Abstract
Recurrent preterm birth is frequently defined as two or more deliveries before 37 completed weeks of gestation. The recurrence rate varies as a function of the antecedent for preterm birth: spontaneous versus indicated. Spontaneous preterm birth is the result of either preterm labor with intact membranes or preterm prelabor rupture of the membranes. This article reviews the body of literature describing the risk of recurrence of spontaneous and indicated preterm birth. Also discussed are the factors which modify the risk for recurrent spontaneous preterm birth (a short sonographic cervical length and a positive cervicovaginal fetal fibronectin test). Patients with a history of an indicated preterm birth are at risk not only for recurrence of this subtype, but also for spontaneous preterm birth. Individuals of African-American origin have a higher rate of recurrent preterm birth. The potential roles of genetic and environmental factors in recurrent preterm birth are considered.
INTRODUCTION
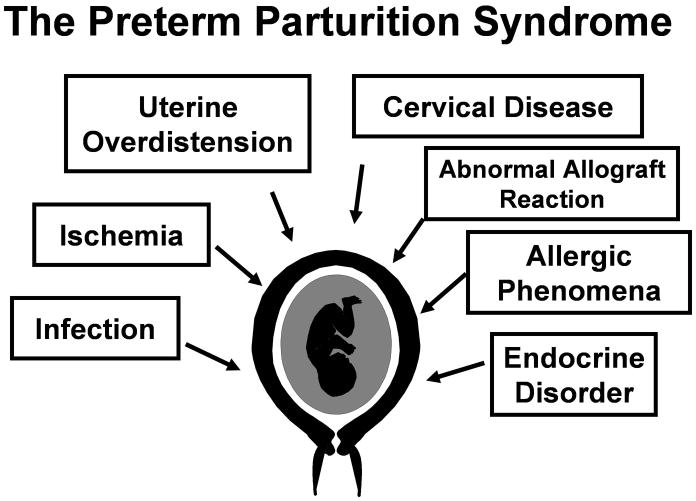
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide.1 A preterm delivery is a risk factor for subsequent preterm birth.2–22 Preterm birth can be the result of three obstetrical circumstances: 1) preterm labor with intact membranes; 2) preterm prelabor rupture of membranes (PROM); and 3) “indicated” preterm birth, which occurs when maternal or fetal indications require delivery before 37 weeks of gestation. The most common indications are preeclampsia and small for gestational age (SGA). Spontaneous preterm parturition is a syndrome caused by multiple etiologies (Figure 1), which are expressed by synchronous or dyssynchronous activation of the common terminal pathway of parturition. The reader is referred to recent reviews for full consideration of this concept.23,24
Figure 1.
Pathological processes implicated in the preterm parturition syndrome. (Reproduced with permission from Romero et al24, with permission.)
Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm
parturition syndrome. In: Critchely H, Bennett P, Thornton S, editors.
Preterm Birth. London: RCOG Press; 2004. p. 28–60.
Although many studies have focused on the rate of preterm birth,25–57 an important consideration is whether these deliveries are the result of spontaneous labor (with intact or ruptured membranes) or “indicated” preterm deliveries. The need for this distinction is based on the premise that the risk factors for recurrent preterm PROM, preterm labor with intact membranes, preeclampsia, and/or SGA are different. However, recent observations suggest that there may be overlap among these conditions,18,19 so that a patient with an “indicated” preterm birth may also be at risk for spontaneous preterm birth.18,19 The converse may also be true (i.e. that a patient with a spontaneous preterm birth is at risk for an “indicated” preterm birth in a subsequent pregnancy).
This review will present a summary of the literature and aims to clarify the risk of recurrent disease and the biological basis for recurrent preterm birth.
Definition of Preterm Birth
Preterm deliveries are those that occur between fetal viability and 37 completed weeks of gestation (menstrual age). However, the lower limit of gestational age used to define a preterm birth has ranged from 13 to 24 weeks of gestation among various reports.21,58,59 Our view is that the delivery of a pre-viable fetus should be considered a spontaneous abortion rather than a spontaneous preterm birth. Otherwise, perinatal and infant mortality estimates in a population or country will be biased by the frequency of late spontaneous abortions.
The precise definition of viability, however, is subject to debate given the increased frequency of survival at very low gestational ages. Clearly, some infants can survive around 24 weeks of gestation, but none at 20 weeks. Therefore, we propose the range of 24 to 36 6/7 weeks of gestation for the definition of preterm birth. If and when technological advances allow substantial survival below 24 weeks of gestation, this definition should be revised.
A birthweight of 500 grams has also been used to define the lower limit of viability.11,60 The popularity of this definition derives from its simplicity. Birthweights can easily be obtained anywhere in the world at a very low cost. The limitations of this approach are that viable neonates born at viable gestational ages and affected by intrauterine growth restriction (IUGR) may have birthweights below 500 grams, and that some pre-viable infants may weigh more than 500 grams. Ideally, gestational age at birth should be used to define viability. There are, however, practical obstacles derived from the uncertainty of gestational age estimation in many countries. This problem can be overcome in areas where ultrasound is performed routinely in early pregnancy, but not elsewhere, including underdeveloped countries. The criteria for the definition of viability have implications for the calculation of vital statistics and comparisons of these among different populations.
RECURRENT PRETERM BIRTH
Recurrent preterm birth is defined as two or more deliveries before 37 completed gestational weeks.2,9,12,59,61,62 However, the definition among studies is not uniform. Criteria that have varied and may affect estimation of the rate of recurrent preterm birth include: 1) gestational age thresholds used for defining the upper (i.e. 35 or 36 weeks)9,12,63,64 and lower (i.e. 13 to 28 weeks)10,59 limits of preterm birth; 2) inclusion of multiple gestations;65–67 3) inclusion of spontaneous, as well as indicated preterm births;11,64 4) the number of preterm births required to fulfill the definition of recurrent preterm birth;8 and 5) the requirement that the preterm births be consecutive. 8
RECURRENT SPONTANEOUS PRETERM BIRTH
The Frequency of Recurrent Spontaneous Preterm Birth
Recurrent spontaneous preterm birth is defined as more than one preterm birth related to spontaneous onset of labor with intact membranes or preterm PROM.
Several studies have consistently indicated that patients with a previous spontaneous preterm birth are at risk for a subsequent spontaneous preterm delivery.2–22 Iams et al9 reported the results of a secondary analysis of the data from the Preterm Prediction Study, conducted under the leadership of Goldenberg et al.68 Among 378 patients with a prior spontaneous preterm birth or spontaneous abortion in the second trimester (gestational age range: 18–36 weeks), the rate of recurrent spontaneous preterm birth (<35 weeks) varied between 14% and 15%, in contrast to the 3% rate of spontaneous preterm birth among 904 parous women with a previous uncomplicated term delivery (Table 1).
Table 1.
Probability and 95% confidence intervals of spontaneous recurrent preterm birth at less than 35 weeks according to the gestational age of the previous spontaneous preterm birth.
| Gestational age at delivery in previous spontaneous preterm birth | Probability (95% CI) of spontaneous recurrent preterm birth at <35 weeks |
|---|---|
| 18–26 weeks | 0.15 (0.05–0.37) |
| 27–31 weeks | 0.15 (0.05–0.38) |
| 32–36 weeks | 0.14 (0.05–0.32) |
| ≥ 37 weeks | 0.03 (0.03–0.03) |
Modified from Iams et al9 with permission.
Iams JD, Goldenberg RL, Mercer BM, Moawad A, Thom E, Meis PJ et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 1998;178:1035–40.
The rate of recurrent preterm birth was modified according to the sonographic cervical length in the index pregnancy and the presence of a positive test for fetal fibronectin in cervicovaginal fluid at 22–24 weeks of gestation.9 Among women with a previous spontaneous preterm birth, the rate of recurrence (<35 weeks) was the highest (64%) among women with a sonographically short cervix (<25 mm) and a positive fetal fibronectin test. The lowest rate of recurrence (7%) occurred among patients with a sonographic cervical length >35 mm and a negative fetal fibronectin test.9
Patients with a positive fibronectin test were at higher risk for spontaneous recurrent preterm birth regardless of cervical length: 28% for patients with a positive test compared to only 7% for patients with a negative test. Similarly, a sonographic short cervix contributed to the risk of recurrent spontaneous preterm birth. Among patients with a history of spontaneous preterm birth and a short cervix, the rate of recurrence was 25% if the fetal fibronectin test was negative and 64% if the test was positive. This information can be used to counsel patients about their risk of spontaneous preterm birth. However, further investigation is required in which a mathematical model is generated to predict the individual risk for preterm birth based upon clinical, sonographic, and biochemical parameters in which results are expressed as continuous variables rather than categorical results.
