Abstract
Background
Obesity is associated with an increased risk of biochemical recurrence (BCR) after radical prostatectomy (RP). It is unclear whether this is due to technical challenges related to operating on obese men or other biologic factors.
Objective
To examine whether obesity predicts higher prostate-specific antigen (PSA) nadir (as a measure of residual PSA-producing tissue) after RP and if this accounts for the greater BCR risk in obese men.
Design, setting, and participants
A retrospective analysis of 1038 RP patients from 2001 to 2010 in the multicenter US Veterans Administration–based Shared Equal Access Regional Cancer Hospital database with median follow-up of 41 mo.
Intervention
All patients underwent RP.
Outcome measurements and statistical analysis
We evaluated the relationship between body mass index (BMI) and ultrasensitive PSA nadir within 6 mo after RP. Adjusted proportional hazards models were used to examine the association between BMI and BCR with and without PSA nadir.
Results and limitations
Mean BMI was 28.5 kg/m2. Higher BMI was associated with higher PSA nadir on both univariable (p = 0.001) and multivariable analyses (p < 0.001). Increased BMI was associated with increased BCR risk (hazard ratio [HR]: 1.06; p = 0.007). Adjusting for PSA nadir slightly attenuated, but did not eliminate, this association (HR: 1.04, p = 0.043). When stratified by PSA nadir, obesity only significantly predicted BCR in men with an undetectable nadir (p = 0.006). Unfortunately, other clinically relevant end points such as metastasis or mortality were not available.
Conclusions
Obese men are more likely to have a higher PSA nadir, suggesting that either more advanced disease or technical issues confound an ideal operation. However, even after adjusting for the increased PSA nadir, obesity remained predictive of BCR, suggesting that tumors in obese men are growing faster. This provides further support for the idea that obesity is biologically associated with prostate cancer progression.
Keywords: Prostate cancer, PSA nadir, obesity, radical prostatectomy, biochemical recurrence
1. Introduction
Obesity is an increasing global concern. Obesity rates increased in the United Kingdom from 6.2% in 1982 to 22.7% in 2002 and in the United States from 13.7% in 1993 to >30% in 2008 [1,2]. Although obesity has been linked with multiple cancers including breast and colon, the relationship with the incidence of prostate cancer (PCa) remains unclear [3–5]. Some studies suggest obesity may be associated with decreased PCa risk; others suggest an increased risk of diagnosis, larger tumors, more aggressive disease, and PCa-related mortality [6–9].
We previously found obese men have an increased risk for developing biochemical recurrence (BCR) and positive margins after radical prostatectomy (RP) [10,11]. One possible explanation for the higher BCR risk in the absence of pathologic differences may relate to technical difficulties in dissecting the prostate in obese men, leading to greater positive margins and residual tumor. Indeed, one study found that even experienced surgeons were more likely to have a capsular incision in obese men, a sign of a less than perfect operation [12]. To address this, we previously examined men undergoing RP with negative margins where obesity remained predictive of BCR [13].
A more accurate way of assessing the amount of residual tumor is to measure postsurgical prostate-specific antigen (PSA) nadir rather than relying on margin status. Residual PSA can result from either advanced disease or poor surgical technique. Regardless, in men undergoing RP, persistently elevated PSA is linked to BCR, disease progression, and overall mortality [14–17]. Importantly, we previously showed that higher BMI predicts higher PSA nadir [15]. As such, obese men have a “headstart” toward developing BCR. Therefore, the purpose of our present study was to evaluate whether higher PSA nadirs among obese men explain the higher BCR rates or whether obesity remains predictive of BCR after adjusting for PSA nadir, which would suggest that tumors in obese men grow faster.
2. Methods
2.1. Study population
Following institutional review board approval, data were collected from men undergoing RP from 1988 to 2010 at four US Veterans Administration hospitals in West Los Angeles and Palo Alto (CA), Augusta (GA), and Durham (NC) and compiled into the Shared Equal Access Regional Cancer Hospital (SEARCH) database [18]. Men treated with preoperative androgen deprivation or radiation therapy were excluded. Within SEARCH, ultrasensitive PSA nadir (detection limit <0.01 ng/ml) was available as of 2001 in two centers, as of 2002 in one center, and as of 2004 in the fourth center. Using these cut-offs, we identified 1224 eligible men. We excluded men missing data for BMI (n = 14), PSA nadir (n = 68), preoperative PSA (n = 11), pathologic prostatic weight (n = 77), Gleason score (n = 2), margin status (n = 4), seminal vesicle invasion (n = 4), and extracapsular extension (n = 6), resulting in a final population of 1038.
