Abstract
Thrombotic thrombocytopenic purpura (TTP) is a fulminant disease characterized by platelet aggregates, thrombocytopenia, renal insufficiency, neurologic changes, and mechanical injury to erythrocytes. Most idiopathic cases of TTP are characterized by a deficiency of ADAMTS13 (a disintegrin and metalloprotease, with thrombospondin-1-like domains) metalloprotease activity. Ironically, use of anti-platelet agents, the thienopyridine derivates clopidogrel and ticlopidine, is associated with drug induced TTP. Data were abstracted from a systematic review of English-language literature for thienopyridine-associated TTP identified in MEDLINE, EMBASE, the public website of the Food and Drug Administration, and abstracts from national scientific conferences from 1991 to April 2008. Ticlopidine and clopidogrel are the two most common drugs associated with TTP in FDA safety databases. Epidemiological studies identify recent initiation of anti-platelet agents as the most common risk factor associated with risks of developing TTP. Laboratory studies indicate that most cases of thienopyridine-associated TTP involve an antibody to ADAMTS13 metalloprotease, present with severe thrombocytopenia, and respond to therapeutic plasma exchange (TPE); a minority of thienopyridine-associated TTP presents with severe renal insufficiency, involves direct endothelial cell damage, and is less responsive to TPE. The evaluation of this potentially fatal drug toxicity can serve as a template for future efforts to comprehensively characterize other severe adverse drug reactions.
Keywords: drug-associated TTP, epidemiology, ADAMTS13
Thrombotic thrombocytopenic purpura (TTP) is a microvascular occlusive disorder characterized by systemic or intrarenal aggregation of platelets, leading to thrombocytopenia and mechanical injury to erythrocytes.1 Conditions and factors associated with TTP include organ transplantation, infectious diseases, and drugs.2 The most common drugs reported to the Food and Drug Administration (FDA) in association with TTP are the thienopyridine-derivative anti-platelet agents, ticlopidine and clopidogrel.3,4 Before 1999, ticlopidine was widely used for prevention of cerebrovascular, cardiovascular, and peripheral vascular complications and following coronary artery stent procedures.5 Since 2000, owing to concerns over ticlopidine-associated agranulocytosis, clinicians switched to clopidogrel in these settings.5-9 Herein, we summarize the clinical, laboratory, and epidemiological information on thienopyridine-associated TTP.
PHARMACOLOGY
Ticlopidine and clopidogrel, thienopyridine derivatives that inhibit platelet aggregation,5 differ structurally by a carboxymethyl side group. Animal studies and in vitro laboratory studies indicate that ticlopidine, but not clopidogrel, is associated with bone marrow toxicity.5 As all clopidogrel metabolites contain the carboxymethyl side group, the two drugs have no common metabolites.10 Ticlopidine and clopidogrel are administered orally, requiring hepatic breakdown to an active metabolite to achieve in vivo activity. The major therapeutic target of the thienopyridines is one of the adenosine diphosphate receptor types on human platelets, P2Y12. Blockade of this receptor impairs adenosine diphosphate-induced platelet aggregation and decreases the propensity for arterial thrombosis.
EPIDEMIOLOGY
Epidemiological investigations identified a strong association of TTP with ticlopidine (Table 1). The first cases of ticlopidine-associated TTP were identified in 1991 at an apheresis center in Paris.11 In 1998, a survey of apheresis centers supplemented by FDA adverse event reports identified 60 cases of ticlopidine-associated TTP; one-third had died from the TTP.12 Most patients had received between 2–12 weeks of ticlopidine.12,13 Subsequently, after the introduction of coronary artery stent procedures, additional ticlopidine-associated TTP cases were identified at interventional cardiology laboratories and therapeutic plasma exchange (TPE) centers.13,14 Two surveys of interventional cardiology laboratories that had placed coronary artery stents in 8000 and 45,000 persons identified rates of TTP after ticlopidine administration of 1 in 1600 and 1 in 5000 patients, respectively.15,16 These findings placed ticlopidine as the drug with the highest reported rate of TTP.
Table 1.
