
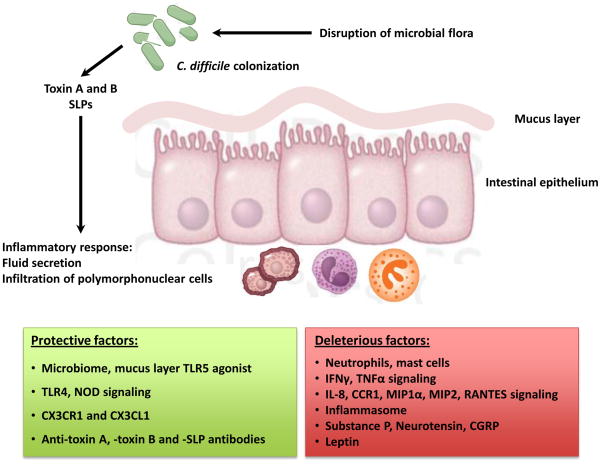
The innate defense mechanisms against C. difficile infection include the endogenous microbial flora (Box 1), the mucus barrier, intestinal epithelial cells, and the mucosal immune system. The C. difficile toxins have multiple effects on all of these innate immune defenses of the body, including stimulating the release of multiple proinflammatory mediators (cytokines, chemokines, neuro-immune peptides) and the recruitment and activation of a variety of innate immune cells (Table 1).
Box 1. Role of the microbiome in C. difficile infection.
The diverse and complex endogenous gut microbial flora likely plays a key role in preventing C. difficile infection. Colonization resistance is a defense mechanism by which the normal, endogenous gastrointestinal flora prevents the establishment of infection, and colonization resistance plays a major role in defense against C. difficile. Disruption of the endogenous gut micro flora is one of the prerequisites for establishing infection. A large percentage of young infants (who do not have an established microbiome) are colonized with C. difficile [86], and as the microbial flora of infants matures colonization with C. difficile is reduced and is almost gone by 2 years of age. It is important to note that despite high levels of colonization, clinical infection in infants is rare, suggesting that host responses are important determinants of disease pathogenesis [86]. Recent studies have also shown that the presence or absence of C. difficile colonization during early infancy is associated with specific alterations in the gut microbiome [87]. In adults, changes in the composition and diversity of the microbial flora by the use of antibiotics makes the host more susceptible to infection [11, 12], and experiments in animal models show that a single dose of antibiotics changes the composition and diversity of microbial flora and makes the host more susceptible to C. difficile infection for at least 10 days [88]. Specifically, changing the microbial environment from Firmicute-dominant to Proteobacteria-dominant using different combinations of antibiotics was associated with clinical disease manifestation in mice [89].
The complex interplay of the metabolic pathways influenced by changing the gut microbiome can play a role in C. difficile disease as well. One such area of study is the role of gut flora in influencing bile salt metabolism. Bile salts play an important role in regulating the transformation of C. difficile spores to vegetative forms. Specifically, studies have shown that some primary bile salts (cholate, taurocholate, glycocholate) can stimulate the germination of spores in vitro [90], whereas others (chenodeoxycholate) can inhibit germination [90]. Interestingly, it has been postulated that one of the mechanisms by which the microbiome could influence disease pathogenesis is by changing the composition of bile salts in the colon, which can then effect the germination of C. difficile spores [91].
The importance of the microbiome during C. difficile infection and disease is further highlighted by reports of successful intestinal microbiota transplantation as a therapy for recurrent infections where standard therapy has failed [92]. Although this therapeutic option has not been extensively studied and is not a standard of care, multiple case reports have shown that this can be used as a treatment for severe, recurrent C. difficile infection when standard treatment has failed. A recent review of 27 clinical reports and case series shows that intestinal microbiota transplantation has high success rates (92%) in resolving disease [92]. However, there is a lack of any randomized controlled trial and the case series and reports vary in terms of patient characteristics, the amount and route of fecal instillation, as well as differences in follow-up to define the cure rates, just to name a few distinction. And although unproven at this time, there is a potential risk of transferring pathogens from donor to recipient. Overall, even though the concept is interesting and shows early promise, more studies are needed before intestinal microbiota transplantation can be used regularly as a therapeutic option for patients with C. difficile infection.
Table 1.
