Abstract
BACKGROUND
Fifty percent of aortic dissections in women younger than 40 years occur in association with pregnancy. Of these, half of type B dissections occur in the postpartum period.
CASE
A 30-year-old woman was status post spontaneous vaginal delivery at 30 weeks of gestation for fetal death, complicated by an eclamptic seizure. On post-partum day 4, she suffered an acute, complicated type B aortic dissection treated with endovascular stent graft placement.
CONCLUSION
Endovascular repair may be an attractive option for the treatment of complicated type B aortic dissections in pregnancy and the peripartum period, with reduced maternal and fetal mortality. This may allow the fetus to remain in situ and avoid the risks of surgery and possible cardiopulmonary bypass, with little radiation risk to the fetus.
Fifty percent of aortic dissections in women younger than 40 years occur in association with pregnancy. In 1944, Schnitker et al reported 49 cases of aortic dissection in women younger than 40 years, including 24 cases associated with pregnancy.1 This association persists in the literature, which identifies pregnancy as an independent risk factor for dissection.2,3 Dissection during pregnancy carries significant maternal and fetal mortality risk; however, optimal treatment has not been established.4
Hemodynamic and hormonal alterations likely contribute to dissections that occur during pregnancy and the postpartum period. Increased blood volume and left ventricular wall mass result in increased cardiac output. When coupled with increased vascular resistance secondary to compression by the gravid uterus, the aorta is subjected to increased shear forces, which ultimately may lead to intimal tears.4
Pregnancy-induced changes include degenerative alterations in the media, perhaps resulting from changing hormone levels, which persist beyond parturition and are implicated in the development of aortic dissections.5 During pregnancy, estrogen and progesterone levels rise, along with the number of receptors in the aortic wall. This change in structure is thought to contribute to a loss of vessel-wall integrity. Relaxin, which enables widening of the symphysis pubis late in pregnancy, also may play a role in altering the characteristics of the aortic wall.5 These alterations increase in degree with progressive gestational age and multiparity.6
Despite differing mechanisms of dissection during pregnancy, the fundamental principles of treatment remain the same, including management of branch ischemia, aortic expansion, or rupture.3 Pregnancy complicates treatment because there are two lives at risk, with variable mortality implications at different gestational ages of the fetus.
The goal of endovascular stent– graft repair of aortic dissection is to exclude the primary tear and to redirect flow through the true lumen, restoring distal perfusion, without a morbid open operation.3 We present a complex type B dissection associated with pregnancy in the postpartum period and propose that endovascular therapy presents an attractive option for the management of dissection when associated with pregnancy or the postpartum period.
CASE
A 30-year-old woman, gravida 2 para 1, was status post spontaneous vaginal delivery at 30 weeks for fetal demise, complicated by an eclamptic seizure. On postpartum day 4, she presented to a community hospital with severe, sudden-onset chest and abdominal pain. Her pain was stabbing in nature, located in the epigastrium, left upper quadrant, and mid-back, and associated with nausea, vomiting, and hematemesis. She noted new left leg numbness and tingling.
Her past obstetric history was remarkable for preeclampsia complicating her first pregnancy, resulting in a 35-week spontaneous vaginal delivery. Her past medical history was unremarkable, with no personal or family history of any aortic aneurysms, dissections, or connective tissue disorders such as Marfan or Ehlers-Danlos syndrome. On physical examination, she was a young, obese woman with significant abdominal pain and in distress. She was hypertensive (146/92), with a normal cardiac and respiratory examination. She complained of mild paresthesia in her left leg, and her abdomen was markedly tender with voluntary guarding. She had palpable and equal pulses in her extremities bilaterally.
Laboratory evaluation revealed a white blood count of 42 k/microliter, a lactic acid level of 2.8 mmol/L, and a base deficit of 6 mmol/L. Computed tomography angiography revealed an acute type B aortic dissection extending from the left subclavian artery to the left common iliac artery. The celiac and superior mesenteric arteries arose from the compressed true lumen. There was a contained rupture of the mid-descending thoracic aorta, and multiple loops of thickened bowel were visualized, suggestive of mesenteric ischemia (Fig. 1).
