Abstract
Background
Making a diagnosis of HIV infection in children aged less than 18 months remains a challenge in low resource settings like Zambia due to the limited availability of gold standard testing with HIV DNA PCR. Clinicians in rural areas have to depend on clinical diagnosis to start HAART as they wait for the dry blood spot (DBS) for DNA PCR results sent from the urban centers.
Methods
This descriptive cross-sectional study was performed at the University Teaching Hospital, Lusaka, Zambia. 299 HIV-exposed children aged less than 18 months were enrolled following a consent procedure. Patients were evaluated for HIV infection based on the World Health Organization’s presumptive diagnostic criteria (WHO-PDC), integrated management of childhood illnesses (IMCI) criteria, select physical exam abnormalities, and CD4% and findings were compared with HIV-DNA PCR results.
Results
Of the 299 exposed patients analyzed, 111(37%) were found to be HIV-positive by DNA PCR. The median CD4% in the infected children was 18%. WHO-PDC used on its own had 23% sensitivity (95% CI 17–32%) and 93% specificity (88–96%), respectively, whereas IMCI criterion had 10% sensitivity (6–17%) and 97% specificity (94–99%), respectively. Multivariate analysis was used to identify the most sensitive predictors when combined with the WHO-PDC and IMCI criterion. WHO-PDC with CD4% improved the sensitivity to 77% (68–83%) with a specificity of 83% (77–88%), positive predictive value (PPV) of 73% (64–80%) and negative predictive value (NPV) of 86% (80–90%). IMCI with CD4% improved sensitivity to 80% (71–87%) with a specificity of 88% (82–92%), PPV 78% (69–85%) and NPV 89% (84–93%). The addition of individual physical exam findings without CD4% improved the sensitivity of WHO-PDC only modestly. When the WHO-PDC, weight<3rd percentile, hepatomegaly, splenomegaly, lymphadenopathy and CD4% were combined, the sensitivity improved to 85% (77–90%), specificity 63% (56–70%), PPV 58% (50–65%) and NPV of 88% (81–92%).
Conclusion
The WHO-PDC clinical algorithm can be improved when combined with a CD4% <25% in children less than 12 months of age and CD4% <20% in those between 12 and 18 months.
Keywords: HIV, early infant diagnosis, diagnosis, Zambia
Introduction
The introduction of combination highly active antiretroviral therapy (HAART) has led to improved outcomes in HIV-infected infants and young children worldwide. In sub-Sahara Africa, countries including Zambia have adopted the WHO Antiretroviral Therapy guidelines when initiating HAART. The 2008 WHO guidelines recommend that HAART should be started in all infants with a positive HIV DNA PCR result.1, 2, 3 This recommendation came from studies showing that in the absence of HAART, there is 30% one-year mortality and 50% two-year mortality.4,5 However, in resource-poor areas where DNA PCR is not readily available, infant HIV diagnosis and treatment are frequently delayed while awaiting PCR results from dried blood spots (DBS) sent to a central referral laboratory.6,7 In light of these limitations, WHO encourages presumptive diagnosis and treatment in children aged less than 18 months with confirmation of HIV status as soon as possible.3
To facilitate this process, WHO introduced presumptive diagnostic criteria (WHO-PDC) based on the WHO clinical paediatric HIV staging system. In addition to the WHO-PDC criteria, other algorithms have been proposed to clinically identify HIV-positive infants and children. The integrated management of childhood illness (IMCI) program is a WHO and UNICEF strategy aimed at improving both case management skills of primary health care providers and community health practitioners for children under 5 years old in places where there are inadequate health care facilities.8
There is insufficient data available regarding these clinical algorithms in conjunction with CD4% or other parameters, and WHO encourages researchers to validate approaches.3 This study evaluated the performance of the WHO clinical algorithm for presumptive diagnosis of HIV infection in HIV-exposed infants and young children. We also evaluated whether or not the addition of CD4% or specific clinical exam findings improves the accuracy of the WHO-PDC.
Methods
Study Design
We conducted a prospective cross-sectional study at the University Teaching Hospital (UTH), a tertiary-care referral hospital in Lusaka, Zambia, with 450 paediatric in-patient beds.
