Abstract
In the cell Mn porphyrins (MnPs) likely couple with cellular reductants which results in a drop of total charge from 5+ to 4+ and dramatically increases their lipophilicity by up to three orders of magnitude depending upon the length of alkylpyridyl chains and type of isomer. The effects result from the interplay of solvation, lipophilicity and stericity. Impact of ascorbate on accumulation of MnPs was measured in E. coli and in Balb/C mouse tumours and muscle; for the latter measurements, the LC/ESI-MS/MS method was developed. Accumulation was significantly enhanced when MnPs were co-administered with ascorbate in both prokaryotic and eukaryotic systems. Further, MnTnHex-2-PyP5+ accumulates 5-fold more in the tumour than in a muscle. Such data increase our understanding of MnPs cellular and sub-cellular accumulation and remarkable in vivo effects. The work is in progress to understand how coupling of MnPs with ascorbate affects their mechanism of action, in particular with respect to cancer therapy.
Keywords: SOD mimics, peroxynitrite scavengers, Mn(III) N-alkylpyridyl porphyrins
Introduction
Mn(III) N-alkylpyridylporphyrins are among the most potent scavengers of superoxide (Table I) and peroxynitrite [1,2]. Besides, they are able to remove other reactive species such as CO3˙−, lipid peroxyl radicals, react with ·NO and judged by the activity of similar Mn porphyrins are likely able to efficiently remove HClO also [1,2].
Table I.
Metal-centred redox potential for MnIII/MnII redox couple, E1/2 and log kcat for O2.− dismutation for Mn(III) N-alkylpyridylporphyrins.
| Porphyrin | E1/2/mV vs NHEa | log kcatb |
|---|---|---|
| MnTM-2-PyP5+ | +220 | 7.79 |
| MnTE-2-PyP5+ | +228 | 7.76 |
| MnTnPr-2-PyP5+ | +238 | 7.38 |
| MnTnBu-2-PyP5+ | +254 | 7.25 |
| MnTnHex-2-PyP5+ | +314 | 7.48 |
| MnTnHep-2-PyP5+ | +342 | 7.65 |
| MnTnOct-2-PyP5+ | +367 | 7.71 |
| MnTM-3-PyP5+ | +52 | 6.61 |
| MnTE-3-PyP5+ | +54 | 6.65 |
| MnTnPr-3-PyP5+ | +62 | 6.69 |
| MnTnBu-3-PyP5+ | +64 | 6.69 |
| MnTnHex-3-PyP5+ | +66 | 6.64 |
Reactive oxygen species (ROS) have been traditionally viewed as by-products of aerobic metabolism or a response to toxic stimuli. Often, superoxide is formed first, followed by a plethora of reactive species: hydrogen peroxide, hydroxyl radical, peroxynitrite, hypochlorus acid and singlet oxygen [3]. In order to cope with excess ROS, cells have a variety of redundant antioxidant defence systems: several superoxide dismutases, gluthatione peroxidases, glutathione reductases, catalase, a family of thioredoxins, peroxiredoxins, a family of glutathione S-transferases, glutaredoxins and others [4]. However, deliberate ROS production by a family of NADPH oxidases (NOX) plays an important role in the signalling pathways under both physiological and pathological conditions [5–8]. There are seven members of the NOX family: NOX1 through NOX5, which are generally considered to produce superoxide, and two hydrogen peroxide releasing DUOX1 and DUOX2 [5]. The first described, phagocytic NOX2, is activated during phagocytosis and produces high levels of superoxide [5]. In contrast, as a response to different stimuli, NOX 1, 3, 4 and 5 in non-phagocytic cells produce only small amounts of ROS, which act as second messengers in a wide variety of cells and under various conditions. NOX1 is expressed in colon epithelial and vascular smooth muscle cells, NOX3 in the inner ear, NOX4 in the kidney and NOX 5 in the testes and spleen [5]. Some of the known stimuli of the non-phagocytic NOX are angiotensin-II, thrombin, receptor tyrosine kinases, insulin, PDGF, bFGF, VEGF, angiopoietin and tumour necrosis factor receptor family [7]. In order to avoid collateral damage to biologically important molecules and cellular compartments, NOX mediated production of superoxide and subsequally ROS are regulated by mechanisms which control site, amount, duration and type of the ROS produced [6].
During tumour development, ROS not only cause genomic instability, which leads to tumour initiation and progression [9], but also activate cellular signalling pathways that, in turn, upregulate tumour proliferation, angiogenesis and metastasis [10]. Tumours can endure higher ROS levels, which would have triggered senescence and apoptosis in normal cells. Moreover, a tumour can utilize excessive oxidative burden to its advantage, i.e. for its progression [8]. Yet, at exceedingly high levels of ROS, tumour growth is suppressed or disabled [8]. Therefore, both antioxidants (that deplete tumour from signalling molecules) and pro-oxidants (that impose excessive oxidative burden upon the tumour) are being explored for the therapeutic potential as anti-cancer agents in the clinics [11].
By removing reactive species, Mn porphyrins decrease the consequences of primary oxidative stress by protecting biological molecules, but also finely tune redox-based cellular transcriptional activity and thus suppress excessive inflammatory and immune responses [12]. Consequently, they proved efficacious in reducing injuries of the central nervous system, radiation damage, diabetes-related disorders, as anticancer agents, etc. [1] and are therefore considered for clinical development. Most perspective candidates have been MnTE-2-PyP5+ (AEOL10113), MnTnHex-2-PyP5+ and MnTDE-2-ImP5+ (AEOL10150) [1]. The most lipophilic among them, MnTnHex-2-PyP5+, appears up to 120-fold more efficacious than either of the other compounds (Figure 1) [1,2,12].
Figure 1.
Structures of ortho and meta Mn(III) N-alkylpyridylporphyrins.
Although excessively hydrophilic, those pentacationic porphyrins are found in all tissues, cells and cellular compartments such as mitochondria [13] and nucleus [12].
Antioxidant potency
The ability to catalyze O2˙− dismutation (log kcat in Table I) best describes the antioxidant potency of the cationic Mn(III) N-alkylpyridylporphyrins [1,2]. The reason lies in the fact that the SOD-like activity parallels the ONOO− scavenging ability and, in turn, the ability of MnPs to finely tune cellular redox-based transcriptional activity [1,2,12,14]. We extensively addressed the antioxidant potency and accumulated a sufficient knowledge on the structure–activity relationship which allows us to accurately identify a drug candidate [1,12]. Due to the ability to easily donate and accept electrons from redox-able biological molecules, MnPs can also produce a therapeutic effect via pro-oxidative mechanism [12,15,16]. Research is in progress to further understand such seemingly ‘opposing’ modes of action/s. In a two-step O2˙− dismutation process, MnP acts both as pro- and anti-oxidant, oxidizing and reducing O2˙−. With efficacious MnP-based SOD mimics that dismute O2˙− at around the potential of SOD enzymes, both processes occur with similar rate constants. Such data clearly suggest that Mn porphyrins may be equally good in acting as anti- and pro-oxidants in other in vivo systems also. These considerations are also reminiscent of anti- and pro-oxidative actions of MnSOD, which have been recently widely contemplated (see Discussion) [11,17].
Indeed, with high endogenous ascorbate levels, MnP may become oxidant and catalyze the oxygen consumption by ascorbate leading to excessive production of peroxide until ascorbate and/or oxygen is consumed [1,2,16,18–21]. We have already reported in a number of our publications the chemical evidence for the formation of peroxide in the presence of MnP and ascorbate [1,2,20,22] (see also in Discussion under Mechanism of action).
Bioavailibility of Mn porphyrins
The in vivo efficacy of MnPs is governed not only by their antioxidant potency, but also by their bioavailability. Recently, we have aimed at understanding which factors affect the bioavailability of Mn porphyrins and, in turn, their efficacy. The bioavailability is governed by lipophilicity, bulkiness, shape, charge, substituents and overall geometry of MnPs. MnP lipophilicity by itself is a fair measure of porphyrin bioavailability [23–25] and has been characterized by either chromatographic retention factor Rf [1,2] or partition of Mn porphyrin between n-octanol and water, log POW [26]. Rf and log POW for Mn(III) N-alkylpyridylporphyrins are linearly related. The determination of Rf is much simpler relative to log POW; in some cases log POW can not be even determined due to excessive hydrophilic character of some MnPs. The log POW can be accurately obtained from Rf vs log POW linear dependence [26]. Few studies showed that Mn porphyrin must possess both fair antioxidant potency and appropriate lipophilicity to be efficacious in vivo [23–26]. The recent comprehensive E. coli study [25] most clearly showed the vast impact of MnP lipophilicity on its efficacy. The series of isomeric ortho and meta isomers (Figure 1) was studied with respect to their cellular accumulation and ability to allow SOD-deficient E. coli to grow aerobically as the wild type. The meta MnTE-3-PyP5+ is ~10-fold less SOD-active, but is 10 fold more lipophilic and accumulated 10-fold more in E. coli than ortho MnTE-2-PyP5+. Its higher accumulation fully compensates for inferior SOD-like activity. Consequently, under the same conditions, MnTE-2-PyP5+ and MnTE-3-PyP5+ were equally able to compensate for the lack of cytosolic superoxide dismutases.