Comparison of basic science, epidemiological, clinical, and pharmacovigilance findings for ticlopidine- versus clopidogrel-associated TTP
| Category | Ticlopidine-associated TTP | Clopidogrel-associated TTP |
|---|---|---|
| Basic science20 | ||
| Probable underlying pathophysiology | Antibody to ADAMTS13 and microvascular endothelial cell damage | Microvascular endothelial cell damage |
| High molecular weight vWF identified during the acute TTP phase | Yes | Yes |
| ADAMTS13 deficiency during the acute TTP phase | Yes | No |
| Functional IgG inhibitors to ADAMTS13 identified during acute phase | Yes | No |
| Clinical20 | ||
| Usual time period for onset | Two to 12 weeks after drug initiation | Within 2 weeks of drug initiation |
| Renal insufficiency | Mild to none | Severe |
| Thrombocytopenia | Severe | Mild |
| Survival after plasma exchange | >90%, usually within days of initiation of plasma exchange | 70%, often takes several weeks of plasma exchange |
| Survival without plasma exchange | 30% | 70% |
| Spontaneous relapse | Occasional | Infrequent |
| Likelihood of relapse occurring with exposure to the other thienopyridine | High | Low |
| Epidemiological | ||
| Epidemiological studies identifying cases of TTP after thienopyridine administration | Surveys of directors of interventional cardiology laboratories as well as directors of therapeutic plasma exchange centers (n=33 cases)26,29 | Surveys of directors of therapeutic plasma exchange centers (n=13 cases)5,31 |
| Estimated incidence based on information included in the FDA-approved package insert | 0.01–0.02%37 | 0.0001%18 (threefold greater than estimated incidence of idiopathic TTP) |
| Population-based case–control studies | None | Recent initiation of anti-platelet agents (clopidogrel, aspirin, or dipyridamole) is associated with 19.8-fold increased risk of developing TTP (n=86 cases; 177 age- and gender-matched controls)41 |
| Pharmacovigilance | ||
| Number of thienopyridine-associated TTP cases identified in the first year of marketing of the relevant drug | 4 (year=1991)11 | 2 (year=1999)38,39 |
| Number of cases included in the largest case series | 98 patients14 | 50 patients3 |
| Year of FDA approval | 1991 (current sales are $100,000)9 | 1998 (current sales are $7.3 billion)9 |
| Time from FDA approval to identification of first cases | 0 years (4 cases) (1991) | 1 year (2 cases) (1999) |
| Time from FDA approval to reporting of first case series | 7 years (1998) | 1.5 years (2000) |
| Rank in FDA MedWatch database in association with drug-associated TTP reports (1998–2006) | First—overall (first in the years 1998 and 1999) | Second—overall (first since 2000) |
| Advisories from the FDA | Package insert warning (1995)40 | Package insert warning (2000)18 |
| Black box warning (1998)37 | ||
| ‘Dear Doctor’ warnings describing drug-associated TTP mailed by the pharmaceutical supplier | 1998 | 2000 |
ADAMTS13, a disintegrin and metalloprotease, with thrombospondin-1-like domains; FDA, Food and Drug Administration; TTP, thrombotic thrombocytopenic purpura.
Recent investigations evaluated the association of clopidogrel with TTP. The first two cases were identified by the directors of apheresis centers in 1998, shortly after the drug received FDA approval.17 In 2000, eleven cases of TTP after administration of clopidogrel were identified at apheresis centers in six cities.17 By 2004, 37 cases of clopidogrel-associated TTP had been reported to the FDA.3 The pharmaceutical supplier reported an estimated incidence rate of 12 TTP cases per million clopidogrel-treated patients,18 three times the background rate for TTP in the general population.19
CLINICAL FEATURES
Thienopyridine-associated TTP is characterized by two clinical syndromes (Figure 1a). Most cases of ticlopidine-associated TTP and a minority of clopidogrel-associated TTP cases present with severe thrombocytopenia, microangiopathic hemolytic anemia, markedly elevated serum levels of lactate dehydrogenase, and normal renal function; and they occur between 2 and 12 weeks after initiation of thienopyridine therapy. Most cases of clopidogrel-associated TTP and a minority of ticlopidine-associated TTP cases present with mild thrombocytopenia, microangiopathic hemolytic anemia, mildly elevated serum levels of lactate dehydrogenase, and marked renal insufficiency. Onset usually occurs within 2 weeks of thienopyridine initiation.3,17,20 Both syndromes differ from those reported for other drug-associated TTP syndromes.2 Thrombotic microangiopathy associated with the calcineurin inhibitors gemcitabine and mitomycin-C is dose dependent, occurs after several weeks or months of use, and is attributed to the cumulative toxic effects on vascular endothelium.2 Renal dysfunction is generally present, with many patients requiring hemodialysis. TPE is not effective.2 Thrombotic microangiopathy developing after organ transplantation is associated with calcineurin inhibitors. The most common treatment strategy is discontinuation of the drug.2 Although quinine-associated TTP/HUS is antibody mediated, the antibodies are directed against granulocytes, lymphocytes, endothelial cells, or platelet glycoprotein Ib/IX or IIb/IIIa complexes.21 The syndrome can occur after ingestion of a single tablet of quinine in previously exposed persons, and is characterized by neurologic complications, thrombocytopenia, and hemolysis. Renal failure is absent occasionally. Treatment includes discontinuation of quinine, TPE, and hemodialysis.