Patients and data collection
All HIV-exposed children aged 18 months or younger admitted to the UTH paediatric wards during a six month period in 2009 were screened for the study. HIV exposure was defined as a positive rapid HIV test using the Determine HIV-1/2 (Abbott Diagnostic Division, Hoofddorp, The Netherlands) with confirmation by a second ELISA test, the Unigold HIV Test (Trinity BioTech, Bray, Ireland). Infants with known HIV DNA PCR results were excluded from the study. Critically ill infants, defined as those who required some form of organ support, including oxygen supplementation, were also excluded due to social constraints. Trained study assistants identified eligible participants and, following a detailed informed consent process, a questionnaire was administered to all consenting participants and 2 milliliters of blood drawn for CD4% and DBS card preparation for DNA PCR testing. All children were examined by a paediatrics Registrar (KMK) who made the final decision regarding physical findings. CD4% was estimated using the BD FACSCalibur™ flow cytometer. HIV DNA PCR was performed with a DBS-based qualitative technique using the Roche AMPLICOR HIV-1 Test, version 1.5 (Roche Diagnostic Systems, Branchburg, NJ).
Algorithms
WHO-PDC
We used the WHO presumptive diagnosis criterion (WHO-PDC) for infants as follows:
-
Confirmed positive HIV antibody test in the infant AND
Presence of any AIDS indicator condition, OR
Symptomatic infant with two or more of the following: oral thrush, severe pneumonia and severe sepsis
Other factors that WHO lists in support of the diagnosis of severe HIV disease (recent HIV-related maternal death, CD4% <20% in children <18 months but >12 months old, CD4% <25% in children <12 months old) are not part of the formal algorithm, and were considered separately.
IMCI CRITERIA
We used the adapted IMCI guidelines for referral in Zambia of children under 5 years old suspected to have HIV. These guidelines include seropositive mother and child and three (3) or more of the following:
chronic otitis media
low weight for age or growth faltering or history of weight loss
recurrent pneumonia
persistent diarrhea
lymphadenopathy in more than two sites (neck, axilla or groin)
oral thrush
parotid enlargement for more than 14 days
health problems in sibling or other parent
Analysis
The data collected was entered in Microsoft Access database and analyzed with SAS version 9.1.3. Analysis included calculation of sensitivity, specificity, positive and negative predictive value of WHO-PDC, IMCI criteria and CD4% and other selected clinical signs including the age of the patient. HIV DNA PCR result was used as the gold standard for presence of HIV disease. WHO-PDC and IMCI criteria are described below. In accordance with WHO criteria, CD4% cutoffs for positive test were set at <25% for children less than 12 months of age and <20% for children 12 to <18 months. Predictors of HIV infection were identified using multivariate analysis. T-test to compare the median CD4% in those with and without confirmed HIV was also applied. This study was approved by the ethics committees of the University of Zambia and the University of Alabama in Birmingham (UAB) and informed consent was obtained from caregivers of all patients recruited.
Results
Enrollment
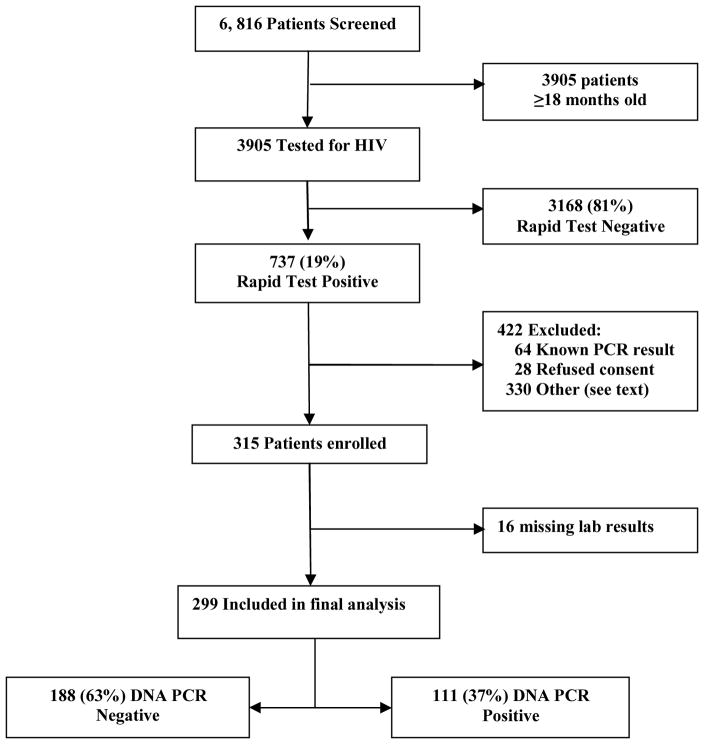
Between May and November 2009, 6,816 children presented to the UTH, of whom 3,905 (57%) were children less than 18 months of age (Figure 1). 737 children <18 months old (19%) tested positive by rapid HIV tests. 422 of the 737 children were excluded for the following reasons: known HIV DNA PCR results, refused consent, were critically ill, were brought by a guardian unfamiliar with the child’s history, or were discharged before they could be consented. 315 children were enrolled into the study. Of these, 16 were excluded from final data analysis due to incomplete laboratory results.