The present study explores the effect of charges on Mn porphyrins lipophilicity and, consequently, on their cellular accumulation. Due to favourable, highly positive E1/2 (Table I), the cationic ortho Mn(III) N-alkylpyridylporphyrins are readily reduced by ascorbate, glutathione, tetrahydrobiopterin and flavoenzymes [2,12,17,27–30]. As the cellular levels of ascorbate and glutathione are high [4], those MnPs would likely be reduced inside the cell. Evidence that this is indeed true was already reported with E. coli [25,31]. Upon reduction, the total charge drops from 5+ to 4+, whereby the single charge at the Mn centre is lost. Since the peripheral charges remain the same, no major impact of charge reduction on lipophilicity has been anticipated. Surprisingly, upon reduction, the MnP lipophilicity increased as much as 850-fold, which has a significant impact on the porphyrin accumulation both in prokaryotic E. coli cell and in eukaryotic mouse tumour and muscle. For comparison, the analogous meta isomer, MnTM-3-PyP5+ was also explored. Such data further our insight into the remarkable in vivo efficacy of ortho isomeric Mn porphyrins, their accumulation in mitochondria and in central nervous system (which is rich in ascorbate) and support their clinical development.
Materials and methods
Materials
Mn porphyrins were synthesized as previously described [2,22,25,26,32]. Acetonitrile was from Fisher Scientific. Plastic-backed silica gel TLC plates (Z122777-25EA), sodium ascorbate and KNO3 were purchased from Sigma-Aldrich. Sodium dithionite was from J. T. Baker.
Thin-layer chromatography
Thin-layer chromatography of Mn porhyrins was performed on silica gel TLC plates (plastic-backed) with KNO3-saturated H2O:H2O:acetonitrile = 1:1:8 mixture as a mobile phase. Typically, 1 µL of ~1 mM samples was applied at ~1 cm of the strip border and the solvent front was allowed to run ~8 cm. The 1 mM ortho porphyrins were reduced with 20 mM ascorbate; ~5 min were allowed for complete reduction. Less reducible MnPs of meta series (see E1/2 values in Table I) were reduced with a much stronger reducing reagent, sodium dithionite. Aerobically, ascorbate reduces MnTM-3-PyP5+ slowly and incompletely. Upon addition of sodium dithionite the MnP was immediately placed on a silica plate. Longer exposure to such strong reductant leads to the reduction of porphyrin ring with its subsequent decomposition observed as the decolouration of solution. The Rf values are sensitive to the degree of the saturation of vapour phase in the TLC chamber and may differ slightly from one to another experiment. Thus, the internal standardization of TLC, using MnTE-2-PyP5+, is required for comparison purposes [33]*. Experimental determination of log POW for the reduced porphyrins is not possible, as ascorbate is hydrophilic and can not be extracted into n-octanol with porphyrin. Without ascorbate MnIIPs are not stable and can not be isolated. Therefore, log POW of the reduced MnPs was calculated from experimentally determined Rf according to equations: log POW = 12.18 · Rf − 7.43 (ortho porphyrins) and log POW = 8.78 · Rf − 7.12 (meta porphyrins) (Table II) [26]. Calculation of log POW from the equation log POW vs nC in the case of the reduced MnPs is not possible, because that equation takes into account only the number of carbon atoms regardless of the Mn centre redox state. In the series of oxidized MnPs, differences in calculated log POW values by both equations are negligible, except for MnIIITM-2-PyP5+ and MnIIITE-3-PyP5+.
Table II.
Lipophilicity of the series of ortho and meta isomeric Mn(III)- and reduced Mn(II) N-alkylpyridylporphyrins, measured by chromatographic retention factor (Rf) and by their partition between n-octanol and water, log POW.
| Porphyrin | Rf (MnIIIP) | log POW(MnIIIP) | Porphyrin | Rf (MnIIP) | log POW(MnIIP) | log POW(MnIIIP) − log POW(MnIIP) |
|---|---|---|---|---|---|---|
| MnIIITE-2-PyP5+ | 0.060 | −6.70a | MnIITE-2-PyP4+ | 0.225 | −4.69a | 2.01 |
| MnIIITnPr-2-PyP5+ | 0.110 | −6.09a | MnIITnPr-2-PyP4+ | 0.340 | −3.29a | 2.80 |
| MnIIITnBu-2-PyP5+ | 0.190 | −5.11b | MnIITnBu-2-PyP4+ | 0.431 | −2.18a | 2.93 |
| MnIIITnHex-2-PyP5+ | 0.380 | −2.76b | MnIITnHex-2-PyP4+ | 0.550 | −0.73a | 2.03 |
| MnIIITnHep-2-PyP5+ | 0.460 | −2.10b | MnIITnHep-2-PyP4+ | 0.578 | −0.39a | 1.71 |
| MnIIITnOct-2-PyP5+ | 0.490 | −1.24b | MnIITnOct-2-PyP4+ | 0.583 | −0.33a | 0.91 |
| MnIIITM-3-PyP5+ | 0.050 | −6.68c | MnIITM-3-PyP4+ | 0.125 | −6.02c | 0.66 |
| MnIIITE-3-PyP5+ | 0.100 | −6.24c | MnIITE-3-PyP4+ | 0.214 | −5.24c | 1.00 |
| MnIIITnPr-3-PyP5+ | 0.220 | −5.00b | MnIITnPr-3-PyP4+ | 0.381 | −3.77c | 1.23 |
| MnIIITnBu-3-PyP5+ | 0.400 | −4.03b | MnIITnBu-3-PyP4+ | 0.548 | −2.31c | 1.72 |
| MnIIITnHex-3-PyP5+ | 0.550 | −2.06b | MnIITnHex-3-PyP4+ | 0.613 | −1.74c | 0.32 |
Rf values were obtained by TLC on plastic-backed silica gel plates in KNO3-saturated H2O:H2O:acetonitrile = 1:1:8.
Calculated according to equation: log POW = 12.18 · Rf − 7.43 [26]. The values are similar to those calculated from log POW vs nC [25]; the deviations are higher with methyl and ethyl analogues.
Determined in water/n-butanol system and converted to water/n-octanol system using equation: log POW = 1.55 (log PBW) − 0.54 [45].
Calculated according to equation: log POW = 8.78 · Rf − 7.12.
In order to gain further insight into the lipophilicity of 4+-carrying ions, we performed TLC under identical conditions for MnIIITE-2-PyP5+ and for tetracationic species: MnIITE-2-PyP4+, metal-free ligand, H2TE-2-PyP5+ [22] and its Zn complex, ZnTE-2-PyP4+. ZnP was synthesized as previously described [2]. MnIIITE-2-PyP5+ was reduced with ascorbate; thus TLC of MnIITE-2-PyP4+ is the only one out of compounds tested to have excess ascorbate in solution. Ascorbate may also act as counterion replacing chloride and/or nitrate ions (the latter coming from excess KNO3 in TLC solvent mixture) and affect Rf value. To account for the effect of ascorbate, the TLC was done with H2TE-2-PyP4+ and ZnTE-2-PyP4+ solutions prepared with and without ascorbate. There was no effect of ascorbate on Rf.
Accumulation of Mn porphyrins in E. coli
A wild type Escherichia coli strain AB1157 was used in this study (F-thr-1; leuB6; proA2; his-4; thi-1; argE2; lacY1; galK2; rpsL; supE44; ara-14; xyl-15; mtl-1; tsx-33) [34]. The experiments were carried out in triplicate, as described in detail in Batinić-Haberle et al. [35]. The accumulation experiment was performed with wild type AB1157 since it grows faster. Deionized water was used throughout the study. The E. coli was grown in flasks in 10 mL of casamino M9CA medium to a density corresponding to A700 ~0.6. Then 5 µM Mn porphyrins alone were added in one experiment and together with 0.5 or 1 mM sodium ascorbate in another one. The cells were kept on a shaker for an additional 60 min. Cells were then rapidly washed with ice-cold PBS, resuspended to a total volume of 1.0 mL and disrupted by sonication. Cytosolic and cell wall fractions were separated by centrifugation. Cell walls are minor fraction of the cell. Due to insufficient material, the quantification of Mn porphyrins is less reliable in cell walls than in cytosols. Spectra were recorded and the absorbances of MnPs measured at their Soret bands [2]. Protein levels were measured by Lowry method [36]. In the growing medium, under aerobic conditions and in the absence of reductants, MnPs are stabilized with Mn being in +3 oxidation state. Due to the reductive cellular environment and particularly when E. coli was growing in the presence of ascorbate, inside the respiring cell MnPs exist predominantly as MnIIPs. However, it is close to impossible to prevent the oxidation of MnIIP to MnIIIP during manipulation of the cells and thus to assess unambiguously the oxidation state of Mn within intact cells. Even after cell disruption, the spectra of processed cytosolic fractions still showed significant levels of reduced MnIIP, which suggests extensive reduction of MnP within the respiring cell. Two maxima were seen, one related to oxidized MnIIIP at ~454 nm for ortho and at 460 nm for meta isomers, the other related to reduced MnIIP at shorter wavelengths of ~440 nm. Upon centrifuging the casamino M9CA medium containing cells and before any manipulation, the cellular pellet was greenish, which is a clear sign of the presence of porphyrin in a reduced state, MnIIP (oxidized MnIIIP is brown-reddish). Two approaches were adopted to calculate the levels of MnPs in cytosolic fractions: (a) using the molar absorptivities and (b) using area below the Soret band. For the latter, the calibration curve was constructed where the area below the Soret band was plotted vs concentration of either ortho or meta porphyrins [25]. Both approaches offered the same conclusions, but the latter appeared to be a more correct strategy as it accounted for both oxidized and reduced porphyrin.