Mercer et al10 performed a secondary analysis of the same dataset to evaluate the association between prior spontaneous preterm birth and subsequent pregnancy outcome. Women with a history of spontaneous preterm delivery (before 37 weeks of gestation) had a 2.5-fold increased risk (95% confidence interval (CI), 1.9–3.2) of spontaneous preterm delivery in a subsequent pregnancy compared to women with no history of spontaneous preterm delivery (21.7% vs. 8.8%, respectively, p<0.001). This risk was particularly high when the analysis focused on recurrent spontaneous preterm delivery before 28 weeks of gestation (relative risk (RR) 10.6 95% CI, 2.9–38.3).10 Moreover, the earlier the gestational age of the first preterm delivery, the greater the risk for recurrent spontaneous preterm birth (RR 2.4, 2.7, and 3.1 for prior delivery at 35–36, 28–34, and 23–27 weeks of gestation, respectively).10
Preterm Prelabor Rupture of Membranes and Recurrent Preterm Birth
PROM is defined as spontaneous rupture of the chorioamniotic membranes before the onset of labor.69 Since the consequences of PROM depend on the gestational age at which the episode occurs, this condition has been classified as preterm PROM or term PROM, depending upon whether the rupture of the membranes occurs before or after 37 weeks of gestation, respectively.70–76 Term PROM occurs in approximately 10% of pregnancies,70–72,74,77,78 whereas the frequency of preterm PROM is 2% to 3.5%.70–72,74,77,78 Preterm PROM accounts for 30% to 40% of preterm deliveries, 70–72,74,77,78 and it is a leading clinically identifiable cause of preterm birth and a major contributor to perinatal morbidity and mortality.21,58,70–78
Analysis of data from the Collaborative Perinatal Project demonstrated that among women with a previous term delivery not complicated by PROM, the frequency of preterm PROM in a subsequent pregnancy is 4%.79 In contrast, among patients with two successive singleton pregnancies in the dataset (n=5,230), the frequency of preterm PROM is 21% if the first pregnancy resulted in a preterm delivery due to preterm PROM.79
Several investigators have confirmed the high recurrence rate of preterm PROM: 1) Asrat et al80 reported a 32% (95% CI: 23.9–40.5) risk of recurrence in 121 patients with a previous episode of preterm PROM; 2) Ekwo et al81 reported that women with preterm PROM in a previous pregnancy had a 5.5-fold higher risk of preterm PROM in a subsequent pregnancy than those in the control group; and 3) Mercer et al58 reported that compared to women with no history of preterm PROM, women with a previous preterm PROM had a higher risk of spontaneous preterm delivery due to preterm PROM in the index pregnancy (13.5% vs. 4.1%, p<0.001; RR: 3.3, 95% CI: 2.1–5.2) as well as a higher risk of preterm PROM at less than 28 weeks (1.8% vs. 0.1%, p<0.01; RR 13.5, 95% CI: 23–80.3).
Mercer et al82 used the Preterm Prediction Study population to determine the risk factors for subsequent preterm birth caused by preterm PROM alone. Preterm PROM at less than 35 weeks of gestation occurred in 2% of patients and at less than 37 weeks in 4.5% of patients. Preterm PROM accounted for 32.6% of all preterm deliveries. Clinical characteristics associated with preterm birth caused by preterm PROM, derived from a multivariable analysis and stratified as preterm PROM <37 and <35 weeks are displayed in Table 2. In nulliparous women, the risk factors for preterm PROM were a cervical length ≤25 mm, working during pregnancy, and the presence of medical complications (the odds ratios (OR) ranged between 3 and 3.7).82 Among multiparous women, a previous preterm birth caused by preterm PROM was the primary risk factor for preterm PROM in a subsequent pregnancy (OR for preterm PROM at <35 weeks: 4.1; <37 weeks: 3.1). Noteworthy is that a previous preterm birth caused by preterm labor with intact membranes was also a risk factor for preterm PROM, although the odds ratios were lower than if the previous preterm birth was the result of preterm PROM (OR for preterm PROM at <35 weeks: 2.6; <37 weeks: 1.8).82
Table 2.
Risk factors associated with preterm PROM (at less than 35 weeks) stratified by parity.
| Nulliparous | Multiparous | |||
|---|---|---|---|---|
| Risk Factor | Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval |
| Cervical length ≤ 25 mm | 9.9 | 3.8–25.9 | 4.2 | 2.0–8.9 |
| Previous preterm birth with preterm PROM | -- | -- | 4.1 | 2.0–8.7 |
| Previous preterm labor with intact membranes | -- | -- | 2.6 | 1.2–5.3 |
| Working during pregnancy | 5.3 | 1.5–18.7 | n.s. | n.s. |
| Medical complications | 4.2 | 1.1–16.0 | n.s. | n.s. |
| FFN (+) | n.s. | n.s. | n.s. | n.s. |
| BV | n.s. | n.s. | n.s. | n.s. |
| FFN (+) and absent BV | n.s. | n.s. | 9.0 | 3.6–22.5 |
| FFN (−) and present BV | n.s. | n.s. | 2.8 | 1.2–6.3 |
FFN = fetal fibronectin; BV = bacterial vaginosis; n.s. = non-significant.
Modified from Mercer et al,82 with permission.
Mercer BM, Goldenberg RL, Meis PJ, Moawad AH, Shellhaas C, Das A et al. The Preterm Prediction Study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 2000;183:738–45.
Interestingly, the only risk factor consistently associated with preterm PROM at <37 and <35 weeks in both nulliparous and multiparous women was a short cervical length. Bacterial vaginosis was not found to be a risk factor for recurrent preterm birth.82
Among multiparous women, a short cervix, a positive fetal fibronectin test, and a history of preterm birth following preterm PROM increased the frequency of recurrent preterm PROM at <35 weeks to 25%. If recurrent preterm PROM was defined as <37 weeks, the combination of these three risk factors increased the risk 7.8-fold over the reference group of multiparous women who had none of these risk factors.82
The mechanisms responsible for the association between previous preterm PROM, short cervix, and positive fetal fibronectin and recurrent preterm birth caused by preterm PROM have not been elucidated. It is likely that an insult during gestation (e.g. intrauterine infection) would be resolved by preterm labor with intact membranes or preterm PROM. We have proposed83 that selection of the specific phenotype may be determined by genetic and/or environmental factors. For example, if patients carry DNA variants which predispose to an excess production of matrix-degrading enzymes, such patients will go into premature labor after rupture of membranes. On the other hand, if the genotype is such that the generation of uterotonic agents rather than matrix-degrading enzymes is favored, then preterm labor with intact membranes will be the clinical expression of the preterm parturition syndrome. The genotype may explain the tendency for the same phenotype to occur in subsequent pregnancies (i.e. preterm PROM).
The relationship between a short cervix and preterm PROM could be due to intrauterine infection.84,85 A long cervix with a well-established mucus plug may serve as an anatomical and biochemical host defense mechanism against ascending intrauterine infection.86–92 A short cervix may predispose to ascending intrauterine infection by shortening the distance between microorganisms in the lower genital tract and the chorioamniotic membranes.89,93 In addition, the process of cervical reducing may lead to the loss of the mucus plug. Cervical mucus has been demonstrated to contain antimicrobial properties, attributed at least in part to antimicrobial peptides such as defensins, lactoferrin, calprotectin, and bacterial permeability factor.86–90
The relationship between a positive cervicovaginal fetal fibronectin test and subsequent preterm PROM has been attributed to the presence of upper genital tract infection.68 This interpretation has been based on the association between a positive fetal fibronectin in the midtrimester and the subsequent demonstration of histologic chorioamnionitis at the time of preterm delivery.68 However, studies in which amniocentesis was performed in women with positive cervicovaginal fetal fibronectin have found that intra-amniotic infection or inflammation is present in less than 2% of patients with a positive fibronectin.94 Because fetal fibronectin is a component of the extracellular matrix located in the chorion leave95 and its abundance in cervicovaginal fluid increases prior to both preterm68,96–109 and term labor110–119, we propose that detection of this protein is a marker of decidual/membrane activation and, therefore, of the common terminal pathway of parturition. Thus, a history of preterm PROM and a positive test for fibronectin in the midtrimester are likely to reflect activation of the decidua/membrane component of the pathway. We propose a sequence of events that may explain the empirical observations reported by Mercer et al.82 A patient with a previous preterm PROM is at risk for subsequent PROM.82 If such a patient has a short cervix, the risk of recurrent preterm PROM would increase because of ascending intrauterine infection. If the infection is such that it activates chorion and decidua, then fetal fibronectin will be positive. Of course, it is also possible that a patient with preterm PROM would have activation of the common terminal pathway (and therefore a positive fetal fibronectin) with a long cervix.
