Abstract
Differences in redox homeostatic control between cancer patients may underlie predisposition to drug resistance and toxicities. To evaluate interindividual differences in redox response among newly diagnosed breast cancer patients undergoing standard chemotherapy, urine samples were collected before (T0), and at 1 (T1) and 24 h (T24) after chemotherapy administration. Oxidative status was assessed by urinary levels of allantoin and four F2-isoprostanes, quantified by LC–MS/MS. In all subjects, biomarker levels increased at T1 and returned to baseline at T24. Analyzing individual responses, two patterns were revealed: 10 subjects showed uniform increases of biomarker levels at T1 (“increase” pattern) and 8 subjects showed mixed (increase/unchanged/decrease) responses for different biomarkers (“mixed” pattern). The increase-pattern group had lower pre-treatment (T0) levels of the biomarkers and showed a sharp increase at T1 (64–141%) with a subsequent decrease at T24. The mixed-pattern group had higher pre-treatment biomarker levels and showed no change in biomarkers either at T1 or at T24. These findings indicate that there may be at least two distinct redox phenotypes with different homeostatic mechanisms balancing oxidative stress in humans. Recognizing redox phenotypes in human populations may lead to more precise assessment of health risks and benefits associated with individual redox make-up, and may also help to identify cancer patients who are especially susceptible to drug resistance and/or drug toxicity.
Keywords: Epidemiology, Oxidative stress, Chemotherapy, Biomarker
Introduction
The major barriers to effective cancer treatment are drug resistance and treatment-induced toxicity [1]. As both of these phenomena have been related to cellular redox (reduction/oxidation) balance [1], it is plausible that differences in redox homeostatic control play an important role in cancer patients’ susceptibility to drug resistance and toxicities. To examine this hypothesis, it is first necessary to identify measurable variables that reflect individual differences in redox homeostatic control. One such variable may be systemic oxidative status measured by biomarkers [2].
Variations of systemic oxidative status in human populations are widely recognized [2, 3]. These variations can be explained by the existing concept that major redox homeostatic mechanisms involve antioxidant defense [4, 5]; that is, according to the concept, humans differ in their oxidative status due to varying ability of their antioxidant system to balance generation of reactive oxygen/nitrogen species (ROS/RNS) by normal metabolism and/or by oxidative exposures. Another implication of this concept is that lower systemic oxidative status (reflected by lower levels of biomarkers) signifies efficient antioxidant defense and robust redox homeostasis. Conversely, individuals with greater oxidative status should have more fragile redox balance and, therefore, experience a more pronounced oxidative swing when exposed to an oxidative challenge.
We previously demonstrated that systemic oxidative status measured by five urinary biomarkers increases in response to chemotherapy administration; specifically, in a group of breast cancer patients undergoing standard adjuvant chemotherapy, the levels of urinary allantoin and four F2-isoprostanes increased at 1 h and returned to pre-treatment levels at 24 h after chemotherapy administration [6]. To explore differences in response between individuals, we hypothesized that cancer patients with higher pre-treatment oxidative status would have a more oxidative internal environment (due to a lower antioxidant defense capacity) and, therefore, would show a greater increase in biomarkers at 1 h after chemotherapy administration.
Materials and methods
Study subjects
We recruited 23 women with newly diagnosed breast cancer scheduled to undergo standard chemotherapy (Doxorubicin 60 mg/m2, Cyclophosphamide 600 mg/m2 × 4 cycles). The recruitment process and eligibility criteria are described elsewhere [6, 7]. The study protocol was approved by the Duke University Medical Center Institutional Review Board.
Urine samples
Urine samples were collected from participants at three time points: immediately before treatment (T0), and at 1 h (T1) and 24 h (T24) after treatment. Samples were stored at −80°C.
Laboratory measurements
All laboratory measurements have been previously described [6, 8]. Four isomers of F2-isoprostanes—iPF(2 alpha)-III; iPF(2 alpha)-VI; 8,12-iso-iPF(2 alpha)-VI; and 2,3-dinor-iPF(2 alpha)-III—were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on Shimadzu 20A series LC and Applied Biosystems API 4000 QTrap MS/MS instruments [6]. Allantoin was quantified using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) with an Acquity UPLC system and TQD triple quadrupole mass spectrometer equipped with an ESI source (Waters Corporation, Milford, MA) [6]. Creatinine was assayed by a fast ESI–MS/MS method [6].