Fig. 1.
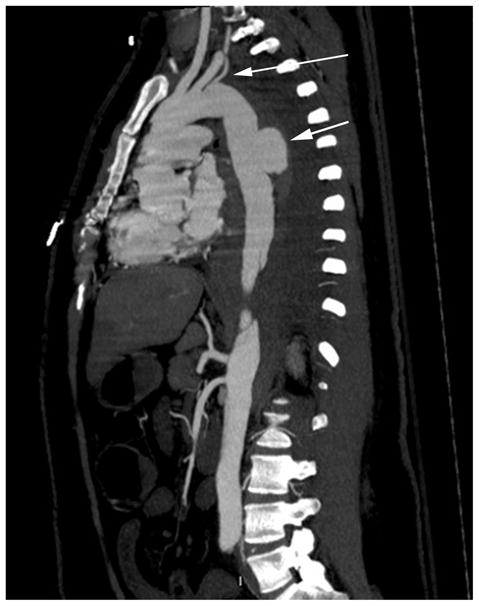
Computed tomography angiogram revealing a complicated type B aortic dissection, contained rupture projecting posteriorly (short arrow), and aberrant left vertebral artery arising distal to the left subclavian artery (long arrow).
Rosenberger. Postpartum Aortic Dissection. Obstet Gynecol 2012.
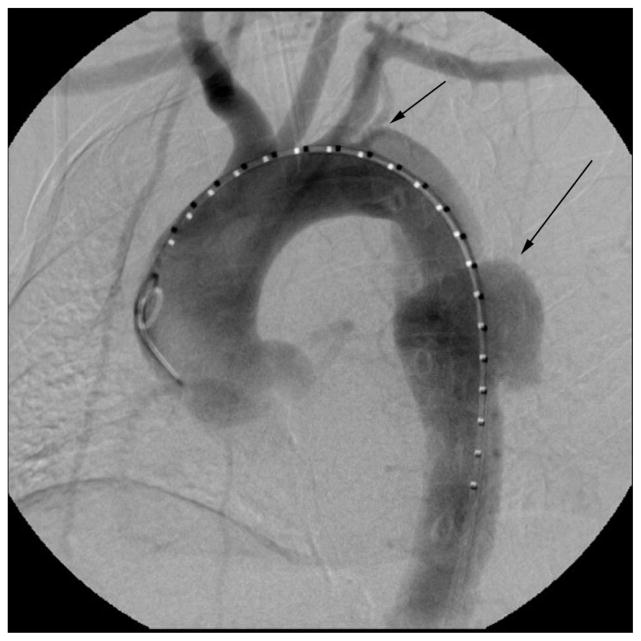
Emergent endovascular repair was planned to cover the primary aortic tear, exclude the contained rupture, and expand the true lumen and thereby improve visceral and lower extremity perfusion. Initial aortogram revealed a proximal entry tear distal to the left subclavian artery (Fig. 2). A lumbar drain was placed for spinal cord protection, and, subsequently, two 26×10-cm endografts (GORE TAG Thoracic Endoprosthesis, W. L. Gore & Associates, Inc.) were deployed in the thoracic aorta without complication. The grafts covered the proximal tear as well as the area of the rupture; both the left subclavian and aberrant left vertebral arteries were, of necessity, covered (Fig. 3). We felt the emergent nature of her problem precluded prior subclavian artery reconstruction. Subsequent abdominal exploration revealed thickened bowel with no necrosis.
Fig. 2.
Initial aortogram revealing a proximal tear just distal to the left subclavian artery (short arrow) and a contained rupture projecting posteriorly (long arrow).
Rosenberger. Postpartum Aortic Dissection. Obstet Gynecol 2012.