2.2. Statistical analysis
BMI was abstracted from preoperative height and weight and categorized as normal (<25 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2). The association between BMI as a categorized variable and baseline clinical and pathologic features were tested using chi square for categorical variables, analysis of variance (ANOVA) for normally distributed continuous variables, and Kruskal-Wallis for non-normally distributed continuous variables. Normally distributed variables are presented as mean plus or minus standard deviation (SD), and non-normally distributed variables are presented as median and interquartile range.
To determine the p trend, BMI was entered into models as a continuous variable with each patient assigned the median BMI of his category to limit undue influence of extreme BMI values. Similarly, PSA nadir within 6 mo after RP was evaluated as a continuous variable of the median nadir within each of the following categories: undetectable, 0.01–0.09, 0.10–0.19, and ≥0.2 ng/ml. Age, RP year, PSA, and prostate weight were evaluated as continuous variables. PSA and prostate weight were logarithmically transformed. Center, race (black, white, other), Gleason scores (2–6, 3 + 4, ≥4 + 3) were analyzed as categorical variables. BCR was defined as a single postoperative PSA value >0.2 ng/ml, two values of 0.2 ng/ml, or secondary treatment for elevated PSA.
The association between BMI and PSA nadir was compared using univariable and multivariable linear regression adjusting for clinical (age, PSA, center, RP year, race) and pathologic variables (Gleason, prostate weight, extracapsular extension, margin status, seminal vesicle invasion, and lymph node status). We tested the relative risk for BCR associated with BMI using a Cox proportional hazards regression model, adjusting for clinicopathologic characteristics with and without PSA nadir. Data were examined as a whole and stratified by PSA nadir (undetectable vs 0.01–0.19 vs ≥0.2 ng/ml). Statistical analyses were performed using Stata v.11.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patient demographics
The mean plus or minus SD BMI was 28.5 ± 4.7 kg/m2, and mean age was 60 ± 6 yr. Of the 1038 men in the study, 591 (57%) had an undetectable PSA nadir, 336 (32%) had a nadir between 0.01 and 0.09, 43 (4%) had a nadir between 0.1 and 0.19, and 68 (7%) had a nadir ≥0.2 ng/ml.
Patients with a higher BMI were younger (p < 0.001), more likely to be treated in recent years (p = 0.019), and had about a 4-mo shorter median follow-up (p = 0.007) as determined by either the ANOVA or Kruskal-Wallis test (Table 1). Additionally, men with a higher BMI had lower PSA levels (p = 0.022) despite larger prostate sizes (p = 0.0003). Finally, there were nonsignificant trends for higher BMI to be associated with higher Gleason scores and seminal vesicle invasion.
Table 1.