Figure 1. Duration of thienopyridine exposure prior to TTP onset.
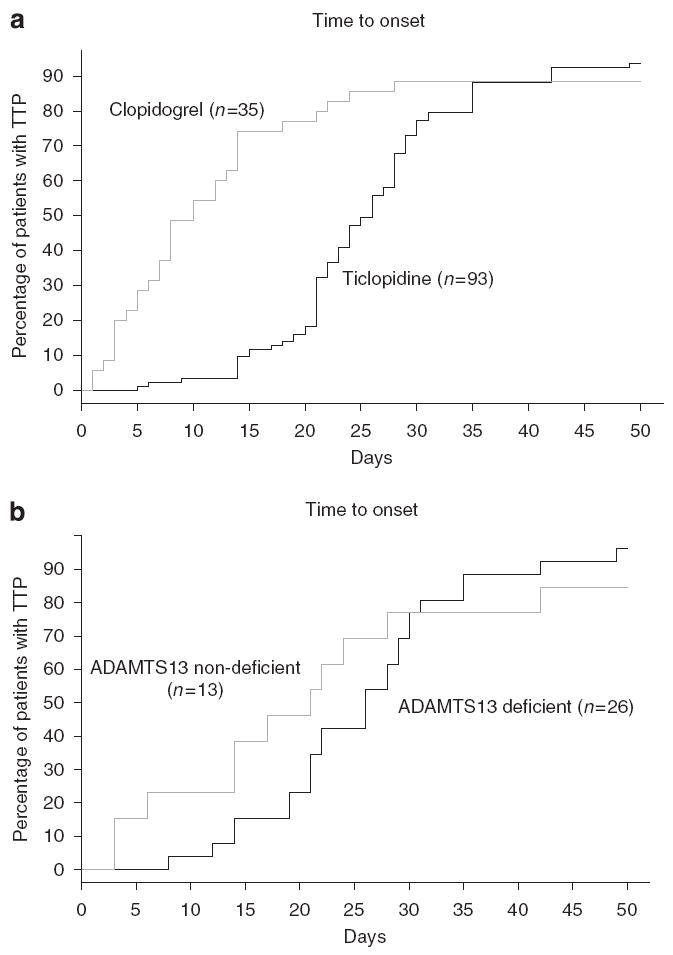
(a) Thienopyridine-associated thrombotic thrombocytopenic purpura (TTP) onset: ticlopidine versus clopidogrel (P = 0.0016). (b) Thienopyridine-associated TTP onset: ADAMTS13 (a disintegrin and metalloprotease, with thrombospondin-1-like domains) deficient (< 15%) versus near-normal levels (> 15%) of ADAMTS13 activity (P > 0.05). This figure has previously been published in Bennett et al.20
PATHOPHYSIOLOGY
For most patients with ticlopidine-associated TTP and a minority of patients with clopidogrel-associated TTP, in vitro assessments of plasma ADAMTS13 activity show severely diminished activity at the time of TTP onset.20 Onset of TTP occurs between 2 and 12 weeks after thienopyridine initiation (Figure 1b). Reduced in vitro ADAMTS13 activity correlates with deficient ADAMTS13 activity near the surface of stimulated endothelial cells that secrete ULVWF multimers. Plasma from six of seven patients with ticlopidine-associated TTP and from two of eleven patients with clopidogrel-associated TTP contained inhibitors to the ADAMTS13 metalloprotease.17,22 Failure to process ULVWF multimers seems to lead to the binding of ULVWF to platelets, systemic platelet aggregation, and TTP.23 After TPE and thienopyridine discontinuation, most patients with ADAMTS13 deficiency, anti-ADAMTS13 autoantibodies, and thienopyridine-associated TTP recover. Plasma exchange may lead to removal of ULVWF multimers, removal of autoantibodies to ADAMTS13, and replacement of the ADAMTS13 with that present in fresh frozen plasma. Clinical findings also require stimulation of endothelial cells to secrete ULVWF. Such a double-insult model is exemplified by the ADAMTS13 knockout mouse, which requires endothelial cell stimulation to evoke a TTP-like microvascular thrombosis.24 In genetically predisposed individuals, thienopyridine may stimulate an autoimmune anti-ADAMTS13 antibody response and microvascular endothelial injury. Ticlopidine and clopidogrel are protein-bound in plasma and can function as haptens capable of eliciting IgE and IgG antibody formation.25 However, they do not directly bind to ADAMTS13 and stimulate production of antibodies that inhibit ADAMTS13 enzyme activity. Anti-ADAMTS13 antibodies generated in a fraction of thienopyridine-treated patients do not require the presence of the drug (or metabolite).17,22,26 Thienopyridine/anti-ADAMTS13 antibodies are analogous to warm auto-antibodies against red blood cell antigens that emerge in a subset of patients treated with the antihypertensive agent, α-methyldopa.27 Binding of thienopyridines to P2Y12 molecules on different cell types may, in a fraction of exposed individuals, initiate anomalous intracellular signaling patterns or provoke antibody production against the haptenic thienopyridine–P2Y12 protein complex on cell surfaces. Malfunction or injury to lymphocytes, CD34 + stem cells, or endothelial cells may result.