Other variables
Data on age, tumor stage, estrogen and progesterone receptor status, height, and weight were obtained from medical records. Data on supplement intake were collected via questionnaire.
Statistical analysis
The descriptive analyses examined correlations in baseline biomarker levels and patient characteristics, using the Wilcoxon test. The analysis of response to oxidative challenge was carried out in three stages. First, we evaluated changes in the mean levels of the biomarkers at the three time points using general linear models that allowed us to control for the covariance structure. As the adjustment for age and other characteristics did not change our estimates, our final models include only the time effect. Second, to examine whether the response at T1 differed between individuals, we considered the T1-T0 change in each biomarker for each person. Variation within 15% (i.e., [−15%]–[+15%]) was categorized as “no change,” and a change in either direction greater than 15% was categorized either as “increase” or as “decrease.” Finally, we classified the pattern of overall response as either an “increase” pattern or a “mixed” pattern and assessed correlations between baseline biomarker levels and the patterns of response, using the Wilcoxon test and logistic regression. In the logistic regression models, the likelihood of the “mixed” versus “increase” pattern was modeled as an outcome, and the odds ratios were scaled to the standard deviation of each biomarker at baseline.
Results
The final analysis included 18 subjects for whom all biomarker measurements at T0, T1, and T24 were available (Table 1). The study population included women from 18 to 63 years of age distributed across all three conventional obesity categories. Most were Caucasian and had been diagnosed with stage I or II breast cancer; five were negative for both estrogen and progesterone receptors (ER negative/PR negative); and seven reported taking antioxidants and/or vitamin and mineral complexes. Pre-treatment levels of the five urinary biomarkers did not correlate with these patient characteristics (not shown).
Table 1.
Study subjects and baseline (T0) levels of the oxidative status biomarkers
| Characteristic | Number (N = 18) |
|---|---|
| Age | |
| 18–39 (pre-menopausal) | 2 |
| 40–54 (peri-menopausal) | 12 |
| 55–63* (menopausal) | 4 |
| Race | |
| African-American | 2 |
| Caucasian | 16 |
| BMI | |
| <25 | 7 |
| 25–29.9 | 4 |
| ≥ 30 | 7 |
| Tumor Stage | |
| I | 4 |
| II + III | 8 |
| Missing | 6 |
| ER/PR status | |
| Neg/Neg† | 5 |
| Neg/Pos | 0 |
| Pos/Neg | 3 |
| Pos/Pos | 10 |
| Use antioxidants and/or vitamin and mineral complexes | 7 |
|
| |
| Oxidative status biomarkers | Mean (SD) |
|
| |
| Allantoin, mmol/mol creatinine | 7.07 (3.83) |
| iPF(2 alpha)-III, ng/mg creatinine | 0.28 (0.14) |
| 2,3-dinor-iPF(2 alpha)-III, ng/mg creatinine | 12.84 (7.46) |
| iPF(2 alpha)-VI, ng/mg creatinine | 3.51 (2.04) |
| 8,12-iso-iPF(2 alpha)-VI, ng/mg creatinine | 8.88 (4.47) |
The oldest patient in this study was 63 years old
Estrogen receptor/Progesterone receptor status, neg negative, pos positive
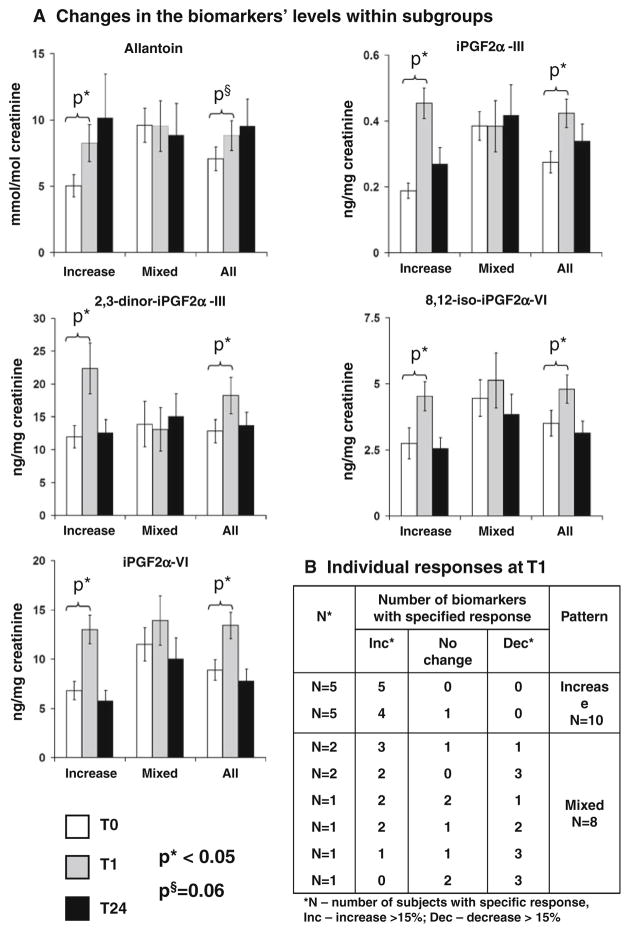
When all 18 subjects were evaluated as one group, the mean levels for all biomarkers increased at T1 (Fig. 1a, All). After categorizing change in biomarker levels at T1 for each participant, however, we found two distinct patterns of response to chemotherapy at T1 (Fig. 1b). Ten participants had an increase in at least four biomarkers and no decrease in any of the biomarkers (“increase” pattern). The other eight participants showed different combinations of biomarker response, including increased levels, no change, or decreased levels (“mixed” pattern). None of the participants’ characteristics were associated with the response pattern (not shown), except initial pre-treatment oxidative status (Table 2).
