SUMMARY
Parkinson’s disease (PD) is typified by the loss of midbrain dopamine neurons, the presence of large proteinaceous α-synuclein-positive intracellular inclusions, oxidatively modified molecules and activated microglia. The etiology of sporadic PD is not fully understood but several lines of evidence suggest that genetic vulnerability and environmental toxicants converge to incite pathology-the multiple hit hypothesis. One gene linked to both familial and sporadic PD is SNCA, which encodes for the protein α-synuclein that has a propensity to misfold into toxic moieties. Here we show that α-synuclein directly activates microglia inciting the production of proinflammatory molecules and altering the expression of Toll-like receptors (TLRs). We discuss the role for α-synuclein-directed TLR expression changes in PD and the therapeutic potential of modifying this response.
Keywords: Microglia, Innate immunity, Classical activation, Glia, Synucleinopathy
1. Introduction
Parkinson’s disease (PD) is the most common synucleinopathy affecting approximately five million people worldwide. Sporadic as well as familial forms of this disease exist; however, the sporadic form accounts for over 90% of all PD patients. This disorder is typified by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), the presence of dystrophic projections to the striatum, intracytoplasmic neuronal proteinaceous inclusions containing large amounts of α-synuclein (Lewy bodies) and activated microglia.
Microglia are highly conserved across evolution and serve immune-like functions in the brain, continuously monitoring and reacting to their microenvironment recognizing and responding to foreign material. Engagement of microglia with foreign substances results in activation mediated by pattern recognition receptors (PRRs) found on the cell surface as well as on endosomal membranes. These receptors which include families of scavenger receptors and Toll-like receptors (TLRs), typically identify and bind pathogen-associated molecular patterns (PAMPs) such as bacterial- and viral-derived carbohydrates, nucleic acids and lipoproteins. Upon activation microglia can release anti-inflammatory (e.g., arginase 1; transforming growth factor β) or pro-inflammatory molecules (e.g., tumor necrosis factor-α [TNF-α]; interleukin-1β [IL-1β]; nitric oxide; superoxide) depending on the activation pathway engaged [1]. When a proinflammatory pathway is activated, microglia contribute to oxidative stress in the microenvironment through release of cytokines and reactive oxygen species that can adversely impact adjacent neurons [2]. As activated microglia are found in the brains of PD patients in increased numbers compared to controls, it is important to understand how they impact dopaminergic neurons as well as contribute to the pathogenesis of the disease.
2. Inflammation in Parkinson’s disease patients and animal models
Signs of inflammation and immune abnormalities have long been reported in PD patients including the presence of activated microglia, but the mechanism and role of this activation remains controversial [3]. In 2001, Nishimura et al. reported that polymorphisms in the TNF-α gene correlated with the early onset of sporadic PD in a Japanese cohort [4]. A recent genome-wide association study (GWAS) identified an association between sporadic PD and a major histocompatibility complex cell surface receptor region on chromosome 6, further supporting a role for innate inflammation in PD pathogenesis [5]. In addition, positron emission tomography with [11C](R)-PK11195 of PD patients exhibiting a reduced dopamine content due to presynaptic terminal loss showed an over six-fold increase in activated microglia compared to control patients [6]. It is intriguing that case reports and epidemiological studies indicate a correlation between the development of PD late in life and early life brain injuries, implying that brain inflammation, and more specifically microglial activation, may play a critical role in the early stages of PD pathogenesis (reviewed in [7]).