Twin Gestation and Recurrent Preterm Birth
There is conflicting data as to whether preterm birth in the context of a multiple gestation is a risk factor for preterm birth in a future singleton pregnancy. Menard et al62 were the first to examine the recurrence rate after preterm birth of a twin gestation. The authors reported the outcomes of 144 women who first delivered twins, followed by a subsequent singleton gestation. Preterm delivery (before 37 weeks of gestation), occurred in 59.7% of twin gestations and in 14.6% of the subsequent singleton pregnancies. Among women who delivered preterm twins, 19.6% delivered preterm in the subsequent singleton pregnancy. Preterm birth in twin gestations was associated with a significantly increased risk of preterm delivery in a subsequent singleton pregnancy (RR 2.87, 95% CI 1.02–8.09). Among the subset of patients that delivered twins before 30 weeks, 42% of the subsequent singleton pregnancies delivered preterm (RR 6.11, 95% CI 2.07–18.02). The RR of preterm birth of a singleton after delivery of twins between 30 and 34 weeks of gestation was 3.63 (95% CI 1.02–12.92). However, women who delivered twins between 34 and 37 weeks of gestation did not have an increased risk for recurrent preterm birth.
In contrast, Rydhstrom59 reported that a preterm twin delivery, regardless of etiology, did not increase the risk of recurrent preterm birth in a subsequent singleton gestation. However, a prior preterm singleton delivery increased the risk of preterm birth in subsequent singleton and twin pregnancies. Bloom et al12 reported that women with a singleton gestation that resulted in preterm birth at less than 35 weeks have an increased risk for recurrence (OR 5.6, 95% CI 4.5–7.0). However, those whose first pregnancy resulted in twins delivered at less than 35 weeks did not have a higher risk of recurrent preterm birth (OR 1.9, 95% CI 0.46–8.14).
Cervical Insufficiency as a Cause of Recurrent Midtrimester Abortion/Preterm Birth
The clinical diagnosis of cervical insufficiency is traditionally made in patients with a history of recurrent mid-trimester spontaneous abortions and/or early preterm deliveries in which “the basic process is thought to be the failure of the cervix to remain closed during pregnancy.”120 Thus, both by definition and clinical practice, the condition now termed, “cervical insufficiency” is recognized as one that recurs in subsequent pregnancies.
Digital examination of the cervix was the method used to determine cervical status (effacement, dilatation, position, and consistency) before the introduction of ultrasound. Cervical sonography has become an objective and reliable method to assess cervical length, which approximates cervical effacement. The shorter the sonographic cervical length in the mid-trimester, the higher the risk of spontaneous preterm labor/delivery.121–125 However, there is no agreement concerning what constitutes a sonographic short cervix. For example, Iams et al122 proposed that a cervix of 26 mm or shorter at 24 weeks of gestation increases the risk for spontaneous preterm delivery (RR: 6.19, 95% CI: 3.84–9.97). The prevalence of spontaneous preterm delivery (defined as less than 35 weeks) in this study was 4.3%, and the positive predictive value was 17.8% for a cervical length ≤ 25 mm at 24 weeks of gestation.122 Other investigators have proposed a cut-off of 15 mm, because a cervical length of 15 mm or less is associated with nearly a 50% rate of spontaneous preterm delivery at 32 weeks of gestation or less, when neonatal morbidity is substantial.123,125
Sonographic cervical length is not a screening test for spontaneous preterm delivery, because only a small fraction of all patients who will have a spontaneous preterm birth had a short cervix in the mid-trimester. Previous studies conducted at our institution have indicated that only 8% of all patients who will have a preterm delivery at less than 32 weeks of gestation have a cervical length of 15 mm or less in the mid-trimester.125 The converse is also true. Among women with a short cervix, some have adverse pregnancy outcomes and others have uncomplicated term deliveries.66,121–123,126–140 Only half of women with a cervical length of 15 mm or less deliver before 32 weeks of gestation.125 This indicates that cervical length is only one of the factors determining the degree of cervical sufficiency and that a short cervix should not be equated with “cervical insufficiency.”
Sonographic cervical length can modify the a priori risk for preterm delivery. For example, in patients with a history of preterm delivery, a twin gestation, or a triplet gestation, a short cervix confers an increased risk for preterm delivery.109,141–149 Indeed, among women with a history of spontaneous preterm birth, the risk of recurrence increases as cervical length shortens.9
The hypothesis that cervical competence or sufficiency represents a spectrum was studied by Parikh and Mehta, who used digital examination of the cervix and concluded that degrees of cervical competence do not exist.150 Iams et al, using sonographic examination of the cervix, suggested that cervical sufficiency/insufficiency is a continuum,66 with a strong relationship between cervical length in the index pregnancy and gestational age at delivery in the first pregnancy. This relationship was nearly linear; patients with a typical history of an incompetent cervix (painless dilatation) do not constitute a separate group from those with a history of spontaneous preterm delivery (preterm labor or preterm PROM).66 Similar results have been reported by Guzman et al.151 Collectively, these studies suggest a relationship between a history of preterm delivery and the cervical length in a subsequent pregnancy. Inasmuch as patients with a short cervix are at increased risk for a mid-trimester pregnancy loss (clinically referred to as “cervical insufficiency”) or spontaneous preterm delivery with intact or ruptured membranes,66,121–123,126–131,133–140,151,152 a short cervix could be considered the expression of a spectrum of cervical diseases or functions.
We have proposed that cervical insufficiency is one of the great “obstetrical syndrome.”153 Cervical ripening in the mid-trimester may be the result of: 1) the loss of connective tissue after a cervical operation such as conization154–156 or LEEP procedure;156 2) a congenital disorder such as cervical hypoplasia after diethylstilbestrol exposure;157–160 3) intrauterine infection;161,162 and 4) a suspension of progesterone action.163 There is experimental evidence that progesterone can reverse cervical compliance induced by the administration of dexamethasone to pregnant sheep.164 Sherman et al165 have also generated evidence that the administration of 17 alpha hydroxyprogesterone may be beneficial in patients with clinically diagnosed “cervical insufficiency” and a cervical disorder that manifests itself with the clinical presentation of “cervical insufficiency.” Each of these causes of the syndrome could be affected by genetic or environmental factors. The possibility of novel and yet-to-be-discovered mechanisms of disease playing a role must also be considered.
A proportion of patients presenting with asymptomatic cervical dilatation in the mid-trimester have microbial invasion of the amniotic cavity (MIAC)161,162 that can be as high as 51.5%.162 Microbial invasion of the amniotic cavity may be due to premature cervical dilatation with the exposure of the chorioamniotic membranes to the microbial flora of the lower genital tract. Microorganisms may gain access to the amniotic cavity by crossing intact membranes.162 Under these circumstances, infection would be a secondary phenomenon to primary cervical disease. An alternative explanation is that primary intrauterine infection (ascending, hematogeneous166), or one caused by activation of microorganisms present within the uterine cavity167 in the second trimester of pregnancy produces myometrial contractility and cervical ripening. Since uterine contractions are usually clinically silent in the mid-trimester of pregnancy, the clinical picture of an infection-induced spontaneous abortion may be indistinguishable from that of an incompetent cervix.65,162 Recently, we have established that 9% (5/57) of women with a short endocervix (less than 25 mm) have microbiologically-proven intra-amniotic infection,168 suggesting that these infections are subclinical and may precede the development of the clinical picture of acute “cervical insufficiency” (dilated and effaced cervix with bulging membranes). The issue of whether subclinical intrauterine infection is a cause of recurrent cervical insufficiency and preterm birth has not been answered.
Women of African-American Origin Have a Greater Risk of Recurrent Preterm Birth than Caucasians
There is a well-established disparity in the rate of preterm birth among ethnic groups in the U.S.8,11,169–176 Individuals of African-American origin are at higher risk for recurrent preterm birth.
A large population-based cohort study11 in the state of Georgia found that among women who delivered between 20 and 31 weeks of gestation in their first pregnancy, African-American women had a higher rate of recurrent preterm birth at 20–31 weeks than did Caucasian women (African-American = 13.4% (95% CI: 11.4–15.6) vs Caucasian = 8.2% (95% CI 6.6–10.1)). The same was the case for deliveries between 32 and 36 weeks of gestation.