Fig. 1.
a Mean levels of biomarkers within the groups with different response patterns and in all participants; error bars present standard error; P-values for the comparisons of mean levels at T1 and T24 with T0 are derived from the generalized linear models.
b Changes in biomarkers’ levels at T1 classified as Increase (>15% increase), Decrease (>15% decrease), or no change (±15%) compared to T0
Table 2.
Baseline levels of oxidative status biomarkers by the response pattern and the association with response
| Biomarkers | Comparison of biomarker mean (Median) by response pattern
|
Association of “Mixed” pattern with increasing levels of biomarkers | ||
|---|---|---|---|---|
| “Increase” N = 10 | “Mixed” N = 8 | OR (95% CI)* | ||
| Allantoin, mmol/mol creatinine | 5.04 (4.18) | P = 0.03† | 9.60 (9.90) | 5.9 (1.8, 31.1) (P = 0.03) |
| iPF(2 alpha)-III, ng/mg creatinine | 0.19 (0.16) | P = 0.01 | 0.39 (0.41) | 14.3 (1.6, 127.8) (P = 0.03) |
| 2,3-dinor-iPF(2 alpha)-III, ng/mg creatinine | 11.99 (10.33) | P = 0.9 | 13.91 (10.98) | 1.3 (0.5, 3.5) (P = 0.6) |
| iPF(2 alpha)-VI, ng/mg creatinine | 2.75 (2.09) | P = 0.1 | 4.46 (4.40) | 2.8 (0.8, 9.1) (P = 0.1) |
| 8,12-iso-iPF(2 alpha)-VI, ng/mg creatinine | 6.79 (6.22) | P = 0.03 | 11.50 (10.10) | 5.6 (0.9, 34.7) (P = 0.07) |
OR scale to SD and shows the odds of “Mixed” pattern compared to the odds of “Increase” pattern
P-values derived from Wilcoxon rank test
Within each pattern of response, we compared the mean biomarker levels at T1 and T24 with the pre-treatment levels (T0) (Fig. 1a). Within the “increase” group, mean levels of all five biomarkers increased at T1 between 64 and 141% (P <0.05), with a subsequent reduction at T24 (P > 0.3). There were no significant changes in biomarker levels (either T1-T0 or T24-T0) within the “mixed” group (P > 0.3). Although we expected to observe sharper increases in biomarker levels in individuals with higher initial levels of oxidative status, the “increase” group had lower pre-treatment levels of four out of five biomarkers (Table 2), whereas the “mixed” pattern was associated with higher T0 levels of four biomarkers.
Discussion
Our findings confirm that oxidative status may define how individuals respond to oxidative stressors, although the observed association operated in the opposite direction to our original hypothesis. Our expectation that an oxidative challenge would cause a greater increase in biomarker levels in the group with initially high oxidative status was based on the dual assumptions: that higher systemic oxidative status reflects lower antioxidant capacity and that antioxidant defense presents the main force in restoring redox balance [2, 5]. However, we found a stronger response to chemotherapy-induced oxidative stress among individuals with lower pre-treatment oxidative status. Individuals with higher pre-treatment oxidative status as a group showed either no response at T1 [allantoin, iPF(2 alpha)-III, and 2,3-dinor-iPF(2 alpha)-III] or small increases that were not statistically significant [iPF(2 alpha)-VI and 8,12-iso-iPF(2 alpha)-VI].
