Summary
Although the pro-apoptotic BH3-only protein, Bim, is required for deletion of autoreactive thymocytes, Bim-deficient mice do not succumb to extensive organ-specific autoimmune disease. To determine whether other BH3-only proteins safeguard tolerance in the absence of Bim, we screened mice lacking Bim alongside other BH3-only proteins. Most strains showed no additional defects, however, mice deficient for both Puma and Bim spontaneously developed autoimmunity in multiple organs and their T-cells could transfer organ-specific autoimmunity. Puma/Bim double-deficient mice had a striking accumulation of mature single positive thymocytes, suggesting a further defect in thymic deletion was the basis for disease. Transgenic mouse models of thymocyte deletion to peripheral neo-antigens confirmed that the loss of Bim and Puma allowed increased numbers of autoreactive thymocytes to escape deletion. Our data show that Puma cooperates with Bim to impose a thymic deletion checkpoint to peripheral self-antigens and cement the notion that defects in apoptosis alone are sufficient to cause autoimmune disease.
Introduction
The property of immunological tolerance is imposed and maintained by a number of mechanisms acting in a serial and overlapping manner. One of the earliest mechanisms proposed and defined experimentally was the deletion of potentially autoreactive lymphocytes by apoptosis. Despite this precedence, the extent to which deletional (apoptotic) mechanisms contribute to tolerance remains an open question (reviewed by (von Boehmer and Melchers, 2010)). Experiments that specifically uncouple the apoptosis of lymphocytes from immunoreceptor stimulation have proven an informative approach to this issue.
During their differentiation in the bone marrow or thymus, immature B- or T-cells bearing a BCR or TCR that recognises self-antigens with an inappropriately high avidity engage the “intrinsic” or “mitochondrial” pathway of apoptosis, regulated by the Bcl-2 family of proteins (Strasser, 2005). Activation of the pro-apoptotic Bcl-2 family members, Bax and Bak, disrupts the mitochondrial outer membrane to allow the release of cytochrome c into the cytosol, leading to formation of the apoptosome and the activation of caspases which dismantle the cell by cleaving hundreds of proteins (Strasser, 2005; Strasser et al., 2011). In healthy cells, Bax and Bak activation is strictly suppressed by the pro-survival members of the Bcl-2 family: Bcl-2, Bcl-xL, Mcl-1, A1 and Bcl-w. Certain cytotoxic stimuli, such as growth factor deprivation or high avidity immunoreceptor signalling, cause the upregulation of a class of pro-apoptotic Bcl-2 family members, the BH3-only proteins. Mice and humans have eight BH3-only proteins - Bad, Bik, Bid, Hrk, Bim, Noxa, Puma and Bmf - all of which can bind to at least some pro-survival Bcl-2 family members and trigger apoptosis when overexpressed (Huang and Strasser, 2000; Strasser et al., 2011). The BH3-only proteins initiate apoptosis by unleashing Bax/Bak from their sequestration by the Bcl-2-like pro-survival proteins and/or by directly activating Bax and Bak (Dewson and Kluck, 2009). Importantly, different cell types have different expression profiles of the pro- and anti-apoptotic Bcl-2 family members and, therefore, similar stimuli may provoke a different life-or-death outcome in distinct cell types.
Bim is required for deletion of autoreactive thymocytes, mature T-cells and B-cells (Bouillet et al., 2002; Davey et al., 2002; Enders et al., 2003). Although there have been some conflicting data on the importance of Bim in superantigen-induced thymocyte deletion (Bouillet et al (2002) and Zhan et al (2011) found that Bim was essential, while (Jorgensen et al., 2007) did not), data from several different TCR transgenic models are in accord. Bim was found to be critical for the apoptosis of autoreactive thymocytes in models that affect deletion at the CD4−CD8− double negative (DN) (Bouillet et al., 2002), the CD4+CD8+ double positive (DP) stage (Hu et al., 2009) and during the DP to CD8+ single positive (SP) transition (Moran et al., 2011; Suen and Baldwin, 2012). The latter studies employed a TCR transgenic negative selection system in which the Autoimmune Regulator (Aire) mediated the presentation of a model peripheral tissue antigen (PTA) produced by medullary thymic epithelial cells (mTEC) (Anderson et al., 2005; Hubert et al., 2011). The survival of Bim-deficient TCR transgenic thymocytes specific to the PTA indicated that Aire-dependent tolerance to PTA also engages Bim-mediated deletion. Curiously, although Bim-deficient mice on a mixed C57BL/6x129SV background succumb to severe SLE-like systemic autoimmunity, they do not develop widespread organ-specific autoimmunity akin to that observed in Aire-deficient mice (Anderson et al., 2002).
This disjunction in the requirement of Bim for deletion and tolerance advances propositions that, 1) apoptotic deletion of developing T- and B-cells is not required for tolerance and that other mechanisms (such as anergy or regulation) are sufficient, and/or 2) that other apoptotic regulators can delete dangerous clones and maintain tolerance. In this study, we probe these possibilities by assaying organ-specific tolerance following the compound loss of Bim and other BH3-only proteins. We found that among the BH3-only proteins, only the additional loss of Puma provoked significant organ-specific autoimmune disease in a sensitized reconstitution model, accompanied by increased T-cell activation and organ-specific autoantibodies. Importantly, spontaneous autoimmunity arose in the peripheral organs of mice deficient in both Puma and Bim, and their T-cells could transfer disease. Moreover, only the compound loss of Puma exacerbated the thymocyte developmental defects observed in Bim-deficent mice and substantially increased the numbers of autoreactive T-cells that escaped thymic deletion. Together, our findings reveal a critical apoptotic checkpoint in thymocytes regulated by Bim and Puma, and compelling evidence that apoptotic deletion of autoreactive thymocytes is indeed important to prevent organ-specific autoimmunity.