Of interest was that teenagers whose first preterm delivery occurred between 20 and 31 weeks of gestation had twice the risk of recurrent preterm birth (20–31 weeks) than that of women 20–49 years of age (Table 3). This observation was significant only among African-American women.
Table 3.
Odds Ratios for Recurrence of Preterm Delivery or Low-Birthweight Newborn by Race in Georgia, 1980–1995*.
| Delivery at 20–31 wk† | Delivery at 32–36 wk† | |||
|---|---|---|---|---|
| Maternal Characteristic in Second Pregnancy | White (n=84) | Black (n=145) | White (n=712) | Black (n=1059) |
| Maternal age (years) | ||||
| 10–17 | 2.3 (0.9–5.6) | 2.0 (1.2–3.5) | 1.3 (0.8–2.0) | 1.3 (1.1–1.7) |
| 18–19 | 1.0 (0.5–2.0) | 1.2 (0.8–2.0) | 1.3 (1.0–1.7) | 1.2 (1.0–1.4) |
| 20–49 | 1.0 | 1.0 | 1.0 | 1.0 |
| Initiation of prenatal care (trimester) | ||||
| First | 1.0 | 1.0 | 1.0 | 1.0 |
| Second, third, or none | 1.2 (0.6–2.2) | 1.2 (0.8–1.7) | 1.1 (0.9–1.4) | 1.1 (1.0–1.3) |
| Interpregnancy interval, months | ||||
| < 6 | 1.1 (0.5–2.1) | 1.4 (0.9–2.3) | 1.0 (0.7–1.3) | 1.2 (1.2–1.5) |
| 6–11 | 1.6 (0.9–2.9) | 0.9 (0.5–1.5) | 1.2 (0.9–1.5) | 1.1 (0.9–1.3) |
| 12–47 | 1.0 | 1.0 | 1.0 | 1.0 |
| > 47 | 1.0 (0.4–2.1) | 0.8 (0.4–1.5) | 0.9 (0.7–1.2) | 0.7 (0.6–0.9) |
| Goodness-of-fit P value§ | 0.72 | 0.33 | 0.29 | 0.93 |
| Smoking during the pregnancy‡ | ||||
| Yes | 0.4 (0.2–1.1) | 1.7 (0.2–14.5) | 0.8 (0.6–1.2) | 0.6 (0.3–1.1) |
| No | 1.0 | 1.0 | 1.0 | 1.0 |
Odds ratio for type of second pregnancy are controlled for all of the other variables in the table except smoking; figures in parenthesis are 95% confidence intervals.
Referent group is delivery in second pregnancy at gestation ≥ 37 weeks.
Goodness-of-fit for model including all variables except smoking
Analysis restricted to second deliveries occurring from 1989 through 1995. Association adjusted for all other variables in the model.
Modified from Adams et al,11 with permission.
Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA 2000;283:1591–96.
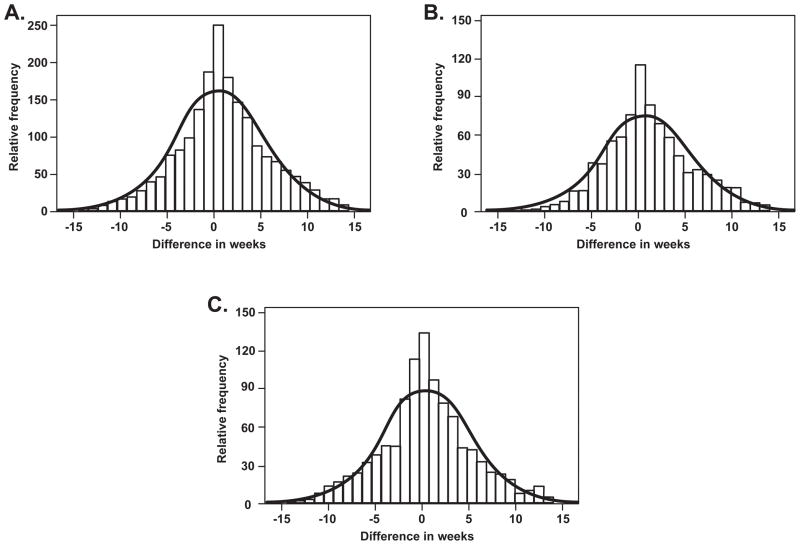
Kitska et al64 used a maternally-linked database from the Missouri Department of Health to study racial disparities and recurrent preterm birth. The study focused on 368,633 mothers who had two or more deliveries between 1978 and 1997. The frequency of recurrent preterm birth was 3.1% among African-Americans and 0.6% among Caucasians (RR, 5.40; 95% CI, 5.06, 5.75). Logistic regression analysis indicated that African-American origin increased the risk for recurrent preterm birth independently of other factors, such as medical complications and low socioeconomic status (adjusted OR, 4.11; 95% CI, 3.78, 4.47). Two additional findings of this study were that: 1) the recurrent preterm birth in women of African-American origin occurred at an earlier median gestational age than in women of Caucasian origin (31 weeks vs. 33 weeks); and 2) the gestational age of the recurrent preterm birth was similar to that of the previous preterm birth and most likely to occur at the same gestational age (Figure 2). This finding was consistent among individuals in both ethnic groups.
Figure 2.
Concordance in timing of preterm (20–34 6/7 weeks’ gestation) birth in Missouri to a mother with previous preterm birth, 1989–1997. The line represents the expected Gaussian curve if concordance in timing is a normally distributed event. The bars represent the timing for each preterm birth after the initial preterm birth for A, all mothers, B, Caucasians, or C, African American in correlation with the expected normal curve. (Reproduced with permission from Kistka et alx, with permission.)
Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, Cole FS et al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol 2007;196:131.
Additional Risk Factors for Recurrent Preterm Birth
Several environmental factors have been implicated in recurrent preterm birth. Cnattingius et al61 studied the association among smoking, previous very early preterm or moderate preterm delivery (before 32 weeks and at 32 to 36 weeks, respectively), and the risk of a subsequent very preterm or moderately preterm delivery in a population-based cohort of 243,858 women in Sweden. The OR for a very early preterm second delivery among the women who smoked 1 to 9 cigarettes per day was 1.4 (95% CI, 1.1, 1.7) and for those who smoked 10 or more cigarettes per day 1.6 (95% CI, 1.3, 2.0), as compared with nonsmokers. Furthermore, women who stopped smoking between pregnancies were not at increased risk for very early or moderate preterm delivery, whereas the women who started to smoke in the second pregnancy had the same risk as those who had continued to smoke.
Merlino et al177 investigated the association between maternal weight loss and recurrent preterm birth in a cohort of 1,241 patients. Women whose body mass index (BMI) decreased more than 5 kg/m2 had more frequent recurrent preterm birth than those whose BMI did not (21.1% vs 9.3%, P ≤ 0.01). For those with a term birth in the first pregnancy, the rate of preterm birth in the subsequent pregnancy was not affected by a decline in BMI. In contrast, women with a preterm birth in the first pregnancy had a higher rate of recurrent preterm birth if BMI decreased more than 5 kg/m2 (80.0% vs 28.2%, P = 0.01). Hence, women whose BMI declines between pregnancies are at increased risk for recurrent preterm birth.
The effect of sexual behavior on the risk of recurrent preterm birth was the subject of a secondary analysis of a multicentric observational study of the association between cervical ultrasound at 16–18 weeks and the risk for recurrent preterm birth. Women (n=187) with singleton gestations who were at high risk for preterm birth because of a prior spontaneous preterm birth at less than 32 weeks of gestation were included.178 A sexual history was obtained by interview at the time of enrollment. Information gathered included the number of sexual partners during the patient’s lifetime, the number of sexual partners during the patient’s pregnancy, and the frequency of sexual intercourse in the preceding month. The greater the number of sexual partners in a woman’s lifetime, the higher the frequency of recurrent preterm birth (one partner 19%, 2 to 3 partners 29%, more than 4 partners 44%, P≤ 0.007). Of interest, neither the frequency of sexual intercourse during early pregnancy nor the number of partners were risk factors for recurrent preterm birth, which is consistent with previous reports. 179–184
RECURRENT INDICATED PRETERM BIRTH
Indicated preterm births are those that result from delivery of patients before term due to complications that place the mother and/or fetus at risk. Various authors include amont those complications hypertension-related disorders, obstetrical hemorrhage (placenta previa, placental abruption, and other causes of antepartum hemorrhage), and all medically induced preterm deliveries.12,40,185 Other investigators18 categorize indicated preterm births into two categories: 1) ischemic placental disease (i.e. preeclampsia, SGA, placental abruption and fetal distress); and 2) miscellaneous (fetal malformations, placenta previa, unexplained vaginal bleeding, chronic hypertension and others).
