Abstract
The myogenic transcription factor Pax3, a member of the paired class homeodomain family of transcription factors, plays an essential role in early skeletal muscle development. We previously demonstrated that Pax3 is phosphorylated at three specific residues (Ser201, Ser205, and Ser209) and that the pattern of phosphorylation at these sites changes throughout early myogenesis. Further, we demonstrated that the protein kinase CK2 phosphorylates Pax3 at Ser205 and that this phosphorylation event is required for the subsequent phosphorylation of Ser201 by GSK3β. However, the kinase that phosphorylates Pax3 at Ser209 has yet to be identified. In the present work we use standard purification methods and in vitro biochemical analyses to provide solid evidence identifying the protein kinase CK2 as phosphorylating Pax3 at Ser209. Further, we qualitatively demonstrate that the phosphorylation of Pax3 at Ser209 by CK2 is enhanced when Ser205 is previously phosphorylated. Taken together, our results allow us to propose a mechanism to describe the ordered phosphorylation of Pax3 throughout early myogenesis.
Keywords: Pax3, Myogenesis, CK2, Phosphorylation
INTRODUCTION
The transcription factor Pax3 is a fundamental player in early skeletal muscle development and is a key component of normal myogenesis [1]. It plays a central role in early vertebrate development and is responsible for the regulation of various aspects of muscle cell growth such as proliferation, differentiation, migration, and survival [2]. Like most transcription factors, recent evidence suggests that Pax3 activity is regulated through a variety of post-translational modifications including acetylation [3], ubiquitination [4], and phosphorylation [5,6]. Along these lines, our previous work demonstrated that Pax3 is phosphorylated at three distinct sites located near the octapeptide domain, a region critical for mediating protein-protein interactions. Phosphorylation at these sites, Ser201, Ser205 and Ser209 changes throughout early differentiation with phosphorylation by CK2 at Ser205 occurring exclusively in proliferating myoblasts [7,8]. This modification acts as a priming event required for the subsequent GSK3β mediated phosphorylation of Ser201, which persists throughout proliferation and differentiation of myoblasts [9]. However, the induction of myogenic differentiation leads to the immediate loss of phosphorylation at Ser205 and a significant gain of phosphorylation at Ser209 [9]. While the kinases that phosphorylate Ser201 and Ser205 have been identified, the kinase responsible for phosphorylating Ser209 is not yet known, thereby preventing a full understanding of the mechanism describing the changing phosphorylation status of Pax3 during early myogenesis.
In this report we utilize total cell extracts from the physiologically relevant mouse primary myoblasts to perform a systematic purification of the endogenous kinase capable of phosphorylating Pax3 at Ser209. Through this process we obtain a >90-fold purification of Ser209-specific kinase activity. Further, consistent with Ser209 being present in a CK2 consensus sequence, we demonstrate that kinase activity present in each stage of the purification is significantly reduced by the CK2-specific inhibitors heparin and DRB and is capable of utilizing GTP as a phosphate donor, all characteristics specific for CK2. Moreover, we demonstrate that while CK2 also phosphorylates Pax3 at Ser205, there are differences in the efficiency with which these two sites are utilized. CK2 preferentially utilizes Ser205 for the de novo phosphorylation of Pax3. However, phosphorylation at Ser205 enhances the subsequent phosphorylation of Ser209. Taken together, the results presented in this report not only identify CK2 as the kinase responsible for phosphorylation Pax3 at Ser209, but also enables us to propose a mechanism describing the ordered and differential phosphorylation of Pax3 throughout early myogenesis.
MATERIALS AND METHODS
Cells and cell culture conditions
Mouse primary myoblasts were isolated from 2 – 4 day old C57/Bl6 mice as previously described [7]. Proliferation medium for the mouse primary myoblasts consisted of Ham's F-10 nutrient medium (Mediatech Cellgro, Herndon, VA) supplemented with 20% FBS (HyClone Laboratories, Inc., Logan, UT), 2.5ng/ml bFGF (Promega Corp., Madison, WI), and 15mM HEPES (HyClone). Differentiation medium consisted of Dulbecco's Modification of Eagle's Medium (DMEM, Gibco BRL) supplemented with 2% horse serum (HyClone). All media contained penicillin G (200U/ml) and streptomycin (200µg/ml). Cells were grown in a humidified incubator at 37°C in 5% CO2. All cells were grown on collagen-coated dishes (Becton Dickinson Labware, Bedford, MA) and were passage-matched to prevent possible differences due to different passage conditions. To induce differentiation of primary myoblasts, the proliferation media was removed, the cells were washed twice with PBS, the media was replaced with 10 ml of differentiation media, and the cells were grown as described above until needed for further analysis.
Creation of expression vector constructs
The GST fusion constructs pGEX-5X-1-Pax3 were a kind gift from Dr. Gerard Grosveld, St. Jude Children's Research Hospital (Memphis, TN) and mutants were created as previously described [9]. The wild-type and point mutant vectors were individually transformed into Rosetta(DE3)pLysS chemically competent bacteria (EMD Chemicals, Gibbstown, NJ) and subsequently used for expression and purification as previously described [7,8,10]. Bacterially expressed and purified GST, GST-Pax3, GST-Pax3(S205A) [SAS], GST-Pax3(S205D) [SDS], GST-Pax3(S201A,S205A) [AAS], and GST-Pax3 (S201A,S209A) [ASA] were used without elution from the resin. Protein expression and purity were confirmed by SDS-PAGE analysis with Coomassie blue staining and the relative protein concentrations on the resin were estimated by comparison to proteins of known concentration (data not shown).