List of innate and adaptive immune responses to C. difficile
| Innate Immunity | Role in C. difficile infection | Effect of inhibition | Refs |
|---|---|---|---|
|
| |||
| Immune Cells | Neutrophils increase the immune response to C. difficile toxin A | Blocking neutrophil recruitment or induction of neutropenia decrease Toxin A mediated disease severity | 31, 34 |
| Mast cells increase activation by C. difficile toxins | Mast cell-deficient mice show decreased inflammation in response to Toxin A | 68, 69 | |
|
| |||
| Cytokines | IFNγ expression is induced by Toxin A | IFNγ−/− mice are protected from toxin A- mediated enteritis | 62 |
|
| |||
| Chemokines and chemokine receptors | A high level of IL-8 production is associated with increased risk of recurrent disease | 65 | |
| CX3CR1 induces heme oxygenase-1 expression after Toxin A challenge | CX3CR1−/− mice have increased fluid accumulation and neutrophil recruitment, as well as more pathological cellular structures, as shown by histology | 66 | |
| Both CCR1 and MIP1α induce Toxin A mediated inflammation | CCR1−;/ − or MIP1α −;/ − mice are protected from toxin A-mediated enteritis | 64 | |
| RANTES induces Toxin A mediated inflammation | RANTES inhibition (met-RANTES) protects from toxin A-mediated enteritis | 64 | |
| MIP2 induces neutrophilic infiltration after Toxin A exposure in rat ileal loops | Anti-MIP2 antibody decreased neutrophil recruitment after toxin A challenge | 63 | |
|
| |||
| Innate immune receptors | TLR4 induces cytokine production in response to SLPs | TLR4−/− mice have worse disease and higher C. difficile burden | 36 |
| TLR5 stimulation decreases C. difficile-induced injury | TLR5 agonist (flagellin) protects from C. difficile colitis and death | 37 | |
| NOD1 recognizes C. difficile and induces neutrophil recruitment | NOD1−/− mice have worse disease and higher bacterial burden | 38 | |
|
| |||
| Neuroinflammatory mediators | Neurotensin (NT) and Substance P induce Toxin A-mediated mast cell activation | NT receptor antagonist inhibits Toxin A- induced enteritis | 73 |
| SP antagonist reduces Toxin A-induced fluid secretion and neutrophil recruitment | 69, 73 | ||
|
| |||
| Other mediators | Toxins A and B induce inflammasome activation and signaling in THP-1 cells and mouse macrophages | ASC−/− and IL-1R antagonism protect from toxin A- and B-mediated intestinal injury, inflammation, and neutrophil recruitment | 39 |
| Heme oxygenase 1 protects from Toxin A- mediated enteritis | HO-1 inhibition (tin-protoporphyrin-IX) enhances Toxin A-mediated enteritis | 66 | |
| Leptin enhances Toxin A-induced enteritis | db/db (LepR−/−) mice are protected from Toxin A-mediated enteritis | 61 | |
|
| |||
| Adaptive immunity | Mediators | Role in immune response | |
|
| |||
| Antibodies to toxins | Anti-Toxin A and anti-Toxin B antibodies | Associated with protection, carrier state, decreased recurrence | 81, 82 |
|
| |||
| Antibodies to non-toxin components | Anti-SLP antibodies | No difference between colonized patients with or without disease | 52, 53 |
The challenge of ileal loops with C. difficile toxin A leads to an intense inflammatory response characterized by fluid accumulation, edema, increased mucosal permeability, mast cell degranulation, epithelial cell death, and neutrophil recruitment. In colonic epithelial cells, toxins have been shown to induce fluid secretion, upregulate production of reactive oxygen intermediates and IL-8 from epithelial cells [55, 56], induce cytokine and chemokine production [56, 57], and downregulate mucin exocytosis from mucin-producing colon cells [58]. In vitro challenge of epithelial cell lines and primary human colonic epithelial cells with toxin A led to cellular rounding, detachment, and apoptosis [55]. Similarly, exposure of lamina propria cells to toxin A in vitro induced apoptosis in macrophages, eosinophils, and T cells [59]. Notably, another study has shown that neutrophils are resistant to C. difficile toxin A-mediated apoptosis [60].
The toxins induce the production of multiple proinflammatory cytokines and chemokines including IL-12, IL-18, IFN-γ, IL-1β, TNF-α, MIP-1α, MIP-2, IL-8, and leptin [61, 62, 63], which propagate inflammation and may be responsible for host damage and many of the histopathologic features of CDAD. IFN-γ-deficient mice had less severe enteritis as compared with wild type mice following toxin A challenge, which manifested as decreased fluid secretion, decreased cellular edema and damage, and decreased myeloperoxidase production (indicating decreased neutrophil activity) [62]. Of note, IFN-γ deficiency was associated with attenuated TNF-α and chemokine secretion [62]. The chemokines RANTES and MIP1α propagate the inflammatory response to toxin A [61]. Blocking RANTES and MIP1α signaling by use of a RANTES inhibitor in MIP1α-deficient mice or CCR-1-deficient mice (CCR1 is the common signaling receptor for RANTES and MIP1α) led to increased inflammatory responses following toxin A challenge [64]. Similarly, the promoter of the human IL-8 gene (functionally equivalent to the mouse neutrophil-attracting chemokine KC/CXCL1) has a particular polymorphism that is associated with increased IL-8 production as well as increased risk of recurrent C. difficile infection [65]. By contrast, mice deficient in the fractalkine receptor (CX3CR1−/ −) had increased inflammation following toxin A challenge [66]. The protective role of this pathway in toxin A-mediated inflammation is likely via induction of heme oxygenase-1 expression in CX3CR1-expressing macrophages [66].