Results
A sensitized screen for organ-specific autoimmunity caused by loss of BH3-only proteins
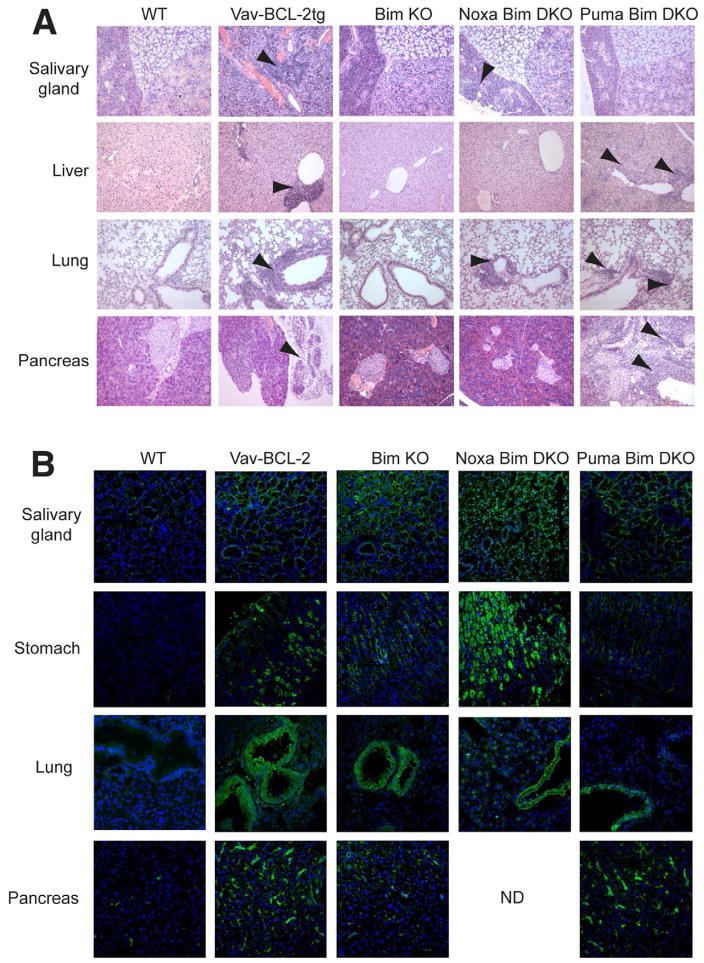
To determine whether the compound loss of Bim and other BH3-only proteins in the hematopoietic system could lead to organ-specific autoimmune disease, we employed a hematopoietic reconstitution system in Rag1•/• mice. The lymphopenic conditions in these hosts can accelerate the development of autoimmunity when provided with immunocompetent precursors from autoimmune-prone donors (Gleeson et al., 1996). As expected, no lymphocytic infiltration was observed in the peripheral organs of Rag1•/• mice (see Experimental Procedures) eight weeks after reconstitution with T-cell depleted bone marrow from wildtype (WT) mice (Figure 1A). By contrast, Rag1•/• mice reconstituted with hematopoietic cells from Vav-BCL-2 transgenic mice, in which the intrinsic pathway of apoptosis is severely impaired (Egle et al., 2004), developed lymphocytic infiltration in the salivary glands, lacrimal glands, stomach, lungs, liver and pancreas (Figure 1A and not shown). Infiltration was accompanied by serum autoantibodies to target tissues, such as the acinar cells of the sub-mandibular salivary gland, bronchiolar epithelium of the lung and parietal cells of the stomach, indicating that an autoimmune response had been instigated (Figure 1B). This outcome indicates that, in the lymphopenic setting, the intrinsic pathway of apoptosis is important for establishing and maintaining tolerance of PTA.
Figure 1. Compound loss of Bim and other BH3-only proteins can cause organ-specific autoimmune disease.
A. H&E stained sections of tissues from lethally irradiated Rag1•/• mice reconstituted with hematopoietic precursors from WT or Vav-BCL-2tg mice, or mice lacking the indicated BH3-only proteins. Arrows indicate areas of lymphocytic infiltration. B. Immunofluorescent staining of Rag1•/• mouse tissues with serum from Rag1•/• mice reconstituted with hematopoietic precursors from WT or Vav-BCL-2tg mice, or mice lacking the indicated BH3-only proteins. Auto-antibody reactivity is shown in green and nuclear DAPI staining in blue. Images are representative of n=4 mice per group.
Nevertheless, mice reconstituted with precursors from Bim-deficient (Bcl2l11•/•) mice did not succumb to organ-specific autoimmunity, although some serum autoantibodies against peripheral organs were elicited (Figure 1A and 1B). Therefore, we next tested precursors from a variety of compound mutants lacking Bim and other BH3-only proteins: Noxa (Pmaip1), Puma (Bbc3), Bmf, Bid or Bad (Hrk is not expressed in lymphocytes and loss of Bik has no impact on lymphocytes (Coultas et al., 2004), even when combined with concomitant loss of Bim (Coultas et al., 2005)). Transfer of hematopoietic progenitors from Bmf•/•Bcl2l11•/•, Bid•/•Bcl2l11•/• and Bad•/•Bcl2l11•/• mice did not elicit organ-specific autoimmunity (data not shown). Mice reconstituted with precursors from Pmaip1•/•Bcl2l11•/• mice occasionally showed signs of mild lymphocytic infiltration of the salivary gland or lung, but other organs were unaffected (Figure 1A). By contrast, mice reconstituted with precursors from Bbc3•/•Bcl2l11•/• mice demonstrated extensive infiltrates in the liver, lungs and pancreas, accompanied by serum autoantibodies targeting these organs (Figure 1A and 1B). Strikingly, the infiltration of the pancreas was associated with exocrine pancreatitis that sometimes completely ablated acinar cells (in 4 of 12 mice), while leaving the endocrine islets intact (Figure 1A). Serum autoantibodies from these mice showed reactivity to the pancreatic vasculature and, occasionally, exocrine acinar cells (Figure 1B). This autoimmune profile is reminiscent of the disease observed in Aire•/• mice (Jiang et al., 2005).
Given this contribution of Puma to organ-specific tolerance with Bim deficiency, we also tested precursors from Bbc3•/•, Bbc3•/•Pmaip1•/• and Trp53•/•Bcl2l11•/• mice (p53 activation can induce Puma (Nakano and Vousden, 2001; Yu et al., 2001)) in this system, but no significant autoimmune pathology was observed (data not shown). Together, these data indicate that, in a lymphopenic setting, Puma cooperates with Bim to establish tolerance to PTA.
Spontaneous organ-specific autoimmunity in mice doubly deficient in Puma and Bim
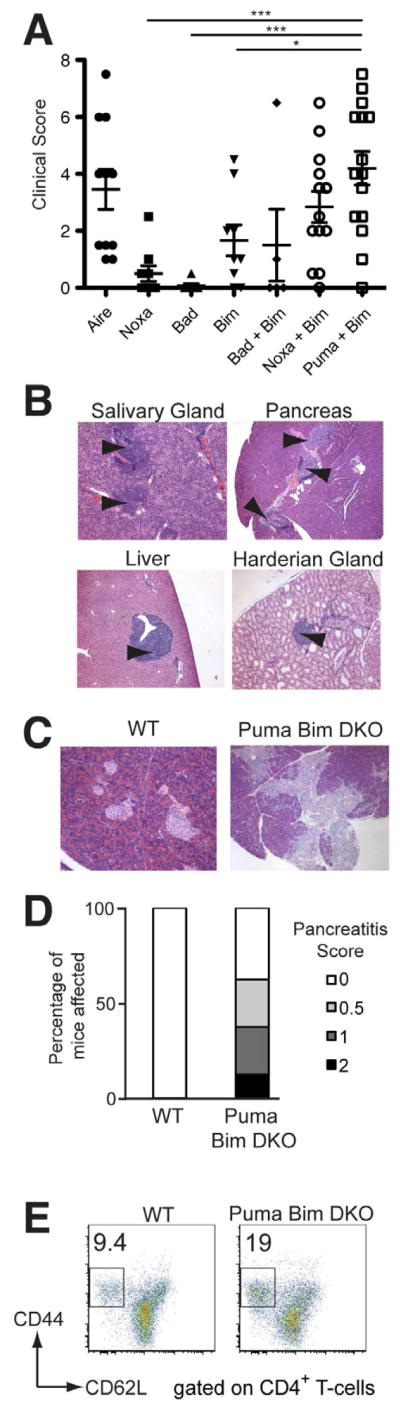
To assess whether this requirement for both Bim and Puma in organ-specific tolerance extended to intact animals, we aged cohorts of mice singly or doubly deficient in key BH3-only proteins and examined a range of tissues for quantification of infiltration to generate a cumulative clinical score of autoimmunity (Gray et al., 2007b). Aged Aire•/• mice served as a positive control, exhibiting autoimmune infiltration in the salivary, prostate and Harderian glands, retina, lungs and ovaries, as reported previously (Jiang et al., 2005) (Figure 2A). Mice lacking either Noxa or Bad had little or no leukocyte infiltration of peripheral organs (Figure 2A). Aged Bcl2l11•/• mice occasionally developed mild infiltration of the lung, but in general, exhibited little autoimmunity (mean ± SEM, 1.7±0.5). By contrast, aged Bbc3•/•Bcl2l11•/• mice developed significantly more autoimmune infiltrates in more organs than Bcl2l11•/• mice (mean ± SEM, 4.2±0.6), with clinical scores comparable to those observed in Aire•/• mice (Figure 2A). The distribution of autoimmune infiltrate in organs from aged Bbc3•/• Bcl2l11•/• mice was similar to that observed in the Rag1•/• reconstitution model, although generally less severe (Figure 2B). The infiltration of the salivary gland was accompanied by fibrinoid necrosis of blood vessels, consistent with vasculitis that was occasionally destructive in this organ. These features did not accompany the lymphocytic infiltrates found in the lung, liver or pancreas (Figure 2B and data not shown). Serum autoantibodies to these tissues were also detected, confirming the autoimmune nature of the lymphocytic infiltrates observed (data not shown).