In vitro kinase assays
GST-Pax3, GST-Pax3[SAS], GST-Pax3[SDS], GST-Pax3[AAS], GST-Pax3[ASA], or GST-Pax3[ AAA] present on the resin (approximately 1µg of protein), prepared as described above, were used for in vitro kinase assays using either purified CK2, total cell extracts derived from primary myoblasts differentiated for 30 minutes, or aliquots from each stage of the purification. For the kinase assay using purified CK2, the GST proteins were individually mixed with 10X CK2 reaction buffer (200mM Tris-HCl [pH 7.5], 500mM KCl, and 100mM MgCl2), 20µM ATP, and 50µCi [γ-32P]-ATP (MP Biomedicals) prior to the addition of 0.02U of purified CK2 enzyme (Calbiochem, LaJolla, CA).
For the kinase assays using differentiated mouse primary myoblast total cell extract, the GST proteins were mixed with 12µl of the 5X kinase stock solution (80mM HEPES, 20mM MgCl2, 100mM KCl, 2mM DTT), 2X phosphatase inhibitor cocktails I and II (Sigma, St. Louis, MO), 12.5X protease inhibitors (Roche) 84µM ATP, and 50µCi [γ-32P]-ATP (MP Biomedicals) prior to the addition of 25µl of total cell extract (2µg/µl), prepared as previously described [7,8,10].
For the kinase assays using fractions obtained from the purification, the GST proteins were mixed with 12µl of the 5X kinase stock solution (80mM HEPES, 20mM MgCl2, 100mM KCl, 2mM DTT), 2X phosphatase inhibitor cocktails I and II, 84µM ATP, and 50µCi [γ-32P]-ATP [MP Biomedicals]) prior to the addition of 25µl of each individual fraction obtained from either the size exclusion or ion exchange chromatography separations. Following the addition of purified CK2, total cell extract, or chromatography fractions, the reaction mixture was incubated at 30°C for 1 hour with periodic gentle agitation. After incubation, the beads were washed two times with 100µl 1X PBS, radiolabeled protein was eluted by boiling in 25µl SDS-PAGE loading buffer, and the proteins were separated by 10% SDS-PAGE. The resulting gels were dried and visualized by autoradiography. Alternatively, the above described assays were performed using 84µM GTP and 50µCi [γ-32P]-GTP (MP Biomedicals) as the phosphate donor.
For the kinase assays performed in the presence of the CK2-specific inhibitors, differentiated mouse primary myoblast total cell extract or fractions obtained from each stage of the purification were incubated at 30°C for 1 hour with 10mM of either 5,6-Dichloro-1-β-D-ribofuranosylbenzimidazole (DRB, Calbiochem) or heparin (Sigma-Aldrich) prior to the addition of unlabeled ATP, [γ-32P]-ATP, 5X kinase buffer and bacterially expressed and purified GST-Pax3[ AAS]. Following the addition of GST-Pax3[AAS], the mixture was allowed to incubate at 30°C for 1 hour with periodic gentle agitation after which the phosphorylated protein was separated by SDS-PAGE. The resulting gels were dried and visualized by autoradiography.
Purification of Pax3 Serine 209 kinase
Preparation of extracts
The endogenous kinase capable of phosphorylating Pax3 at Ser209 was purified from mouse primary myoblasts induced to differentiate for 30 minutes. Cells were grown up to 80% confluency (≈9.5×107 cells), induced to differentiate for 30 minutes and harvested to make total cell extracts using 1.5 ml of Myoblast Lysis Buffer (50mM Tris-Hcl pH7.4, 150mM NaCl, 1mM EDTA, 1% Triton X-100) supplemented with 2X phosphatase inhibitor cocktail I and II (Sigma) and 1X protease inhibitor cocktail (Roche). Total cell extracts were then filtered using a 0.22 micron filter and then used as the starting material for protein purification.
Size exclusion chromatography
A Superose 6 10/300 GL Tricorn™ (10 X 300mm, 24ml bed volume, GE Healthcare) high performance column was used for the first step of purification. All operations were carried out at room temperature. The column was operated through the ÄKTA FPLC liquid chromatography system (Amersham Biosciences) using the UNICORN™ software package. The column was equilibrated with 50 ml (2 column volumes) of 20mM Tris-HCl (pH 8.0), which was used as the eluent. The filtered myoblast total cell extract (0.5 ml) was injected and allowed to run through the column at a constant flow rate of 0.5ml/min. 1 ml fractions were collected and the presence of kinase activity was determined in each fraction using the in vitro kinase assay described above and GST-Pax3[AAS] as the substrate. The fractions containing kinase activity were pooled and used for the subsequent ion exchange chromatography separation.