Tumour and muscle levels of MnTnHex-2-PyP5+
The tumours and muscle, provided from a 4T1 breast cancer mouse study (publication in preparation, few data available in refs [1] and [16]) were used to determine the levels of Mn porphyrins. All in vivo procedures were conducted in accordance with a protocol approved by the Duke University Institutional Animal Care and Use Committee. Four groups of Balb/C female mice weighing on average 20 g with 15 mice per group were studied: (1) 400 µL of PBS ip; (2) sodium ascorbate 2 g/kg twice daily ip; (3) MnTnHex-2-PyP5+ 1 mg/kg twice daily sc; (4) MnTnHex-2-PyP5+ 1 mg/kg twice daily sc and sodium ascorbate 2 g/kg twice daily ip. In the 4th group, sodium ascorbate was delivered 1 h after the injection of MnTnHex-2-PyP5+. Tumours were established from a 100 µL single cell suspension of 1 × 106 cells/mL injected subcutaneously into the right flank of mice. The doses of MnP and ascorbate chosen were based on in vivo experiments [37,38]. Mice were sacrificed 12 h after the last drug injection and tumours and the normal muscle from the non-tumour bearing leg were excised for MnP analysis. Given the data from pharmacokinetic studies [39], 12 h after the injection of the drug, no significant levels are supposed to be in the blood. Thus, blood content did not affect MnP tumour and muscle levels.
LC/ESI-MS/MS analysis
Tumours and muscles were homogenized (1/3 g tissue/mL water), proteins removed from tissue with 1% acetic acid in methanol (1/2 homogenate/methanol), solvent evaporated and residue reconstituted in mobile phase A (see below). Analyses were performed at Duke Comprehensive Cancer Center, Clinical Pharmacology Laboratory.
LC conditions
Shimadzu 20A series HPLC; column: Phenomenex 4 × 3 mm, C18 guard cartridge only; column temperature: 35 °C; mobile phase A: 95:5 H2O:acetonitrile (0.1% heptafluorobutyric acid, HFBA); mobile phase B: acetonitrile (0.1%, HFBA); elution gradient: 0–0.2 min, 0–90% B, 0.2–0.7 min, 90% B, 0.7–0.75 min, 90–0% B; run time: 4 min; injection volume: 10 µL.
MS/MS conditions
Applied Biosystems MDS Sciex 3200 Q Trap or 4000 Q Trap ESI-MS/MS; MRM transitions: MnTnHex-2-PyP5+ at m/z 825.5/611.5 (parent ion [MnP5+ + 3HFBA−]2+/2) and MnTnHep-2-PyP5+ (internal standard) at m/z 853.5/639.5. Calibration samples in 0.1–30 µM range were prepared by adding known amounts of MnTnHex-2-PyP5+ into homogenates of tumours and muscles of untreated mice and were run along with study samples. Linear response was observed in the concentration range measured.
Results and discussion
Ascorbic acid
Along with glutathione, ascorbate is our main endogenous low molecular-weight antioxidant. Its major role is to assure the action of α-tocopherol in preventing lipid peroxidation (via reduction of α-tocopherol radical back to α-tocopherol) and, thus, in turn, maintains the cell membrane integrity. After intestinal apsorption, ascorbate reaches plasma concentrations of 40–60 µM. The highest levels are found in the brain (2–4 mM) and adrenal gland (10 mM), where there is an abundance of lipids and α-tocopherol protection is critical. Concentration of ascorbate in the liver is 0.8–1 mM and in the muscle 0.4 mM [40–42]. Ascorbate cellular concentrations are up to two orders of magnitude higher than plasma levels.
Mn porphyrins
In vivo, due to easy reducibility of ortho isomeric Mn porphyrins (Table I), and significantly higher cellular levels of small molecular-weight endogenous antioxidants (mM levels [4]) than of the reactive species (nM to µM [4]), MnPs would readily couple with cellular reductants while removing O2˙− and peroxynitrite. Upon reduction from MnIIIP to MnIIP, they would reduce O2˙− or ONOO− in a subsequent step. Such removal of O2˙− is reminiscent of the action of superoxide reductase, such as rubredoxin oxidoreductase (desulphoferrodoxin) [43]. The reduction of ONOO− with MnIIP may happen one- or two-electronically, leading to the production of ˙NO2 radical or benign nitrite, NO2−, respectively [44].
Mn porphyrins + ascorbate
Reduction of ortho Mn porphyrins is involved not only in the elimination of reactive species, but may seemingly affect their cellular accumulation. Upon reduction of MnP with ascorbate, its charge drops for only one unit, from 5+ to 4+. The single charge gets lost from the Mn centre. Since peripheral charges remain unchanged, we expected no major impact of such charge reduction on lipophilicity. Surprisingly, the lipophilicity increased as much as 850-fold, depending upon the length of the alkyl chain and the type of the isomer (Table II).
The reduction of less reducible meta MnTM-3-PyP5+ (more negative E1/2, Table I) was performed with sodium dithionite.
The log POW vs Rf
We previously showed that log POW of the oxidized MnIIIP is linearly related to the chromatographic Rf value [26]. Log POW of the reduced MnIIP can not be experimentally assessed (see under Experimental). Thus, it was calculated from the Rf using reported equations and tabulated (Table II) [26]. Rf is much easier to determine and thus we use it to conveniently and routinely characterize the lipophilicity of Mn porphyrins [26].
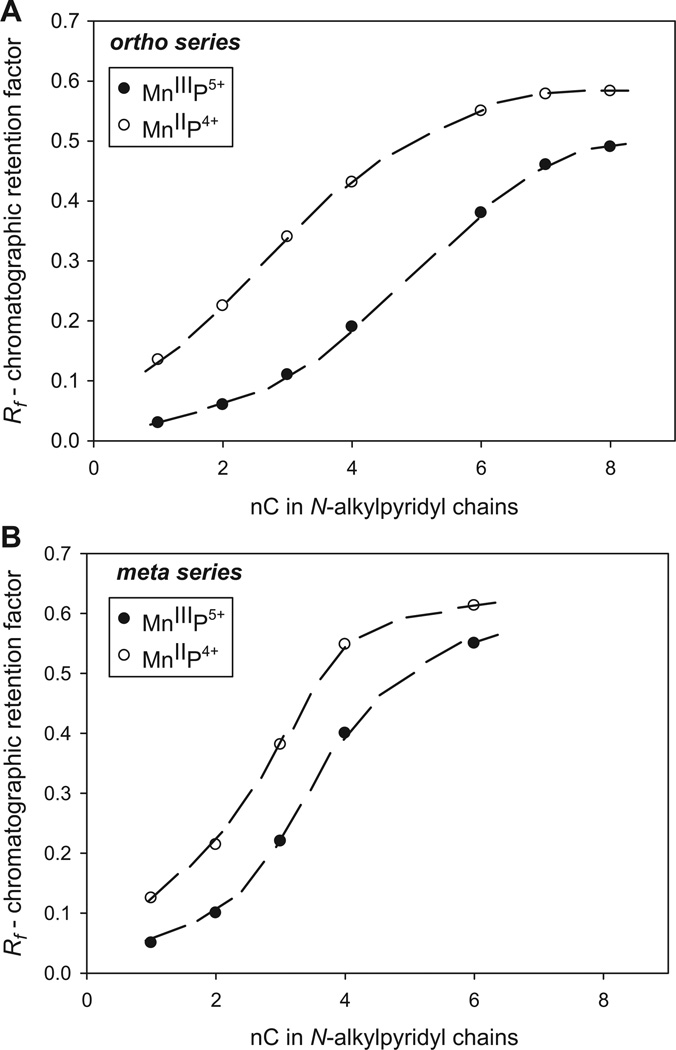
The Rf vs alkyl chain length
We previously showed that lipophilicity of ortho and meta MnIIIPs increases as the N-alkylpyridyl chains lengthen from methyl to octyl in a linear fashion [26]. Herein we showed that this relationship is valid for both oxidized MnIIIP and reduced MnIIP porphyrins and for both ortho and meta isomers (Figure 2). Figure 2, however, shows that as the alkyl chains lengthen beyond hexyl, the effect of the Mn site solvation (degree of which determines porphyrin hydrophilicity/lipophilicity) becomes increasingly suppressed and the porphyrin may eventually cease to sense whether the single charge resides on Mn or not. With short methyl chains the difference in lipophilicity between MnIIIP and MnIIP is also smaller. There is seemingly an optimal length of alkyl chains that is critical for the creation of cavity which can support substantial MnP solvation.
Figure 2.
Relationship between the chromatographic retention factor (Rf) and the number of carbon atoms (nC) in alkyl chains for the series of ortho (A) and meta (B) oxidized (MnIIIP5+) and reduced (MnIIP4+) Mn N-alkylpyridylporphyrins. Filled circles represent MnIIIPs, while empty circles represent MnIIPs, respectively.
Data in Figure 2 further show that the meta isomers are less sensitive to the loss of a single charge than ortho porphyrins (see discussion in next paragraph). To note, small differences in Rf values in Figure 2 correspond to large differences in log POW values (Table II) [26].