Clinical and pathologic features of 1038 men in the SEARCH database with available prostate-specific antigen nadir data, sorted by body mass index
| Features | Normal weight <25 kg/m2 | Overweight 25.0–29.9 kg/m2 | Obese ≥30 kg/m2 | p* |
|---|---|---|---|---|
| No. of patients (%) | 228 (22) | 473 (46) | 337 (32) | |
|
| ||||
| Year of surgery, median (IQR) | 2005 (2003–2007) | 2005 (2003–2007) | 2006 (2004–2007) | 0.0185† |
|
| ||||
| Follow-up, mo, median (IQR) | 44.1(19.5–69.9) | 42.1(20.7–66.4) | 40.4(15.6–61.8) | 0.0071† |
|
| ||||
| Age, yr, mean ± SD | 61.1 ± 6.0 | 60.9 ± 6.0 | 59.5 ± 5.9 | 0.0008‡ |
|
| ||||
| Race (%) | 0.293 | |||
| White | 103 (45) | 235 (50) | 156 (46) | |
| Black | 113 (50) | 208 (44) | 168 (50) | |
| Other | 12 (5) | 30 (6) | 13 (4) | |
|
| ||||
| PSA, ng/ml, median (IQR) | 6.7 (5.0–10.0) | 5.9 (4.5–8.4) | 5.8 (4.5–8.6) | 0.0219† |
|
| ||||
| Pathologic Gleason sum (%) | 0.077 | |||
| 2–6 | 80 (35) | 181 (38) | 97 (29) | |
| 3 + 4 | 102 (45) | 193 (41) | 165 (49) | |
| ≥4 + 3 | 46 (20) | 99 (21) | 75 (22) | |
|
| ||||
| Prostate weight, g, median (IQR) | 37.8 (30.0–48.3) | 40.0 (32.1–51.4) | 43.0 (33.4–55.0) | 0.0003† |
|
| ||||
| Positive surgical margin (%) | 101 (44) | 213 (45) | 158 (47) | 0.8048 |
|
| ||||
| Capsular penetration (%) | 36 (16) | 83 (18) | 56 (17) | 0.8352 |
|
| ||||
| Seminal vesicle invasion (%) | 10 (4) | 42 (9) | 25 (7) | 0.104 |
|
| ||||
| Lymph node involvement (%) | 1 (0.4) | 7 (1.5) | 10 (3.0) | 0.208 |
|
| ||||
| PSA nadir, ng/ml | 0.055 | |||
| 0 (undetectable) | 140 (61) | 269 (57) | 182 (54) | |
| 0.01–0.09 | 72 (32) | 156 (33) | 108 (32) | |
| 0.1–0.19 | 10 (4) | 19 (4) | 14 (4) | |
| ≥0.2 | 6 (3) | 29 (6) | 33 (10) | |
IQR = interquartile range; SD = standard deviation; PSA = prostate-specific antigen.
p value by chi square, except where noted.
p value by Kruskal-Wallis.
p value by analysis of variance.
3.2. Obesity and prostate-specific antigen nadir
When grouped by categories, there was a nonsignificant trend for higher BMI to be associated with higher PSA nadir (p = 0.055; chi square) (Table 1). However, when BMI and PSA nadir were treated as continuous variables using the median of each category, there was a clear association between higher BMI and greater PSA nadir using linear regression (p = 0.001) (Table 2). This association remained after adjustment for multiple demographic and clinicopathologic characteristics (p < 0.001).
Table 2.
Linear regression: predictors of prostate-specific antigen nadir relative to normal weight
| Factor | Coefficient | 95% CI | p trend |
|---|---|---|---|
| Univariable | 0.001† | ||
| Overweight (BMI 25.0–29.9 kg/m2) | 0.018 | −0.002 to 0.039 | |
| Obese (BMI ≥30 kg/m2) | 0.038 | 0.016–0.060 | |
| Multivariable* | <0.001† | ||
| Overweight (BMI 25.0–29.9 kg/m2) | 0.023 | 0.003–0.043 | |
| Obese (BMI ≥30 kg/m2) | 0.048 | 0.026–0.069 |
CI = confidence interval; BMI = body mass index.
Adjusting for year of surgery, center, age, race, prostate-specific antigen, pathologic prostatic weight, Gleason score, extracapsular extension, seminal vesicle invasion, margin status, and nodal status.
p trend calculated using median BMI of each category as a continuous variable.
3.3. Biochemical recurrence
Mean and median follow-up in men without BCR was 44 and 41 mo, respectively. During this time, 262 patients (25%) developed BCR with a median recurrence time of 10 mo. Using Cox proportional hazard models, increased BMI was associated with increased BCR risk (hazard ratio [HR]: 1.06; p < 0.007; Fig. 1) on multivariable analysis when not adjusted for PSA nadir (Table 3). Adjusting for PSA nadir mildly attenuated, but did not eliminate, this association (HR: 1.04; p = 0.043).