Although proinflammatory molecules are secreted from neurons, microglia and astrocytes, here we focus on inflammation driven by microglia and the vulnerability of the SNpc dopamine neurons. Microglia are more abundant in the SN than in other brain regions and the enriched oxidative environment of the SNpc is thought to enhance this region’s susceptibility to neurodegeneration. Furthermore, the evidence that microglia are activated in mouse, rat and non-human primate models of PD prior to frank neuron death is substantial. Both in vitro and in vivo studies demonstrate that compared with neurons in other brain regions, SNpc neurons are more sensitive to inflammation-dependent damage and this sensitivity is correlated with the quantity of microglia present. For example, in the LPS animal model of PD, there is selective degeneration of dopaminergic neurons in the SNpc while GABAergic and serotonergic neurons remain intact. Additionally, this damage results after a single LPS insult, demonstrating that brief exposure to an initiating event can lead to permanent and progressive cell loss [8]. Other neurotoxicant and genetic animal models of PD also exhibit microglial activation prior to cell death suggesting that this event is important for disease progression [9]. What we do not fully understand are the mechanisms leading to inflammation in this neurodegenerative disorder. Here we suggest that a key protein in PD, α-synuclein, incites microglial activation early in disease and later, along with the innate immune response, this protein plays a critical role in the progression of PD.
3. Relationship between α-synuclein and microglial activation
One major component of Lewy bodies and Lewy neurites is fibrillar α-synuclein [10]. This protein was first implicated in PD pathogenesis when point mutations and overexpression of the α-synuclein gene, SNCA, were associated with familial forms of this disorder. Importantly, GWAS have linked SNCA polymorphisms with an increased risk for developing sporadic PD [5]. Moreover, evidence suggests that PD pathogenesis is closely linked with a toxic gain-of-function of α-synuclein due to this protein’s tendency to change conformation.
α-Synuclein misfolds into protofibrils and higher order oligomers following changes in pH and ionic strength, increases in molecular crowding, and interactions with lipid membranes as well as secondary modification such as dopamine adduction, nitrosylation and phosphorylation (reviewed in [11]). Studies suggest that the pathological role of α-synuclein is linked to this ability to misfold and self-assemble into higher-order structures. In cell culture models, α-synuclein-induced cell death has been associated with the formation of oligomeric α-synuclein, increased cell membrane conductance, mitochondrial, lysosomal and proteasomal dysfunction, and microglial activation [9,12–14]. A major consequence of these synuclein-mediated perturbations is an overall increase in oxidative stress which can result from neuronal production of reactive oxygen species (ROS), decreased anti-oxidant responses as well as increased ROS from surrounding activated microglia [2]. Importantly, α-synuclein can be released from cells where it would be available to interact with surveilling microglia [15]. An important lingering question is how does α-synuclein activate microglia? In an in vitro system utilizing microglia derived from mice devoid of the pattern recognition receptor, CD36, α-synuclein-directed activation of microglia was diminished [9]. The partial inhibition of activation could be due to an inability of CD36 knockout mice to mount a “normal” innate immune response, the ability of microglia to employ other PRRs, and/or the propensity for α-synuclein to misfold into a variety of conformations each of which may be capable of binding different PRRs. We suggest that the identification of the specific PRRs engaged following exposure to defined conformations of α-synuclein would enable the development of clinically relevant therapeutics for synucleinopathies including PD. We have demonstrated that misfolded α-synuclein composed of monomers, dimers and oligomers of ~250 kDa causes direct microglial activation with classical cytokine upregulation, morphological changes, increased expression of antioxidant response enzymes and alterations in TLR gene expression (Figure 1; Table 1; ref. [12]). To date, few studies have addressed the mechanism by which different conformations of α-synuclein directly activate the innate immune system (e.g., microglia and the complement system).
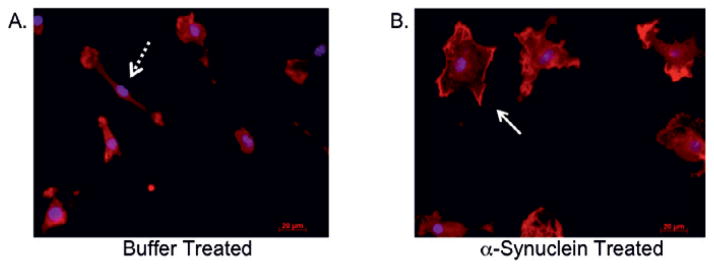
Fig. 1.