Figure 2. Spontaneous organ-specific autoimmunity in Bbc3•/•Bcl2l11•/• mice.
A. Graph of the cumulative clinical scores of 10 organs quantifying autoimmune infiltration and destruction in aged mice deficient in the proteins indicated. Groups were compared using an ANOVA with a multiple comparison Tukey’s post-test. * p<0.05, *** p<0.001. Mean ages at time of analysis were: Aire•/• d237 (n=11); Pmaip1•/• d147 (n=9); Bad•/• d165 (n=7); Bcl2l11•/• d141 (n=8); Bad•/•Bcl2l11•/• d166 (n=5); Pmaip1•/•Bcl2l11•/• d153 (n=13); Bbc3•/•Bcl2l11•/• d143 (n=15). B. Representative images of H&E stained sections of tissues taken from aged Bbc3•/•Bcl2l11•/• mice, with arrows indicating lymphocytic infiltration. C. Images of H&E stained sections of pancreas taken from Rag1•/• mice that had been injected with T-cells from WT or healthy Bbc3•/•Bcl2l11•/• mice, with arrows indicating lymphocytic infiltration and tissue destruction. D. Graph of the incidence and severity of pancreatitis in Rag1•/• mice that had been injected with T-cells from WT or Bbc3•/•Bcl2l11•/• mice (n=8). E. CD44 vs. CD62L expression on CD4+ splenic T-cells, with the mean proportion of activated CD44hiCD62Llo/− T-cells shown.
To determine whether T-cells mediated the autoimmunity observed, we performed adoptive transfer of T-cells from WT or young Bbc3•/•Bcl2l11•/• mice into Rag1•/• mice. After eight weeks we assayed infiltration of the pancreas, a signature target organ in mice deficient in both Puma and Bim. T-cells transferred from WT mice did not cause pathology in the pancreas, whereas those from Bbc3•/•Bcl2l11•/• mice elicited mild to severe exocrine pancreatitis (Figure 2C and 2D). Consistent with a critical role for T-cells in mediating the organ-specific autoimmunity observed in Puma and Bim double-deficient mice, we found increased levels of CD44highCD62Llow effector-memory T-cells in the spleen and lymph nodes, also a feature of Aire•/• mice (Figure 2E) (Anderson et al., 2002). Together, these data show that mice deficient in both Puma and Bim spontaneously develop T-cell driven autoimmunity that is not observed in Bim-deficient mice.
Among BH3-only proteins, only the additional loss of Puma worsens the thymocyte developmental defects in Bim-deficient mice
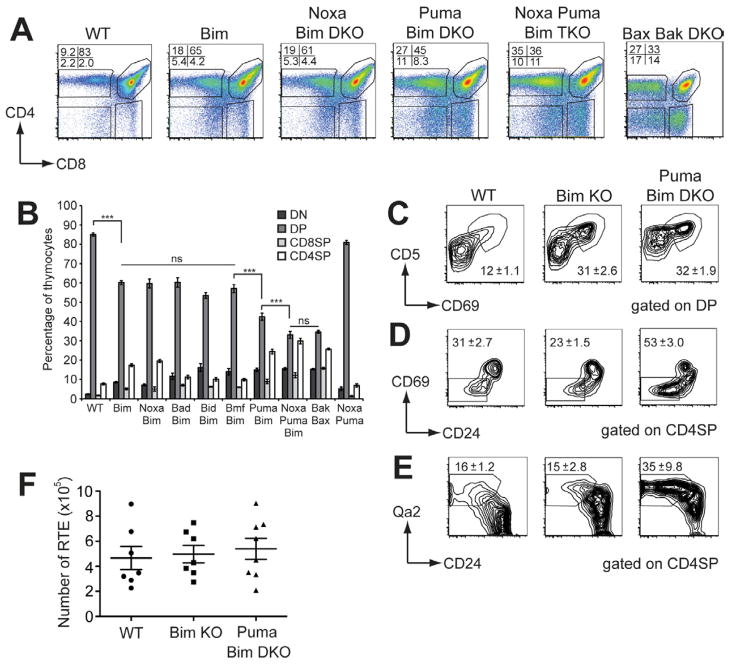
Thymocyte differentiation in Bcl2l11•/• mice is abnormal, with a marked reduction in DP thymocytes and corresponding increases in CD4SP and CD8SP T-cells, consistent with autoreactive cells evading thymic deletion (Bouillet et al., 2002). Previous studies showed that the single loss of any other BH3-only protein does not disrupt thymocyte differentiation ((Kaufmann et al., 2007; Labi et al., 2008; Ranger et al., 2003; Villunger et al., 2003) and data not shown). However, the compound loss of both Bim and Puma impaired thymocyte differentiation markedly beyond what is observed in Bcl2l11•/• mice (Erlacher et al., 2006), indicating that there are other roles for BH3-only proteins in thymocyte apoptosis. For this reason, and the T-cell dependent organ-specific autoimmunity observed in mice deficient in both Puma and Bim, we performed a deeper analysis of thymocyte differentiation in the absence of Bim and other BH3-only proteins.
We established BM chimeras in C57BL/6.Ly5.1 (Ly5.1) mice using the various compound mutant mice as donors and analysed thymocyte differentiation 8 weeks later. In contrast to the Rag1•/• chimeras, Ly5.1 mice reconstituted with precursors from Vav-BCL-2 transgenic or Bbc3•/•Bcl2l11•/• mice showed no signs of autoimmunity at this time point (median survival is extended to 206 days; K. Mason, D.H.D.G., A.S. and L.A.O. unpublished observations), thereby excluding any possible impact of peripheral autoimmunity on thymocyte subset composition. As expected, Bim deficiency significantly reduced the proportion and number of DP thymocytes, while increasing the CD4SP and CD8SP mature subsets compared to WT controls (Figure 3A, B and Figure S1)(Bouillet et al., 1999). Mice reconstituted with precursors from Pmaip1•/•Bcl2l11•/•, Bad•/•Bcl2l11•/•, Bid•/•Bcl2l11•/• or Bmf•/•Bcl2l11•/• mice exhibited no differences in thymocyte differentiation compared to the Bcl2l11•/• chimeras (Figure 3A and 3B). Consistent with the observations of Erlacher, et al. (Erlacher et al., 2006), mice reconstituted with precursors deficient in both Bim and Puma showed a further, substantial reduction in the percentage of DP thymocytes compared to Bcl2l11•/• chimeras, as the number and proportion of mature CD4SP and CD8SP increased (Figure 3A, 3B and Figure S1B). This exacerbation of the defects in thymocyte differentiation caused by hematopoietic deficiency of both Puma and Bim was reflected by the reduced ratio of DP:SP thymocytes (2.7±0.1 in Bcl2l11•/•; 1.3±0.1 in Bbc3•/•Bcl2l11•/•; (mean±SEM) Student’s t-test p>0.0001). Importantly, the loss of Puma alone had no discernible impact on thymocyte differentiation ((Erlacher et al., 2006) and data not shown). Together, these data show that Puma is unique among the BH3-only proteins in synergizing with Bim to execute the apoptosis of thymocytes at the critical DP to SP transition when negative selection primarily occurs.