Figure 1.
Enrollment and Outcomes
Patient characteristics
Biological mothers accounted for 94% of the respondents. The majority of caregivers were aged between 25–35 years (57.4%) and unemployed (61%). 227 (75.9%) children enrolled in the study were under 6 months of age. Only 13 (4.3%) of the children were aged between 12–18 months. Pneumonia (41.8%) and sepsis (35.1%) were the most common admission diagnoses. 118 (49.0%) children were receiving co-trimoxazole for pneumocystis Jerovici prophylaxis as outpatients. Baseline characteristics of the children are summarized in Table 1.
Table 1.
Baseline characteristics of 299 children included
| Age | |
| <6 months | 227 (75.9) |
| 6 to <12 months | 59 (19.7) |
| 12 to 18 months | 13 (4.3) |
|
| |
| Male | 159 (53.2) |
|
| |
| Mother deceased | 6 (2.0) |
|
| |
| Sibling deceased | 67 (22.5) |
|
| |
| CD4%, median (IQR) | |
| <12 months of age | 32 (20–44) |
| 12–18 months of age† | 20 (13–27) |
|
| |
| Hospital diagnosis^ | |
| Pneumonia | 125 (41.8) |
| Sepsis | 105 (35.1) |
| Acute diarrheal disease | 44 (14.7) |
| Oral thrush | 35 (11.7) |
| Excessive irritability/fever | 19 (6.4) |
| Severe malnutrition | 7 (2.3) |
| URTI | 7 (2.3) |
| Meningitis | 6 (2.0) |
| Chronic diarrhea | 4 (1.3) |
| Other | 27 (9.0) |
|
| |
| Received antiretrovirals for PMTCT* | |
| None | 122 (43.3) |
| Mother only | 21 (7.4) |
| Mother and infant | 139 (49.3) |
|
| |
| Breastfed | 181 (60.5) |
|
| |
| Co-trimoxazole prophylaxis | 118 (49.0) |
All values reported as n (%) except CD4%
All 13 children ≥12 months of age were DNA PCR positive
Total is more than 100% due to presence of concurrent diagnoses.
PMTCT – Prevention of mother-to-child transmission; PMTCT information available for 282 mother-infant pairs
HIV results
Positive HIV DNA PCR was noted in 111 (37%) of the 299 infants analyzed. The proportion of HIV-exposed children with positive PCR increased with age (Table 2). All of the 13 children between 12 and 18 months of age tested PCR positive. Median CD4% was 18% (IQR 12–26%) among HIV-positive children and 40% (IQR 31–50%) in HIV-negative children. Antiretroviral PMTCT (Odds ratio 0.62; 95% CI 0.37 – 1.03) and breastfeeding (OR 1.32; 95% CI 0.79 – 2.21) were not significantly associated with HIV infection.
Table 2.