For most clopidogrel-associated and a minority of ticlopidine-associated TTP patients, the syndrome is characterized by mild thrombocytopenia, microangiopathic hemolytic anemia, and marked renal insufficiency.20,28 Onset of TTP is generally within 2 weeks of thienopyridine initiation (Figure 1a). Most cases have ULVWF in their plasma and near-normal levels of plasma ADAMTS13 metalloprotease activity during the acute phase of the syndrome, suggesting endothelial cell injury or stimulation with release of ULVWF.20 Thienopyridines may bind to P2Y12 receptors (with or without anti-thienopyridine antibodies) on CD34 + stem cells, altering cell proliferation and differentiation. Interaction of thienopyridines with endothelial cells has been shown to result in nitric oxide and possibly prostacyclin (PGI2) generation.29,30 At least some thienopyridine binding to human endothelial cells is likely to be through the P2Y12 receptors on these cells. In a few thienopyridine-treated patients, the endothelial cell response to the thienopyridine (with or without antibody) attachment may be a combination of cell injury, excessive secretion of ULVWF multimeric strings, or apoptosis.23
THERAPY
All patients who develop thienopyridine-associated TTP should have prompt plasma exchange.1,3,12,17,31,32 Plasma exchange is continued until the goals of resolution of neurologic symptoms, improvement of LDH to near normal, and achieving and maintaining for 2–3 days a platelet count of 150,000/mm3 are achieved.33 After this, plasma exchange may be either discontinued or reduced in frequency.32 Among thienopyridine-associated TTP patients who have antibody-mediated ADAMTS13 deficiency, a few days of plasma exchange are required. Although patients generally recover without permanent organ damage, a spontaneous relapse occurs occasionally. Among thienopyridine-associated TTP patients without autoantibodies against ADAMTS13, several weeks of plasma exchange are often required and spontaneous relapses are rare.3,14,17,20 One report describes a patient with a drug-eluting coronary artery stent who developed TTP within days of clopidogrel initiation. After the TTP resolved with TPE and clopidogrel discontinuation, the patient was re-challenged with ticlopidine and did not experience a TTP relapse.34
CONCLUSIONS
Thienopyridine-associated TTP has served as the focus of intensive scientific investigation over the past two decades.12,14,15,17,20,22,23,26,35,36 As with idiopathic TTP, most thienopyridine-associated TTP cases are associated with auto-antibodies that inhibit the plasma metalloprotease, ADAMTS13. For these individuals, TTP onset usually occurs within 2–12 weeks after initiation of ticlopidine (rarely clopidogrel) and resolves rapidly with TPE. For a minority of cases of thienopyridine-associated TTP, autoantibodies directed against ADAMTS13 metalloprotease have not been implicated. For these persons, the syndrome occurs within 2 weeks of initiating clopidogrel (rarely ticlopidine) and is less responsive to TPE. Prominent warnings in FDA-approved labels for ticlopidine and clopidogrel describe clinical findings, and the importance of timely initiation of plasma exchange for patient who develop this toxicity. The clinical, laboratory, and epidemiological evaluation and related pharmaceutical safety interventions for thienopyridine-associated TTP can serve as a template for future efforts to investigate and protect patients against harm from other severe adverse drug reactions.