Figure 3. Only the additional loss of Puma exacerbates defects in thymocyte development in Bim-deficient mice.
A. CD4 vs. CD8 expression profiles of thymocytes from lethally irradiated mice reconstituted with hematopoietic progenitors lacking the BH3-only proteins indicated and analyzed 8 weeks later. Profiles are representative of 4 experiments (total n=6–13). B. Thymocyte subset proportions in mice reconstituted with hematopoietic progenitors from mice lacking the BH3-only proteins indicated. Statistically significant differences among the proportions of DP thymocytes are shown, tested with a one-way ANOVA with multiple comparison Tukey’s post-test (*** p<0.0001). C. CD5 vs. CD69 expression on DP thymocytes with the mean (±SEM) proportion of CD69+CD5hi thymocytes shown. D. CD69 vs. CD24 expression on CD4SP thymocytes with the mean (±SEM) proportion of mature CD69−CD24lo/− thymocytes shown. E. Graph of the numbers of FITC+ RTE recovered from the spleens of mice 24 h following intra-thymic FITC injection, with means±SEM shown.
To further investigate how the intrinsic pathway of apoptosis shapes thymocyte differentiation, we also constructed chimeras from Bbc3•/•Pmaip1•/•Bcl2l11•/• mice (Figure 3A, 3B and Figure S1). An additional impact on the DP to SP transition was observed, showing that, although Noxa can affect some thymocyte apoptosis, Bim and/or Puma can fulfil this role. Furthermore, Bbc3•/•Pmaip1•/• chimeras showed no thymocyte defects compared to WT controls, indicating that Bim alone is sufficient to mediate the pro-apoptotic functions exerted by Puma and Noxa during thymocyte differentiation (Figure 3B and Figure S1B). We also compared the thymocyte phenotype of Bbc3•/•Pmaip1•/•Bcl2l11•/• chimeras with that of Bax•/•Bak•/• chimeras, in which the intrinsic pathway of apoptosis is completely disabled. There were no differences between these two groups (Figure 3B), indicating that the loss of the three pro-apoptotic BH3-only proteins Puma, Noxa and Bim causes maximal impairment of thymocyte apoptosis in vivo.
Altered thymocyte selection in mice deficient in both Puma and Bim
The exacerbated accumulation of SP thymocytes in Bbc3•/•Bcl2l11•/• mice suggested that further impairment of deletion was the basis of the organ-specific autoimmunity observed in these mice. We therefore investigated the expression of a range of markers associated with thymocyte selection events.
When thymocytes express an αβTcR that engages self-MHC:peptide complexes in the thymic cortex with moderate affinity, they are rescued from apoptosis and commence differentiation into SP cells while they migrate to the thymic medulla. To assess whether the loss of BH3-only proteins affected the efficiency of DP positive selection, we examined the upregulation of CD69 and CD5, early indicators of TCR signaling. Surprisingly, we found that the proportion of CD69+CD5hi DP thymocytes was increased in the Bcl2l11•/• chimeras compared to recipients of WT BM, consistent with an increased rate of positive selection (Figure 3C). Bbc3•/•Bcl2l11•/• chimeras had a similar proportion and number of CD69+ DP as the Bcl2l11•/• chimeras (Figure 3C and Figure S1C & S1D), suggesting that altered positive selection was not the basis for the organ-specific autoimmunity.
In the absence of further TCR stimulation, positively selected thymocytes downregulate CD69 following differentiation into CD8SP or CD4SP cells. As SP thymocytes mature, they downregulate CD24 and upregulate CD62L and Qa2 prior to their S1P1-mediated egress from the thymus (Love and Bhandoola, 2011). However, in thymocytes expressing a TCR that interacts with self peptide:MHC complexes with high avidity, this maturation is interrupted and they are usually induced to undergo apoptosis (deletion) (Hogquist et al., 2005). Analysis of Bcl2l11•/• chimeras revealed a normal distribution of CD69+CD24hi semi-mature and CD69−CD24lo/− mature CD4SP thymocytes, but Bbc3•/•Bcl2l11•/• chimeras had an increased proportion and number of mature CD69−CD24lo/−CD4SP cells (Figure 3D and Figure S1E and S1F). The increase in mature SP was confirmed by the detection of increased proportions of Qa2+/CD24lo/− and CD62L expressing cells in Bbc3•/•Bcl2l11•/• compared to Bcl2l11•/• chimeras (Figure 3E and Figure S1G). The increase in mature SP in Bbc3•/•Bcl2l11•/• compared to Bcl2l11•/• chimeras suggests that the deletion of semi-mature SP had been impaired, allowing further maturation of thymocytes normally deleted in a Puma-dependent manner. Importantly, we observed no reduction in thymic dendritic cell (DC) numbers, proportions or MHC II expression in Bim- deficient or Puma and Bim-deficient mice (data not shown), ruling out defects in DC as an explanation for the aberrant thymocyte differentiation in these animals.
In light of this apparent disruption in SP maturation, it was important to assess T-cell export from the thymus of Bbc3•/•Bcl2l11•/• chimeras. Therefore, we performed intrathymic FITC injections of chimeras and analysed FITC+ recent thymic emigrants in the spleen 24 hours later. Comparable numbers of T-cells were exported from the thymi of WT, Bcl2l11•/• or Bbc3•/•Bcl2l11•/• chimeras (Figure 3F), indicating that no global alterations in thymic output had occurred. Therefore, despite extensive perturbations to thymic selection, similar numbers of T-cells were exported in mice deficient in both Bim and Puma.
Together with the autoimmunity observed in peripheral organs of Bbc3•/•Bcl2l11•/• mice, these data implicated a failure of deletion in the medulla. Therefore, we went on to directly examine whether deletion to PTA was impaired in mice deficient in both Puma and Bim.
Loss of Puma, in addition to loss of Bim, causes increased persistence of autoreactive thymocytes and T-cells
To address whether there is an auxiliary role for Puma supporting Bim in the deletion of thymocytes reactive to PTA we turned to two well-established TCR transgenic models of thymic deletion driven by a neo-peripheral antigen (Anderson et al., 2005; Hubert et al., 2011). RIP-mOVA transgenic mice express a membrane-bound form of ovalbumin (mOVA) under the control of a rat insulin promoter (RIP) which enforces expression in the pancreatic beta cells, the kidney and medullary epithelial cells of the thymus (Gallegos and Bevan, 2004). Therefore, when thymocytes expressing the OT-I TCR (reactive to a fragment of mOVA presented by H2-Kb molecules) develop in this setting, extensive deletion of OT-I CD8SP ensues in the medulla (Anderson et al., 2005; Gallegos and Bevan, 2004; Hubert et al., 2011). The transcriptional regulator, Aire is required for this deletion, affecting the expression (Hubert et al., 2011) and/or presentation (Anderson et al., 2005) of mOVA in the thymus.