The incidence of indicated preterm birth has been reported to range from 1% to 5.5% of all deliveries.12,176,186,187 However, indicated preterm birth accounts for approximately one-third of all preterm births.40,185,187
Meis et al185 reported a study examining the risk factors for indicated preterm birth using the Preterm Prediction Study data set. A history of a previous indicated preterm delivery was associated with an OR of 2.8 (95% CI 1.5–5.4; p=0.002; multivariable analysis by logistic regression including other risk factors for indicated preterm birth).
Bloom et al12 reported the largest study conducted today in a single unit and concluded that an indicated preterm delivery in singleton gestations is associated with an OR of 5.4 (95% CI 3.1–9.2) for recurrent preterm birth at less than 35 weeks of gestation in comparison to patients who delivered at term in their first pregnancy. Patients who had an indicated preterm birth between 24 and 28 weeks had an OR of 12.5 (95% CI 3.8–40.7) for recurrent preterm delivery and 10 (95% CI 4.8–20.8) if they were delivered between 29 and 32 weeks of gestation. In contrast, patients who were delivered between 33 and 34 weeks did not have a higher risk for recurrent preterm birth.
Patients with one prior preterm birth had an OR of 2.4 (95% CI 1.5–4.1) for indicated preterm delivery compared to women with no history of preterm birth. Moreover, the OR increases to 5.2 (95% CI 2.2–11.9) when the patients had 2 or more previous preterm deliveries.176 Collectively, these studies suggest that indicated preterm birth is not an isolated event and puts the patient at risk for a subsequent indicated, as well as spontaneous, preterm birth.
The subjects of recurrent preeclampsia and SGA are discussed elsewhere in this issue of the Seminars.
IS THE RECURRENCE RISK FOR PRETERM BIRTH DIFFERENT FOR SPONTANEOUS VERSUS INDICATED PRETERM BIRTH?
Preterm births have been classified as “spontaneous” or “indicated” because of the implicit assumption that preterm labor with intact membranes and preterm PROM share pathophysiologic features and clinical presentation (spontaneous onset of labor). Preterm preeclampsia, fetal distress, and severe IUGR – the most common causes of indicated preterm birth186 – usually occur in the absence of spontaneous parturition. Thus, the presence or absence of spontaneous parturition has been a sharp dividing line between the phenotypes of indicated and non-indicated preterm birth.
One can also argue that the mechanisms of disease responsible for the phenotypes are shared within the conditions responsible for spontaneous preterm birth and within the conditions responsible for indicated preterm birth. For example, MIAC with bacteria is common in preterm labor188–204 and preterm PROM,93,205–211 but rare in preeclampsia and IUGR. In contrast, “failure of physiologic transformation of the spiral arteries” can be observed in all of these four conditions,212,213 but is more prevalent and severe in preeclampsia and IUGR214–220 than in preterm labor and preterm PROM.212,213
Ananth et al18 provided evidence in support of this view, but also noted that spontaneous preterm birth in the first pregnancy may be followed by an indicated preterm birth in the subsequent pregnancy and vice-versa. The observations are derived from a population-based retrospective cohort study of births in Missouri in which analyses were restricted to women who delivered their first 2 consecutive singleton live births during the study period of 1989–1997.18 The key observation was that if the first pregnancy resulted in a spontaneous or indicated preterm birth, then women were more likely to have the same type of preterm birth (spontaneous or indicated) in the second pregnancy. Indeed, women with spontaneous preterm birth before 35 weeks of gestation in the first pregnancy had an OR of 3.6 (95% CI 3.2–4.0) for preterm birth before 35 weeks in the second pregnancy. However, the risk for a medically indicated preterm birth was also increased (OR 2.5, 95% CI 2.1–3.0).18
Similarly, women who delivered at less than 35 weeks because of a medical indication in the first pregnancy were much more likely to have an indicated preterm birth at less than 35 weeks of gestation in their subsequent pregnancy (OR 10.6, 95% CI 9.1–12.4). However, these patients were also at increased risk of having a spontaneous preterm birth (OR 1.6, 95% CI 1.3–2.1), although that risk was lower than the risk of having an indicated preterm birth.18 Similar findings were evident in pregnancies that ended at less than 32 weeks (Table 4). The greatest risk for recurrence of preterm birth was observed when women delivered their first preterm birth at less than 28 weeks of gestation. The magnitude of the risk for recurrence of preterm birth decreased progressively as gestational age at delivery of the first preterm birth increased.18
Table 4.
Recurrence of preterm birth at < 37, < 35 and < 32 weeks and subtypes in second pregnancy based on preterm birth at less than 37, less than 35, and less than 32 weeks in the first pregnancy, respectively: Missouri, 1989 to 1997
| Preterm birth in the first pregnancy | Preterm birth in second pregnancy, adjusted OR (95% CI) | ||
|---|---|---|---|
| All preterm births | Spontaneous births | preterm Medically preterm indicated births | |
| Preterm birth at < 37 wks | |||
| Preterm birth at < 37 wks | 2.9 (2.8, 3.0) | 2.7 (2.5, 2.9) | 3.3 (3.1, 3.6) |
| Spontaneous preterm birth | 2.8 (2.7, 3.0) | 3.2 (3.1, 3.4) | 1.7 (1.5, 1.9) |
| Medically indicated preterm birth | 3.0 (2.8, 3.3) | 1.0 (0.9, 1.2) | 7.7 (7.0, 8.5) |
| Preterm birth at < 35 wks | |||
| Preterm birth at < 35 wks | 3.6 (3.4, 3.9) | 3.1 (2.8, 3.4) | 4.8 (4.3, 5.4) |
| Spontaneous preterm birth | 3.3 (3.0, 3.6) | 3.6 (3.2, 4.0) | 2.5 (2.1, 3.0) |
| Medically indicated preterm birth | 4.6 (4.0, 5.2) | 1.6 (1.3, 2.1) | 10.6 (9.1, 12.4) |
| Preterm birth at < 32 wks | |||
| Preterm birth at < 32 wks | 4.9 (4.2, 5.7) | 4.1 (3.4, 4.9) | 6.5 (5.2, 8.0) |
| Spontaneous preterm birth | 4.5 (3.8, 5.4) | 4.6 (3.7, 5.6) | 4.3 (3.1, 5.8) |
| Medically indicated preterm birth | 5.8 (4.5, 7.4) | 2.7 (1.8, 4.1) | 11.3 (8.4, 15.1) |
ORs are adjusted for maternal age (second birth), education (second birth), marital status, race/ethnicity, smoking and alcohol use, prepregnancy body mass index, and lack of or late initiation of prenatal care and interpregnancy interval. OR, Odds ratio; CI, confidence interval.
Modified from Ananth et al,18 with permission.
Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am. J. Obstet. Gynecol. 2006;195:643–50.
ISSUES ON THE MANAGEMENT AND PREVENTION OF A PATIENT WITH A HISTORY OF PRETERM BIRTH
Prevention of Recurrent Preterm Birth
Progesterone Administration
Progesterone plays a central role in pregnancy. The proposed functions of progesterone include maintenance of myometrial quiescence, down-regulation of gap-junction formation, and inhibition of cervical ripening.221–223
Prevention of recurrent preterm birth by progesterone administration has been a matter of debate in the literature for decades.224–242 This section will review the results of randomized clinical trials and meta-analyses published recently.
da Fonseca et al,243 reported a randomized, double-blind, placebo-controlled trial of vaginal progesterone versus placebo in decreasing the rate of spontaneous preterm birth. Patients with at least one previous spontaneous preterm birth, a prophylactic cervical cerclage, or a uterine malformation (n=142). Patients were allocated to receive either daily progesterone (100 mg) or placebo by vaginal suppository from 24 to 34 weeks of gestation. The rates of preterm delivery at both less than 37 weeks and less than 34 weeks were lower in the progesterone group than in the placebo group. [For 37 weeks; progesterone: 13.8% (10/72) vs. placebo: 28.5% (20/70); p=0.03 and for 34 weeks; progesterone: 2.8% (2/72) vs. placebo: 18.6% (13/70); p=0.002].