Acknowledgments
This work was supported by grants from the National Heart Lung and Blood Institute (1R01 HL—096 717 and R01 CA102713 to CLB; P30 CA60553 to JMM). This work was supported by a grant from the Ministry and Welfare of Japan for Blood Coagulation Abnormalities (H17-02 to YF), and by a grant from the Mary Gibson Foundation (to JLM).
AZ received grant support from the CDC/MCHB. JLM received grant support from the Mary R. Gibson Foundation. NB received consulting fees from Aldagen, Inc. DWR received grant support from GlaxoSmithKline, Savient Pharmaceuticals, and Abraxis. TJR received grant support from the NIH/NHLB1. XLZ received grant support from NIH.
Footnotes
DISCLOSURE
The remaining authors have declared no financial interests.
References
- 1.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 2.Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost. 2005;31:681–690. doi: 10.1055/s-2005-925474. [DOI] [PubMed] [Google Scholar]
- 3.Zakarija A, Bandarenko N, Pandey DK, et al. Clopidogrel-associated TTP: an update of pharmacovigilance efforts conducted by independent researchers, pharmaceutical suppliers, and the Food and Drug Administration. Stroke. 2004;35:533–537. doi: 10.1161/01.STR.0000109253.66918.5E. [DOI] [PubMed] [Google Scholar]
- 4.Saste VV, Terrell DR, Vesely SK, et al. Drug-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome (TTP–HUS): frequency, presenting features, and clinical outcomes. ASH Annu Meet Abstr. 2007;110:1315. [Google Scholar]
- 5.Sharis PJ, Cannon CP, Loscalzo J. The antiplatelet effects of ticlopidine and clopidogrel. Ann Intern Med. 1998;129:394–405. doi: 10.7326/0003-4819-129-5-199809010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Berger PB, Bell MR, Grill DE, et al. Frequency of adverse clinical events in the 12 months following successful intracoronary stent placement in patients treated with aspirin and ticlopidine (without warfarin) Am J Cardiol. 1998;81:713–718. doi: 10.1016/s0002-9149(97)01005-9. [DOI] [PubMed] [Google Scholar]
- 7.Hass WK, Easton JD, Adams HP, Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 8.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 9.IMS Health, Inc. Leading Products by Global Pharmaceutical Sales, 2007. Vol. 2008. IMS Health, Inc.; 2008. [10 December 2008]. Available at http://www.imshealth.com/deployedfiles/imshealth/Global/Content/StaticFile/TopLine_Data/Top10GlobalProducts.pdf. [Google Scholar]
- 10.Savi P, Beauverger P, Labouret C, et al. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett. 1998;422:291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 11.Page Y, Tardy B, Zeni F, et al. Thrombotic thrombocytopenic purpura related to ticlopidine. Lancet. 1991;337:774–776. doi: 10.1016/0140-6736(91)91383-6. [DOI] [PubMed] [Google Scholar]
- 12.Bennett CL, Weinberg PD, Rozenberg-Ben-Dror K, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine. A review of 60 cases. Ann Intern Med. 1998;128:541–544. doi: 10.7326/0003-4819-128-7-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Bennett CL, Davidson CJ, Green D, et al. Ticlopidine and TTP after coronary stenting. JAMA. 1999;282:1717. [PubMed] [Google Scholar]
- 14.Bennett CL, Davidson CJ, Raisch DW, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch Intern Med. 1999;159:2524–2528. doi: 10.1001/archinte.159.21.2524. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Kiss JE, Weinberg PD, et al. Thrombotic thrombocytopenic purpura after stenting and ticlopidine. Lancet. 1998;352:1036–1037. doi: 10.1016/s0140-6736(05)60079-7. [DOI] [PubMed] [Google Scholar]
- 16.Steinhubl SR, Tan WA, Foody JM, et al. Incidence and clinical course of thrombotic thrombocytopenic purpura due to ticlopidine following coronary stenting. EPISTENT Investigators. Evaluation of platelet IIb/IIIa inhibitor for stenting. JAMA. 1999;281:806–810. doi: 10.1001/jama.281.9.806. [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med. 2000;342:1773–1777. doi: 10.1056/NEJM200006153422402. [DOI] [PubMed] [Google Scholar]
- 18.Clopidogrel (Plavix) [package insert] Bristol-Myers Squibb and Sanofi-Synthelabo; New York, NY: 2006. [Google Scholar]