Ion Exchange Chromatography
A MonoQ 5/50 GL Tricorn™ high performance column (5 X 50mm, 1ml bed volume, GE Healthcare) anion exchange column was used for the second phase of purification. All operations were carried out at room temperature. Start buffer (Buffer A) was 20mM Tris-HCl pH 8.0, and the elution buffer (Buffer B) was 20mM Tris-HCl pH 8.0 containing 1M NaCl. The column was prewashed and equilibrated with 5 ml distilled water at a flow rate of 1ml/min followed by washing the column with 5 ml Buffer A (20mM Tris-HCl pH 8.0). This was followed by a wash with 5 ml Buffer B (20mM Tris-HCl pH 8.0 and 1M NaCl) with final equilibration with 5 ml Buffer A maintaining a constant flow rate at 1ml/min in all applications. The pooled kinase positive fractions obtained from size exclusion chromatography (5 ml) was injected and allowed to run through the column at a constant flow rate of 1ml/min and collecting 1ml fractions. After washing with approximately 2 column volumes of Buffer A, proteins were eluted with stepwise (10% vol/vol) increments of Buffer B (0.1M NaCl steps) up to 100% Buffer B. The collected fractions were subsequently used in in vitro kinase assays as described above. Kinase positive fractions were pooled and stored at −80 °C.
RESULTS AND DISCUSSION
Isolation of kinase activity specific for Pax3 at Ser209
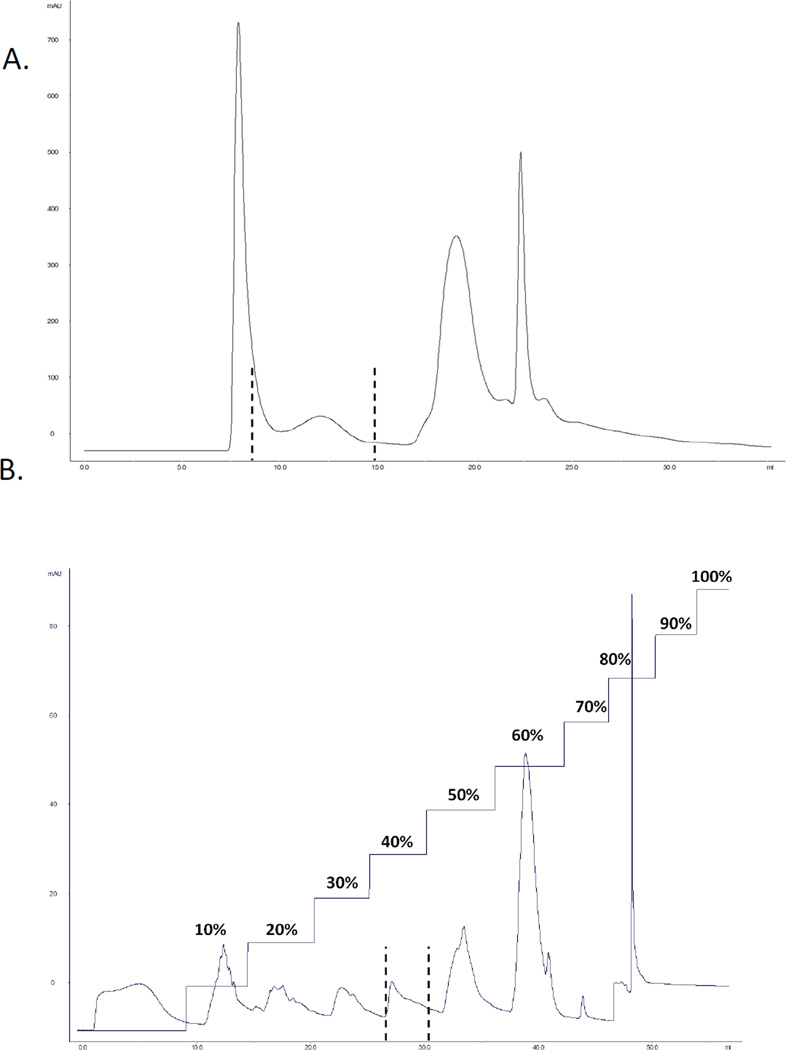
We previously established that the myogenic transcription factor Pax3 is phosphorylated at three residues in vivo and in vitro, Ser201, Ser205 and Ser209 [7,10]. Further, we identified that phosphorylation of Ser205 is carried out by the protein kinase CK2 [8], which is then required for the subsequent phosphorylation of Ser201 by GSK3β [10]. However, the kinase responsible for the phosphorylation of Ser209 has not yet been identified. In order to isolate the kinase that phosphorylates Pax3 at Ser209, we used standard protein chromatographic methods including size exclusion and ion exchange chromatography methods. Total cell lysates prepared from primary myoblast differentiated for 30 minutes, a time period where we observe maximal phosphorylation of Ser209 [9], were used as the starting material for the purification of the endogenous kinase. Total cell lysates were loaded onto a Superose 6 10/300 GL size exclusion column and fractions obtained were tested for kinase activity using our in vitro kinase assay with a form of Pax3 that is capable of being phosphorylated only at Ser209 [Pax3(AAS)]. Of the 36 fractions collected, fractions 8 – 15 contained Ser209-specific kinase activity (Figure 1A). Further, we observed no phosphorylation of a phospho-incompetent form of Pax3 (Pax3[AAA]) or GST only (data not shown), proving that the kinase present in these fractions is indeed specific for phosphorylating Ser209 of Pax3. Kinase positive fractions were then pooled and run over a MonoQ 5/50 GL Tricorn™ anion exchange column with step elution in 10% (0.1M) increments. Ser209-specific kinase activity was specifically eluted with 0.4M NaCl treatment (Figure 1B). This overall purification resulted in >90-fold purification of protein containing kinase activity capable of specifically phosphorylating Pax3 at Ser209.