α-Synuclein activates primary microglia. Primary microglia derived from C57/BL6 mouse cortices were treated with buffer (A) or 50nM α-synuclein (B) for 24 hr as previously described [12]. Cells were subsequently fixed and subjected to immunocytochemistry using anti-Iba-1 primary antibody (1:750/Wako Chemicals) followed by incubation with goat anti-rabbit fluorescent secondary antibody. 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nuclei. The dashed arrow (A) points to a ramified microglia prototypic of a non-activated cell while the solid arrow (B) points to an amoeboid shaped cell, the typical morphology of classically activated microglia. All animals used (Figures 1, 2; Table 1) were maintained and treated in accordance with the regulatory standards of the Animal Welfare Act and approved for use by the Georgetown University Animal Care and Use Committee.
Table 1.
α-Synuclein-mediated effects on microglia
| Protein/Gene | Cell Typea | α-synuclein induced effectsb |
|---|---|---|
| TNF-α | PMG | 2163 pg/mlc |
| MMP-9 | PMG | 1.3 pg/mlc |
| HO-1 | BV-2 | 20-fold increase |
| NO | BV-2 | 71μMc |
| IL-1β | PMG | 240-fold upregulation |
| TNF-α | PMG | 16-fold upregulation |
| TLR1 | PMG | 9-fold upregulation |
| TLR2 | PMG | 7-fold upregulation |
| TLR3 | PMG | 4-fold upregulation |
| TLR4 | PMG | 1.7-fold downregulation |
| TLR6 | PMG | No change |
| TLR7 | PMG | 1.4-fold upregulation |
| TLR9 | PMG | No change |
| MYD88 | PMG | 3-fold upregulation |
Primary microglia (PMG) or BV-2 microglia were treated with 50 nM of α-synuclein or buffer for 24hr following methods previously described [12].
Outcome measures include: ELISA to determine TNF-α and MMP-9 secretion; Greiss reagent assay to determine nitric oxide (NO) release; Western blot followed by densitometry to determine HO-1protein levels in cells; quantitative rtPCR to assess gene expression changes for TNF-α, IL-1β, and TLRs relative to buffer control treated microglia.
Values were obtained following α-synuclein treatment of the defined cell type, PMG or BV-2 and are significantly greater than cells treated with buffer control (p < 0.05). The data in this table was adapted from our previously published work [12] except for the MMP-9 data which appears here for the first time.
4. TLRs as therapeutic targets for microglial activation
TLRs are activated by PAMPs but more recently sterile non-pathogen related forms of inflammation in which endogenous disease-related signals (damage-associated molecular patterns; DAMPs) drive microglial activation have also been associated with TLRs. As discussed above, α-synuclein is an established activator of microglia inducing the expression and release of molecules most closely associated with classical microglial proinflammation including a subset of TLRs. However, the precise temporal progression of inflammation in relationship to neuronal death has yet to be fully established for PD. In fact, some level of microglial activation is beneficial to the brain enabling the removal of cellular debris from dying neurons and/or glia. It is perhaps this duality of function that has led to the many disparate findings when pan anti-inflammatory drugs were used as potential therapeutics for neurodegenerative diseases. We suggest that characterization of the particular microglial pathways activated during pathogenesis including establishing the earliest signals (e.g., TLR activation; α-synuclein misfolding) will facilitate the development of targeted effective therapies for neurodegenerative disorders like PD.
In Figure 2, we outline our hypothesis that early activation of microglia during neurodegenerative disease pathogenesis is mediated by an upregulation of TLRs. We have recently shown that extracellular α-synuclein may act as a DAMP for microglia, increasing the expression of TLR-1, -2, -3 and -7, MyD88, MMP-9, TNFα and IL-1β (Table 1; ref. [12]), whereas Zhang et al. demonstrated that extracellular misfolded α-synuclein enhanced prostaglandin E2 levels but not TNF-α [16]. The discrepancy between these studies could arise from the different types of α-synuclein used which we believe need to be standardized for the α-synuclein field to move forward. Importantly, in vivo studies demonstrate an early increase in various markers of classical microglial activation in both transgenic mice that overexpress human α-synuclein and following viral transduction of the substantia nigra dopamine neurons with a recombinant adeno-associated virus vector expressing human α-synuclein [9,17]. Both of these studies suggest that early inflammation may be important for disease progression; however, the form of α-synuclein that is responsible for microglial activation in these studies is not known.