C. difficile toxins activate both surface and intracellular innate immune sensors, including the inflammasome and the TLR4, TLR5, and NOD1 signaling pathways [36, 37, 38, 39]. TLR4- and MyD88-dependent signaling pathways lead to enhanced inflammatory response [36], and blocking of these pathways leads to increased bacterial burden and worsening of the disease [36]. In the case of TLR5 signaling, although deficiency was not associated with any change in survival, exogenous stimulation of TLR5 signaling with flagellin was protective against C. difficile infection [37]. The TLR5-mediated protection is likely secondary to protective effects on the intestinal epithelial layer [37].
The intracellular innate immune sensors NOD1 and the IL-1β/inflammasome are also activated after C. difficile infection [38, 39]. C. difficile-induced NOD1 activation led to increased chemokine production and NOD1−/ − mice have lower chemokine production, less neutrophil recruitment, and more severe disease [38]. Similar to TLR4−/ − mice, NOD1−/− mice have a higher C. difficile burden [38]. C. difficile toxins stimulate IL-1β release by activating inflammasomes in both mouse macrophages and human colon biopsy specimens [39]; however, unlike TLR4 and NOD1 signaling, blocking both the inflammasome signaling by using IL-1 receptor antagonist or ASC−/− mice is associated with less toxin-mediated inflammation and damage [39]. It is important to note that whereas the studies looking at the role of TLR4, TLR5, and NOD1 signaling pathways used an infection model of C. difficile, the IL-1β/inflammasome pathway was studied in the context of purified toxin injection in an ileal loop model.
Activation of the innate immune sensors and the release of cytokine and chemokine mediators is followed by an intense local neutrophilic infiltration [31]. This neutrophilic infiltration is one of the major pathologic findings after C. difficile infection, and it is believed that neutrophils play a major role in disease pathogenesis. Both local recruitment and systemic proliferation of neutrophils is seen in CDAD [22, 31]. Antibody-mediated blocking of neutrophil migration in rabbits significantly reduced disease severity following challenge with C. difficile toxin A [31]. Similarly in rats, induction of neutropenia (loss of neutrophils) was associated with less severe disease [34]. These studies using toxin A-mediated enteritis would suggest that neutrophils enhance the inflammatory response and lead to host damage.
Intestinal mast cells also play an important role in the toxin-mediated inflammatory responses. Both toxins A and B lead to activation, degranulation, and the release of inflammatory mediators from mast cells [67]. In vitro studies showed that inhibiting mast cell degranulation and blocking mast cell-derived histamine was associated with a decrease in inflammatory responses to toxin A [68]. Mast cell-deficient mice have less severe inflammation and neutrophilic infiltration as compared with wild-type mice in response to C. difficile toxin A [69]. These studies suggest that like neutrophils, mast cells propagate the inflammatory response in CDAD. At least part of the toxin A-mediated neutrophil recruitment in rat ileal loops is dependent on mast cell activation [69].
The role of other immune cells, including macrophages, monocytes, and dendritic cells, has generally been extrapolated from in vitro and ex vivo studies using human and mouse cell lines, human monocytes, and monocyte-derived dendritic cells. These studies have shown that C. difficile toxins can stimulate the release of proinflammatory cytokines and chemokines from these cells in a MAPK- and p38-dependent manner [70, 71], and toxin A induces NFκB-mediated IL-8 production from human monocytes [72]. Whereas all of these studies using ex vivo toxin challenge would suggest that inflammatory cell recruitment is associated with worse disease, at least one recent study has shown that lack of neutrophils could lead to poor control of bacterial burden and thus more C. difficile-mediated disease [38].