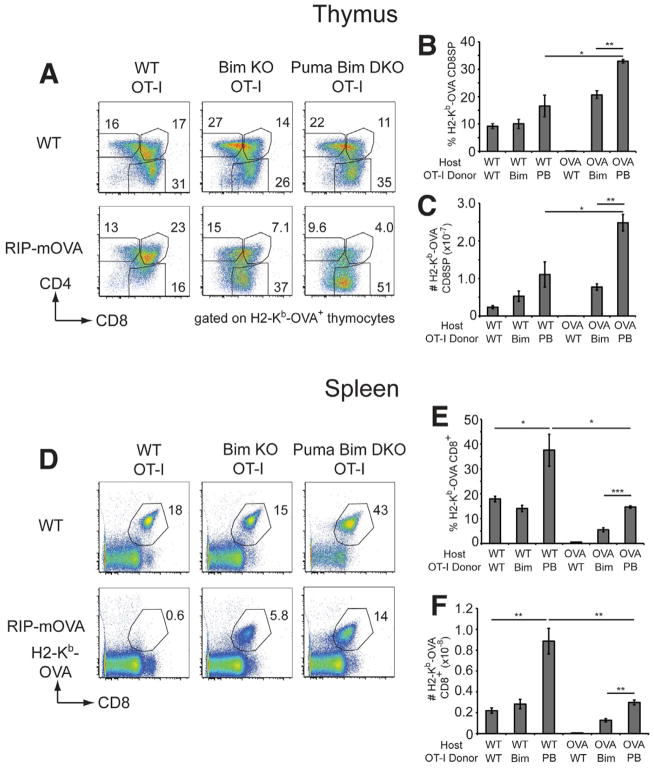
We constructed a series of hematopoietic chimeras using T-cell depleted BM from WT OT-I, Bcl2l11•/• OT-I or Bbc3•/•Bcl2l11•/• OT-I mice introduced into C57BL/6 (B6) mice (to assay positive selection) or RIP-mOVA+ hosts (to assay deletion). Analysis of thymocytes expressing the OT-I TCR using an H2-Kb-OVA tetramer revealed normal positive selection of OT-I CD8SP thymocytes in the B6 chimeras, regardless of the donor genotype (Figure 4A). As expected, there was complete deletion of OVA-reactive CD8SP thymocytes in WT OT-I => RIP-mOVA+ chimeras due to their encounter of cognate antigen in the thymic medulla (Figure 4A) (Gallegos and Bevan, 2004). In accord with recent studies (Moran et al., 2011; Suen and Baldwin, 2012), we observed that Bim deficiency rescued many of these thymocytes from deletion (Figure 4A). We found equivalent numbers of Bcl2l11•/• OT-I thymocytes in both RIP-mOVA− and RIP-mOVA+ hosts, suggesting that most OT-I thymocytes that would otherwise have been deleted by antigen encounter had been rescued (Figure 4B & C).
Figure 4. Bim and Puma are required for deletion of OT-I thymocytes in RIP-mOVA+ mice.
A. Representative dot plots of CD4 and CD8 expression gated on H2-Kb-OVA tetramer+ thymocytes from WT or RIP-mOVA+ chimeras that had been reconstituted with BM from OT-I transgenic mice on WT, Bim-deficient or Puma and Bim double-deficient backgrounds. The percentages of DP, CD4SP and CD8SP gated on H2-Kb-OVA+ thymocytes are shown. Mean percentages (B.) and cell numbers (C.) of H2-Kb-OVA+ CD8SP thymocytes (±SEM) from the various chimeras. D. H2-Kb-OVA versus CD8 staining on splenocytes from the various chimeras, with the proportion of H2-Kb-OVA+ CD8+ T-cells shown. Mean percentages (E.) and numbers (F.) of H2-Kb-OVA+ CD8+ splenocytes (±SEM) from the various chimeras. PB denotes Puma and Bim double-deficient. Groups composed of 3–4 mice were compared using student’s t-test; * p<0.05, ** p<0.01, *** p<0.001. Data from one of two experiments are shown.
Remarkably, Bbc3•/•Bcl2l11•/• OT-I => RIP-mOVA+ chimeras contained almost twice the proportion and three times the number of autoreactive OT-I CD8SP recovered from the Bcl2l11•/• OT-I => RIP-mOVA+ chimeras (Figure 4B & C). The proportion and numbers of OT-I thymocytes in the Bbc3•/•Bcl2l11•/• OT-I => RIP-mOVA+ chimeras exceeded those observed in the Bbc3•/•Bcl2l11•/• OT-I => B6 hosts, indicating that, in the presence of antigen, autoreactive thymocytes deficient in both Puma and Bim were able to further expand (Figure 4B & C).
The changes to thymocyte selection observed with the compound loss of Puma and Bim were reflected in the periphery. Under positive selecting conditions, similar numbers of OT-I CD8+ T-cells appeared in the spleens of B6 mice receiving WT or Bcl2l11•/• OT-I precursors (Figure 4D–F). Bbc3•/•Bcl2l11•/• OT-I => B6 chimeras had an increased percentage and number of OT-I CD8+ T-cells in their periphery. Under deleting conditions, essentially no OT-I T-cells escaped the thymus of WT OT-I => RIP-mOVA+ chimeras. By contrast, a significant proportion and number of splenic OT-I T-cells appeared with Bim deficiency and these doubled when Puma was also absent (Figure 4D–F).
Interestingly, the OT-I CD8SP thymocytes that escaped deletion in the RIP-mOVA+ chimeras receiving either Bcl2l11•/• OT-I or Bbc3•/•Bcl2l11•/• OT-I BM had downregulated the CD8 co-receptor and αβTCR compared to their B6 controls (Figure S2A & S2B). This downregulation of the αβTCR and the co-receptor was also apparent in a population of SP thymocytes in non-transgenic Bcl2l11•/• and Bbc3•/•Bcl2l11•/• mice ((Bouillet et al., 2002) and Figure 3A).