Meis et al244 reported the results of a double-blind placebo-controlled clinical trial comparing the effects of intramuscular 17-OH P versus placebo. Patients with a history of spontaneous preterm delivery (n=463) were enrolled at 16 to 20 weeks of gestation and randomly assigned in a 2:1 ratio to receive weekly injections of 250 mg of 17-OH P or an inert oil placebo until either delivery or 36 weeks of gestation. Treatment with 17-OH P significantly reduced the rate of preterm delivery at less than 37 weeks [17-OH P 36.3% vs. placebo 54.9%, RR 0.66 (95% CI 0.54–0.81)] and less than 35 weeks of gestation [17-OH P 20.6% vs. placebo 30.7%, RR 0.67 (95% CI 0.48–0.93)]. Moreover, neonates born to women treated with 17-P had significantly lower rates of necrotizing enterocolitis [17-P 0% vs. placebo 2.6%, RR could not be calculated], intraventricular hemorrhage [17-OH P 1.3% vs. placebo 5.2%, RR 0.25 (95% CI 0.8–0.82)] and need for supplemental oxygen [17-OH P 14.9% vs. placebo 23.8%, RR 0.62 (95% CI 0.42–0.92)].
Of interest, the effect of 17-OH P in preventing recurrent preterm delivery was demonstrated only in patients whose previous preterm delivery had occurred between 20 and 33.9 weeks of gestation.245 Moreover, it has been estimated that 4.7 women would need to be treated to prevent one recurrent preterm delivery among patients who had delivered between 20–27.9 weeks of gestation in a previous pregnancy. The number needed to treat is similar for women who had delivered at 28–33.9 weeks. Of note, 17-OH P was not associated with a reduction in the rate of recurrent preterm deliveries in patients whose previous preterm delivery had occurred between 34–36.9 weeks of gestation.
The efficacy of 17-OH P in singleton gestations was not demonstrated in twin gestations.246 In this trial, no significant differences were found between the groups in the rates of spontaneous or indicated preterm birth.
In a 2006 Cochrane review, Dodd et al247 reported the results of six randomized trials including 988 patients randomized to receive either 17-OH P or placebo. The administration of 17-OH P was associated with reduced risks for preterm delivery before 37 weeks of gestation (six studies, RR 0.65 95%CI 0.54–0.79) and before 34 weeks gestation (one study, RR 0.15 95% CI 0.04–0.64). Moreover, treatment with progesterone was associated with lower risks for birthweight below 2500 grams (four studies, RR 0.63 95%CI 0.49–0.81) and intraventricular hemorrhage (one study, RR 0.63 95% CI 0.08–0.82). No difference in perinatal death was found between treatment groups (five studies, RR 0.66 95% CI 0.37–1.19). There were no interactions between the dose of progesterone (>500 mg vs. <500 mg 17-OH P weekly) or gestational age at commencing progesterone administration and the reported outcomes (i.e. preterm delivery, neonatal morbidity and mortality). These results were in accord with a previous meta-analysis by this group.248 Additionally, the authors stated that there is currently insufficient information concerning the safety of progesterone supplementation.
Sanchez-Ramos et al249 reported another meta-analysis including ten randomized clinical trails and a total of 1339 patients; eight trials used 17-OH P and two used other progestational agents for the prevention of recurrent preterm birth or recurrent abortion. Patients who were treated with progestational agents had a reduced risk of preterm delivery compared to patients in the placebo group (OR 0.45 95% CI 0.25–0.80). The number needed to treat to prevent a single preterm delivery was 10 (95% CI 6–24). A similar effect was observed when only trials using 17-OH P were included (OR 0.45 95% CI 0.25–0.80); and the number needed to treat was eight (95% CI 5–19).
Odibo et al250 performed a cost-effectiveness analysis of the treatment with 17-OH P for the prevention of preterm birth. The cost savings per quality-adjusted life year gained by using 17-OH P was $3,090 in women with a prior preterm delivery at <32 weeks and $2,963 in women who had delivered at 32–37 weeks of gestation. Moreover, the cost per additional preterm delivery avoided with the use of 17-OH P was $35,319 in women with a previous preterm delivery at <32 weeks and $36,093 in women who had delivered at 32–37 weeks of gestation.
In a recent meeting of the Prematurity Interest Group of the Society for Maternal-Fetal Medicine, da Fonseca et al reported the results of a randomized clinical trial of vaginal progesterone administration to women with a short cervix (<15 mm). A 40% reduction in the rate of preterm birth by was found in women treated with vaginal progesterone (da Fonseca E, Nicolaides K, personal communication). In contrast, the largest randomized clinical trial of vaginal progesterone in women with a history of previous preterm delivery did not demonstrate a beneficial effect of vaginal progesterone (Lewis D et al, personal communication). The FDA has raised questions about a safety signal.251 However, this concern was not identified in the trial in twins.246 A review of embryo toxicity in animals has been recently published.252
In summary, it seems that the administration of progesterone may be an effective intervention to reduce the rate of preterm delivery in a subset of women with a previous preterm delivery. Women with a short cervix may benefit from this intervention.
Treatment of Bacterial Vaginosis
Treatment of patients with bacterial vaginosis and a history of preterm birth is controversial. While some investigators have argued strongly in favor of treatment, 253 others believe that this intervention is not justifiable.254–258 Controversy over the choice of antibiotic also exists, with evidence that early treatment with clindamycin is preferable to treatment with metronidazole.259–262 There is no evidence that treatment of patients with a previous preterm delivery with interconceptional antibiotics will prevent a subsequent preterm birth.263,264
The Use of Cerclage
The clinical value of cervical cerclage has been subject of many observational and randomized clinical trials14,131,133,134,138,265–286 and the studies have been the topic of several systematic reviews.287–289 The evidence suggests the following conclusions:
Cervical cerclage in women with a sonographic short cervix (15 mm or less) and a low risk for preterm delivery (by history) does not reduce the rate of spontaneous preterm birth.286
The effectiveness of cervical cerclage in women with a sonographic short cervix and a high risk (by history) for the prevention of preterm delivery is controversial.14,136,277,278,290
The role of prophylactic cerclage in high-risk patients without a sonographic short cervix for the prevention of preterm delivery/midtrimester abortion (by history) is unclear.269–271,278,285 While the largest trial conducted prior to the introduction of ultrasound evaluation of the cervix suggested a modest beneficial effect,271 other trials269,270 and systematic reviews120 prior to the use of ultrasound have indicated that the evidence of effectiveness is either weak or non-existent.
In patients at risk for preterm delivery, serial sonographic examination of the cervix followed by cerclage in those who shortened the cervix is a reasonable alternative to prophylactic placement of a cerclage based upon uncontrolled studies.131,282,291
This evidence indicates that only patients with the clinical presentation of “acute cervical insufficiency” and those with a pregnancy history consistent with “cervical insufficiency” and progressive shortening of the cervix demonstrated with ultrasound may benefit from cerclage placement. However, important to consider is that each conclusion is based on the results of only one randomized clinical trial.278,283 Sakai et al demonstrated that the inflammatory status of the endocervix may be an additional criterion to distinguish those patients who would benefit from cerclage placement from those in whom this intervention may be ineffective or harmful.292
Summary
The evaluation of a patient with a previous preterm birth begins with an examination of the obstetrical circumstances responsible for this complication. If the preterm birth was “indicated,” then the risk of recurrence is related to the specific condition, such as preterm preeclampsia, preterm severe IUGR, placenta previa, etc. The reader is referred to the relevant articles in this volume of the Seminars for details about recurrence rate, monitoring of the index pregnancy, and interventions.
If the previous preterm birth was the result of spontaneous labor (with intact or ruptured membranes), the information provided in this article can be used to counsel the patient about the likelihood of recurrence. In general, most patients with a previous spontaneous preterm birth will deliver at term in a subsequent pregnancy.2–22 However, the earlier the gestational age of the preterm birth, the higher the likelihood of recurrence. It is important to be aware that recurrent preterm births tend to occur at the same gestational age.10,12,18 Counseling should ideally be conducted prior to conception, and efforts should be made to identify potentially treatable causes such as a Mullerian duct abnormalities. However, the attributable risk of these conditions for preterm birth is extremely low.