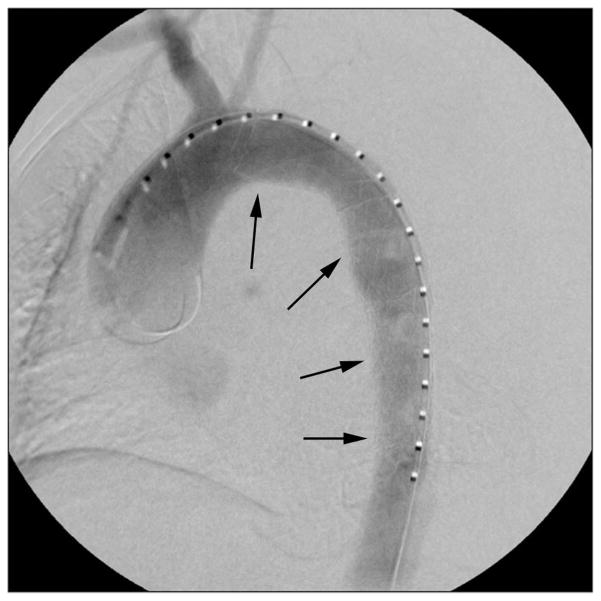
Fig. 3.
Poststent aortogram revealing stent in place (arrows) with satisfactory exclusion of the rupture and rapid flow into the true lumen of the aorta.
Rosenberger. Postpartum Aortic Dissection. Obstet Gynecol 2012.
Although initially stable, on postoperative day 2 the patient’s condition deteriorated, with sudden, profound hypotension requiring emergency resuscitation. Repeat imaging revealed a new left hemothorax and increased filling of the false lumen of the aorta, distal to the stent-grafts, through a distal fenestration. Two additional 26×10-cm GORE TAG Thoracic Endoprosthesis grafts were deployed to extend the existing graft coverage to just above the celiac artery. Aortography revealed a significant increase in true lumen diameter and excellent perfusion of the visceral arteries.
A pelvic arteriogram revealed occlusion of the left common iliac artery. Stents (GORE VIABAHN Endoprosthesis, W. L. Gore & Associates, Inc.) were deployed bilaterally in a kissing fashion and extended distally, with a second VIABAHN on the left and an iCast Covered Stent (Atrium) on the right. Postdeployment arteriography revealed widely patent iliac arteries. The patient progressed well thereafter and was discharged on hospital day 31. Imaging obtained 28 months postoperatively revealed graft patency without aneurysmal changes and favorable remodeling of the thoracic aorta. Postoperatively, the patient continues to do well, without any pain or claudication.
COMMENT
Aortic dissection remains a rare event, affecting 2,000 patients per year in the United States, of whom 5.3% are women of reproductive age.7 Of these women, between 14% and 50% have Marfan syndrome and 34% to 71% have hypertension.7,8 According to the Stanford classification system, a type A dissection involves the ascending aorta with or without involvement of the descending portion, whereas a type B dissection involves the descending aorta alone.9 A literature review by Immer et al analyzed 57 cases of aortic dissection associated with pregnancy, of which 45 cases (78.9%) were acute type A dissections and 12 (21.1%) were type B dissections.4 These rates are consistent with those reported by Konishi and colleagues— 89% and 11% of type A and type B, respectively.10
The treatment for uncomplicated type B dissections classically has been aggressive medical management. Complicated type B dissections, defined by rupture, end organ ischemia, aneurysmal aortic expansion, dissection extension, or continued pain, classically have required open surgical intervention. Rates of in utero fetal demise remain high, likely due to partial occlusion of internal iliac artery branches resulting in decreased placental perfusion. In the large review by Immer and colleagues, type B dissections diagnosed prepartum resulted in a 43% fetal mortality rate.4 However, type B aortic dissections frequently occur in the early postpartum period. In the review by Immer et al, 41.7% of the type B dissections occurred postpartum (5/12), which is consistent with our present case.4 The median time from symptoms to surgery has been reported to be 21.6 hours.7 It is unlikely that the type of repair, operative compared with endovascular, plays a substantial role in reducing the time to reperfusion, but it does reduce maternal and fetal morbidity and mortality associated with premature delivery and operative intervention.
A paucity of data exists regarding endovascular repair of aortic dissections associated with pregnancy or the postpartum period. Although the patient was no longer pregnant at presentation, we believe endovascular repair also should be considered as an option for the treatment of dissection with the fetus in situ.