Figure 1.
Isolation of Ser209-specific kinase activity. (A) Primary myoblasts were induced to differentiation for 30 minutes, a time at which we observe maximal phosphorylation at Ser209. Total cell extracts were made and the filtered extract was loaded onto a Superose 6 10/300 GL Size Exclusion chromatography column previously equilibrated with 20mM Tris-HCl [pH 8.0] (500 l/run). Separation was run at a flow rate of 0.5 ml/min collecting 1 ml fractions. (B) Fractions from the size exclusion column were pooled and loaded onto a Mono Q anion exchange column equilibrated with the same buffer. Separation was run at a flow rate of 0.5 ml/min collecting 2 ml fractions. Proteins were step eluted with 10% (0.1 M) increments with 1M NaCl. In both panels, kinase activity was determined by incubating bacterially expressed and purified GST-Pax3[AAS] with 25 l of each individual fraction and 25 l of a 2X kinase buffer (80 mM HEPES, 20 mM MgCl2, 100 mM KCl, and 2 mM DTT) containing 84 M ATP and 50 Ci of [ -32P] ATP. The reaction was incubated at 30°C for 30 minutes, washed extensively with PBS, the radiolabeled proteins were separated by 10% SDS-PAGE, and the dried gel visualized by autoradiography. In both panels, the dotted lines indicate the fractions containing kinase activity.
Identification of Pax3 S209 kinase
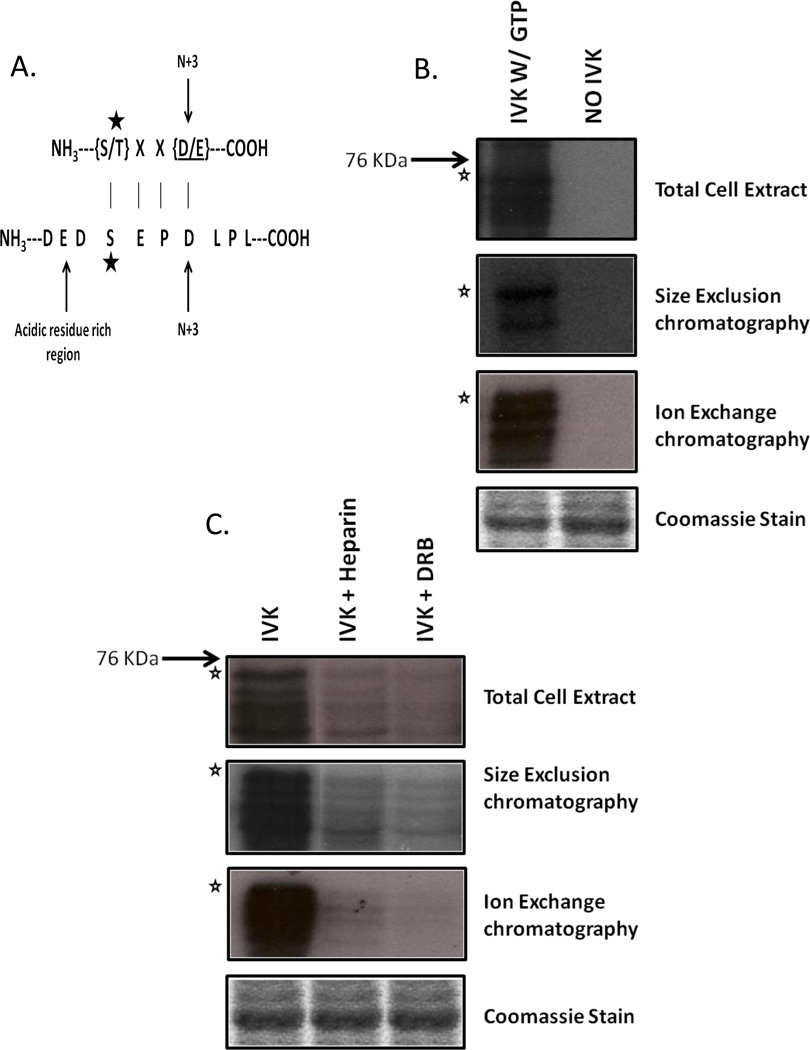
Multiple attempts at using mass spectral analysis to identify the kinase present in the final purified fraction proved to be unsuccessful (data not shown). However, a close examination of the amino acid sequence surrounding Pax3 Ser209 was found to have strong similarities to the known CK2 consensus sequence (Figure 2A). CK2 prefers to phosphorylate residues that are surrounded by acidic amino acids, especially at the N+3 position where N is the residue to be phosphorylated. (Figure 2A) [11]. Additionally, unlike other kinases CK2 possesses the unique property of being able to utilize both GTP and ATP as phosphate donors [12]. Hence, to test whether CK2 is the kinase present in the final purified fraction, we performed an in vitro kinase assay using GST-Pax3[AAS] as the substrate in the presence of radiolabelled GTP. We utilized total cell extracts from cells differentiation for 30 minutes and aliquots from each stage of the purification. We observed the ability of the kinase present in the total cell extract and each stage of the purification to utilize GTP to specifically label Pax3 at Ser209 (Figure 2B), thereby providing strong evidence to support the idea that CK2 is the kinase present in the final purified fraction.