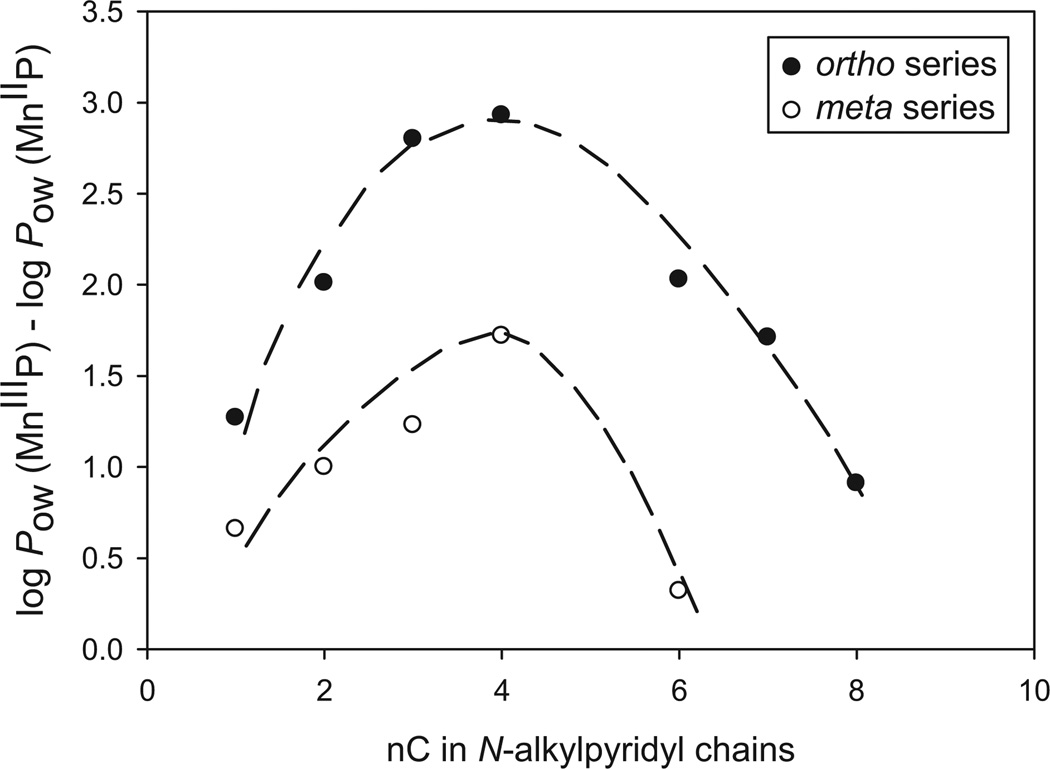
MnP reduction-based gain in lipophilicity as a function of alkyl chain length and type of isomer
We have further observed that the change in the lipophilicity upon MnP reduction is related to the length of alkyl chains and the type of the isomer. This relationship has a bell shape (Figure 3). Upon reduction, the change in lipophilicity of MnPs first increases as the alkyl chain lengthens up to butyl, but then decreases as the alkyl chain grows further (Figure 3). The effect is the consequence of the interplay of MnP lipophilicity, solvation and steric effects. As the chains lengthen from methyl to butyl, the cavity they form around the metal site gets larger and facilitates the Mn site solvation. When the chain lengthens beyond butyl, the impact of long lipophilic alkyl chains prevails, shielding the solvation effects arising from the single charge residing on a Mn site. A 17-fold stronger effect was observed with ortho than with meta isomers: with meta isomers the lipophilicity changes upon reduction by up to ~50-fold and with ortho by up to ~850-fold (Table II). The largest change in lipophilicity was observed with ortho and meta butyl compounds, MnTnBu-2(or 3)-PyP5+. The difference between the gain in lipophilicity of ortho and meta isomers fades away as the chains become shorter. Small substituents, such as methyl groups, hardly create any cavity that would have enhanced metal centre solvation differently with ortho than with meta isomers.
Figure 3.
The effect of MnIIIP5+ to MnIIP4+ reduction on lipophilicity (described as log POW) as influenced by the length of N-alkylpyridyl chains (described as the number of carbon atoms in the chains, nC).
Due to sterical crowding between alkyl chains and β-pyrrolic hydrogens of ortho porphyrin, the alkyl chains are fixed above and below the porphyrin plane and they define the cavity within which counterion and/or solvent molecules reside in a first and second Mn co-ordination sphere. With the meta isomer, there is no sterical hindrance; consequently, the alkyl chains freely rotate around pyridyl groups. Further, as the electron-withdrawing cationic pyridyl nitrogens are placed further away from the porphyrin core, the meta Mn(III) centre is more electron rich than the ortho Mn(III) site (see E1/2 in Table I) and thus does not favour axial ligation and solvation as much as does the ortho analogue. The differences in electron richness/deficiency of isomers, which defines the solvation of the Mn site, are clearly seen in their mass spectra (MS) [46]. The major ions in the MS of ortho MnTIIIM-2-PyP5+ have one or two chlorides axially bound to oxidized or reduced Mn site, while insignificant contribution of ions bearing two chlorides was found in the MS of meta, MnTIIIM-3-PyP5+. For such reasons: (1) the oxidized ortho Mn(III) N-alkylpyridylporphyrins are 10-fold more hydrophilic than are the meta species; (2) the ortho isomers on average sense 10-fold more the loss of single charge than do the meta analogues.
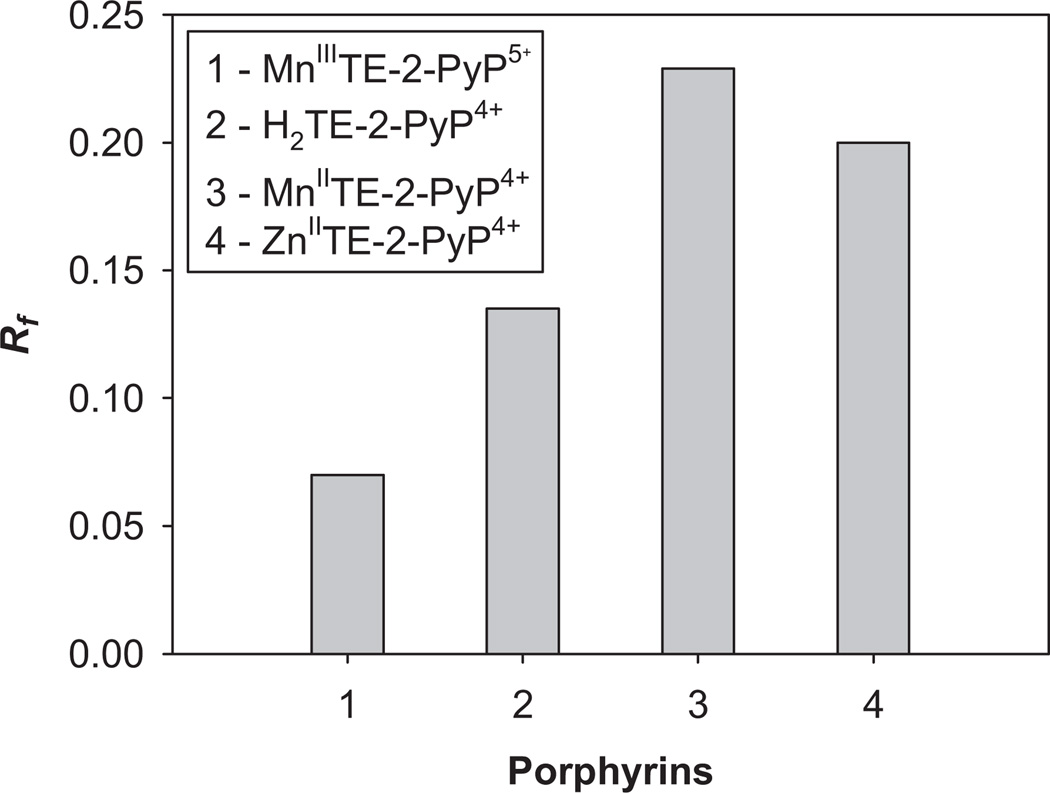
Lipophilicity of pentactionic MnIIITE-2-PyP5+ vs different tetracationic species: MnIITE-2-PyP4+, ZnTE-2-PyP4+, H2TE-2-PyP4+
The effect of the metal site or the lack of it on MnP lipophilicity was clearly seen when pentacationic MnIIITE-2-PyP5+ was compared to tetracationic MnIITE-2-PyP4+, H2TE-2-PyP4+ and ZnTE-2-PyP4+. During redox cycling MnIITE-2-PyP4+ is formed, which is much less stable than MnIIITE-2-PyP5+ and may lose metal to some extent whereby metal-free ligand would emerge. All tetracationic porphyrins, as expected, were more lipophilic than pentacationic, MnIIITE-2-PyP5+. The most lipophilic is the reduced MnIITE-2-PyP4+, followed by ZnIITE-2-PyP4+. Out of tetracationic species, the metal-free ligand, H2TE-2-PyP4+ appears the most solvated compound (Figure 4).
Figure 4.
The effect of the type of porphyrin bearing the same tetracationic charge on its lipophilicity. All tetracationic compounds are more lipophilic than pentacationic MnIIITE-2-PyP5+. The most lipophilic was reduced MnIITE-2-PyP4+.
To further the insight into the effect of lipophilicity upon cellular accumulation we studied two in vivo models: prokaryotic E. coli and eukaryotic mouse. In plasma and in E. coli growing medium, MnPs presumably exist predominantly in their oxidized Mn(III) form. The concentration of ascorbate in the plasma of mammals is lower than in the cell and there is none in the cellular medium where prokaryotic E. coli grows. We aimed here to see if co-administration of ascorbate with porphyrins would enhance their in vivo accumulation, due to the gain in lipophilicity upon MnIIIP to MnII P reduction.