According to the American College of Radiology practice guidelines regarding imaging in pregnancy, there are no in utero radiation-induced effects at doses lower than 50 mGy.11 Between 50 and 100 mGy, there are no effects after 18 weeks of gestation and effects are probably too subtle to be detectable clinically before 17 weeks. Higher than 100 mGy of radiation, there is risk of spontaneous abortion (at 3– 4 weeks of gestation), malformations (5–10 weeks), and intelligence quotient deficits (11–17 weeks). After 17 weeks of gestation, there are no detectable deficits at 100 mGy of radiation.11 A large patient-safety review revealed that the effective dose of radiation for emergency endovascular repairs, including preoperative computed tomography, endovascular stent– graft repair, and 1 year postoperative surveillance, had a mean total of 47.2 mSv (mGy).12 This is well within safe ranges for pregnant patients as established by the American College of Radiology.
Endovascular repair may be an attractive option for the treatment of complicated type B aortic dissections in pregnancy, with reduced maternal and fetal mortality. Endovascular repair may allow the fetus to remain in situ and avoid the risks associated with premature delivery, an open operative repair, and possible cardiopulmonary bypass, with little radiation risk to the fetus.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Schnitker M, Bayer CA. Dissecting aneurysm in young individuals, particularly in association with pregnancy: with report of case. Ann Intern Med. 1944;20:486–511. [Google Scholar]
- 2.Karthikesalingam A, Holt PJ, Hinchliffe RJ, Thompson MM, Loftus IM. The diagnosis and management of aortic dissection. Vasc Endo Surg. 2010;44:165–9. doi: 10.1177/1538574410362118. [DOI] [PubMed] [Google Scholar]
- 3.Lin PH, Huynh TT, Kougias P, Huh J, LeMaire SA, Coselli JS. Descending thoracic aortic dissection: evaluation and management in the era of endovascular technology. Vasc Endo Surg. 2009;43:5–24. doi: 10.1177/1538574408318475. [DOI] [PubMed] [Google Scholar]
- 4.Immer FF, Bansi AG, Immer-Bansi AS, McDougall J, Zehr KJ, Schaff HV, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003;76:309–14. doi: 10.1016/s0003-4975(03)00169-3. [DOI] [PubMed] [Google Scholar]
- 5.Abbas MA, Stone WM, Fowl RJ, Gloviczki P, Oldenburg WA, Pairolero PC, et al. Splenic artery aneurysms: two decades experience at Mayo Clinic. Ann Vasc Surg. 2002;16:442–9. doi: 10.1007/s10016-001-0207-4. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RA, Fineron PW. Aortic dissection in pregnancy: importance of pregnancy-induced changes in the vessel wall and bicuspid aortic valve in pathogenesis. Br J Obstet Gynaecol. 1994;101:1085– 8. doi: 10.1111/j.1471-0528.1994.tb13589.x. [DOI] [PubMed] [Google Scholar]
- 7.Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD) J Am Coll Cardiol. 2004;43:665–9. doi: 10.1016/j.jacc.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 8.Oskoui R, Lindsay J., Jr Aortic dissection in women <40 years of age and the unimportance of pregnancy. Am J Cardiol. 1994;73:821–3. doi: 10.1016/0002-9149(94)90888-5. [DOI] [PubMed] [Google Scholar]
- 9.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237– 47. doi: 10.1016/s0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 10.Konishi Y, Tatsuta N, Kumada K. Dissecting aneurysm during pregnancy and the puerperium. Jpn Circ J. 1980;44:726–33. doi: 10.1253/jcj.44.726. [DOI] [PubMed] [Google Scholar]
- 11.ACR practice guideline for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation. American College of Radiology; 2008. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/dx/Pregnancy.aspx. Retrieved October 31, 2011. [Google Scholar]
- 12.Jones C, Badger SA, Boyd CS, Soong CV. The impact of radiation dose exposure during endovascular aneurysm repair on patient safety. J Vasc Surg. 2010;52:298–302. doi: 10.1016/j.jvs.2010.03.004. [DOI] [PubMed] [Google Scholar]