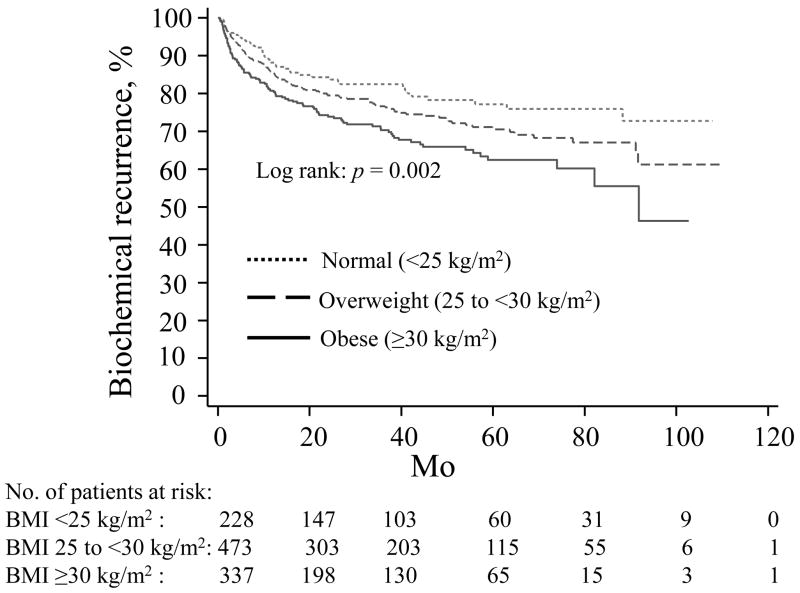
Fig. 1.
Time until biochemical recurrence stratified by body mass index (BMI). Obese men with BMI ≥ 30 kg/m2 (solid line) and overweight men with BMI 25.0–29.9 kg/m2 (dashed line) had a greater risk of biochemical recurrence compared with normal weight men with BMI <25 kg/m2 (dotted line).
Table 3.
Multivariable models of time to biochemical recurrence after radical prostatectomy by body mass index relative to normal weight
| Cox proportional hazards | HR | 95% CI | p trend |
|---|---|---|---|
| Multivariable model not including PSA nadir | 0.007* | ||
| Normal weight (BMI ≤25 kg/m2) | 1.00 | – | |
| Overweight (BMI 25.0–29.9 kg/m2) | 1.21 | 0.81–1.79 | |
| Obese (BMI ≥30 kg/m2) | 1.73 | 1.12–2.66 | |
| Multivariable model including PSA nadir | 0.043* | ||
| Overweight (BMI 25.0–29.9 kg/m2) | 1.10 | 0.74–1.64 | |
| Obese (BMI ≥30 kg/m2) | 1.49 | 0.97–2.30 |
HR = hazard ratio; CI = confidence interval; BMI = body mass index; PSA = prostate-specific antigen.
All analyses adjust for age, race, PSA, surgical center, year of surgery, pathologic prostatic weight, Gleason sum, margin status, extraprostatic extension, and seminal vesicle invasion.
p trend calculated using median BMI of each category as a continuous variable.
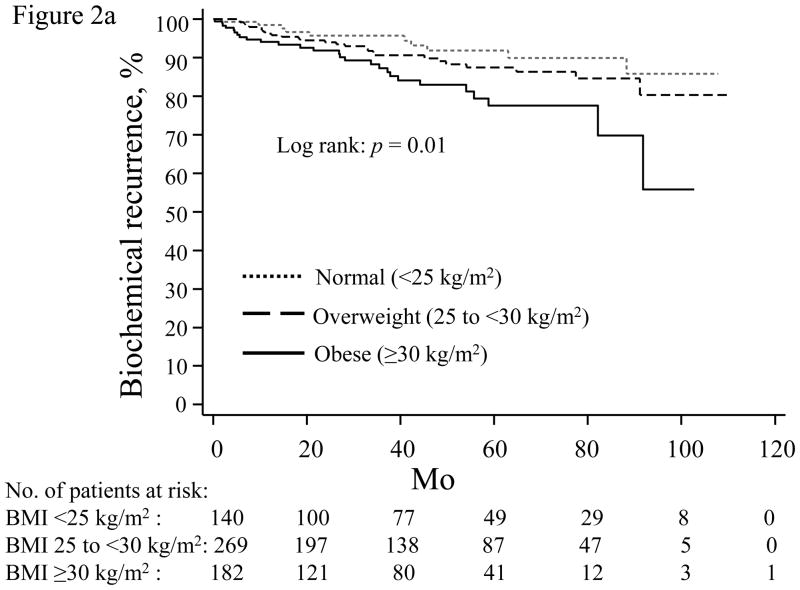
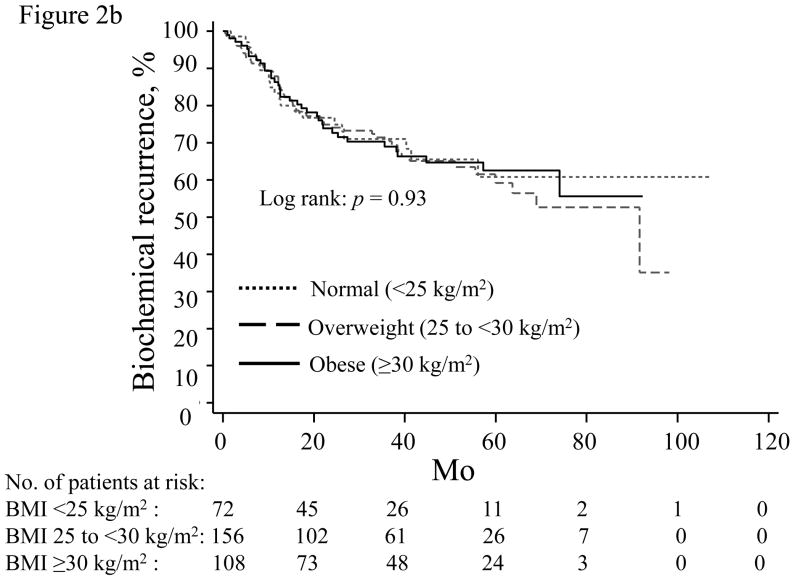
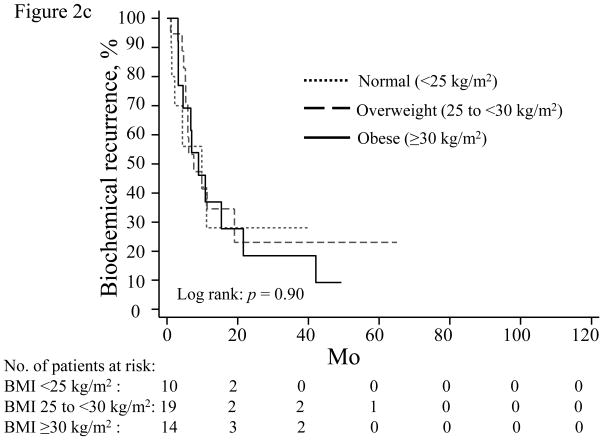