E. coli study
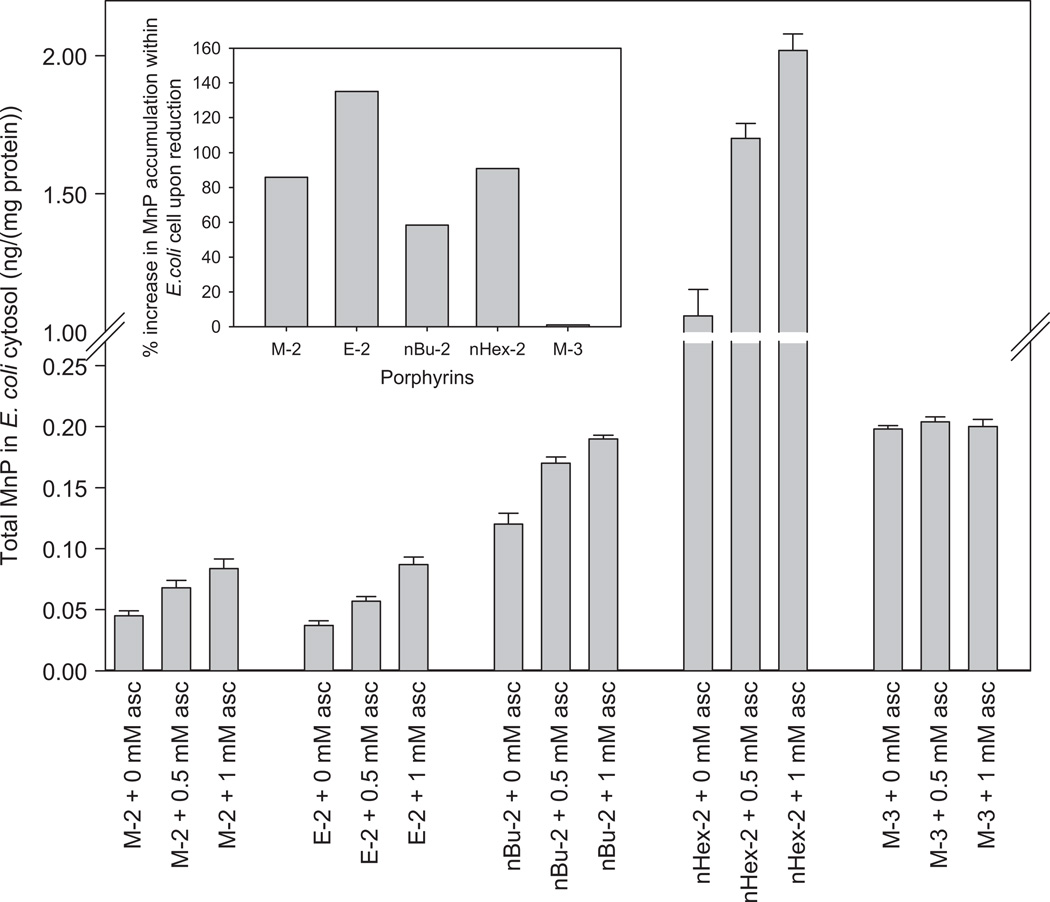
We were able to significantly enhance the accumulation of ortho MnPs within E. coli, when ascorbate was present in the growing medium. Yet, with the less reducible meta isomer MnTM-3-PyP5+, no effect was observed (Figure 5). This is consistent with the data on equally reducible para isomer, MnTM-4-PyP5+. The E1/2 of MnTM-4-PyP5+ is +60 mV vs NHE while of MnTM-3-PyP5+ is +52 mV vs NHE. In a study on submitochondrial particles, MnTM-4-PyP5+ was not readily reduced with components of mitochondrial respiration [27]. A 130% enhancement of MnP accumulation by ascorbate was observed with ortho ethyl porphyrin, MnTE-2-PyP5+ (Figure 5, Inset).
Figure 5.
Cytosolic accumulation of Mn N-alkylpyridylporphyrins. E. coli (AB1157) grew in casamino M9CA medium for 1 h in the presence of 5 µM MnPs and in the presence or absence of 0.5 and 1 mM sodium ascorbate. Inset: The effect of the reduction of MnP on E. coli cytosolic accumulation. The effect depends upon MnP/ascorbate ratio.
Mouse study
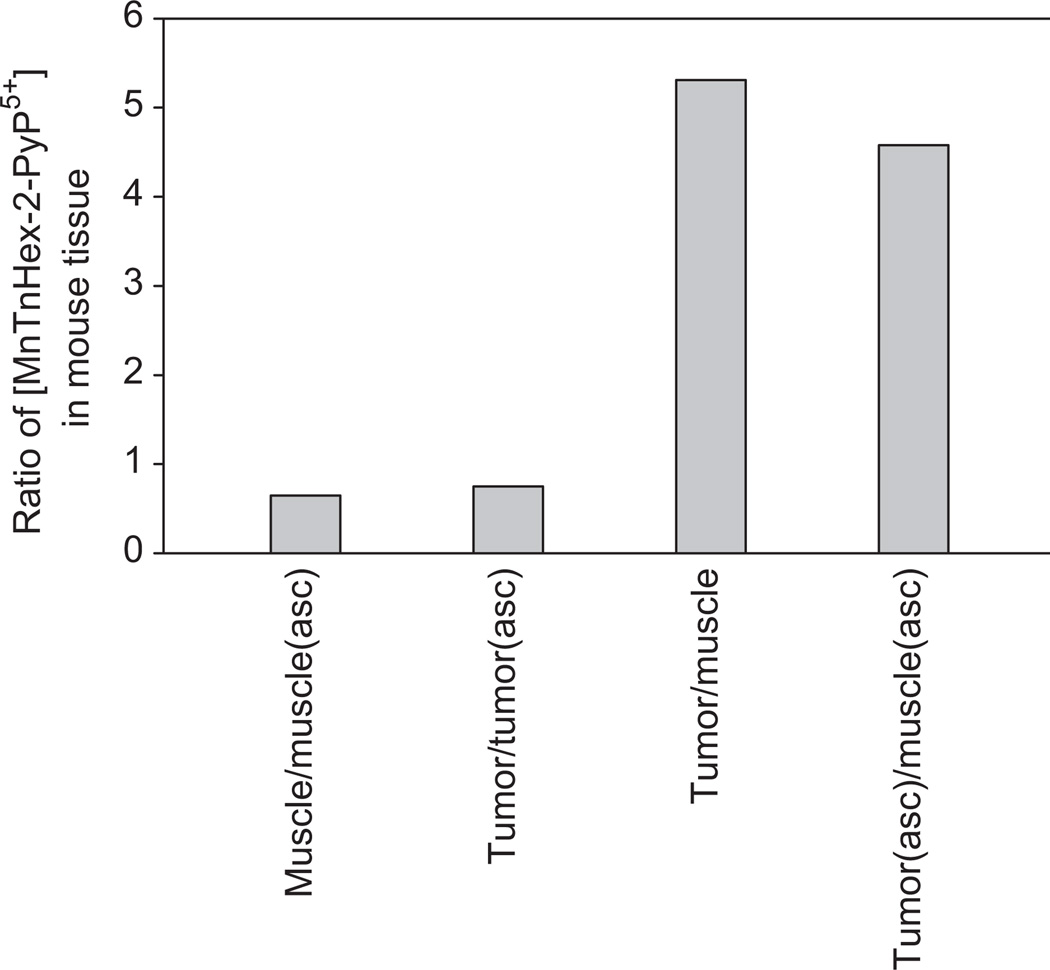
In addition to E. coli, we also analysed the distribution of MnTnHex-2-PyP5+ in a mouse tumour and a muscle after 2 weeks of daily administration of MnP alone and along with ascorbate (Figure 6 and Table III). Two sets of observations were made: (1) MnTnHex-2-PyP5+ accumulates ~5-fold more in tumour than in muscle when administered either alone or with ascorbate; (2) Regardless of the type of tissue, ascorbate enhances MnP accumulation and does so to a similar extent: ~33% [(2.20–1.65)/1.65] in tumour and 54% [(0.48–0.31)/0.31] in muscle (Table III and Figure 6). Higher tumour accumulation of MnP may be due to leaky tumour vasculature [47] and to other effects discussed under Accumulation of MnPs in tumour.
Figure 6.
The ratios of the levels of MnTnHex-2-PyP5+ in muscle and tumour when administered with and without ascorbate. The first two bars indicate the enhancement in MnP accumulation as influenced by ascorbate. The last two bars show the preferred accumulation of MnP in tumour compared to muscle regardless of the presence of ascorbate in plasma.
Table III.
Levels of MnTnHex-2-PyP5+ in mouse tumour and muscle expressed in µM in tissue determined by LC/ESI-MS/MS method. Tumours and muscle tissues were collected in a 4T1 mouse breast tumour study. Tumours were grown in Balb/c mice (n = 15). Mice were injected twice daily with MnP and ascorbate for 2 weeks from the moment the tumour size reached ~100–200 mm3, until its volume increased ~5-fold.
| PyP5+ | MnTnHex-2-PyP5+/ µM in tissue (mean) |
SD | Range |
|---|---|---|---|
| Tumor (MnP) | 1.65 | 1.29 | 0.735 – 5.89 |
| Muscle (MnP) | 0.31 | 0.07 | 0.166 – 0.417 |
| Tumour (MnP + asc) | 2.20 | 0.84 | 1.01 – 3.81 |
| Muscle (MnP + asc) | 0.48 | 0.16 | 0.231 – 0.849 |
A new LC/ESI-MS/MS method was developed for the determination of MnTnHex-2-PyP5+ in tumours and muscle tissue. It is less time- and labour-consuming than the HPLC/fluorescence method that we previously employed [13,38,39]. The HPLC/fluorescence method involves the MnIIIP reduction with ascorbate, followed by Mn to Zn exchange and ZnP fluorescence detection. With longer alkyl chain analogues, such as hexyl, it is nearly impossible to fully reduce MnP in order to exchange Mn for Zn. Thus, the LC/ESI-MS/MS method has wider applicability and, importantly, higher sensitivity where as low as 1 nM levels of MnPs in tissue homogenates may be measured.
The in vivo implications of MnP reduction-based enhancement in lipophilicity
It appears that the same factors that provide ortho isomers with high antioxidant potency and determine their mechanism/s of action—electron-deficiency, cationic charge next to the metal site—also provide them with high gain in lipophilicity upon their reduction (the step that is a part of their catalytic antioxidant-based action) and thus favourably affect their tissue and sub-cellular accumulation.