Figure 2.
Identification of CK2 as the Ser209-specific kinase. (A) Ser209 is present in the context of a CK2 recognition site. The star indicates the phosphorylated amino acid. (B) Bacterially expressed GST-Pax3[AAS] was incubated with 25 l of total cell extracts or pooled fractions from size exclusion, or ion exchange separations in the presence of radiolabeled GTP. In vitro kinase assays were carried out, as described in the Materials and Methods. (C) Aliquots from total cell extracts, size exclusion chromatography, or anion exchange chromatography were pre-incubated with the CK2-specific inhibitors Heparin or 5,6-dichloro-1- -D-ribofuranosyl- 1H- benzimidazole (DRB) after which in vitro kinase assays were carried out, as described in the Materials and Methods. In all panels the star indicates the mobility of the GST-Pax3[AAS] with lower bands representing commonly seen degradation products. Equal loading is indicated by Coomassie staining.
To provide further evidence to support the idea that CK2 is the kinase responsible for phosphorylating Pax3 at S209, we utilized total cell extracts from cells differentiated for 30 minutes or aliquots from each stage of the purification to determine the ability of the CK2- specific inhibitors heparin or DRB to alter phosphorylation at Ser209. All samples were preincubated for one hour with previously established optimum levels of CK2 specific inhibitors heparin or DRB [8]. This incubation was followed by the addition of ATP and bacterially expressed and purified GST-Pax3[AAS]. As seen in Figure 2C, the pre-incubation of myoblast total cell extracts as well as all steps of the purification with the CK2 inhibitors significantly decreased the phosphorylation of Pax3 at Ser209, providing further evidence to support the idea that CK2 is the kinase responsible for the phosphorylation of Pax3 Ser209 upon the induction of myogenic differentiation.
The ability of the kinase present in the all stages of the purification to efficiently utilize GTP and to be specifically inhibited by both heparin and DRB provide solid evidence supporting the idea that CK2 is the kinase responsible for phosphorylating Pax3 at Ser209. However, despite our strong experimental data, other kinases are inhibited by DRB including CK1, cyclin-dependent kinase 7 (CDK7), CDK8 and CDK9 [13,14,15]. However, we show the ability of Ser209 to be phosphorylated in the presence of GTP as well as ATP with nearly equal efficiency throughout the entirety of the purification process. Besides CK2, CDK9 is also capable of utilizing GTP as a phosphate donor. However, unlike CK2, which uses ATP and GTP with equal efficiency, CDK9 uses GTP with an almost 10-fold decreased efficiency [16]. Moreover, the Ser209 peptide sequence very closely resembles the recognition sequence known for CK2 (S*- X-X-D/E where the asterix represents the residue being phosphorylated), a sequence that is significantly and distinctly different from the identified consensus sequence for CDK9 (Y-S-P-TS*- P-S).
Multiple attempts were made to determine the in vivo role of CK2 in the phosphorylation of Pax3 at Ser209 by the treatment of primary myoblast cultures with the CK2-specific inhibitors heparin and DRB. However, treatment of primary myoblasts with these chemicals led to extensive cell death within one hour of incubation (data not shown), thereby preventing any form of in vivo analysis. This observation is consistent with previous reports demonstrating that the chemical inhibition of CK2 within primary cells leads to apoptosis within the first 24 hours of treatment [17,18]. Therefore, based on our in vitro inhibition studies, demonstration of the utilization of GTP as a phosphate donor, combined with what is known about the biochemical characteristics of CK2 and other kinases, we conclude that CK2 is the kinase that phosphorylates Pax3 at Ser209.