Another interesting facet of C. difficile toxin A-mediated inflammation is the induction of a strong neuroinflammatory response via secretion of various neuropeptides: neurotensin (NT), substance P (SP), calcitonin gene-related peptide (CGRP) [73], corticotropin-releasing hormone (CRH) [74], and melanin-concentrating hormone [75]. Blocking enteric neural transmission by the local administration of lidocaine or systemic administration of hexamethonium abrogated the toxin A-mediated inflammatory response [76]. This was further shown to be dependent on the release of SP, CGRP, and NT in the intestine [73, 76]. Both an SP receptor antagonist (SR-48,692) and an NT antagonist (CP-96,345) reduced C. difficile toxin A-mediated intestinal inflammation, mast cell degranulation, and proinflammatory cytokine secretion [73, 76].
Adaptive immune responses
Antibodies to C. difficile toxins are naturally present in up to 60% of healthy adults and older children [53, 77]. In experimental models, passive immunization of mice and hamsters with antibodies to C. difficile can be protective [78, 79]. In the case of patients infected with C. difficile, systemic antibodies can be detected against both the toxins and various non-toxin antigens [53, 80, 81]. The presence of anti-toxin A antibodies is strongly associated with asymptomatic carriage of C. difficile, and levels of anti-toxin A IgM during an acute episode of C. difficile infection correlate with the risk of developing recurrent disease [80, 81] (Table 1). Patients that had recurrent infections had lower anti-toxin A antibody levels during the initial episode as compared to patients with a single episode of disease [81]. Recent studies have shown that this holds true for anti-toxin B antibodies as well–lower levels of anti-toxin B antibodies correlate with a higher risk of developing recurrent infections [82]. This suggests that an antibody-mediated immune response to C. difficile toxins has an important role in determining asymptomatic carriage and predisposition to recurrent infections. Furthermore, passive immunization studies in both humans [83] and hamsters [84] have shown that anti-toxin antibodies reduce recurrence (human studies and animal models) and enhance protection in primary disease (animal models).
Antibody responses to non-toxin components of C. difficile, such as the SLPs, are also observed in C. difficile disease. However, the role of antibodies to non-toxin components in protecting against C. difficile infection is unclear. Although serum IgG and IgM antibodies to SLPs have been observed in both C. difficile patients and asymptomatic carriers [52, 53], there was no difference in the antibody response between colonized patients with or without symptomatic disease. Cases of C. difficile diarrhea did have a higher anti-SLP IgG levels as compared to asymptomatic carriers and controls, showing an increased antibody response during infection; however, the exact role of this response in controlling the disease manifestations is still unclear. Recent data from animal models suggests that immunization of hamsters using Cwp84 protease as an antigen increased protection against C. difficile infection [85], but more studies are needed to clarify the role of SLPs and the immune response to these antigens during infection.
Concluding remarks
With the widespread use (and misuse) of antibiotics and emergence of the hypervirulent NAP1 strain, C. difficile has become a major nosocomial pathogen. The bacterium remains susceptible to metronidazole and vancomycin, but the incidence of recurrent infections is on the rise and novel therapeutic strategies are urgently needed. The hallmark of C. difficile infection is an intense inflammatory response characterized by recruitment of polymorphonuclear lymphocytes to the colon. However, it remains unclear whether the inflammatory response (Table 1, Figure 2) is beneficial or harmful to the host. It is also very interesting that the enteric nervous system and neuropeptides play an important role in directing inflammatory responses during this disease (Table 1). The studies discussed in this review suggest that whereas the adaptive immune response (in the form of antibodies to toxins and non-toxin antigens) may have a beneficial effect on the outcome of infection, the innate immune responses may enhance disease by initiating and propagating an inflammatory cascade. Many lines of evidence suggest that dampening the host response can ameliorate the severity of C. difficile toxin-mediated disease. However, some recent data from animal models of C. difficile infection suggests that dampening the immune response could lead to a higher pathogen burden. This could partly be a feature of the model systems used: toxin A-mediated enteritis versus infection with C. difficile. One can postulate that in the absence of bacterium (toxin A-induced enteritis) blocking the robust inflammatory response helps control host damage, whereas during infection with C. difficile blocking the immune response limits immune-mediated host damage but this control exposes the host to a higher bacterial burden, leading to bacteria-mediated damage.
Figure 2.
Pathogenesis of C. difficile-associated disease.
Given the current morbidity and mortality associated with C. difficile disease despite antibiotic therapy, one could argue that it is time to take a different approach. Targeting specific pathways in the inflammatory cascade that follows C. difficile infection could be an adjunct to antibiotic therapy. A clearer understanding of the nature and character of the host immune responses to C. difficile will be critical step in developing novel host-targeted therapies for this truly difficult to treat disease.