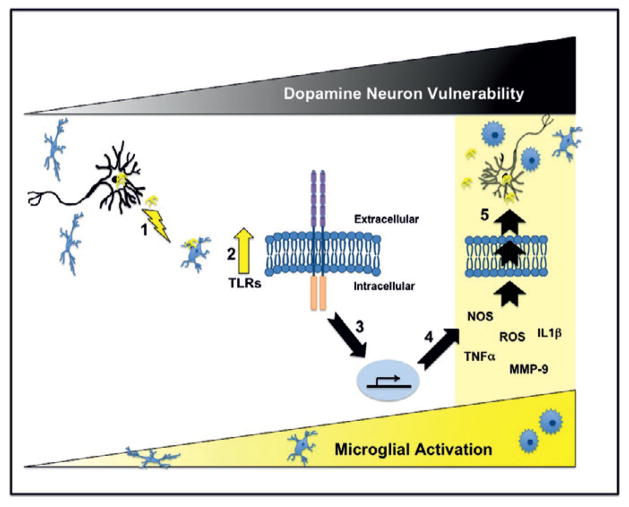
Fig. 2.
α-Synuclein incites microglial activation via toll-like receptor activation. This schematic diagram depicts our current hypothesis that α-synuclein released from neurons and/or presynaptic terminals (e.g., activity dependent release or as a result of ongoing neurodegeneration) activates microglia [1]. We have previously demonstrated that α-synuclein-mediated activation of microglia results in increased expression of TLRs (2; lightning bolt; Table 1 and ref. [12]). Importantly TLRs can mediate downstream pathways that result in the translocation of NFκB to the nucleus, which in turn causes increased expression of proinflammatory molecules (3 & 4; Table 1) that are detrimental to dopamine neurons (5; black triangle “Dopamine Neuron Vulnerability”). Microglial activation is often accompanied by a change in morphology from highly branched cells (ramified) to rounded cells with little branching (amboid) as indicated in the lower yellow triangle. Our data indicates that 24-hours post-exposure α-synuclein-directed microglial activation results in increased expression of TNF-α, NOS, IL-1β, MMP-9, ROS and morphological changes consistent with enhanced classical inflammation (Table 1 and Figure 1). If this proinflammatory milieu continues progressive death of dopamine neurons releasing α-synuclein would ensue followed by continued activation of microglia. However, since TLRs can also promote cell growth and survival it will be important in future studies to establish the specific TLRs that are altered by cognate α-synuclein conformers and subsequently to determine the exact downstream TLR pathways activated (e.g., MyD88 and/or PI3K) so that targeted therapies can be developed.