The estimates of risk of recurrence for spontaneous preterm birth can be improved by performing a sonographic examination of the uterine cervix and a fetal fibronectin test. A long cervix and a negative fetal fibronectin test reduce the risk just as a short cervix and a positive fetal fibronectin test increase the risk.9,122
No intervention has been proven effective in reducing the rate of preterm birth in patients with a positive fetal fibronectin test.293,294 Similarly, the management of patients with a short cervix remains controversial. Evidence suggests that cervical cerclage does not prevent preterm birth in women with a short cervix who do not have a history of previous preterm birth.286 Similarly, a prophylactic cerclage has not been effective in reducing the rate of preterm birth in patients at risk for midtrimester abortion or spontaneous preterm birth.138,277,282 In contrast, one randomized clinical trial of patients with risk factors or symptoms of cervical insufficiency and a shortened cervix (<25 mm before 27 weeks of gestation) in the index pregnancy found a benefit of “therapeutic cerclage.”278 Though further studies are required to identify effective interventions and the patients who will benefit from them, monitoring cervical length with ultrasound and offering cerclage based on individual risk assessment is a reasonable management strategy.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Carr-Hill RA, Hall MH. The repetition of spontaneous preterm labour. Br J Obstet Gynaecol. 1985;92:921–28. doi: 10.1111/j.1471-0528.1985.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 3.Ekwo EE, Gosselink CA, Moawad A. Previous pregnancy outcomes and subsequent risk of preterm rupture of amniotic sac membranes. Br J Obstet Gynaecol. 1993;100:536–41. doi: 10.1111/j.1471-0528.1993.tb15304.x. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen J, Langhoff-Roos J, Kristensen FB. Implications of idiopathic preterm delivery for previous and subsequent pregnancies. Obstet Gynecol. 1995;86:800–04. doi: 10.1016/0029-7844(95)00275-V. [DOI] [PubMed] [Google Scholar]
- 5.Gibb DM, Salaria DA. Transabdominal cervicoisthmic cerclage in the management of recurrent second trimester miscarriage and preterm delivery. Br J Obstet Gynaecol. 1995;102:802–06. doi: 10.1111/j.1471-0528.1995.tb10846.x. [DOI] [PubMed] [Google Scholar]
- 6.Guinn DA, Goldenberg RL, Hauth JC, Andrews WW, Thom E, Romero R. Risk factors for the development of preterm premature rupture of the membranes after arrest of preterm labor. Am J Obstet Gynecol. 1995;173:1310–15. doi: 10.1016/0002-9378(95)91377-7. [DOI] [PubMed] [Google Scholar]
- 7.Kramer MS, Coates AL, Michoud MC, Dagenais S, Moshonas D, Davis GM, et al. Maternal asthma and idiopathic preterm labor. Am J Epidemiol. 1995;142:1078–88. doi: 10.1093/oxfordjournals.aje.a117561. [DOI] [PubMed] [Google Scholar]
- 8.Ekwo E, Moawad A. The risk for recurrence of premature births to African-American and white women. J Assoc Acad Minor Phys. 1998;9:16–21. [PubMed] [Google Scholar]
- 9.Iams JD, Goldenberg RL, Mercer BM, Moawad A, Thom E, Meis PJ, et al. The Preterm Prediction Study: recurrence risk of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1998;178:1035–40. doi: 10.1016/s0002-9378(98)70544-7. [DOI] [PubMed] [Google Scholar]
- 10.Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, Das AF, et al. The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1999;181:1216–21. doi: 10.1016/s0002-9378(99)70111-0. [DOI] [PubMed] [Google Scholar]
- 11.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283:1591–96. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 12.Bloom SL, Yost NP, McIntire DD, Leveno KJ. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol. 2001;98:379–85. doi: 10.1016/s0029-7844(01)01466-1. [DOI] [PubMed] [Google Scholar]
- 13.Koike T, Minakami H, Izumi A, Watanabe T, Matsubara S, Sato I. Recurrence risk of preterm birth due to preeclampsia. Gynecol Obstet Invest. 2002;53:22–27. doi: 10.1159/000049406. [DOI] [PubMed] [Google Scholar]
- 14.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311–17. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Yost NP, Owen J, Berghella V, Macpherson C, Swain M, Dildy GA, III, et al. Number and gestational age of prior preterm births does not modify the predictive value of a short cervix. Am J Obstet Gynecol. 2004;191:241–46. doi: 10.1016/j.ajog.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Tekesin I, Eberhart LH, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet Gynecol. 2005;26:699–706. doi: 10.1002/uog.2633. [DOI] [PubMed] [Google Scholar]
- 17.Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193:1170–74. doi: 10.1016/j.ajog.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 18.Ananth CV, Getahun D, Peltier MR, Salihu HM, Vintzileos AM. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195:643–50. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 20.Meis PJ, Klebanoff M, Dombrowski MP, Sibai BM, Leindecker S, Moawad AH, et al. Does progesterone treatment influence risk factors for recurrent preterm delivery? Obstet Gynecol. 2005;106:557–61. doi: 10.1097/01.AOG.0000174582.79364.a7. [DOI] [PubMed] [Google Scholar]
- 21.Mercer B, Milluzzi C, Collin M. Periviable birth at 20 to 26 weeks of gestation: proximate causes, previous obstetric history and recurrence risk. Am J Obstet Gynecol. 2005;193:1175–80. doi: 10.1016/j.ajog.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 22.Mercer BM, Macpherson CA, Goldenberg RL, Goepfert AR, Haugel-De MS, Varner MW, et al. Are women with recurrent spontaneous preterm births different from those without such history? Am J Obstet Gynecol. 2006;194:1176–84. doi: 10.1016/j.ajog.2006.01.069. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchely H, Bennett P, Thornton S, editors. Preterm Birth. London: RCOG Press; 2004. pp. 28–60. [Google Scholar]
- 25.Joseph KS, Kramer MS, Marcoux S, Ohlsson A, Wen SW, Allen A, et al. Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. N Engl J Med. 1998;339:1434–39. doi: 10.1056/NEJM199811123392004. [DOI] [PubMed] [Google Scholar]
- 26.Preterm singleton births--United States, 1989–1996. MMWR Morb Mortal Wkly Rep. 1999;48:185–89. [PubMed] [Google Scholar]
- 27.Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA. The impact of prenatal care in the United States on preterm births in the presence and absence of antenatal high-risk conditions. Am J Obstet Gynecol. 2002;187:1254–57. doi: 10.1067/mob.2002.127140. [DOI] [PubMed] [Google Scholar]
- 28.Little RE, Gladen BC, Birmingham K, Shkyryak-Nyzhnyk ZA, Chyslovska N. Preterm birth rates in Avon County, England, and urban Ukraine. Eur J Obstet Gynecol Reprod Biol. 2004;113:154–59. doi: 10.1016/S0301-2115(03)00372-5. [DOI] [PubMed] [Google Scholar]
- 29.Morken NH, Kallen K, Hagberg H, Jacobsson B. Preterm birth in Sweden 1973–2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstet Gynecol Scand. 2005;84:558–65. doi: 10.1111/j.0001-6349.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 30.Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Papiernik E. Is the high rate of preterm birth in the United States linked to previous induced abortions? Pediatrics. 2006;118:795–96. doi: 10.1542/peds.2006-0603. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs K, Wapner R. Elective cesarean section and induction and their impact on late preterm births. Clin Perinatol. 2006;33:793–801. doi: 10.1016/j.clp.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119:345–60. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 34.Collins JW, Jr, David RJ, Simon DM, Prachand NG. Preterm birth among African American and white women with a lifelong residence in high-income Chicago neighborhoods: an exploratory study. Ethn Dis. 2007;17:113–17. [PubMed] [Google Scholar]