Puma is required for the deletion of semi-mature SP thymocytes
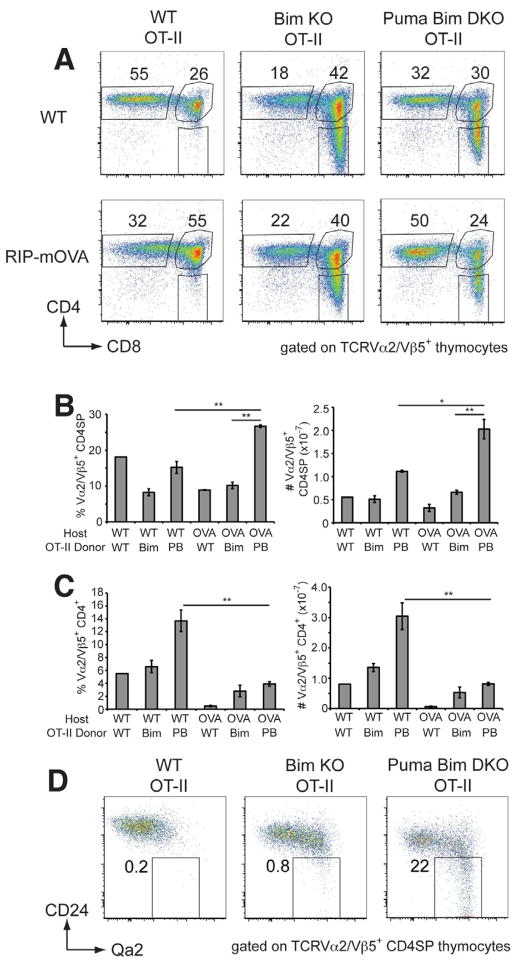
To confirm and extend these findings to an MHC II-restricted system, we performed similar experiments using the OT-II TCR transgenic model. Irradiated RIP-mOVA transgenic or B6 mice received T-cell depleted BM from WT OT-II, Bcl2l11•/• OT-II or Bbc3•/•Bcl2l11•/• OT-II mice and 8 weeks later, OT-II T-cell differentiation was analyzed in these chimeras with antibodies to the Vα2/Vβ5 components of the OT-II TCR. Under the positive selection conditions of the B6 chimeras, only the mice deficient in both Puma and Bim had increased numbers of Vα2/Vβ5+ CD4SP (Figure 5A & B).
Figure 5. Both Bim and Puma are required for the deletion of mature CD4SP OT-II thymocytes in RIP-mOVA+ mice.
A. Representative dot plots of CD4 and CD8 expression gated on Vα2/Vβ5+ thymocytes from RIP-mOVA− or RIP-mOVA+ chimeras that had been reconstituted with BM from OT-II transgenic mice on WT, Bim-deficient or Puma and Bim double-deficient backgrounds. The percentages of Vα2/Vβ5+ CD4SP are shown. Mean percentages and cell numbers of Vα2/Vβ5+ CD4SP thymocytes (B.) or Vα2/Vβ5+ CD4+ splenocytes (C.) (±SEM) from the various chimeras. D. Qa2 versus CD24 expression on Vα2/Vβ5+ CD4SP from the various chimeras. Groups composed of 3–4 mice were compared using student’s t-test; * p<0.05, ** p<0.01. Data from one of two experiments are shown.
Under negative selection conditions in the RIP-mOVA+ chimeras, the proportion and number of CD4SP in recipients of WT OT-II BM were approximately half of those observed in C57BL/6 chimeras, as the Vα2/Vβ5high cells were deleted (Figure 5A & B and data not shown). In RIP-mOVA+ chimeras, Bim deficiency restored the numbers of OT-II CD4SP to those observed under conditions of positive selection. This increase was accompanied by significant numbers of splenic Vα2/Vβ5+ CD4+ T-cells (Figure 5C), indicating substantial impairment of thymic deletion in the absence of Bim.
In accord with the OT-I system, Bbc3•/•Bcl2l11•/• OT-II => RIP-mOVA+ chimeras generated approximately twice the number of OT-II CD4SP compared to B6 recipients (Figure 5A & B). In contrast to recipients of WT or Bcl2l11•/• OT-II BM, many of the CD4SP in Bbc3•/•Bcl2l11•/• OT-II => RIP-mOVA+ chimeras exhibited a mature, CD24lowQa2+ phenotype (Figure 5D). These data, and similar findings in the OT-I system (data not shown), are consistent with the specific expansion of mature SP thymocytes observed in non-transgenic Bbc3•/•Bcl2l11•/• mice (Figure 3E). Analysis of BrdU incorporation 16 h following injection revealed that all chimeras had a similar, low level of dividing OT-II CD4SP, indicating that the expansion of this subset in Bbc3•/•Bcl2l11•/• chimeras was not caused by increased proliferation (Figure S3A). By contrast, thymocytes from mice deficient in both Puma and Bim were more resistant to apoptosis provoked by in vitro TCR stimulation than thymocytes from Bim-deficient (or WT) mice, measured by both Annexin V/PI and active-caspase-3 staining (Figure S3B–E). We also found that TCR-stimulated thymocytes from Bbc3•/•Bcl2l11•/• mice had considerably lower Bak activation, measured using an antibody that recognises the active form of Bak (data not shown). Together, these results suggest that the loss of Puma (in addition to Bim) rescues CD4SP OT-II thymocytes that are normally deleted at the CD24highQa2− semi-mature stage of differentiation via activation of the intrinsic pathway of apoptosis.
In summary, these data suggest that although Bim deficiency alone can inhibit the deletion of PTA-reactive thymocytes, compound loss of Bim plus Puma substantially enhances the rescue of autoreactive thymocytes, allowing a greater number to complete thymic maturation and emigrate to the periphery.
Discussion
Bim is required for thymocyte deletion in a range of settings, however, the absence of organ-specific autoimmunity in Bim-deficient mice suggests that other apoptotic regulators play a role in PTA-mediated deletion. This prediction was borne out by our observations of organ-specific autoimmunity in Rag1•/• mice reconstituted with precursors from Vav-BCL-2 transgenic mice, in which the intrinsic pathway of apoptosis is potently blocked. By reconstituting Rag1•/• mice with hematopoietic precursors from a range of compound mutant mice lacking Bim and other BH3-only proteins, we found that only the combined loss of Puma and Bim provoked a similar pathology in this sensitized assay of autoimmunity. Importantly, aged Bbc3•/•Bcl2l11•/• mice spontaneously developed organ-specific autoimmunity (albeit with a reduced severity), indicating that Puma was unique among BH3-only proteins in synergizing with Bim to impose deletional tolerance of peripheral tissues.
The autoimmunity in Bbc3•/•Bcl2l11•/• mice was reminiscent of that observed in Aire•/• mice, but there are important differences in the pathologies and, presumably, the auto-antigens involved. For instance, retinopathy and serum autoantibodies directed against the interphotoreceptor layer develop in Aire•/• mice (Anderson et al., 2002) but were not observed in mice deficient in both Puma and Bim. Conversely, the lymphocytic infiltration of the pancreas in Bbc3•/•Bcl2l11•/• mice (which became destructive in reconstituted Rag1•/• mice) was not observed in C57BL/6.Aire•/• mice (Jiang et al., 2005). These findings suggest that Puma and Bim are not required for deletional tolerance of all Aire-regulated PTA, and conversely, can affect tolerance to Aire-independent PTA.
Defects in T-cell tolerance underpinned the autoimmunity we observed in Bbc3•/•Bcl2l11•/• mice. Their T-cells showed evidence of increased activation and could transfer autoimmunity to Rag1•/• recipients. In the thymus, Puma was again unique amongst the BH3-only proteins in preventing further accumulation of SP thymocytes in the absence of Bim. In fact, increased numbers of mature CD69−CD24low/−CD62L+Qa2+ SP thymocytes was a prominent, distinguishing feature of Bbc3•/•Bcl2l11•/• mice in both the polyclonal and TCR transgenic settings. Evidence that this SP defect reflected impaired tolerance came from the TCR transgenic models where compound deficiency in Puma and Bim in the OT-I and OT-II models significantly increased the escape of PTA-reactive thymocytes beyond that in Bcl2l11•/• mice. Importantly, the increase could not be attributed to an increased proliferative response to the antigen because there was no change in BrdU incorporation by these cells. Rather, our data indicate that Puma is required for normal deletion of semi-mature CD69+CD24hiCD62L−Qa2− SP thymocytes following high avidity TCR stimulation, extending upon Bim’s function in this process (Villunger et al., 2004).