Ordered phosphorylation of Ser205 and Ser209 by CK2
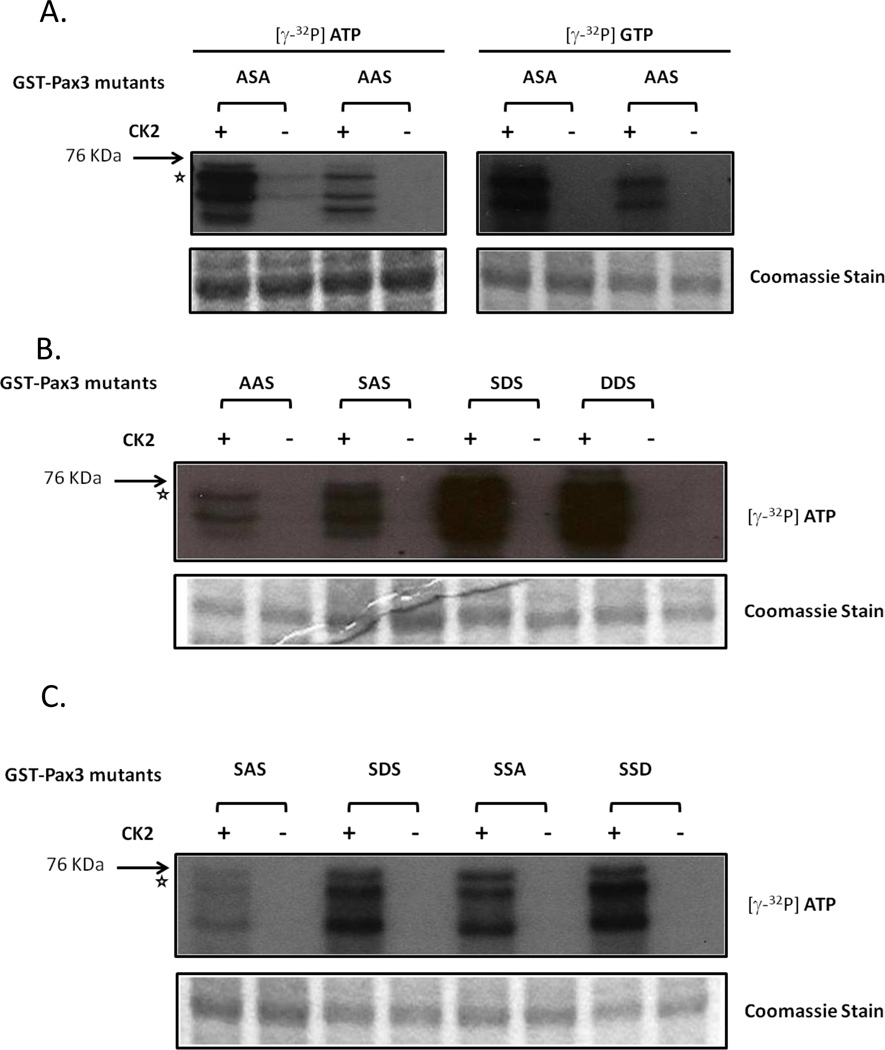
We previously showed that Pax3 is phosphorylated by CK2 at Ser205 in proliferating myoblasts [8] and that this event is required for the phosphorylation of Ser201 by GSK3β [10]. Surprisingly, in addition to the utilization of CK2 to phosphorylate Pax3 at Ser205, our results described above indicate the involvement of CK2 in the phosphorylation of Ser209. In order to compare the extent of phosphorylation of Ser209 and Ser205 by CK2 we used bacterially expressed and purified GST-Pax3[ASA] and GST-Pax3[AAS] in in-vitro kinase assays using purified CK2 in the presence of radiolabelled ATP or GTP. Results show that while Ser209 is phosphorylated by purified CK2 in the presence of ATP or GTP, the extent of phosphorylation is significantly reduced compared to that seen at Ser205 (Figure 3A). These results, along with evidence from previous studies [8], suggest that phosphorylation of Pax3 at Ser205 by CK2 is the primary de novo event in proliferating primary myoblasts.
Figure 3.
Extent of phosphorylation of Ser205 and Ser209 by CK2. In Vitro kinase assays were conducted using bacterially expressed and purified (A) GST-Pax3[ASA] or GST-Pax3[AAS] with either ATP or GTP; (B) GST-Pax3[SAS], GST-Pax3[SDS], GST-Pax3[SSA], GST-Pax3[SSD] and only ATP; or (C) GST-Pax3[AAS], GST-Pax3[SAS], GST-Pax3[SDS] and GST-Pax3[DDS] and only ATP. In all cases the in vitro kinase assay was performed as described in the Materials and Methods using 0.02U of commercially available, purified CK2. The star indicates the mobility of the GST-Pax3 protein with lower bands representing commonly seen degradation products. A Coomassie stained gel demonstrates the presence of equal amounts of GST proteins in all assays.
To further understand the relationship between CK2-dependent phosphorylation of Ser209 and Ser205, we conducted in vitro kinase assays using purified CK2, radiolabelled ATP and bacterially expressed and purified GST-Pax3 [AAS, SAS, SDS and DDS]. These GST-Pax3 mutants mimic a form of Pax3 “permanently” locked into a phosphorylated or nonphosphorylated state at Ser201 and/or Ser205. Results from this experiment show that while the extent of phosphorylation of GST-Pax3[AAS] and GST-Pax3[SAS] are similar (Figure 3B.), CK2-dependent phosphorylation of Ser209 on GST-Pax3[SDS], a form “permanently” phosphorylated at Ser205, is nine-fold greater than that of GST-Pax3[SAS], a form that mimics a Ser205 nonphosphorylated state (Figure 3B and C). This increase in phosphorylation of Ser209 in the presence of a permanently phosphorylated Ser205 indicates that Ser205 phosphorylation positively influences phosphorylation of Pax3 at Ser209. Additionally, the similar extent of phosphorylation of GST-Pax3[DDS], a form in which both Ser201 and Ser205 are present in a “permanently” phosphorylated state, and GST-Pax3[SDS] by CK2 indicates that the phosphorylation status of Ser201 does not influence the ability of CK2 to phosphorylate Ser209 (Figure 3C.). Conversely, the phosphorylation of Ser209 does not seem to greatly affect the ability of CK2 to phosphorylate Ser205 (Figure 3C.). Moreover, we see no differences between the phosphorylation of GST-Pax3[SSA], GST-Pax3[SSD], GST-Pax3[SDS], and GSTPax3[ DDS], which confirms that the observed changes in the extent of phosphorylation are not a consequence of subtle mutations in this region of the protein. Thus we conclude that while CK2 phosphorylates both Ser205 during myoblast proliferation and Ser209 during myogenic differentiation, the phosphorylation of Ser209 is more efficient in the presence of a previously phosphorylated Ser205 and is unaffected by the phosphorylation of S201.