In our model, α-synuclein released early in disease or after nigrostriatal degeneration acts as a DAMP, activating local microglia (Figure 2; Step 1). Activation of microglia is a dynamic process involving morphological and cellular changes that results in an augmentation of TLR expression (Step 2; Table 1 and Figure 1). In turn, TLR activation initiates downstream molecular pathways resulting in the translocation of NFκB to the nucleus and subsequent upregulation of proinflammatory molecule expression (Step 3 & 4; Table 1). These molecules are then released from the activated microglia into the local milieu enhancing the oxidative stress of the surrounding SNpc dopamine neurons (Step 5) resulting in injury and finally death. If relentless microglial activation is a driving force in PD pathogenesis and microglia are also necessary for maintaining a homeostatic environment for the SNpc dopamine neurons then we suggest that dampening all microglial responses would not be beneficial. In contrast, we suggest that targeted highly specific therapies are required. One therapy would involve interfering with the misfolding and/or release of α-synuclein, which should prevent the early inflammatory events seen in PD animal models as well as subsequent toxicity due to protein misfolding (reviewed in [18]). A second approach entails manipulating TLR expression. Targeting TLRs is an area of interest for several disorders including cardiovascular diseases, cancer, autoimmune diseases and as we suggest here, synucleinopathies. Effective and specific antagonists and agonists for TLRs are commercially available or under development (reviewed in [19]). We speculate that a combination of specific TLR agonist and antagonist would be beneficial in the setting of microglial activation as complex as that which occurs in PD. This is certainly true for other diseases; for instance in myocardial ischemia/reperfusion injury models ligand agonists of TLR2 and antagonists of TLR4 are cardioprotective suggesting that blockade of the MyD88 pathway and enhancement the serine/threonine protein kinase Akt pathway are both beneficial (reviewed in [20]). In our model, interfering with TLRs that promote proinflammation would be predicted to decrease NFκB-directed transcription of damaging cytokines (e.g. TNF-α and IL-1β) leading to an overall reduction of oxidative stress in the environment enveloping the SNpc dopamine neurons. Meanwhile, augmentation of TLRs that mediate the Akt pathway would be predicted to dampen the innate immune response and also promote SNpc dopamine neuron survival. Before such treatments can become a reality however, these complex pathways need to be delineated. Therefore one of the future challenges for TLR-mediated PD therapy is to decode the TLR pathways altered by specific α-synuclein conformers.
Acknowledgments
This work was supported by NIEHS (R01ES014470 to K.M.Z.). We thank Dr. Katherine Conant for help with the MMP-9 ELISA. Special thanks to Dr. Howard J. Federoff for many hours of scientific discussion on this topic. Due to space limitations we are unable to cite all of the excellent studies that are relevant to this field. We apologize to those authors whose work is not included.
Footnotes
Conflict of interests
The authors have no conflict of interest.
References
- 1.Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):174–91. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 2.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007 Jan;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988 Aug;38(8):1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, et al. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson’s disease. Neurosci Lett. 2001 Sep 21;311(1):1–4. doi: 10.1016/s0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- 5.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010 Sep;42(9):781–5. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009 Dec;15(Suppl 3):S200–4. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 7.Chade AR, Kasten M, Tanner CM. Nongenetic causes of Parkinson’s disease. J Neural Transm Suppl. 2006;70:147–51. doi: 10.1007/978-3-211-45295-0_23. [DOI] [PubMed] [Google Scholar]
- 8.Herrera AJ, Castano A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000 Aug;7(4):429–47. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- 9.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008 Nov;29(11):1690–701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997 Aug 28;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 11.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008 Oct;9(5):507–40. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 12.Beraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, et al. alpha-Synuclein alters Toll-like receptor expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng LR, Federoff HJ, Vicini S, Maguire-Zeiss KA. Alpha-synuclein mediates alterations in membrane conductance: a potential role for alpha-synuclein oligomers in cell vulnerability. Eur J Neurosci. 2010 Jul;32(1):10–7. doi: 10.1111/j.1460-9568.2010.07266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Zhang Y, Yuan Y, Chen N. A new perspective in Parkinson’s disease, chaperone-mediated autophagy. Parkinsonism Relat Disord. 2011 May;17(4):231–5. doi: 10.1016/j.parkreldis.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Angot E, Brundin P. Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson’s disease. Parkinsonism Relat Disord. 2009 Dec;15(Suppl 3):S143–7. doi: 10.1016/S1353-8020(09)70802-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005 Apr;19(6):533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 17.Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008 Dec;67(12):1149–58. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maguire-Zeiss KA. alpha-Synuclein: a therapeutic target for Parkinson’s disease? Pharmacol Res. 2008 Nov-Dec;58(5–6):271–80. doi: 10.1016/j.phrs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010 Apr;9(4):293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 20.Ha T, Liu L, Kelley J, Kao R, Williams D, Li C. Toll-like receptors: new players in myocardial ischemia/reperfusion injury. Antioxid Redox Signal. 2011 Oct 1;15(7):1875–93. doi: 10.1089/ars.2010.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]