Transport across the plasma membrane
We are assuming that in plasma at physiological concentrations of endogenous ascorbate, the Mn porphyrins are in (oxidized) +3 Mn oxidation state. The following calculation supports such speculation. If the blood volume of a 20 g mouse is 1.6 mL [48], then plasma concentration of MnTnHex-2-PyP5+ (given in a dose of 2 mg/kg daily) would be in the range of 10–62.5 µM. The first number is based on the mouse pharmacokinetic study of MnTE-2-PyP5+ [39] where 16% of the injected amount of 1 mg/kg reaches plasma. The second number (of 100% resorption) was based on 13500-fold higher lipophilicity of MnTnHex-2-PyP5+ which in turn suggests much higher blood levels. Ascorbate mouse plasma concentration of 42 µM (0.83 mg/100 mL) [49] is similar to a human plasma level of 40–60 µM [40,41]) and similar to MnP plasma concentration. Our previous data show that 1:1 ratio of ascorbate:MnP is insufficient to reduce MnP aerobically [22]; routinely we need 20-fold excess of ascorbate over MnP [22]. With a dose of 2 g/kg, ascorbate mouse plasma concentration reaches 126 mM; > 2000-fold excess of ascorbate, which ensures the reduction of Mn3+ to Mn2+ and, in turn, increases the MnP lipophilicity and facilitates the transport across the plasma membrane.
Accumulation of MnPs within mitochondria
Within the cell, regardless of the exogenous ascorbate, the abundance of endogenous ascorbate and other cellular reductants stabilize MnP in its reduced state. It is well accepted now that positive charge and lipophilicity drive compounds to the mitochondria [13,50]. A skin carcinogenesis study [51] indicated that MnTE-2-PyP5+ mimics MnSOD and suggests its likely accumulation in the mitochondrial matrix. The following study on submitochondrial particles indicated that MnTE-2-PyP5+ gets reduced by components of the mitochondrial respiratory chain to MnTE-2-PyP4+ which in turn scavenges ONOO− [27]. Thus, MnTE-2-PyP4+ binds ONOO− in a first step and reduces it to innocent NO2− in a subsequent step [18]. The mouse study, which followed, showed that indeed MnTE-2-PyP5+ accumulates in mouse heart mitochondria and at levels enough to protect it against ONOO−-mediated damage [1,2,13,27]. The accumulation is greatly affected by MnP lipophilicity and is correlated with the alkyl-chain length; MnTnHex-2-PyP5+ accumulates significantly more in mitochondria relative to cytosol than MnTE-2-PyP5+ (Spasojevic, St Clair et al. unpublished). Such data correlate well with yeast mitochondria study on Mn(III) N-alkylpyridylporphyrins of different alkyl chain length [52]. The yeast study offers additional insight into why otherwise excessively hydrophilic MnTE-2-PyP5+ and to a much higher extent lipophilic MnTnHex-2-PyP5+ accumulate in the mitochondria: the cytosolic-based reduction, accompanied by a remarkable gain in lipophilicity, may be at least in part a driving force for their mitochondrial accumulation.
Accumulation of MnPs in tumours
Along with MRI imaging data [53], this set of data clearly show preferred accumulation of MnPs in tumour than in a normal tissue.
Higher tumour accumulation of cationic MnPs may be due to several reasons. In general, and as already stated, the tumour leaky vasculature facilitates higher accumulation of any administered drug [47]. Further, cancer cells have a higher surface area than the normal cell, because of the higher number of microvilli and more chances to interact with a drug [54,55]. Finally, in contrast to the normal cell, which expresses mostly neutral zwitterionic phospholipids and sterols on its surface [56], cancer cells have a net negative surface charge, due to the higher expression of anionic molecules, such as phosphatidylserine, O-glycosylated mucins, sialilated gangliosides and heparin sulphates [57]. These negatively charged molecules facilitate interaction and penetration of the cationic species through the membrane [55–59]. It has been shown that some cationic porphyrins bind to anionic regions of proteins, such as human serum albumin, which transports them into the tumour [60–62]. Porphyrins studied were para isomers, MnTM-4-PyP5+ and Mn(III) meso-tetrakis (4-N, N, N-trimethylanilinium) porphyrin. Datta-Gupta et al. [61] showed that metal-free analogues of N-alkylpyridylporphyrins we studied herein (the isomeric metal-free N-methylpyridylporphyrins ortho, meta and para H2TM-(2 or 3 or 4)-PyP4+) bind on the surface of albumin, at a site near to tryptophan residue, rather than sliding in a crevise of human serum albumin.
Mechanism of action
Most cells have abundant levels of ascorbate. In the presence of ascorbate, redox-able MnPs may act as pro-oxidants and catalyse oxygen consumption of ascorbate resulting in the production of O2˙−/H2O2 [22], which in turn may cause cancer cell death [16,19,21]. Under physiological conditions with an abundance of peroxide-removal systems, such redox cycling of ascorbate (which should have otherwise been catalysed by endogenous Fe porphyrins [19]) may not be damaging. In general, integrity of tumour is compromised; it is under constant oxidative stress and deficient in endogenous antioxidant defenses relative to normal cells [4]. Tumour employs such deficiency to its advantage to support its aggressive proliferation [4]. We have already shown that MnTE-2-PyP5+ closely mimics MnSOD in skin carcinogenesis; the anti-oxidative action was suggested [51]. The same action was proposed for the role of MnTE-2-PyP5+ in a mouse 4T1 breast cancer study [38]. While MnP itself is not toxic to tumour cells [16], the data suggest that it suppresses tumour growth via anti-angiogenic mode of action [38]. Such anti-oxidative action was explained to result fom the removal of signaling reactive species and suppression of HIF-1α activation and VEGF expression, which in turn results in suppression of tumour vasculature growth [38]. When coupling with endogenous or exogenous ascorbate, MnP could increase cellular oxidative burden and eventually suppress tumour growth via pro-oxidative action in a way already suggested and fairly justified for the enzyme MnSOD itself [11,63–68]. Kim et al. [17] provided convincing evidence that overexpression of MnSOD in liver cells (or by the analogy the administration of MnTE-2-PyP5+) increases oxidative burden. Yet, the cells seemingly counterfight with adaptive response, i.e. subsequent upregulation of endogenous antioxidant defenses. In turn, the signs of suppressed oxidative stress may be observed and ascribed to the anti-oxidative action of MnSOD. By analogy, the mimic of MnSOD may be operating in similar ways. Future work on mechanistic aspects of SOD mimics is in progress.
The most obvious case of the pro-oxidative action of MnP, under biologically relevant reducing conditions, was observed in a very simple and straightforward system, the aerobic growth of SOD-deficient E. coli. The E. coli growth assay is superoxide-specific [1]. It has been shown in numerous studies that the SOD-deficient E. coli, which lacks cytosolic SODs, grows very poorly aerobically. However, it grows as well as wild type when the medium is supplied with efficacious SOD mimic. Yet, if the medium contains both MnTE-2-PyP5+ and ascorbate, the growth of SOD-deficient E. coli was fully suppressed; the same has been observed with wild type. Another obvious case is the growth of different cancer cells in the presence of MnP/ascorbate [16]. Neither MnP nor ascorbate were toxic under concentrations where the combined treatment killed four different cancer cells ([16], Fels et al. unpublished). The pro-oxidative mode of action was also seen in our in vivo 4T1 breast cancer mouse study when MnTnHex-2-PyP5+ was co-administerd with ascorbate ([16], Fels et al. unpublished); our data are in agreement with the Levine et al. [19] study where ascorbate administration killed different tumours via peroxide formation catalyzed by endogenous antioxidants. The combined use of MnP/ascorbate may have dual benefit in cancer therapy: (1) the gain in lipophilicity upon reduction would facilitate MnP transport into the cell and critical sub-cellular compartments (mitochondria, nucleus etc), whereas (2) MnP-driven catalysis of oxygen reduction by ascorbate would enhance tumour growth suppression via excessive pro-oxidative damage.
The mere fact that MnP can easily attain four oxidation states in vivo (+2, +3, +4 and +5), each of them with its own protonation/deprotonation equilibria (detailed in [69] and [70]), suggests a complex biology of Mn porphyrins. Further studies are needed to comprehend the role of endogenous and exogenous antioxidants in cancer therapy.
Ortho vs meta MnTE-2-PyP5+
When given ip at 10 mg/kg to mouse, within 30 min MnTE-2-PyP5+ reaches ~20 µM plasma levels (6-fold less than injected) [39]; i.e. similar to the levels of ascorbate. Under the same conditions, meta MnIIITE-3-PyP5+ would cross the plasma membrane more readily than ortho, as it is more lipophilic (Table II) [25]. In the cell, where ortho and meta are equally reducible, their lipophilicity would have increased 100- and 10-fold, respectively, and they would have had the same probability to reach mitochondria. However, Ferrer-Sueta et al. [27] showed that MnTM-4-PyP5+ analogue of similar reducibility as MnTE-3-PyP5+ (Table I) [1,2,12,27] can not be as readily reduced in vivo as ortho MnTE-2-PyP5+. Further, antioxidant capacity, conveniently expressed as kcat (O2˙−), is 10-fold lower for meta than for ortho. Based on this evidence, once both isomers cross the plasma membrane, ortho analogue may have a higher chance to reach mitochondria and scavenge ROS/RNS. Unless lipophilicity of meta MnIIITE-3-PyP5+ is a critical factor that controls the extent to which the MnP crosses plasma membrane and reaches cell interior and therefore presides over its destiny within the cell.
Conclusions
Endogenous reductants + MnP
Within the cell, the ortho Mn porphyrins get easily reduced with cellular reductants. Such reduction is likely coupled to a catalytic scavenging of reactive oxygen and nitrogen species. Upon reduction, they lose a single charge from Mn site, whereby they become up to three orders more lipophilic. Such gain in lipophilicity facilitates their cellular and sub-cellular accumulation. The increase in lipophilicity upon reduction was observed with both ortho and meta isomers. Its magnitude is related to the length of alkyl chain and the type of isomer in a bell shape fashion as a consequence of the interplay between the solvation, steric and lipophilicity effects. All effects are more pronounced with ortho than with meta isomers.