Performance of WHO-PDC for HIV-1 infections in infants by age
| Age Group in months | Total n | HIV+ n (%) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| 0 to <6 | 227 | 57 (25) | 0.21 (0.11 – 0.34) | 0.93 (0.88 – 0.96) |
| 6 to <12 | 59 | 41 (69) | 0.24 (0.12 – 0.40) | 0.89 (0.65 – 0.99) |
| 12 to <18 | 13 | 13 (100) | 0.23 (0.05 – 0.54) | - |
| All Children | 299 | 111 (37) | 0.23 (0.17 – 0.32) | 0.93 (0.88 – 0.96) |
Performance of Diagnostic Algorithms
Sensitivities, specificities, and positive and negative predictive values of WHO-PDC and the other various diagnostic algorithms are displayed in Tables 2 and 3. Ninety-five percent confidence intervals are included in the tables. The sensitivity of the WHO-PDC was 23% and the specificity 93%. WHO-PDC performance did not vary with age (Table 2). When combined with CD4%, WHO-PDC sensitivity increased to 77%, though specificity dropped to 83%. CD4% alone had a sensitivity and specificity of 74% and 88%, respectively.
Table 3.
Sensitivity, Specificity and predictive values for HIV-1 infection in infants using Different Algorithms
| Criteria | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| WHO-PDC | 0.23 (0.17–0.32) | 0.93 (0.88–0.96) | 0.65 (0.50–0.78) | 0.67 (0.61–0.73) |
| CD4%* | 0.74 (0.65–0.82) | 0.88 (0.83–0.92) | 0.77 (0.67–0.84) | 0.87 (0.81–0.91) |
| WHO –PDC+ CD4%* | 0. 77 (0.68–0.83) | 0.83 (0.77–0.88) | 0.73 (0.64–0.80) | 0.86 (0.80–0.90) |
| WHO-PDC + CD4%* + clinical findings** | 0. 85 (0.77–0.90) | 0.63 (0.56–0.70) | 0.58 (0.50–0.65) | 0.88 (0.81–0.92) |
| IMCI | 0.10 (0.06–0.17) | 0.97 (0.94–0.99) | 0.69 (0.44–0.86) | 0.65 (0.59–0.70) |
| IMCI + CD4%* | 0. 80 (0.71–0.87) | 0.88 (0.82–0.92) | 0.78 (0.69–0.85) | 0.89 (0.84–0.93) |
95% confidence intervals in parentheses.
CD4 cutoffs: <25% if age <12 months, <20% if 12–18 months
Clinical findings = weight <3rd percentile, hepatomegaly, splenomegaly, lymphadenopathy
The following physical exam findings when individually added to the WHO-PDC slightly improved the sensitivity: splenomegaly (35%, 95%CI 26–45%), weight <3rd percentile (36%, 95%CI 27–46%), hepatomegaly (40%, 95%CI 31–49%), and lymphadenopathy (41%, 95% CI 33–51%). Specificities ranged from 85–91%. The addition of all four examination findings to WHO-PDC, and CD4% yielded the highest sensitivity (85%) with specificity of 64%, positive and negative predictive values of 57% and 86%, respectively (see Table 3). The addition of CD4% to IMCI criteria raised its sensitivity from 10% to 80% but lowered the specificity to 88%. Receiver operating characteristic (ROC) curves for selected algorithms are displayed in Figure 2. Area under the ROC curves was greatest for IMCI plus CD4% (0.84).
Figure 2.
Receiver Operating Curves for HIV-1 Diagnostic Algorithms
Discussion
This study assessed the performance of the WHO-PDC for diagnosing HIV in children less than 18 months of age. WHO-PDC was compared with the DNA PCR, which is the current gold standard for the diagnosis of HIV in children younger than 18 months. The HIV prevalence was 37% in this group of 299 seropositive children. The WHO-PDC was found to be only 23% sensitive, with a specificity of 93%. A low CD4% on its own or combined with the WHO-PDC was found to be highly predictive of HIV infection. Clinical signs were 33% to 41% sensitive when combined with WHO-PDC with specificities above 85%. Use of the IMCI guidelines also showed an increased sensitivity (80%, from 10%) when combined with the CD4%.
The sensitivity of the WHO-PDC algorithm (23%) was markedly lower than reported in other studies. In Kenya, Inwani et al found the WHO-PDC to be 43% sensitive and 88% specific.9 Peltier et al evaluated the algorithm in Rwanda and documented 77% sensitivity but only 63% specificity.10 Both of these studies had higher detection rates for stage 4 conditions. In Rwanda, 52% of all patients had severe malnutrition, a stage 4 diagnosis that was relatively uncommon in our study population. A notable difference in our study design that likely contributed to the decreased sensitivity of WHO-PDC was the exclusion of critically ill infants. A non-critically ill population such as ours is less likely to present with multiple PDC criteria despite being HIV-positive, and it is possible that many of the HIV-positive infants who presented with stage 4 diseases were excluded because of critical illness.