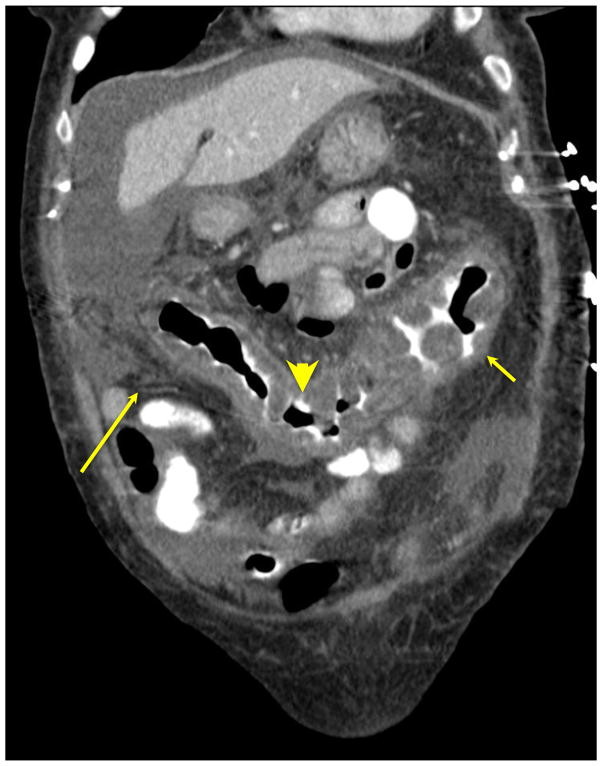
Figure 1. Compound tomography (CT) scan of patient with C. difficile colitis showing diffuse thickening along the entire colon.
CT findings in patients with C. difficile colitis include colonic thickening, peri-colonic stranding edema, and ‘accordion sign’ (oral contrast material trapped between edematous haustral folds).
Box 2. Outstanding Questions.
Is the C. difficile toxin-induced inflammatory response beneficial or harmful to the host?
Are there differences between the host response to C. difficile toxin A-mediated disease and infection with bacterium?
What is the role of innate immune responses during C. difficile colitis?
Can we target the host inflammatory response as an adjunct therapy to ameliorate C. difficile-associated disease?
Can the gut microbial communities be manipulated as a novel strategy for curing C. difficile infection?
Glossary
- Colonization resistance
defines the concept that indigenous bacterial flora (particularly gut anaerobes) limit the growth and concentration of potentially pathogenic bacterial species
- Pathogenicity locus
a 19.6 kb region in the C. difficile genome that encodes for various toxins and virulence factors
- Large clostridial toxins
are a family of related exotoxin molecules that act as major virulence factors. These include Toxins A and B of C. difficile, lethal and hemorrhagic toxin of C. sordellii, and the α-toxin of C. novyi
- Toll-like Receptors (TLRs)
are extracellular pathogen recognition receptors that recognize harmful stimuli and play an important role in innate immune host defense
- NOD signaling pathway
nucleotide-binding oligomerization domain (NOD) proteins are part of the innate immune system and bind to NOD-like receptors (NLRs), which are cytoplasmic receptors that triggers various inflammatory and apoptotic pathways
- Inflammasome
a multi-protein complex whose formation is triggered by specific stimuli, promoting the maturation of proinflammatory mediators interleukin (IL)-1β and IL-18
- Leukocytosis
above average white blood cell count in the blood, often indicative of an inflammatory response
- Lamina propria
thin layer of connective tissue below the surface cellular layer (epithelium) of mucosal surfaces of the body
- Pseudo-membranous colitis
infection of the large intestine that presents with yellow exudates (pseudo-membranes) in the colon, usually associated with severe C. difficile infection
- Fulminant colitis
severe, rapidly progressive infection of the large intestine (colon) that often leads to systemic shock and illness
- Myeloperoxidase
enzyme that is expressed at high levels in neutrophilic granules and participates in host defense mechanisms against pathogens
- Neutrophil
most abundant type of white blood cells in the body which play an important role in innate responses
- Mast cell
granular cells of the immune system that reside in various tissues and mediate early inflammatory responses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall ICOTE. Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. American Journal of Diseases of Children. 1935;49:390–402. [Google Scholar]
- 2.Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–4. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–82. [PubMed] [Google Scholar]
- 4.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409–15. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–90. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 6.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 7.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 8.Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011;11:194. doi: 10.1186/1471-2334-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deneve C, Janoir C, Poilane I, Fantinato C, Collignon A. New trends in Clostridium difficile virulence and pathogenesis. Int J Antimicrob Agents. 2009;33(Suppl 1):S24–8. doi: 10.1016/S0924-8579(09)70012-3. [DOI] [PubMed] [Google Scholar]
- 11.Thibault A, Miller MA, Gaese C. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak. Infect Control Hosp Epidemiol. 1991;12:345–8. doi: 10.1086/646354. [DOI] [PubMed] [Google Scholar]
- 12.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 13.McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162:678–84. doi: 10.1093/infdis/162.3.678. [DOI] [PubMed] [Google Scholar]
- 14.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170:772–8. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 15.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 16.Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012–9. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653–9. doi: 10.1086/501989. [DOI] [PubMed] [Google Scholar]