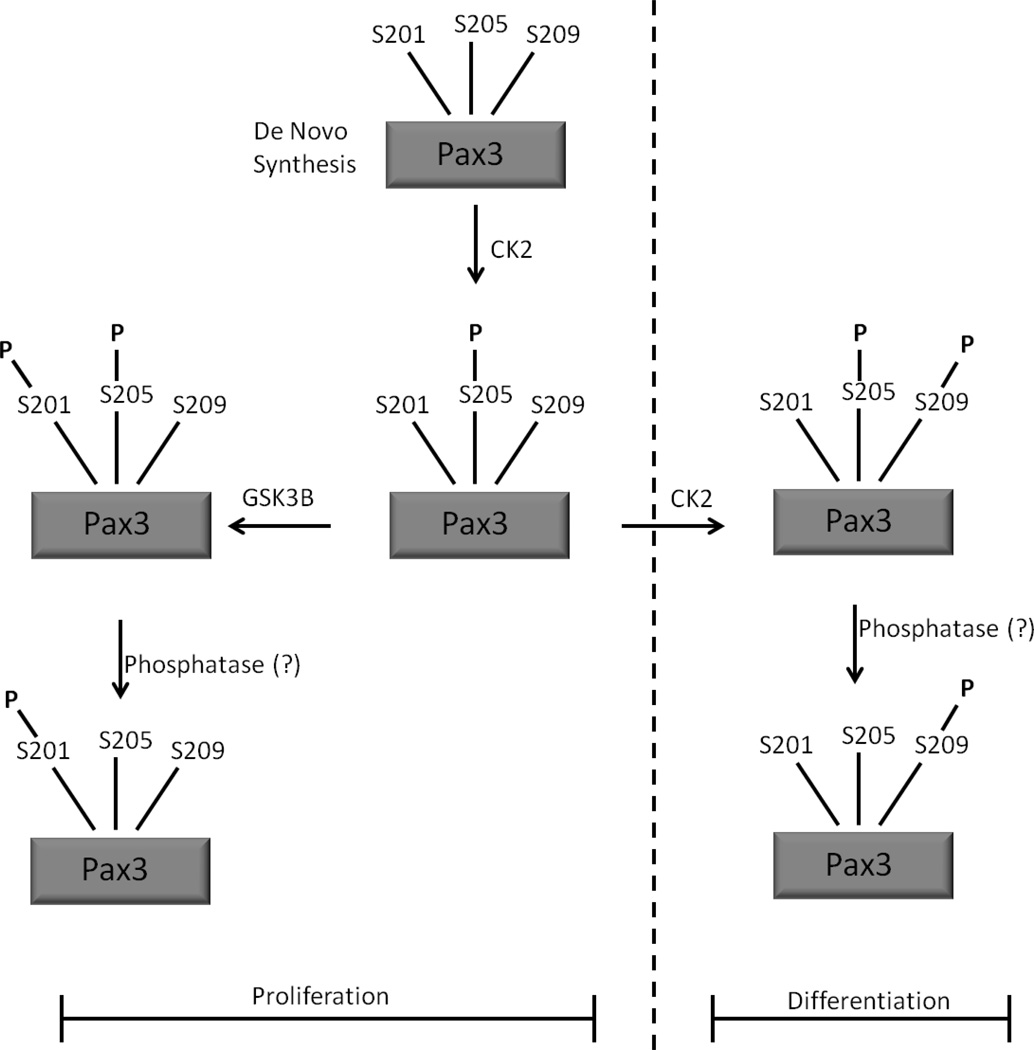
Model to describe the ordered phosphorylation of Pax3 during early Myogenesis
Taken together, the results presented here, along with previously published results [7,8,10], allow us to propose a model to describe the ordered phosphorylation of Pax3 during early myogenesis (Figure 4). Our results, along with literature evidence support the idea that during proliferation, the initial phosphorylation event on de novo translated Pax3 is at Ser205, an event that is mediated by CK2. This phosphorylation then primes Pax3 for subsequent additional post-translational modification [7,8] or for the subsequent phosphorylation of Ser201 by GSK3β. However, since we observed no dual phosphorylation of Ser201 and Ser205 in proliferating cells [10], it is likely that an as of yet unidentified phosphatase dephosphorylates Pax3 at Ser205 to generate a form of Pax3 solely phosphorylated at Ser201.
Figure 4.
A schematic of a model demonstrating the mechanism to describe the ordered and varying phosphorylation of Pax3 throughout early myogenic differentiation.