- 19.Torok TJ, Holman RC, Chorba TL. Increasing mortality from thrombotic thrombocytopenic purpura in the United States—analysis of national mortality data, 1968–1991. Am J Hematol. 1995;50:84–90. doi: 10.1002/ajh.2830500203. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CL, Kim B, Zakarija A, et al. Two mechanistic pathways for thienopyridine-associated thrombotic thrombocytopenic purpura: a report from the SERF-TTP Research Group and the RADAR Project. J Am Coll Cardiol. 2007;50:1138–1143. doi: 10.1016/j.jacc.2007.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojouri K, Vesely SK, George JN. Quinine-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: frequency, clinical features, and long-term outcomes. Ann Intern Med. 2001;135:1047–1051. doi: 10.7326/0003-4819-135-12-200112180-00008. [DOI] [PubMed] [Google Scholar]
- 22.Tsai HM, Rice L, Sarode R, et al. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med. 2000;132:794–799. doi: 10.7326/0003-4819-132-10-200005160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauro M, Zlatopolskiy A, Raife TJ, et al. Thienopyridine-linked thrombotic microangiopathy: association with endothelial cell apoptosis and activation of MAP kinase signalling cascades. Br J Haematol. 2004;124:200–210. doi: 10.1046/j.1365-2141.2003.04743.x. [DOI] [PubMed] [Google Scholar]
- 24.Motto DG, Chauhan AK, Zhu G, et al. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camara MG, Almeda FQ. Clopidogrel (Plavix) desensitization: a case series. Catheter Cardiovasc Interv. 2005;65:525–527. doi: 10.1002/ccd.20433. [DOI] [PubMed] [Google Scholar]
- 26.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carstairs KC, Breckenridge A, Dollery CT, et al. Incidence of a positive direct Coombs test in patients on alpha-methyldopa. Lancet. 1966;2:133–135. doi: 10.1016/s0140-6736(66)92422-6. [DOI] [PubMed] [Google Scholar]
- 28.Evens AM, Kwaan HC, Kaufman DB, et al. TTP/HUS occurring in a simultaneous pancreas/kidney transplant recipient after clopidogrel treatment: evidence of a nonimmunological etiology. Transplantation. 2002;74:885–887. doi: 10.1097/00007890-200209270-00026. [DOI] [PubMed] [Google Scholar]
- 29.Ziemianin B, Olszanecki R, Uracz W, et al. Thienopyridines: effects on cultured endothelial cells. J Physiol Pharmacol. 1999;50:597–604. [PubMed] [Google Scholar]
- 30.Jakubowski A, Chlopicki S, Olszanecki R, et al. Endothelial action of thienopyridines and thienopyrimidinones in the isolated guinea pig heart. Prostagland Leukot Essent Fatty Acids. 2005;72:139–145. doi: 10.1016/j.plefa.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 32.George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood. 2000;96:1223–1229. [PubMed] [Google Scholar]
- 33.Szczepiorkowski ZM, Bandarenko N, Kim HC, et al. Guidelines on the use of therapeutic apheresis in clinical practice: evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2007;22:106–175. doi: 10.1002/jca.20129. [DOI] [PubMed] [Google Scholar]
- 34.Patel TN, Kreindel M, Lincoff AM. Use of ticlopidine and cilostazol after intracoronary drug-eluting stent placement in a patient with previous clopidogrel-induced thrombotic thrombocytopenic purpura: a case report. J Invasive Cardiol. 2006;18:E211–E213. [PubMed] [Google Scholar]
- 35.Furlan M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolyticuremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 36.Trontell AE, Honig PK. Clopidogrel and thrombotic thrombocytopenic purpura. N Engl J Med. 2000;343:1191–1192. [PubMed] [Google Scholar]
- 37.Ticlid (ticlopidine HCl) [package insert] Roche Laboratories Inc.; Nutley, NJ: 2006. [Google Scholar]
- 38.Connors JG, Robson S, Churchill WH, et al. Clopidogrel associated TTP. Transfusion. 1999;39:56S. [Google Scholar]
- 39.Carwile J, Laber DA, Soltero ER, et al. Thrombotic thrombocytopenic purpura occurring after exposure to clopidogrel. Blood. 1999;94:78b. [Google Scholar]
- 40.Wysowksi DK, Bacsanyi J. Blood dycrasias and hematologic reactions in ticlopidine users. JAMA. 1996;276:952. [PubMed] [Google Scholar]
- 41.Zakarija A, Bennett CL, Kwaan HC, et al. Idiopathic thrombotic thrombocytopenic purpura: final results from the surveillance, epidemiology, and risk factors for TTP (SERF-TTP) Blood (under review) [Google Scholar]