- 18.Cloud J, Noddin L, Pressman A, Hu M, Kelly C. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin Gastroenterol Hepatol. 2009;7:868–73. e2. doi: 10.1016/j.cgh.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, Pasculle AW, Paterson DL, Saul M, Harrison LH. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 20.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000;95:3137–41. doi: 10.1111/j.1572-0241.2000.03284.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:220–8. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 23.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 24.Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28:1–9. doi: 10.1097/MOG.0b013e32834bc9a9. [DOI] [PubMed] [Google Scholar]
- 25.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 26.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–42. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, Godin D, Bourassa C. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 28.Pepin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102:2781–8. doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 29.Taylor NS, Thorne GM, Bartlett JG. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981;34:1036–43. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang TW, Bartlett JG, Taylor NS. Clostridium difficile toxin. Pharmacol Ther. 1981;13:441–52. doi: 10.1016/0163-7258(81)90025-5. [DOI] [PubMed] [Google Scholar]
- 31.Kelly CP, Becker S, Linevsky JK, Joshi MA, O’Keane JC, Dickey BF, LaMont JT, Pothoulakis C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest. 1994;93:1257–65. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–63. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60:1070–9. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 34.Qiu B, Pothoulakis C, Castagliuolo I, Nikulasson S, LaMont JT. Participation of reactive oxygen metabolites in Clostridium difficile toxin A-induced enteritis in rats. Am J Physiol. 1999;276:G485–90. doi: 10.1152/ajpgi.1999.276.2.G485. [DOI] [PubMed] [Google Scholar]
- 35.von Eichel-Streiber C, Laufenberg-Feldmann R, Sartingen S, Schulze J, Sauerborn M. Comparative sequence analysis of the Clostridium difficile toxins A and B. Mol Gen Genet. 1992;233:260–8. doi: 10.1007/BF00587587. [DOI] [PubMed] [Google Scholar]
- 36.Ryan A, Lynch M, Smith SM, Amu S, Nel HJ, McCoy CE, Dowling JK, Draper E, O’Reilly V, McCarthy C, O’Brien J, Ni Eidhin D, O’Connell MJ, Keogh B, Morton CO, Rogers TR, Fallon PG, O’Neill LA, Kelleher D, Loscher CE. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 2011;7:e1002076. doi: 10.1371/journal.ppat.1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor 5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun. 2011;79:1498–503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Nunez G, Inohara N. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol. 2011;186:4872–80. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- 39.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology. 2010;139:542–52. 52, e1–3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Lyerly DM, Saum KE, MacDonald DK, Wilkins TD. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–52. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyras D, O’Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–3. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 43.Carter GP, Awad MM, Kelly ML, Rood JI, Lyras D. TcdB or not TcdB: a tale of two Clostridium difficile toxins. Future Microbiol. 2011;6:121–3. doi: 10.2217/fmb.10.169. [DOI] [PubMed] [Google Scholar]
- 44.Carter GP, Rood JI, Lyras D. The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol. 2012;20:21–9. doi: 10.1016/j.tim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Drudy D, Fanning S, Kyne L. Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis. 2007;11:5–10. doi: 10.1016/j.ijid.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Carter GP, Douce GR, Govind R, Howarth PM, Mackin KE, Spencer J, Buckley AM, Antunes A, Kotsanas D, Jenkin GA, Dupuy B, Rood JI, Lyras D. The anti-sigma factor TcdC modulates hypervirulence in an epidemic BI/NAP1/027 clinical isolate of Clostridium difficile. PLoS Pathog. 2011;7:e1002317. doi: 10.1371/journal.ppat.1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, Wehland J, Aktories K. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geric B, Carman RJ, Rupnik M, Genheimer CW, Sambol SP, Lyerly DM, Gerding DN, Johnson S. Binary toxin-producing, large clostridial toxin-negative Clostridium difficile strains are enterotoxic but do not cause disease in hamsters. J Infect Dis. 2006;193:1143–50. doi: 10.1086/501368. [DOI] [PubMed] [Google Scholar]