Is the increased production of PTA-reactive T-cells escaping Puma- and Bim-mediated deletion driving the autoimmunity observed in these mice? Defects in thymic deletion cause the autoimmunity observed in Aire•/• mice (reviewed in (Taniguchi and Anderson, 2011)), without any requirement for “danger” signals or inflammation (Gray et al., 2007b). The PTA-mediated deletion of OT-I and OT-II thymocytes was even more severely affected in Bbc3•/• Bcl2l11•/• mice than in Aire•/• mice (Anderson et al., 2005; Hubert et al., 2011), suggesting the lesion to central tolerance caused by crippling the intrinsic pathway of apoptosis would be sufficient to explain the autoimmunity we observed. In addition, there is good evidence that dominant tolerance imposed by Treg remains intact in Bbc3•/•Bcl2l11•/• mice (indeed, it may be amplified); CD25+ Treg from mice with deficiencies in the intrinsic pathway of apoptosis function normally and are significantly increased in number (Zhan et al., 2011), while conventional T-cells from Bbc3•/•Bcl2l11•/• mice can be efficiently suppressed (Szymczak-Workman et al., 2011). Therefore, the thymic production of PTA-reactive T-cells in mice deficient in Puma and Bim is likely to be sufficient to provoke autoimmunity, overwhelming peripheral tolerance mechanisms.
By contrast to Aire•/• mice, thymocytes that avoid deletion in Bbc3•/•Bcl2l11•/• mice show evidence of antigen encounter in the thymus (increased CD69 and reduced co-receptor expression). This stimulation would presumably increase the scope for the induction of non-deletional negative selection mechanisms, which may explain the finding that Bbc3•/•Bcl2l11•/• OT-I => RIP-mOVA chimeras did not develop diabetes and exhibited peripheral OT-I T-cells with an anergic phenotype (consistent with the data in Bcl2l11•/• mice from (Suen and Baldwin, 2012)). Therefore, among the PTA-reactive clones that escape to the periphery, only some will cause organ-specific autoimmunity, while others may become anergic or be diverted into Treg. The next challenge is to elucidate the most important synergies between non-deletional pathways and the physiological mediators of deletion we have identified here.
The reason Puma is unique among BH3-only proteins in providing an ancillary function in central tolerance is probably because it can, like Bim, engage in strong interactions with all of the pro-survival Bcl-2 proteins (Chen et al., 2005; Kuwana et al., 2005). The increased expression of the anti-apoptotic Bcl-2 family members throughout late thymocyte differentiation would require potent antagonism from BH3-only proteins for efficient induction of apoptosis following high avidity TCR interactions in the medulla. The selective interactions of the other BH3-only proteins (e.g. Noxa, Bmf and Bad) with the prosurvival Bcl-2 family members, or the requirement for death receptor engagement (for Bid activation) might render them ineffective for PTA-mediated thymocyte deletion. It is also possible that the requirement for Puma and Bim in combination might be for direct activation of Bax and Bak (Dewson and Kluck, 2009). However, we also found that the loss of Noxa, Puma and Bim together was required to phenocopy the Bax•/•Bak•/• chimeras. Given that the combined loss of Bax and Bak completely cripples the intrinsic apoptotic pathway, this observation indicates that Bim, then Puma, and then Noxa are the major BH3-only proteins involved in the life and death decisions that shape thymocyte differentiation.
Our results also demonstrate that this important pathway is extremely robust. The likelihood that the combination of loss-of-function mutations necessary to completely ablate the intrinsic pathway of apoptosis (i.e. to Noxa, Puma and Bim or Bax and Bak) would manifest in thymocytes is extremely low. It is more probable that partial defects to deletion and anergy or T-cell regulation could cooperate to drive autoimmune disease (e.g. (Teh et al., 2010)). Understanding the molecular basis to those tolerance mechanisms that synergise with deletion should reveal better ways to deal with autoreactivity and autoimmune disease.
Experimental Procedures
Mice
All mice were housed at WEHI under SPF conditions and experiments were carried out in accordance with the Melbourne Directorate Animal Ethics Committee guidelines. Some of the gene-targeted mice (Pmaip1•/•, Bbc3•/•, Bid•/•, Bmf•/•) were generated on an inbred C57BL/6 background, using C57BL/6 derived ES cells. Other mice (Bad•/•, Bcl2l11•/•, Trp53•/•) were generated on a mixed C57BL/6x129SV background and then backcrossed to the C57BL/6 background >10 times prior to intercrossing. The BM donor strains Bcl2l11•/• and Trp53•/• were backcrossed 14 and 46 times to the C57BL/6 background, respectively. The Vav-BCL-2 tg mice were generated on an inbred C57BL/6 background (Ogilvy et al., 1999). Bcl2l11•/• (Bouillet et al., 1999), Pmaip1•/• (Villunger et al., 2003), Bbc3•/• (Villunger et al., 2003), Bid•/• (Kaufmann et al., 2007), Bmf•/• (Labi et al., 2008), Bad•/• (Ranger et al., 2003), Bax•/• (Knudson et al., 1995), Bak•/• (Lindsten et al., 2000), and Trp53•/• (Jacks et al., 1994) were intercrossed to generate the various compound mutant mice. Vav-BCL-2 (Ogilvy et al., 1999), OT-I (Hogquist et al., 1994), OT-II (Barnden et al., 1998) and RIP-mOVA (Kurts et al., 1996) transgenic mice (all generated on a C57BL/6 background) have been described and Rag1•/• and C57BL/6.Ly5.1 mice were obtained from WEHI Bioservices. For the assessment of spontaneous autoimmunity in aged mice, compound mutants were taken when moribund for any reason (usually due to glomerulonephritis), excluding leukemia. Bad•/• or Pmaip1•/• mice were taken when their double knockout littermates became sick.
Hematopoietic reconstitution
To generate hematopoietic chimeras, adult Rag1•/• or B6.Ly5.1 mice were irradiated with 2 doses of 5.5 Gy 3 h apart and reconstituted by i.v. injection of 0.5–1.0 × 107 T-cell depleted bone marrow cells. T-cell depletion was performed on an AutoMACS (Miltenyi Biotec, Germany) using biotinylated anti-Thy-1 mAb (clone T24) and streptavidin or anti-biotin microbeads according to the manufacturer’s instructions. Mice received 100 μg of anti-Thy1 mAb (clone T24) i.p. 24 h following injection of bone marrow (BM) cells to eliminate residual donor T-cells and were then left for 8 weeks prior to analysis.
Adoptive transfer
Splenic T-cells from either WT or healthy Bbc3•/•Bcl2l11•/• mice were isolated by staining with biotinylated anti-Thy-1 (clone T24), followed by incubation with streptavidin microbeads and positive selection on an AutoMACS according to the manufacturer’s instructions. Rag1•/• mice received 1×106 T-cells and after 8 weeks, organs were harvested for histological analysis and flow cytometric analysis of blood and spleen was performed to confirm that only donor-derived T-cells were present.