When stratified by PSA nadir, increasing BMI was most strongly associated with higher BCR risk in men with an undetectable PSA nadir (Table 4; Fig. 2a). For men with a detectable PSA nadir, higher BMI trended toward increased recurrence risk for those with a PSA nadir of 0.01–0.09 (Table 4; Fig. 2b), but not among men with a nadir of 0.10–0.19 ng/ml (Table 4; Fig. 2c).
Table 4.
Hazard ratio of time to biochemical recurrence after radical prostatectomy stratified by prostate-specific antigen nadir and body mass index
| Cox proportional hazards | HR | 95% CI | p trend |
|---|---|---|---|
| PSA nadir 0 (undetectable) (n = 591) | 0.006* | ||
| Overweight, BMI 25.0–29.9 kg/m2 | 1.23 | 0.57–2.65 | |
| Obese, BMI ≥30 kg/m2 | 2.62 | 1.18–5.83 | |
| PSA nadir 0.01–0.09 (n = 336) | 0.090* | ||
| Overweight, BMI 25.0–29.9 kg/m2 | 1.48 | 0.82–2.67 | |
| Obese, BMI ≥30 kg/m2 | 1.80 | 0.94–3.42 | |
| PSA nadir 0.10–0.19 (n = 43) | 0.914* | ||
| Overweight, BMI 25.0–29.9 kg/m2 | 0.43 | 0.09–2.20 | |
| Obese, BMI ≥30 kg/m2 | 0.85 | 0.19–3.81 |
HR = hazard ratio; CI = confidence interval; BMI = body mass index; PSA = prostate-specific antigen.
All analyses adjust for age, race, PSA, surgical center, year of surgery, pathologic prostatic weight, Gleason sum, margin status, extraprostatic extension, and seminal vesicle invasion.
p trend calculated using median BMI of each category as a continuous variable.