- 49.Bianco M, Fedele G, Quattrini A, Spigaglia P, Barbanti F, Mastrantonio P, Ausiello CM. Immunomodulatory activities of surface-layer proteins obtained from epidemic and hypervirulent Clostridium difficile strains. J Med Microbiol. 2011;60:1162–7. doi: 10.1099/jmm.0.029694-0. [DOI] [PubMed] [Google Scholar]
- 50.Calabi E, Ward S, Wren B, Paxton T, Panico M, Morris H, Dell A, Dougan G, Fairweather N. Molecular characterization of the surface layer proteins from Clostridium difficile. Mol Microbiol. 2001;40:1187–99. doi: 10.1046/j.1365-2958.2001.02461.x. [DOI] [PubMed] [Google Scholar]
- 51.Calabi E, Calabi F, Phillips AD, Fairweather NF. Binding of Clostridium difficile surface layer proteins to gastrointestinal tissues. Infect Immun. 2002;70:5770–8. doi: 10.1128/IAI.70.10.5770-5778.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pechine S, Gleizes A, Janoir C, Gorges-Kergot R, Barc MC, Delmee M, Collignon A. Immunological properties of surface proteins of Clostridium difficile. J Med Microbiol. 2005;54:193–6. doi: 10.1099/jmm.0.45800-0. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez-Hurtado K, Corretge M, Mutlu E, McIlhagger R, Starr JM, Poxton IR. Systemic antibody response to Clostridium difficile in colonized patients with and without symptoms and matched controls. J Med Microbiol. 2008;57:717–24. doi: 10.1099/jmm.0.47713-0. [DOI] [PubMed] [Google Scholar]
- 54.Tasteyre A, Barc MC, Collignon A, Boureau H, Karjalainen T. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect Immun. 2001;69:7937–40. doi: 10.1128/IAI.69.12.7937-7940.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahida YR, Makh S, Hyde S, Gray T, Borriello SP. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–47. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He D, Sougioultzis S, Hagen S, Liu J, Keates S, Keates AC, Pothoulakis C, Lamont JT. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology. 2002;122:1048–57. doi: 10.1053/gast.2002.32386. [DOI] [PubMed] [Google Scholar]
- 57.Kim JM, Kim JS, Jun HC, Oh YK, Song IS, Kim CY. Differential expression and polarized secretion of CXC and CC chemokines by human intestinal epithelial cancer cell lines in response to Clostridium difficile toxin A. Microbiol Immunol. 2002;46:333–42. doi: 10.1111/j.1348-0421.2002.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 58.Branka JE, Vallette G, Jarry A, Bou-Hanna C, Lemarre P, Van PN, Laboisse CL. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology. 1997;112:1887–94. doi: 10.1053/gast.1997.v112.pm9178681. [DOI] [PubMed] [Google Scholar]
- 59.Mahida YR, Galvin A, Makh S, Hyde S, Sanfilippo L, Borriello SP, Sewell HF. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T cell apoptosis. Infect Immun. 1998;66:5462–9. doi: 10.1128/iai.66.11.5462-5469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dailey DC, Kaiser A, Schloemer RH. Factors influencing the phagocytosis of Clostridium difficile by human polymorphonuclear leukocytes. Infect Immun. 1987;55:1541–6. doi: 10.1128/iai.55.7.1541-1546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mykoniatis A, Anton PM, Wlk M, Wang CC, Ungsunan L, Bluher S, Venihaki M, Simeonidis S, Zacks J, Zhao D, Sougioultzis S, Karalis K, Mantzoros C, Pothoulakis C. Leptin mediates Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 2003;124:683–91. doi: 10.1053/gast.2003.50101. [DOI] [PubMed] [Google Scholar]
- 62.Ishida Y, Maegawa T, Kondo T, Kimura A, Iwakura Y, Nakamura S, Mukaida N. Essential involvement of IFN-gamma in Clostridium difficile toxin A-induced enteritis. J Immunol. 2004;172:3018–25. doi: 10.4049/jimmunol.172.5.3018. [DOI] [PubMed] [Google Scholar]
- 63.Castagliuolo I, Keates AC, Wang CC, Pasha A, Valenick L, Kelly CP, Nikulasson ST, LaMont JT, Pothoulakis C. Clostridium difficile toxin A stimulates macrophage-inflammatory protein-2 production in rat intestinal epithelial cells. J Immunol. 1998;160:6039–45. [PubMed] [Google Scholar]
- 64.Morteau O, Castagliuolo I, Mykoniatis A, Zacks J, Wlk M, Lu B, Pothoulakis C, Gerard NP, Gerard C. Genetic deficiency in the chemokine receptor CCR1 protects against acute Clostridium difficile toxin A enteritis in mice. Gastroenterology. 2002;122:725–33. doi: 10.1053/gast.2002.31873. [DOI] [PubMed] [Google Scholar]
- 65.Garey KW, Jiang ZD, Ghantoji S, Tam VH, Arora V, Dupont HL. A common polymorphism in the interleukin-8 gene promoter is associated with an increased risk for recurrent Clostridium difficile infection. Clin Infect Dis. 2010;51:1406–10. doi: 10.1086/657398. [DOI] [PubMed] [Google Scholar]