Exogenous reductants + MnP
The ascorbate levels in plasma and extracellular fluids are significantly lower than within the cell and may not be enough to stabilize MnPs in the reduced state. Thus, their administration with ascorbate would facilitate their reduction in plasma, whereby lipophlicity and, in turn, transport across the plasma membrane gets enhanced.
Relevance to anti-cancer therapy
Our data further show that MnP accumulates 5-fold more in tumour than in surrounding muscle, which was enhanced with exogenously added ascorbate and strengthens their development as anti-cancer drugs and tumour imaging agents. Future studies will address (1) if MnPs anti-cancer efficacy could be enhanced when they are co-administered with reductants; (2) whether co-administration with ascorbate affects the MnP mechanism of action and if this is dependent upon the cancer cell oxidative burden (relative to normal cell) and (3) which are better drug candidates: ortho or meta isomers.
Acknowledgments
IBH, ZR, IK, KL, XY and MWD acknowledge the financial help from Wallace H. Coulter Translational Partners Grant Program and from the National Institutes for Allergy and Infectious Diseases (U19AI0 677989). IBH is also grateful to R01 DA 024074. IS thanks NIH/NCI Duke Comprehensive Cancer Center Core Grant (5-P30-CA14236-29). DF, CD and MWD are thankful to CA40355-25, LB to Kuwait University, grant MB03/07, ZV to R01 CA098452 and U19AI0677989, and JSR to Universidade Federal da Paraíba. XDY is very grateful to the financial support from Hefei National Laboratory for Physical Sciences at the Microscale at University of Science and Technology of China during his stay in Professor Kam W. Leong group at Duke University.
Abbreviations
- Asc
sodium ascorbate
- CO3•−
carbonate radical
- E1/2
half-wave reduction potential
- HFBA
heptafluorobutyric acid
- H2O2
hydrogen peroxide
- HClO
hypochlorous acid
- ip
intraperitoneal
- kcat
rate constant for O2•− dismutation
- meso
5,10,15,20 positions on porphyrin ring
- MnP
Mn porphyrin
- Herein
oxidized MnP relates to complexes (MnIIIP) where Mn is in + 3 oxidation state, relative to complexes (MnIIP) where Mn is in +2 oxidation state
- MnTalkyl-2(or 3 or 4)-PyP5+
Mn(III) meso-tetrakis(N-alkylyridinium-2(or 3 or 4)-yl)porphyrin, alkyl being methyl (M), ethyl (E), n-propyl (nPr, also Pr), n-butyl (nBu, also Bu), n-hexyl (nHex, also Hex), n-heptyl (nHep, also Hep), n-octyl (nOct, also Oct)
- 2, 3 and 4 relate to ortho
meta and para isomers, respectively
- MnTM-2-PyP+ (AEOL10112)
MnTE-2-PyP5+ (AEOL10113, FBC-007), MnTDE-2-ImP5+, Mn(III) meso-tetrakis(N,N’-diethylimidazolium-2-yl)porphyrin, AEOL10150
- H2TM-2(or 3 or 4)-PyP5+
meso-tetrakis(N-methylpyridinium-2(or 3 or 4)-yl)porphyrin
- MS
mass spectroscopy
- LC/ESI-MS/MS
liquid chromatography/electrospray ionization-tandem mass spectrometry
- MRI
magnetic resonance imaging
- NHE
normal hydrogen electrode
- •NO
nitric oxide
- O2•−
superoxide
- ONOO−
peroxynitrite, ONOO− + ONOOH, given its pKa = 6.6 at pH 7.8 peroxynitrite exists predominantly as ONOO−
- POW
partition coefficient between n-octanol and water
- Rf
thin-layer chromatographic retention factor
- sc
subcutaneous
- SOD
superoxide dismutase
- TLC
thin-layer chromatography
- ZnP
Zn porphyrin
- ZnTE-2-PyP4+
Zn(II) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin
- bFGF
basic fibroblast growth factor
- DUOX
dual oxidase
- NOX
NADPH oxidase
- PDGF
platelet-derived growth factor
- VEGF
vascular endothelial growth factor
Footnotes
The best approach would be to run in parallel TLC of all compounds of interest.
Declaration of interest
References
- 1.Batinić-Haberle I, Rebouças JS, Spasojević I. Superoxide dismitase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batinić-Haberle I, Reboucas JS, Benov L, Spasojevic I. Chemistry, biology and medical effects of water soluble metalloporphyrins. In: Kadish KM, Smith KM, Guillard R, editors. Handbook of porphyrin science. Vol11. World Scientific; 2010. [Google Scholar]
- 3.Kietzmann T. Intracellular redox compartments: mechanisms and significances. Antioxid Redox Signal. 2010;13:395–398. doi: 10.1089/ars.2009.3001. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. New York: Oxford University Press; 2007. pp. 79–186. [Google Scholar]
- 5.Takeya R, Sumimoto H. Regulation of novel superoxide-producing NADP(H) oxidases. Antioxid Redox Signal. 2006;8:1523–1532. doi: 10.1089/ars.2006.8.1523. [DOI] [PubMed] [Google Scholar]
- 6.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petry A, Weitnauer M, Görlach A. Receptor activation of NADPH oxidases. Antioxid Redox Signal. 2010;13:467–487. doi: 10.1089/ars.2009.3026. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerutti PA. Pro-oxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 10.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 11.Holley AK, Dhar SK, Xu Y, St. Clair DK. Manganese superoxide dismutase: beyond life and death. Amino Acids. 2010 doi: 10.1007/s00726-010-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batinić-Haberle I, Spasojević I, Tse HM, Tovmasyan A, Rajic Z, St. Clair DK, Vujasković Z, Dewhirst MW, Piganelli JD. Design of Mn porhyrins for treating oxidative stress injuries and their redox-based regulation of celluler transcriptional activities. Amino Acids. 2010 doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spasojević I, Yumin C, Noel T, Yu I, Pole MP, Zhang L, Zhao Y, St Clair DK, Batinić-Haberle I. Mn porphyrin-based SOD mimic, MnTE-2-PyP5+ targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrer-Sueta G, Vitturi D, Batinić-Haberle I, Fridovich I, Goldstein S, Czapski G, Radi R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J Biol Chem. 2003;278:27432–27438. doi: 10.1074/jbc.M213302200. [DOI] [PubMed] [Google Scholar]
- 15.Jaramillo MC, Frye JB, Crapo JD, Briehl MM, Tome ME. Increased manganese superoxide dismutase expression or treatment with manganese porphyrin potentiates dexamethasone-induced apoptosis in lymphoma cells. Cancer Res. 2009;69:5450–5457. doi: 10.1158/0008-5472.CAN-08-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Fels D, Dedeugd C, Dewhirst MW, Leong K, Batinić-Haberle I. The in vitro cytotoxic effects of Mn(III) alkylpyridylporphyrin/ascorbate system on four tumor cell lines. Free Radic Biol Med. 2009;47:136. [Google Scholar]
- 17.Kim A, Joseph S, Khan A, Epstein CJ, Sobel R, Huang T-T. Enhanced expression of mitochondrial superoxide dismutase leads to prolonged in vivo cell cycle progression and up-regulation of mitochondrial thioredoxin. Free Radic Biol Med. 2010;48:1501–1512. doi: 10.1016/j.freeradbiomed.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spasojević I, Batinić-Haberle I, Fridovich I. Nitrosylation of manganese(II) tetrakis(N-ethylpyridinium-2-yl)porphyrin. Nitric Oxide. 2000;4:526–533. doi: 10.1006/niox.2000.0303. [DOI] [PubMed] [Google Scholar]
- 19.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3703–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spasojevic I, Colvin OM, Warshany KR, Batinic-Haberle I. New approach to the activation of anti-cancer pro-drugs by metalloporphyrin-based cytochrome P450 mimics in all-aqueous biologically relevant system. J Inorg Biochem. 2006;100:1897–1902. doi: 10.1016/j.jinorgbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Rajic Z, Benov L, Kos I, Tovmasyan A, Batinic-Haberle I. Cationic Mn porphyrins change their action from anti- to pro-oxidative in the presence of cellular reductants. Relevance to understanding beneficial therapeutic effects of SOD mimics in vivo. Free Radic Biol Med. 2010;49S Book of Abstracts. [Google Scholar]
- 22.Batinić-Haberle I, Spasojević I, Stevens RD, Ham-bright P, Fridovich I. Manganese(III) meso tetrakis ortho N-alkylpyridylporphyrins: synthesis, characterization and catalysis of O2·−dismutation. J Chem Soc Dalton Trans. 2002:2689–2696. [Google Scholar]
- 23.Okado-Matsumoto A, Batinić-Haberle I, Fridovich I. Complementation of SOD deficient Escherichia coli by manganese porphyrin mimics of superoxide dismutase. Free Radic Biol Med. 2004;37:401–410. doi: 10.1016/j.freeradbiomed.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Pollard JM, Rebouças JS, Durazo A, Kos I, Fike F, Panni M, Gralla EB, Valentine JS, Batinić-Haberle I, Gatti RA. Radio-protective effects of manganese-containing superoxide dismutase mimics on ataxia telangiectasia cells. Free Radic Biol Med. 2009;47:250–260. doi: 10.1016/j.freeradbiomed.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kos I, Benov L, Spasojević I, Rebouças JS, Batinić-Haberle I. High lipophilicity of meta Mn(III) N-alkylpyridylporphyrin-based SOD mimics compensates for their lower antioxidant potency and makes them equally effective as ortho analogues in protecting SOD-deficient E. coli. J. Med Chem. 2009;52:7868–7872. doi: 10.1021/jm900576g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kos I, Rebouças JS, DeFreitas-Silva G, Salvemini D, Vujasković Z, Dewhirst MW, Spasojević I, Batinić-Haberle I. Lipophilicity of potent porphyrin-based antioxidants: comparison of ortho and meta isomers of Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2009;47:72–78. doi: 10.1016/j.freeradbiomed.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrer-Sueta G, Hannibal L, Batinić-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles and the catalytic redox cycle of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N’-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Batinić-Haberle I, Spasojević I, Fridovich I. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn(III) ortho-tetrakis (N-ethylpyridinium-2-yl)porphyrin. Free Radic Biol Med. 2004;37:367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 30.Ferrer-Sueta G, Batinić-Haberle I, Spasojević I, Fridovich I, Radi R. Catalytic scavenging of peroxynitrite by isomeric Mn(III) N-methylpyridylporphyrins in the presence of reductants. Chem Res Toxicol. 1999;12:442–449. doi: 10.1021/tx980245d. [DOI] [PubMed] [Google Scholar]