Fig. 2.
Time until biochemical recurrence in men stratified by prostate-specific antigen nadir: (a) undetectable, <0.01 ng/ml; (b) 0.01–0.09 ng/ml; (c) 0.10–0.19 ng/ml; normal weight (dotted line), overweight (dashed line), obese (solid line). BMI = body mass index.
4. Discussion
Obese men are at a higher BCR risk following RP [10,19,20]. This may be due, in part, to poor surgical technique and a greater risk of positive margins [11,12]. However, in men undergoing RP with negative margins, obesity remained a predictor of BCR [13]. Thus the degree to which technical difficulties of operating on obese men lead to higher BCR rates is unknown. In this study, we used ultrasensitive PSA nadir rather than margin status as a more accurate way of evaluating the effect of obesity on our ability to remove all PSA-producing tissue, accounting for both more advanced disease and poor technique. Although we found obesity predicted higher PSA nadirs, increased PSA nadir does not completely account for the higher BCR risk in men with higher BMI. These data support the idea that obesity is associated with faster growing tumors and more aggressive PCa.
Obesity affects >30% of adults in the United States. In 2010, it was estimated that >217 000 new cases of PCa would be diagnosed, translating to approximately 64 000 obese men with PCa, assuming no major effect of obesity on PCa incidence [21]. Likewise, >380 000 new cases of PCa were diagnosed in Europe in 2008, with obesity rates >20% in the United Kingdom [22]. There are reasons to suspect obesity may hinder an ideal operation: Excess abdominal and pelvic fat may interfere with the surgeon’s ability to visualize and remove the entire prostate and tumor. If obesity does present a technical challenge to an ideal operation, it potentially has an impact on thousands of obese patients diagnosed with PCa annually.
Obesity is associated with higher rates of positive margins in the absence of other adverse pathologic features [11,20]. Although surgical skill is known to have an impact on outcomes [23], obesity was also shown to be positively associated with capsular incision, a pathologic surrogate of a technically inferior operation, even in experienced hands [12]. This suggests the greater likelihood of a less ideal operation in obese patients.
We examined whether obesity was associated with a technically inferior operation and/or more advanced disease using ultrasensitive PSA nadir, a more accurate way of measuring residual tumor after surgery. In men undergoing RP from 2001 to 2008 in the SEARCH database, we previously reported that higher BMI was significantly predictive of PSA persistence (PSA nadir ≥0.03 ng/ml) [15]. Using data updated through 2010, we confirmed that when evaluated as a continuous variable, higher BMI predicts increased PSA nadir. Various studies, including results from SEARCH, found that persistently elevated PSA is associated with BCR and overall mortality [14,24]. We found that being obese compared with normal weight predicts an approximately 0.05 ng/ml increase in PSA nadir. Comparatively, Eisenberg et al [25] and Moreira et al [15] reported that PSA levels persistently >0.05 and ≥0.03 ng/ml, respectively, were associated with increased BCR risk. Others have also linked persistently detectable PSA with other markers of high-risk disease including higher Gleason scores, positive margins, extracapsular extension, seminal vesicle invasion, and even increased PCa-specific mortality [14,26]. Thus simply being obese increases the risk for poor prognostic disease due to the increased likelihood of higher PSA nadirs compared with patients with normal BMI. Consistent with prior studies, we also found that obesity was associated with BCR [10,19]. However, given the association between obesity and higher PSA nadir and between elevated PSA nadir and BCR, it is not known whether the higher nadir accounts for the increased BCR rates. We tested this by subsequently controlling for PSA nadir in our model, which attenuated but did not eliminate the association between BMI and increased BCR risk. This suggests that although obesity may present technical challenges during surgery or be associated with more advanced disease (ie, micrometastases) as evidenced by higher PSA nadir levels, it does not fully explain the greater BCR rates among men with higher BMI. As such, these data provide further support for the idea that obesity is biologically associated with faster tumor growth and PCa progression.