Flow cytometry
Single cell suspensions of various organs were stained with fluorochrome or biotin conjugated antibodies to the following markers (produced in our laboratory except where indicated): CD4 (BioLegend, clone GK1.5), CD8 (clone YTS.169), TCRβ (clone H57-597), TCRVβ5 (BD Biosciences, clone MR9-4), TCRVα2 (BD Biosciences, clone B20.1), CD44 (clone IM7), CD62L (BioLegend, clone MEL-14), CD5 (BioLegend, clone 53-7.3), CD69 (BioLegend, clone H1.2F3), CD24 (BioLegend, clone M1/69), Ly5.1 (clone A20) and Qa2 (BioLegend, clone 695H1-9-9). H-2Kb-OVA monomer was purchased from Dr. Andrew Brooks (University of Melbourne) and tetramerized with streptavidin-PE (BD Biosciences). Bromodeoxyuridine (BrdU) (Sigma) was administered via intraperitoneal injection (1mg) and detected with Alexa Fluor-647 conjugated anti-BrdU (Invitrogen, clone MoBU-1) as described (Gray et al., 2007a). Apoptosis was measured by Annexin V and PI staining, or by active-caspase-3 (BD Biosciences) staining of fixed and permeabilised thymocytes. Sample data were acquired on an LSRII flow cytometer (BD Biosciences) and analysed using FloJo software (Treestar).
Histology
Tissues were fixed in formalin, embedded, sectioned and stained with hematoxylin and eosin (H&E), and infiltration was scored as described (Gray et al., 2007b), with scores 0, 0.5, 1, 2 and 3 indicating none, trace, mild, moderate or severe lymphocytic infiltration and tissue destruction, respectively. The organs analyzed included the eyes, Harderian glands, salivary glands, thyroid glands, prostate glands or ovaries, stomach, pancreas, kidney, liver and lungs. For serum immunohistology, 8 μm sections of the indicated organs were prepared from 7–10 wk old Rag1•/• mice (to preclude background caused by endogenous Ig) and stained with 1:100 dilutions of sera, followed by FITC-conjugated goat anti-mouse IgG antibodies (Cappel) and DAPI (Sigma). Immunofluorescent images were acquired using an LSM5 system (Zeiss).
Quantification of recent thymic emigrants
C57BL/6.Ly5.1 hematopoietic chimeras grafted with BM cells from WT, Bcl2l11•/• or Bbc3•/• Bcl2l11•/• mice were subjected to intra-thymic FITC injection as described (Berzins et al., 1998). Mice were then killed by CO2 asphyxiation 24hrs later and single cell suspensions of lymphoid organs were stained with antibodies to Ly5.1, CD4 and CD8 for analysis on an LSRII flow cytometer. Recent thymic emigrants were identified as FITC+ cells expressing either CD4 or CD8 among live-gated cells and the numbers of emigrants corrected for the extent of FITC labeling of thymocytes (typically 50–90%).
Statistics
Statistical comparisons were made using 1-way ANOVA with a Tukey’s post-test for multiple comparisons or a 2-tailed Student’s t-test with Prism v5.0 (Graphpad). P-values <0.05 were considered to indicate a statistically significant difference.
Supplementary Material
Acknowledgments
We gratefully acknowledge C. Thompson (Memorial Sloan-Kettering Cancer Center, New York, NY, USA) for the kind gift Bak•/• mice, N. Danial (Dana Farber, Boston, MA, USA) for Bad•/• mice and T. Jacks (MIT, Cambridge, MA, USA) for Trp53•/• mice; K. Mason and D. Huang for the provision of Bax•/•Bak•/• mouse fetal livers; G. Dewson for the provision of antibodies; G. Siciliano, K. Vella, K. Trueman, G-F. Dabrowski for animal care; B. Helbert, C. Young, A. Policheni and the WEHI Cytometry and Histology Facilities for technical assistance. This work was supported by the Australian National Health and Medical Research Council (NH&MRC Project Grants #637332 and #1009145, Australia Fellowship to A.S., Career Development Fellowship (#637353) to D.H.D.G) and the Juvenile Diabetes Foundation; the Leukemia and Lymphoma Society (New York; SCOR grant #7413), the National Cancer Institute (NIH, US; CA 43540). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA- based a- and b-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, Strasser A. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. Embo J. 2005;24:3963–3973. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol. 2004;24:1570–1581. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, Carbone FR, Heath WR. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. Journal of Experimental Medicine. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 2009;122:2801–2808. doi: 10.1242/jcs.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bath ML, O’Reilly L, Cory S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 2004;103:2276–2283. doi: 10.1182/blood-2003-07-2469. [DOI] [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Laabi V, Manzl C, Bock G, Tzankov A, Haecker G, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. Journal of Experimental Medicine. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson PA, Toh BH, van Driel IR. Organ-specific autoimmunity induced by lymphopenia. Immunol Rev. 1996;149:97–125. doi: 10.1111/j.1600-065x.1996.tb00901.x. [DOI] [PubMed] [Google Scholar]
- Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007a;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DH, Gavanescu I, Benoist C, Mathis D. Danger-free autoimmune disease in Aire-deficient mice. Proc Natl Acad Sci U S A. 2007b;104:18193–18198. doi: 10.1073/pnas.0709160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Hu Q, Sader A, Parkman JC, Baldwin TA. Bim-Mediated Apoptosis Is Not Necessary for Thymic Negative Selection to Ubiquitous Self-Antigens. J Immunol. 2009;183:7761–7767. doi: 10.4049/jimmunol.0902181. [DOI] [PubMed] [Google Scholar]
- Huang DCS, Strasser A. BH3-only proteins – essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen TN, McKee A, Wang M, Kushnir E, White J, Refaeli Y, Kappler JW, Marrack P. Bim and Bcl-2 mutually affect the expression of the other in T cells. J Immunol. 2007;179:3417–3424. doi: 10.4049/jimmunol.179.6.3417. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A. The BH3-Only Protein Bid Is Dispensable for DNA Damage- and Replicative Stress-Induced Apoptosis or Cell-Cycle Arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Tung KSK, Tourtellotte WG, Brown GAJ, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 Domains of BH3-Only Proteins Differentially Regulate Bax-Mediated Mitochondrial Membrane Permeabilization Both Directly and Indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, Strasser A, Villunger A. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11:469–477. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci U S A. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen AY, Baldwin TA. Proapoptotic protein Bim is differentially required during thymic clonal deletion to ubiquitous versus tissue-restricted antigens. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1114834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak-Workman AL, Delgoffe GM, Green DR, Vignali DA. Cutting Edge: Regulatory T Cells Do Not Mediate Suppression via Programmed Cell Death Pathways. J Immunol. 2011;187:4416–4420. doi: 10.4049/jimmunol.1100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi RT, Anderson MS. The role of Aire in clonal selection. Immunol Cell Biol. 2011;89:40–44. doi: 10.1038/icb.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh CE, Daley SR, Enders A, Goodnow CC. T-cell regulation by casitas B-lineage lymphoma (Cblb) is a critical failsafe against autoimmune disease due to autoimmune regulator (Aire) deficiency. Proc Natl Acad Sci U S A. 2010;107:14709–14714. doi: 10.1073/pnas.1009209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Marsden VS, Zhan Y, Erlacher M, Lew AM, Bouillet P, Berzins S, Godfrey DI, Heath WR, Strasser A. Negative selection of semimature CD4(+)8(−)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc Natl Acad Sci U S A. 2004;101:7052–7057. doi: 10.1073/pnas.0305757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Zhang Y, Gray D, Carrington EM, Bouillet P, Ko HJ, O’Reilly L, Wicks IP, Strasser A, Lew AM. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol. 2011;187:1566–1577. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.