Upon the induction of differentiation, CK2 then phosphorylates Ser209 with a preference for utilizing the form of Pax3 solely phosphorylated at Ser205. As with the transiently present, dual phosphorylation of Pax3 at Ser201/Ser205 in proliferating cells, it can be conjectured that the transient dual phosphorylation of Pax3 at Ser205 and Ser209 promotes the dephosphorylation at Ser205, by potentially the same unknown phosphatase, leaving Pax3 solely phosphorylated at Ser209 in early differentiation. Alternatively, it is possible that the dual phosphorylation events, either of Ser201/Ser205 in proliferating cells or Ser205/Ser209 in differentiated cells, if not effectively converted to the single phosphorylated species, promotes the rapid degradation of Pax3 such that only singly phosphorylated Pax3 species remain. At present, the biological signals that promote the CK2-dependent phosphorylation of Pax3 at Ser209 upon the induction of myogenic differentiation are not known. However, the understanding of how the kinases CK2 and GSK3β contribute to the ordered and varied phosphorylation states of Pax3 during the switch from proliferation to early differentiation will allow a better understanding of how the changing phosphorylation status regulates the biological activity of Pax3 and various downstream targets important for the myogenic process.
Biochemical isolation of a kinase that specifically phosphorylates Pax3 at Ser209
Biochemical identification of the isolated kinase as being CK2
Phosphorylation at Ser205 enhances the phosphorylation of Pax3 at Ser209
Mechanism describing the orderd phosphorylation of Pax3 in early myogenesis
ACKNOWLEDGEMENTS
Funding for this work was provided by grant number 1 R01 CA138656 from the National Cancer Institute (NCI), a component of the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bober E, Franz T, Arnold HH, Gruss P, Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994;120:603–612. doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- 2.Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovasc Res. 1997;36:163–173. doi: 10.1016/s0008-6363(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 3.Ichi S, Boshnjaku V, Shen YW, Mania-Farnell B, Ahlgren S, Sapru S, Mansukhani N, McLone DG, Tomita T, Mayanil CS. Role of Pax3 acetylation in the regulation of Hes1 and Neurog2. Mol Biol Cell. 2011;22:503–512. doi: 10.1091/mbc.E10-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutet SC, Disatnik MH, Chan LS, Iori K, Rando TA. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 5.Amstutz R, Wachtel M, Troxler H, Kleinert P, Ebauer M, Haneke T, Oehler-Janne C, Fabbro D, Niggli FK, Schafer BW. Phosphorylation regulates transcriptional activity of PAX3/FKHR and reveals novel therapeutic possibilities. Cancer Res. 2008;68:3767–3776. doi: 10.1158/0008-5472.CAN-07-2447. [DOI] [PubMed] [Google Scholar]
- 6.Miller PJ, Hollenbach AD. The oncogenic fusion protein Pax3-FKHR has a greater posttranslational stability relative to Pax3 during early myogenesis. Biochim Biophys Acta. 2007;1770:1450–1458. doi: 10.1016/j.bbagen.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller PJ, Dietz KN, Hollenbach AD. Identification of serine 205 as a site of phosphorylation on Pax3 in proliferating but not differentiating primary myoblasts. Protein Sci. 2008;17:1979–1986. doi: 10.1110/ps.035956.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietz KN, Miller PJ, Hollenbach AD. Phosphorylation of serine 205 by the protein kinase CK2 persists on Pax3-FOXO1, but not Pax3, throughout early myogenic differentiation. Biochemistry. 2009;48:11786–11795. doi: 10.1021/bi9012947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz KN, Miller PJ, Iyengar AS, Loupe JM, Hollenbach AD. Identification of serines 201 and 209 as sites of Pax3 phosphorylation and the altered phosphorylation status of Pax3-FOXO1 during early myogenic differentiation, Int J. Biochem Cell Biol. 2011 doi: 10.1016/j.biocel.2011.03.010. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz KN, Miller PJ, Iyengar AS, Loupe JM, Hollenbach AD. Identification of serines 201 and 209 as sites of Pax3 phosphorylation and the altered phosphorylation status of Pax3-FOXO1 during early myogenic differentiation. Int J Biochem Cell Biol (Revision submitted) 2011 doi: 10.1016/j.biocel.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 12.Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–323. doi: 10.1096/fasebj.9.5.7896000. [DOI] [PubMed] [Google Scholar]
- 13.Meggio F, Shugar D, Pinna LA. Ribofuranosyl-benzimidazole derivatives as inhibitors of casein kinase-2 and casein kinase-1. Eur J Biochem. 1990;187:89–94. doi: 10.1111/j.1432-1033.1990.tb15280.x. [DOI] [PubMed] [Google Scholar]
- 14.Rickert P, Corden JL, Lees E. Cyclin C/CDK8 and cyclin H/CDK7/p36 are biochemically distinct CTD kinases. Oncogene. 1999;18:1093–1102. doi: 10.1038/sj.onc.1202399. [DOI] [PubMed] [Google Scholar]
- 15.Schang LM. Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother. 2002;50:779–792. doi: 10.1093/jac/dkf227. [DOI] [PubMed] [Google Scholar]
- 16.Pei Y, Shuman S. Characterization of the Schizosaccharomyces pombe Cdk9/Pch1 protein kinase: Spt5 phosphorylation, autophosphorylation, and mutational analysis. J Biol Chem. 2003;278:43346–43356. doi: 10.1074/jbc.M307319200. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–1043. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells--a potential approach to cancer therapy. Mol Cell Biochem. 2005;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]