One explanation for this counterintuitive finding is offered by the hypothesis that different redox homeostatic mechanisms are responsible for the two observed response patterns. The “increase” pattern can be explained by the conventional concept of antioxidant defense [5], which postulates that an initial increase of ROS mobilizes anti-oxidant response to restore redox balance. Two of our earlier reported findings support this explanation [7]. Both exogenous and endogenous antioxidants (plasma tocopherols and glutathione levels in erythrocytes, respectively) showed a statistically significant increase (glutathione) or decrease (tocopherols) in their levels 24 h after chemotherapy administration; no statistically significant changes in antioxidant levels were observed at T1. This indicates that antioxidant defense was mobilized within 24 h after the oxidative challenge but was not fully working at T1, suggesting a complementary dynamic between antioxidants and oxidative status markers.
In the “mixed” group, four subjects showed a greater than 15% decrease in the levels of three biomarkers (Fig. 1b). Even if we postulate that no change in biomarker levels reflects a strong antioxidant reserve, this does not explain the simultaneous decrease of three biomarkers. Instead, biomarker decreases suggest that something in addition to antioxidant defense is capable of regulating redox homeostasis. We therefore speculate that redox balance can be restored not only by enhancement of anti-oxidant defenses but also by reduction in ROS production. Homeostatic redox control of mitochondrial ROS generation is one possible mechanism, where ROS generation is proportional to transmembrane potential and is downregulated by proton leak [9, 10]. Regulation of the Nox family of NADPH oxidases, the sole function of which is ROS generation [11], offers another axis for redox control. The evidence that ROS generation by mitochondria and NADPH oxidases is integrated by redox homeostasis corroborates these assumptions [12–15]. Finally, several functional polymorphisms in the Nox family have been identified, suggesting different phenotypic responses to oxidative stress when Nox enzymes are involved [11].
To further explain the intriguing correlation between high oxidative status and the “mixed” pattern, a plausible mechanistic link between redox characteristics may reside within the signaling network of the AMP-activated protein kinase (AMPK) and the peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1-α) [16, 17]. Activation of AMPK/PGC-1-α signaling pathways increases mitochondrial metabolism with a pronounced rise in fat oxidation and (probably as a defense from increased ROS generation) proton leak; in many instances proton leak is upregulated through induction of uncoupling proteins (UCPs) [18–21]. Moreover, increased fat oxidation, as suggested elsewhere [22], raises systemic oxidative status, connecting high oxidative status to active AMPK/PGC-1-α signaling and proton leak upregulation. Where the mechanism for proton leak is in place, individuals should experience greater protection from oxidative stress through a quick reduction of mitochondrial ROS generation [9, 10]. Besides UCP expression, the PGC-1 family of transcription coactivators also induces antioxidant enzymes, such as manganese superoxide dismutase, peroxiredoxins 3 and 5, thioredoxin 2, thioredoxin reductase 2, glutaredoxin 2a, and glutathione peroxidase 4 [17, 23, 24]. If indeed the “mixed” pattern is at least partially determined by AMPK/PGC-1-α signaling, such relationships highlight the likelihood that increased oxidative status (in the absence of exposure to a stressor) does not signify a low antioxidant capacity. Supporting this assumption, no correlation was found between antioxidant indices and the urinary biomarkers of oxidative damage [6, 7].
In summary, the two response patterns observed in our study (Fig. 1b) suggest that at least two protection mechanisms may be operating—induction of antioxidant defense and downregulation of mitochondrial ROS generation. Which mechanism dominates may be determined by an individual’s oxidative status, the set point of the inner redox environment. We speculate that among individuals with lower oxidative status, the contribution of antioxidant defense is larger, whereas the contribution of ROS downregulation is greater among those with higher oxidative status. Recognizing these distinct redox phenotypes, characterized by levels of systemic oxidative status and corresponding reactions to oxidative stressors, represents an important step in understanding the role of redox biology in human health. Capturing phenotypic differences may provide an avenue for further physiological and genotypic comparisons leading to more precise assessment of health risks and benefits associated with individual redox make-up.