- 66.Inui M, Ishida Y, Kimura A, Kuninaka Y, Mukaida N, Kondo T. Protective roles of CX3CR1-mediated signals in toxin A-induced enteritis through the induction of heme oxygenase-1 expression. J Immunol. 2011;186:423–31. doi: 10.4049/jimmunol.1000043. [DOI] [PubMed] [Google Scholar]
- 67.Meyer GK, Neetz A, Brandes G, Tsikas D, Butterfield JH, Just I, Gerhard R. Clostridium difficile toxins A and B directly stimulate human mast cells. Infect Immun. 2007;75:3868–76. doi: 10.1128/IAI.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurose I, Pothoulakis C, LaMont JT, Anderson DC, Paulson JC, Miyasaka M, Wolf R, Granger DN. Clostridium difficile toxin A-induced microvascular dysfunction. Role of histamine. J Clin Invest. 1994;94:1919–26. doi: 10.1172/JCI117542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 1998;114:956–64. doi: 10.1016/s0016-5085(98)70315-4. [DOI] [PubMed] [Google Scholar]
- 70.Sun X, He X, Tzipori S, Gerhard R, Feng H. Essential role of the glucosyltransferase activity in Clostridium difficile toxin-induced secretion of TNF-alpha by macrophages. Microb Pathog. 2009;46:298–305. doi: 10.1016/j.micpath.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest. 2000;105:1147–56. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jefferson KK, Smith MF, Jr, Bobak DA. Roles of intracellular calcium and NF-kappa B in the Clostridium difficile toxin A-induced up-regulation and secretion of IL-8 from human monocytes. J Immunol. 1999;163:5183–91. [PubMed] [Google Scholar]
- 73.Castagliuolo I, Wang CC, Valenick L, Pasha A, Nikulasson S, Carraway RE, Pothoulakis C. Neurotensin is a proinflammatory neuropeptide in colonic inflammation. J Clin Invest. 1999;103:843–9. doi: 10.1172/JCI4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anton PM, Gay J, Mykoniatis A, Pan A, O’Brien M, Brown D, Karalis K, Pothoulakis C. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci U S A. 2004;101:8503–8. doi: 10.1073/pnas.0402693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kokkotou E, Espinoza DO, Torres D, Karagiannides I, Kosteletos S, Savidge T, O’Brien M, Pothoulakis C. Melanin-concentrating hormone (MCH) modulates C difficile toxin A-mediated enteritis in mice. Gut. 2009;58:34–40. doi: 10.1136/gut.2008.155341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castagliuolo I, LaMont JT, Letourneau R, Kelly C, O’Keane JC, Jaffer A, Theoharides TC, Pothoulakis C. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology. 1994;107:657–65. doi: 10.1016/0016-5085(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 77.Viscidi R, Laughon BE, Yolken R, Bo-Linn P, Moench T, Ryder RW, Bartlett JG. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 78.Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, Thomas WD., Jr Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun. 1999;67:527–38. doi: 10.1128/iai.67.2.527-538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyerly DM, Bostwick EF, Binion SB, Wilkins TD. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–8. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–7. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 81.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–93. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 82.Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI) Vaccine. 2010;28:965–9. doi: 10.1016/j.vaccine.2009.10.144. [DOI] [PubMed] [Google Scholar]
- 83.Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD, Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 84.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD., Jr Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun. 2006;74:6339–47. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pechine S, Deneve C, Le Monnier A, Hoys S, Janoir C, Collignon A. Immunization of hamsters against Clostridium difficile infection using the Cwp84 protease as an antigen. FEMS Immunol Med Microbiol. 2011;63:73–81. doi: 10.1111/j.1574-695X.2011.00832.x. [DOI] [PubMed] [Google Scholar]
- 86.Collignon A, Ticchi L, Depitre C, Gaudelus J, Delmee M, Corthier G. Heterogeneity of Clostridium difficile isolates from infants. Eur J Pediatr. 1993;152:319–22. doi: 10.1007/BF01956743. [DOI] [PubMed] [Google Scholar]
- 87.Rousseau C, Levenez F, Fouqueray C, Dore J, Collignon A, Lepage P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol. 2011;49:858–65. doi: 10.1128/JCM.01507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145–58. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol. 2010;192:4983–90. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giel JL, Sorg JA, Sonenshein AL, Zhu J. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010;5:e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]