The median CD4% in HIV-infected children was 18%, compared with 40% in the HIV-negative children. Various studies have shown that CD4% is lower in HIV-1-infected children, and a low CD4% is highly predictive of disease progression, even in young infants.11–12 Our study roughly agreed with an earlier study done in Zambia that showed a median CD4% of 35% in HIV-negative children less than 2 years old.13 We used CD4% cut-offs according to WHO immunological criteria for severe immunodeficiency by age:less than 25% in those under 12 months and less than 20% in those ≥12 months but <18 months.3 Based on these cutoffs, CD4% used alone or in combination with the WHO-PDC was found to strongly predict the chances of a positive DNA PCR. Analyzing WHO-PDC and CD4% criteria would have identified 83 (75%) of the 111 HIV-infected children without having to wait for DNA PCR results, with only 30 false positives. The performance of CD4% in our study exceeded that seen in Kenya (81% sensitive, 39% specific) and Rwanda (56% sensitive, 79% specific). CD4 percentages were generally higher in the Rwandan study despite severe malnutrition. We could not establish if the prevailing infections in our study had a marked reductive effect on the T-lymphocytes. It would have also been useful to assess CD4:CD8 ratios, which have been a more sensitive predictor of HIV infection in exposed children in other areas.14–17
Although clinical exam abnormalities, such as nappy rash, lymphadenopathy, and hepatomegaly, are more common in HIV-infected children18, individual exam abnormalities, added to WHO-PDC, were not particularly sensitive indicators of HIV infection. When the WHO-PDC, weight <3rd percentile, hepatomegaly, splenomegaly, lymphadenopathy and CD4% were combined, the sensitivity improved to 85%, with a specificity 63%. However, the area under the ROC curve was lower with this combination than with WHO-PDC plus CD4 count or with CD4 count alone.
Although WHO-PDC criteria were insensitive, IMCI guidelines were even worse when used alone, yielding 10% sensitivity when compared to DNA PCR. In combination with CD4%, IMCI sensitivity improved to 80% with a specificity of 88%. Thus, the IMCI tool in conjunction with CD4% would be useful in situations where caregivers are more familiar with the IMCI guidelines as opposed to the WHO-PDC. In fact, in our study IMCI plus CD4% was the best performing algorithm as measured by area under ROC curve.
There were several limitations to this study. As previously mentioned, the exclusion of critically ill patients likely diminished the sensitivity of the WHO-PDC. Additionally, the study site was a tertiary care referral hospital and thus the study population was not representative of the general population seen at primary care settings.
Despite these limitations, several conclusions can be drawn from our findings. The addition of one or multiple physical exam findings increases the sensitivity of the WHO-PDC only marginally. Adding CD4% testing to the WHO-PDC improves the sensitivity of the algorithm considerably but is not any better than CD4% alone. Clinical diagnostic algorithms are generally not reliable enough to conclusively diagnose HIV infection. Improving the access to dry blood spot PCR with timely reporting of results would be the optimal means of addressing the problem of early diagnosis in infants. In settings where timely PCR results are not possible but CD4% is available, we recommend the use of CD4% in any diagnostic algorithm.
Acknowledgments
Support was provided by NIH/FIC grant R24TW007988-03 (Fogarty International Clinical Research Scholars Program) and a CDC interagency agreement with NIH, D43TW001035-11-S1 (Vanderbilt-CIDRZ AIDS International Training and Research Program).