Our study also raises the important and plausible question of whether redox phenotypes relate to chemotherapy resistance and susceptibility to toxicity. Although the limited sample size and available data precluded a full-scale analysis, our study allowed for preliminary exploration of the hypothesis that neutropenia (<2 × 109 neutrophils count, registered ≥ 2 times during treatment) is associated with response pattern. The finding that the “increase” pattern was associated with neutropenia (OR = 6.5, P = 0.1) provides some support for the hypothesized connection between redox phenotypes and treatment-related toxicities. This association may be explained by the different time scales of redox restoration within the two response patterns. Whereas induction of antioxidant defense within the “increase” pattern takes hours or days, swift reduction of oxidative stress by ROS downregulation (“mixed” pattern) may protect normal tissues from oxidative damage faster, lowering individuals’ susceptibility to chemotherapy toxicity. A handful of other studies also have linked ROS downregulation to cancer treatment outcomes. For example, activation of AMPK/PGC-1-α pathways increased cancer cell sensitivity to chemotherapeutic cytotoxicity, thereby reducing drug resistance [16, 25], and the functional polymorphisms of NADPH oxidases have been shown to increase the risk of doxorubicin-related cardiotoxicity [26] and decrease event-free survival in non-Hodgkin’s lymphoma [27]. Alternatively, patients with initially high systemic oxidative status may have developed adaptive responses to oxidative stress; such adaptive responses have been shown to result in drug resistance [28–31]. From this perspective, investigation of redox phenotypes not only addresses an interesting scientific question but may help to identify the subpopulation of cancer patients who are especially susceptible to drug resistance and/or drug toxicity.
Acknowledgments
This research was funded by a Duke Comprehensive Cancer Center pilot study award, NIH SPORE Grant 5P50 CA68438, the Department of Defense Breast Cancer Research Program (BCRP) predoctoral traineeship award BC083154, and Anna Merills’ Fund for Down Syndrome Research Foundation grant.
Abbreviations
- AMPK
AMP-activated protein kinase
- LC–MS/MS
liquid chromatography-tandem mass spectrometry
- PGC-1-α
peroxisome proliferator-activated receptor-γ coactivator-1α
- T0
time point of urine collection before administration of doxorubicin
- T1
time point of urine collection 1 h after administration of doxorubicin
- T24
time point of urine collection 24 h after administration of doxorubicin
- UCPs
uncoupling proteins (1–6)
- UPLC–MS/MS
ultra performance liquid chromatography-tandem mass spectrometry
Footnotes
Disclosure Authors of this manuscript, Dora Il’yasova, Kelly Kennedy, Ivan Spasojevic, Frances Wang, Adviye A. Tolun, Karel Base, Sarah P. Young, P Kelly Marcom, Jeffrey Marks, David S. Millington, and Mark Dewhirst, have neither actual nor potential commercial associations that might create a conflict of interest in connection with submitted manuscripts.
Contributor Information
Dora Il’yasova, Email: dora.ilyasova@duke.edu, Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC 27710, USA.
Kelly Kennedy, Department of Pathology, Duke University Medical Center, Durham, NC, USA.
Ivan Spasojevic, Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC 27710, USA.
Frances Wang, Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC 27710, USA.
Adviye A. Tolun, Department of Pediatrics, Medical Genetics Division, Duke University Medical Center, Durham NC, USA
Karel Base, Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC 27710, USA.
Sarah P. Young, Department of Pediatrics, Medical Genetics Division, Duke University Medical Center, Durham NC, USA
P. Kelly Marcom, Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC 27710, USA.
Jeffrey Marks, Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC 27710, USA.