Interestingly, when stratified by PSA nadir, increasing BMI was most strongly associated with higher BCR risk in patients with an undetectable PSA nadir. Meanwhile, in men with a detectable nadir, higher BMI trended toward increased recurrence risk for men with a PSA nadir of 0.01–0.09 (p = 0.09) but not among men with a nadir of 0.10–0.19 ng/ml. We previously noted that men with a PSA nadir of 0.10–0.19 ng/ml are ≥11 times more likely to develop BCR than men with an undetectable nadir [15]. Recurrence rates for these men approached 80% by 4 yr. Thus it is possible that with such a high-risk group, the addition of obesity, a modest risk factor for aggressive disease, adds little to risk. Alternatively, men with an undetectable nadir have a favorable prognosis with a 5-yr recurrence risk <20%. For these men, the addition of even a modest risk factor like obesity can lead to significantly poorer outcome. In other words, although obesity is indeed associated with aggressive disease, the association is less strong than other risk factors such as nadir PSA. However, this does not negate the importance of BMI because >55% of men had an undetectable nadir, and for these men, BMI predicts poor outcome. Ultimately, longer follow-up will be intriguing to determine whether BMI increases risk of more distant events such as metastases and PCa-related death even for the so-called high-risk group. Several mechanisms have been proposed to account for the increased risk of aggressive PCa in obese patients. One hypothesis suggests that excess adipose tissue affects the hormonal axis by decreasing free testosterone and sex hormone-binding globulin, which has been associated with worse pathologic stage in men with PCa [27,28]. Elevated blood insulin levels and insulin growth factors secondary to increased abdominal obesity may facilitate PCa growth [29]. Delayed diagnosis in obese men may lead to higher grade disease at diagnosis. Given the modern dependence on PSA-based screening, lower PSA levels due to PSA hemodilution in obese men is one possible mechanism for delayed diagnosis [30]. Obese men also have larger prostates, which may make it more difficult to find the PCa on biopsy [7]. Ultimately, the fact that obesity has been linked with PCa mortality since the 1960s [9], which predates PSA screening and the widespread use of RP, provides compelling data for a biologic link, although the exact mechanisms by which obesity exerts its effects are not fully understood.
Our study has several limitations including the retrospective nature of the cohort. Our analysis was limited to patients who underwent RP, who represent a selected group of men with PCa. Thus we cannot assess the relationship between obesity and advanced PCa. Also, the rate of positive margins was high. How this may have influenced our results is unknown, but it requires validation in centers with lower positive margin rates. Furthermore, although we correlated PSA nadir and obesity to BCR, not all patients who develop recurrence will metastasize and die from PCa. Future studies should be directed at using other clinically relevant end points including metastasis, PCa-specific mortality, and overall mortality. Finally, these results, like all results, are subject to type 1 error and residual confounding, and thus they require validation in other data sets. These limitations, however, are balanced by a key strength of limiting our sample to men who were managed using ultrasensitive PSA nadir, with a sensitivity limit <0.01 ng/ml, allowing us to detect even modest associations between BMI and PSA nadir.
5. Conclusions
Obese men are more likely to have higher PSA nadir, suggesting either more advanced disease or technical issues confounding an ideal operation. However, even after adjusting for the increased PSA nadir, obesity remained predictive of BCR, suggesting tumors in obese men are growing faster and providing further support for the idea that obesity is biologically associated with PCa progression.
Take-home message.
Obesity appears to be related to either more advanced disease or inferior operative technique. Despite these factors, tumors are likely faster growing in obese men, supporting a biological association between obesity and prostate cancer progression.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by the US Department of Veterans Affairs, US Department of Defense Prostate Cancer Research Program, the American Urological Association Foundation/Astellas Rising Star in Urology Award, National Institutes of Health Specialized Programs of Research Excellence Grant P50 CA58236, the Georgia Cancer Coalition, National Institutes of Health R01CA100938, and National Institutes of Health Specialized Programs of Research Excellence Grant P50 CA92131-01A1. Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ho.
Acquisition of data: Ho, Gerber, Aronson, Terris, Presti, Kane, Amling, Freedland.
Analysis and interpretation of data: Ho, Freedland.
Drafting of the manuscript: Ho, Freedland.
Critical revision of the manuscript for important intellectual content: Ho, Gerber, Aronson, Terris, Presti, Kane, Amling, Freedland.
Statistical analysis: Ho, Freedland.