References
- 1.HIV paediatric Prognostic Markers Collaborative Study Group. Short-term risk of disease progression in HIV-1 infected children receiving no antiretroviral therapy or Zidovudine monotherapy: a meta-analysis. Lancet. 2003 Nov 15;362(9396):1605–11. doi: 10.1016/s0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 2.HIV paediatric Prognostic Markers Collaborative Study Group. Current CD4 cell count and short term risk of AIDS and death before availability of effective antiretroviral therapy in adults and children; a meta-analysis. J Infect Dis. 2008 Feb 1;197(3):398–404. doi: 10.1086/524686. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. ART of HIV infection in infants and children in low resource setting. Geneva: WHO Press; 2006. [Google Scholar]
- 4.Violari A, Cotton M, Gibb D, et al. Children with HIV Early Antiretroviral Therapy (CHER study) N Engl J Med. 2007;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faye A, Le Chenadec J, Dollfus C, Thuret, et al. Early versus Deferred Antiretroviral multidrug therapy in HIV type 1. Clin Infect Dis. 2004;39:1692–98. doi: 10.1086/425739. [DOI] [PubMed] [Google Scholar]
- 6.Horwood C, Liebeschuetz S, Blaauw D, Cassol S, Qazi S. Diagnosis of paediatric HIV infection in a primary Health Care Setting with a clinical Algorithm. Bull World Health Organ. 2003;81(12):858–66. [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SA, Shermann GG, Coovadia AH. Can Clinical Algorithms Deliver an Accurate diagnosis of HIV Infection in infancy? Bull World Health Organ. 2005 Jul;83(7):559–60. [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, United Nations Children’s fund. Integrated management of childhood illnesses complimentary course on HIV/AIDS. Geneva: WHO; 2006. Jan, [Google Scholar]
- 9.Inwani I, Mbori-Ngacha D, Nduati R, Obimbo E, et al. Performance of Clinical Algorithms for HIV-1 Diagnosis and Antiretroviral Initiation Among HIV-1 Exposed Children Aged less than 18 months in Kenya. JAIDS. 2009;50:492–98. doi: 10.1097/QAI.0b013e318198a8a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltier CA, Omes C, Ndimubanzi PC, et al. Validation of 2006 WHO Prediction Scores for True HIV Infection in Children Less than 18 Months with a Positive Serological HIV Test. PLoS One. 2009;4:e5312. doi: 10.1371/journal.pone.0005312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaspan H, et al. Clinical and immunological characteristics of very young infants with HIV infection; Children with HIV Early Antiretroviral Study. 5th conference on Retroviruses and opportunistic infections; Boston. 2008. p. Abstract 76. [Google Scholar]
- 12.Amadi B, Kelly P, Mwiya M, Mulwazi E, Sianongo S, Changwe F, Thomson M, Hachungula J, Watuka A, Walker-Smith J, Chintu C. Intestinal and Systemic Infection, HIV, and Mortality in Zambian Children with Persistent Diarrhea and Malnutrition. J Pediatr Gastroenterol Nutr. 2001 May;32:550–54. doi: 10.1097/00005176-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Ndhlovu Z, Ryon J, Griffin E, et al. CD4+ and CD8+ T- lymphocyte Subsets in Zambian Children. J Trop Pediatr. 2004;50(2):94–97. doi: 10.1093/tropej/50.2.94. [DOI] [PubMed] [Google Scholar]
- 14.Shearer WT, Patiwa S, Read JS, Chen J, Wijayawardana SR, Palumbo p, et al. CD4/CD8 T-cell ratio predicts HIV infection in infants; The National Heart, Lung and Blood Institute P2C2 study. J of Allergy and Clinical Immunology. 2007 Dec;120(6):1149–56. doi: 10.1016/j.jaci.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moodley D, Bobat RA, Coovadia HM, Doovasamy T, Munsamy S, Gouws E. Lymphocyte subset changes between 3 and 15 months of age in infants born to HIV seropositive women in South Africa. Trop Med Int Health. 1997 May;2(5):415–21. [PubMed] [Google Scholar]
- 16.Zijenah LS, Katzenstein DA, Nathoo KJ, et al. T lymphocytes among HIV-1 infected and uninfected infants:CD4/CD8 ratio as a potential tool in diagnosis of infection in children under 2 years. J Transl Med. 2005;3:3–6. doi: 10.1186/1479-5876-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Embree J, Bwayo J, Nagelkerke N, et al. Lymphocyte subsets in human immunodeficiency virus type 1 infected and uninfected children in Nairobi. Pediatr Infect Dis J. 2001;20:397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Jaspan H, et al. Clinical and immunological characteristics of very young infants with HIV infection; Children with HIV Early Antiretroviral Study. 15th conference on Retroviruses and opportunistic infections; Boston. 2008. p. Abstract 76. [Google Scholar]