David S. Millington, Department of Pediatrics, Medical Genetics Division, Duke University Medical Center, Durham NC, USA
Mark W. Dewhirst, Radiation Oncology Department and Duke Comprehensive Cancer Center, Duke University Medical Center, Durham, NC, USA
References
- 1.Doroshow JH. Redox modulation of chemotherapy-induced tumor cell killing and normal tissue toxicity. J Natl Cancer Inst. 2006;98:223–225. doi: 10.1093/jnci/djj065. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford University Press; Oxford: 2007. [Google Scholar]
- 5.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 6.Il’yasova D, Spasojevich I, Wang F, Tolun AA, Base K, Young SP, Marcom PK, Marks J, Mixon G, Di Giulio R, Millington DS. Urinary biomarkers of oxidative status in a clinical model of oxidative assault. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1506–1510. doi: 10.1158/1055-9965.EPI-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Il’yasova D, Mixon G, Wang F, Marcom PK, Marks J, Spasojevich I, Craft N, Arredondo F, DiGiulio R. Markers of oxidative status in a clinical model of oxidative assault: a pilot study in human blood following doxorubicin administration. Biomarkers. 2009;14:321–325. doi: 10.1080/13547500902946757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolun AA, Zhang H, Il’yasova D, Sztbray J, Young SP, Millington DS. Allantoin in human urine quantified by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Biochem. 2010;402:191–193. doi: 10.1016/j.ab.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 13.Wosniak J, Jr, Santos CX, Kowaltowski AJ, Laurindo FR. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 14.Lopes LR, Dagher MC, Gutierrez A, Young B, Bouin AP, Fuchs A, Babior BM. Phosphorylated p40PHOX as a negative regulator of NADPH oxidase. Biochemistry. 2004;43:3723–3730. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- 15.Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005;4:1367–1373. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- 16.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Chan SH, Wu CA, Wu KL, Ho YH, Chang AY, Chan JY. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ Res. 2009;105:886–896. doi: 10.1161/CIRCRESAHA.109.199018. [DOI] [PubMed] [Google Scholar]
- 19.Handschin C. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha in muscle links metabolism to inflammation. Clin Exp Pharmacol Physiol. 2009;36:1139–1143. doi: 10.1111/j.1440-1681.2009.05275.x. [DOI] [PubMed] [Google Scholar]
- 20.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Lombardi A, de Lange P, Silvestri E, Busiello RA, Lanni A, Goglia F, Moreno M. 3, 5-Diiodo-L-thyronine rapidly enhances mitochondrial fatty acid oxidation rate and thermogenesis in rat skeletal muscle: AMP-activated protein kinase involvement. Am J Physiol Endocrinol Metab. 2009;296(3):E497–E502. doi: 10.1152/ajpendo.90642.2008. [DOI] [PubMed] [Google Scholar]
- 22.Il’yasova D, Morrow JD, Wagenknecht LE. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obes Res. 2005;13:1638–1644. doi: 10.1038/oby.2005.201. [DOI] [PubMed] [Google Scholar]
- 23.Olmos Y, Valle I, Borniquel S, Tierrez A, Soria E, Lamas S, Monsalve M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, JSger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Kong X, Fan H, Liu X, Wang R, Liang J, Gupta N, Chen Y, Fang F, Chang Y. Peroxisome proliferator-activated receptor gamma coactivator-1alpha enhances antiproliferative activity of 5′-deoxy-5-fluorouridine in cancer cells through induction of uridine phosphorylase. Mol Pharmacol. 2009;76:854–860. doi: 10.1124/mol.109.056424. [DOI] [PubMed] [Google Scholar]
- 26.Wojnowski L, Kulle B, Schirmer M, Schluter G, Schmidt A, Rosenberger A, Vonhof S, Bickeboller H, Toliat MR, Suk EK, Tzvetkov M, Kruger A, Seifert S, Kloess M, Hahn H, Loeffler M, Nurnberg P, Pfreundschuh M, Trumper L, Brockmoller J, Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann M, Schirmer MA, Tzvetkov MV, Kreuz M, Ziepert M, Wojnowski L, Kube D, Pfreundschuh M, Trumper L, Loeffler M, Brockmoller J. A functional polymorphism in the NAD(P)H oxidase subunit CYBA is related to gene expression, enzyme activity, and outcome in non-Hodgkin lymphoma. Cancer Res. 2010;70:2328–2338. doi: 10.1158/0008-5472.CAN-09-2388. [DOI] [PubMed] [Google Scholar]
- 28.Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701–2716. doi: 10.1089/ars.2009.2692. [DOI] [PubMed] [Google Scholar]
- 29.Pennington JD, Wang TJ, Nguyen P, Sun L, Bisht K, Smart D, Gius D. Redox-sensitive signaling factors as a novel molecular targets for cancer therapy 1. Drug Resist Updat. 2005;8:322–330. doi: 10.1016/j.drup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Yokomizo A, Ono M, Nanri H, Makino Y, Ohga T, Wada M, Okamoto T, Yodoi J, Kuwano M, Kohno K. Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide 1. Cancer Res. 1995;55:4293–4296. [PubMed] [Google Scholar]
- 31.Mitchell JB, Russo A. The role of glutathione in radiation and drug induced cytotoxicity 1. Br J Cancer Suppl. 1987;8:96–104. [PMC free article] [PubMed] [Google Scholar]