Obtaining funding: Ho, Freedland.
Administrative, technical, or material support: Gerber.
Supervision: Freedland.
Other (specify): None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Global database on body mass index. [Accessed January 25, 2011.];World Health Organization. Web site http://apps.who.int/bmi/index.jsp.
- 3.Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–42. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laake I, Thune I, Selmer R, Tretli S, Slattery ML, Veierod MB. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev. 2010;19:1511–22. doi: 10.1158/1055-9965.EPI-09-0813. [DOI] [PubMed] [Google Scholar]
- 5.Nomura AM. Body size and prostate cancer. Epidemiol Rev. 2001;23:126–31. doi: 10.1093/oxfordjournals.epirev.a000777. [DOI] [PubMed] [Google Scholar]
- 6.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–9. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12:259–63. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003;95:1240–4. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–53. [PubMed] [Google Scholar]
- 10.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol. 2005;174:919–22. doi: 10.1097/01.ju.0000169459.78982.d7. [DOI] [PubMed] [Google Scholar]
- 12.Freedland SJ, Grubb KA, Yiu SK, et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 2005;174:1798–801. doi: 10.1097/01.ju.0000177077.53037.72. [DOI] [PubMed] [Google Scholar]
- 13.Freedland SJ, Terris MK, Presti JC, Jr, et al. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172:520–4. doi: 10.1097/01.ju.0000135302.58378.ae. [DOI] [PubMed] [Google Scholar]
- 14.Moreira DM, Presti JC, Jr, Aronson WJ, et al. Natural history of persistently elevated prostate specific antigen after radical prostatectomy: results from the SEARCH database. J Urol. 2009;182:2250–5. doi: 10.1016/j.juro.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Moreira DM, Presti JC, Jr, Aronson WJ, et al. Definition and preoperative predictors of persistently elevated prostate-specific antigen after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2010;105:1541–7. doi: 10.1111/j.1464-410X.2009.09016.x. [DOI] [PubMed] [Google Scholar]
- 16.Rogers CG, Khan MA, Craig Miller M, Veltri RW, Partin AW. Natural history of disease progression in patients who fail to achieve an undetectable prostate-specific antigen level after undergoing radical prostatectomy. Cancer. 2004;101:2549–56. doi: 10.1002/cncr.20637. [DOI] [PubMed] [Google Scholar]
- 17.Shen S, Lepor H, Yaffee R, Taneja SS. Ultrasensitive serum prostate specific antigen nadir accurately predicts the risk of early relapse after radical prostatectomy. J Urol. 2005;173:777–80. doi: 10.1097/01.ju.0000153619.33446.60. [DOI] [PubMed] [Google Scholar]
- 18.Moreira DM, Presti JC, Jr, Aronson WJ, et al. The effect of race on the discriminatory accuracy of models to predict biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. Prostate Cancer Prostatic Dis. 2010;13:87–93. doi: 10.1038/pcan.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–45. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 20.Freedland SJ, Sun L, Kane CJ, et al. Obesity and oncological outcome after radical prostatectomy: impact of prostate-specific antigen-based prostate cancer screening: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. BJU Int. 2008;102:969–74. doi: 10.1111/j.1464-410X.2008.07934.x. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 22.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 24.Naselli A, Introini C, Andreatta R, Spina B, Truini M, Puppo P. Prognostic factors of persistently detectable PSA after radical prostatectomy. Int J Urol. 2009;16:82–6. doi: 10.1111/j.1442-2042.2008.02198.x. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg ML, Davies BJ, Cooperberg MR, Cowan JE, Carroll PR. Prognostic implications of an undetectable ultrasensitive prostate-specific antigen level after radical prostatectomy. Eur Urol. 2010;57:622–9. doi: 10.1016/j.eururo.2009.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta S, Christensen CM, Zincke H, et al. Detectable PSA between 60 and 120 days following RP for prostate cancer: natural history and prognostic significance. J Urol. 2006;176:559–63. doi: 10.1016/j.juro.2006.03.086. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer? J Urol. 2000;163:824–7. [PubMed] [Google Scholar]
- 28.Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–4. doi: 10.1016/0026-0495(91)90199-7. [DOI] [PubMed] [Google Scholar]
- 29.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–5. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 30.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]