- 31.Batinić-Haberle I, Benov L, Spasojević I, Fridovich I. The ortho effect makes manganese (III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin (MnTM-2-PyP) a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 32.Batinić-Haberle I, Benov L, Spasojević I, Hambright P, Crumbliss AL, Fridovich I. The relationship between redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vitro and in vivo superoxide dismutase activities of manganese(III) and iron(III) cationic and anionic porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 33.Rebouças JS, Kos I, Vujasković Z, Batinić-Haberle I. Determination of residual manganese in Mn porphyrin-based superoxide dismutase (SOD) and peroxynitrite reductase mimics. J Pharm Biomed Anal. 2009;50:1088–1091. doi: 10.1016/j.jpba.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imlay JA, Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987;169:2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.B atinić-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojević I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two different models of oxidative stress injuries, SOD-specific E. coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 37.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as pro-oxidant and desrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabbani ZN, Spasojević I, Zhang X, Moeller BJ, Haberle S, Vasquez-Vivar J, Dewhirst MW, Vujasković Z, Batinić-Haberle I. Antiangiogenic action of redox-modulating Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin, MnTE-2-PyP5+, via suppression of oxidative stress in a mouse model of breast tumor. Free Radic Biol Med. 2009;47:992–1004. doi: 10.1016/j.freeradbiomed.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spasojević I, Chen Y, Noel TJ, Fan P, Zhang L, Rebouças JS, St Clair DK, Batinić-Haberle I. Pharmacokinetics of the potent redox modulating manganese porphyrin, MnTE-2-PyP5+ in plasma and major organs of B6C3F1 mice. Free Radic Biol Med. 2008;45:943–949. doi: 10.1016/j.freeradbiomed.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miele M, Fillenz M. In vivo determination of extracellular brain ascorbate. J Neurosci Methods. 1996;70:15–19. doi: 10.1016/S0165-0270(96)00094-5. [DOI] [PubMed] [Google Scholar]
- 41.Schenk JO, Miller E, Gaddis R, Adams RN. Homeostatic control of ascorbate concentration in CNS extracellular fluid. Brain Res. 1982;253:353–356. doi: 10.1016/0006-8993(82)90709-0. [DOI] [PubMed] [Google Scholar]
- 42.Mandl J, Szarka A, Banhegyi G. Vitamin C: update on physiology and pharmacology. Brit J Pharmacol. 2009;157:1097–1110. doi: 10.1111/j.1476-5381.2009.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eric D, Culter ED, Emerson JP, Kurtz DM, Jr, Cabelli DE. Superoxide reactivity of rubredoxin oxidoreductase (desulfoferrodoxin) from Desulfovibrio vulgaris: a pulse radiolysis study. J Am Chem Soc. 2000;122:11555–11556. [Google Scholar]
- 44.Ferrer-Sueta G, Quijano C, Alvarez B, Radi R. Reactions of manganese porphyrins and manganese-superoxide dismutase with peroxynitrite. Methods Enzymol. 2002;349:23–37. doi: 10.1016/s0076-6879(02)49318-4. [DOI] [PubMed] [Google Scholar]
- 45.Engelmann FM, Rocha SVO, Toma HE, Araki K, Baptista MS. Datermination od n-octanol/water partition and membrane binding of cationic porphyrins. Int J Pharm. 2007;329:12–18. doi: 10.1016/j.ijpharm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Batinic-Haberle I, Stevens R, Fridovich I. Electrospray mass spectrometry of isomeric tetrakis-(N-alkylpyridyl)porphyrins and their manganese(III) and iron(III) complexes. J Porphyrins Phthalocyanines. 2000;4:217–227. [Google Scholar]
- 47.Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;197:3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 48.Riches AC, Sharp JG, Brynmor Thomas D, Vaughan Smith S. Blood volume determination in the mouse. J Physiol. 1973;228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsao CS, Leung PY, Young M. Effect of dietary ascorbic acid intake on tissue vitamin C in mice. J Nutrition. 1987;117 doi: 10.1093/jn/117.2.291. 291-197. [DOI] [PubMed] [Google Scholar]
- 50.Murphy MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta. 2008;177:1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Chaiswing L, Oberley TD, Batinić-Haberle I, St Clair W, Epstein CJ, St Clair DK. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 52.Spasojevic I, Li AM, Tovmasyan A, Rajic Z, Salvemini D, St. Clair D, Valentine JS, Vujaskovic Z, Gralla EB, Batinic-Haberle I. Accumulation of porphyrin-based SOD mimics in mitochondria is proportional to their lipophilicity. S. cerevisiae study of ortho Mn(III) N-alkylpyridylporphyrins. Free Radic Biol Med. 2010;49S Book of abstracts. [Google Scholar]
- 53.Lascola CD, Batinić-Haberle I, Venkatraman T, Amrheim T, Mouraviev V, Wang H. Mn-porphyrins SOD mimetics as novel NMR imaging probes. 6th International Conference on Porphyrins and Phthalocyanines, ICPP-6; New Mexico. 2010. [Google Scholar]
- 54.Chan SC, Hui L, Chen HM. Enhancement of the cytolytic effect of anti-bacterial peptide cecropin by the microvilli of cancer cells. Anticancer Res. 1998;18:4467–4474. [PubMed] [Google Scholar]
- 55.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 56.Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778:357–375. doi: 10.1016/j.bbamem.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 58.Kornguth SE, Kalianke T, Robins HI, Cohen JD, Turski P. Preferential binding of radiolabeled poly-L-lysine to C6 and U87 MG glioblastomas compared to endothelial cells in vitro. Cancer Res. 1989;49:6390–6395. [PubMed] [Google Scholar]
- 59.Jensen TJ, Vicente MGH, Luguya R, Norton J, Fronczek FR, Smith KM. Effect of overall charge and charge distribution on cellular uptake, distribution and phototoxicity of cationic porphyrins in Hep2 cells. J Photochem Photobiol B: Biol. 2010;100:100–111. doi: 10.1016/j.jphotobiol.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou B, Zhang Z, Zhang Y, Li R, Xiao Q, Liu Y, Li Z. Binding of cationic porphyrin to human serum albumin studied using comprehensive spectroscopic methods. J Pharm Sci. 2009;98:105–113. doi: 10.1002/jps.21413. [DOI] [PubMed] [Google Scholar]
- 61.Datta-Gupta N, Malakar D, Walters E, Thompson B. Binding studies of three water-soluble polycationic porphyrins with human serum albumin. Res Commun Chem Pathol Pharmacol. 1988;60:347–360. [PubMed] [Google Scholar]
- 62.Ma H-M, Chen X, Zhang N, Han Y-Y, Wu D, Du B, Wei Q. Spectroscopic studies on the interaction of a water-soluble cationic porphyrin with proteins. Spectrochim Acta, Part A: Mol Biomol Spectroscopy. 2009;72:465–469. doi: 10.1016/j.saa.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Li S, Yan T, Yang J-Q, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 64.Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2 O2 in cells and therby their biological state. Free Radic Biol Med. 2006;41:1338–1350. doi: 10.1016/j.freeradbiomed.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitohondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H2 O2. Free Radic Biol Med. 2000;29:801–813. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 66.Darby Weydert CJ, Smith BB, Xu L, Kregel KC, Ritchie JM, Davis CS, Oberley LW. Inhibition of oral cancer cell growth by adenovirus MnSOD plus BCNU treatment. Free Radic Biol Med. 2003;34:316–329. doi: 10.1016/s0891-5849(02)01245-5. [DOI] [PubMed] [Google Scholar]
- 67.Ridnour LA, Oberley TD, Oberley LW. Tumor supressive effects of MnSOD overexpression may involve imbalance in peroxide generation versus peroxide removal. Antiox Redox Signal. 2004;6:501–512. doi: 10.1089/152308604773934260. [DOI] [PubMed] [Google Scholar]
- 68.Amstad P, Moret R, Cerutti P. Glutathione peroidase compensates for the hypersensitivity of Cu,Zn-superoxide overproducers to oxidant stress. J Biol Chem. 1994;269:1606–1609. [PubMed] [Google Scholar]
- 69.Budimir A, Smuc T, Weitner T, Batinic-Haberle I, Birus M. Thermodynamics and electrochemistry of manganese tetra (ortho-n-butylpyridyl)porphyrin in aqueous solutions. J Coord Chem. 2010;63:2750–2765. [Google Scholar]
- 70.Weitner T, Budimir A, Kos I, Batinić-Haberle I, Biruš M. Acid-base and electrochemical properties of manganese meso(ortho- and meta-ethylpyridyl)porphyrins: potentiometric, spectrophotometric and spectroelectrochemical study of protolytic and redox equilibria. Dalton Trans. 2010 doi: 10.1039/c0dt00585a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]