- 35.Gazaway P, Mullins CL. Prevention of preterm labor and premature rupture of the membranes. Clin Obstet Gynecol. 1986;29:835–49. doi: 10.1097/00003081-198612000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Nwaesei CG, Young DC, Byrne JM, Vincer MJ, Sampson D, Evans JR, et al. Preterm birth at 23 to 26 weeks’ gestation: is active obstetric management justified? Am J Obstet Gynecol. 1987;157:890–97. doi: 10.1016/s0002-9378(87)80080-7. [DOI] [PubMed] [Google Scholar]
- 37.Mueller-Heubach E, Guzick DS. Evaluation of risk scoring in a preterm birth prevention study of indigent patients. Am J Obstet Gynecol. 1989;160:829–35. doi: 10.1016/0002-9378(89)90298-6. [DOI] [PubMed] [Google Scholar]
- 38.Roberts WE, Morrison JC, Hamer C, Wiser WL. The incidence of preterm labor and specific risk factors. Obstet Gynecol. 1990;76:85S–9S. [PubMed] [Google Scholar]
- 39.Yan JS, Yin CS. No decline in preterm birth rate over three decades. Int J Gynaecol Obstet. 1991;34:1–5. doi: 10.1016/0020-7292(91)90530-i. [DOI] [PubMed] [Google Scholar]
- 40.Tucker JM, Goldenberg RL, Davis RO, Copper RL, Winkler CL, Hauth JC. Etiologies of preterm birth in an indigent population: is prevention a logical expectation? Obstet Gynecol. 1991;77:343–47. [PubMed] [Google Scholar]
- 41.Kramer MS, McLean FH, Eason EL, Usher RH. Maternal nutrition and spontaneous preterm birth. Am J Epidemiol. 1992;136:574–83. doi: 10.1093/oxfordjournals.aje.a116535. [DOI] [PubMed] [Google Scholar]
- 42.Multicenter randomized, controlled trial of a preterm birth prevention program. Collaborative Group on Preterm Birth Prevention. Am J Obstet Gynecol. 1993;169:352–66. doi: 10.1016/0002-9378(93)90087-y. [DOI] [PubMed] [Google Scholar]
- 43.Hobel CJ, Ross MG, Bemis RL, Bragonier JR, Nessim S, Sandhu M, et al. The West Los Angeles Preterm Birth Prevention Project. I. Program impact on high-risk women. Am J Obstet Gynecol. 1994;170:54–62. doi: 10.1016/s0002-9378(94)70384-1. [DOI] [PubMed] [Google Scholar]
- 44.Harbert GM., Jr Efforts to reduce low birth weight and preterm births: a statewide analysis (Virginia) Am J Obstet Gynecol. 1994;171:329–38. doi: 10.1016/s0002-9378(94)70031-1. [DOI] [PubMed] [Google Scholar]
- 45.Lubarsky SL, Schiff E, Friedman SA, Mercer BM, Sibai BM. Obstetric characteristics among nulliparas under age 15. Obstet Gynecol. 1994;84:365–68. [PubMed] [Google Scholar]
- 46.Kristensen J, Langhoff-Roos J, Kristensen FB. Idiopathic preterm deliveries in Denmark. Obstet Gynecol. 1995;85:549–52. doi: 10.1016/0029-7844(95)00002-9. [DOI] [PubMed] [Google Scholar]
- 47.Gardner MO, Goldenberg RL, Cliver SP, Tucker JM, Nelson KG, Copper RL. The origin and outcome of preterm twin pregnancies. [DOI] [PubMed] [Google Scholar]
- 48.Graf RA, Perez-Woods R. Trends in preterm labor. J Perinatol. 1992;12:51–58. [PubMed] [Google Scholar]
- 49.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 993(15):414–43. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 50.Heaman MI, Sprague AE, Stewart PJ. Reducing the preterm birth rate: a population health strategy. J Obstet Gynecol Neonatal Nurs. 2001;30:20–29. [PubMed] [Google Scholar]
- 51.Mauldin JG, Newman RB. Preterm birth risk assessment. Semin Perinatol. 2001;25:215–22. doi: 10.1053/sper.2001.26419. [DOI] [PubMed] [Google Scholar]
- 52.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133:1592S–6S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- 53.Wen SW, Smith G, Yang Q, Walker M. Epidemiology of preterm birth and neonatal outcome. Semin Fetal Neonatal Med. 2004;9:429–35. doi: 10.1016/j.siny.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Bibby E, Stewart A. The epidemiology of preterm birth. Neuro Endocrinol Lett pl. 1:43–47. [PubMed] [Google Scholar]
- 55.Higgins RD, Delivoria-Papadopoulos M, Raju TN. Executive summary of the workshop on the border of viability. Pediatrics. 2005;115:1392–96. doi: 10.1542/peds.2004-1989. [DOI] [PubMed] [Google Scholar]
- 56.Raju TN. Epidemiology of late preterm (near-term) births. Clin Perinatol. 2006;33:751–63. doi: 10.1016/j.clp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Steer P. The epidemiology of preterm labor--a global perspective. J Perinat Med. 2005;33:273–76. doi: 10.1515/JPM.2005.053. [DOI] [PubMed] [Google Scholar]
- 58.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178–93. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 59.Rydhstroem H. Gestational duration in the pregnancy after a preterm twin delivery. Am J Obstet Gynecol. 1998;178:136–39. doi: 10.1016/s0002-9378(98)70640-4. [DOI] [PubMed] [Google Scholar]
- 60.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147–48. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Cnattingius S, Granath F, Petersson G, Harlow BL. The influence of gestational age and smoking habits on the risk of subsequent preterm deliveries. N Engl J Med. 1999;341:943–48. doi: 10.1056/NEJM199909233411303. [DOI] [PubMed] [Google Scholar]
- 62.Menard MK, Newman RB, Keenan A, Ebeling M. Prognostic significance of prior preterm twin delivery on subsequent singleton pregnancy. Am J Obstet Gynecol. 1996;174:1429–32. doi: 10.1016/s0002-9378(96)70584-7. [DOI] [PubMed] [Google Scholar]
- 63.Krymko H, Bashiri A, Smolin A, Sheiner E, Bar-David J, Shoham-Vardi I, et al. Risk factors for recurrent preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2004;113:160–63. doi: 10.1016/j.ejogrb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, Cole FS, et al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol. 2007;196:131. doi: 10.1016/j.ajog.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 65.Romero R, Mazor M, Gomez R, Gonzalez R, Galasso M, Cotton D. Cervix, incompetence and premature labor. The Fetus. 1993;3:1–10. [Google Scholar]
- 66.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 67.Rydhstrom H. Twin pregnancy and the effects of prophylactic leave of absence on pregnancy duration and birth weight. Acta Obstet Gynecol Scand. 1988;67:81–84. doi: 10.3109/00016348809004173. [DOI] [PubMed] [Google Scholar]
- 68.Goldenberg RL, Mercer BM, Meis PJ, Copper RL, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:643–48. doi: 10.1016/0029-7844(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 69.Keirse MJ, Ohlsson A, Treffers PE, Kanhani HHH. Prelabour rupture of the membranes preterm. In: Chalmers I, Enkin M, Keirse MJ, editors. Effective care in pregnancy and childbirth. Oxford: Oxford University Press; 1989. p. 666. [Google Scholar]
- 70.Sachs M, Baker TH. Spontaneous premature rupture of the membranes. Am J Obstet Gynecol. 1967;97:888. doi: 10.1016/0002-9378(67)90512-1. [DOI] [PubMed] [Google Scholar]
- 71.Gunn GC, Mishell DR, Jr, Morton DG. Premature rupture of the fetal membranes. A review. Am J Obstet Gynecol. 1970;106:469–83. doi: 10.1016/0002-9378(70)90378-9. [DOI] [PubMed] [Google Scholar]
- 72.Christensen KK, Christensen P, Ingemarsson I, Mardh PA, Nordenfelt E, Ripa T, et al. A study of complications in preterm deliveries after prolonged premature rupture of the membranes. Obstet Gynecol. 1976;48:670–77. [PubMed] [Google Scholar]
- 73.Johnson JW, Daikoku NH, Niebyl JR, Johnson TR, Jr, Khouzami VA, Witter FR. Premature rupture of the membranes and prolonged latency. Obstet Gynecol. 1981;57:547–56. [PubMed] [Google Scholar]
- 74.Daikoku NH, Kaltreider DF, Khouzami VA, Spence M, Johnson JW. Premature rupture of membranes and spontaneous preterm labor: maternal endometritis risks. Obstet Gynecol. 1982;59:13–20. [PubMed] [Google Scholar]
- 75.Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstet Gynecol. 1982;60:671–79. [PubMed] [Google Scholar]
- 76.Shubert PJ, Diss E, Iams JD. Etiology of preterm premature rupture of membranes. Obstet Gynecol Clin North Am. 1992;19:251–63. [PubMed] [Google Scholar]
- 77.Lebherz TB, Hellman LP, Madding R, Anctila A, Arje Sl. Double-Blind Study of Premature Rupture of the Membranes. A Report of 1,896 Cases. Am J Obstet Gynecol. 1963;87:218–25. doi: 10.1016/0002-9378(63)90502-7. [DOI] [PubMed] [Google Scholar]
- 78.Fayez JA, Hasan AA, Jonas HS, Miller GL. Management of premature rupture of the membranes. Obstet Gynecol. 1978;52:17–21. [PubMed] [Google Scholar]
- 79.Naeye RL. Factors that predispose to premature rupture of the fetal membranes. Obstet Gynecol. 1982;60:93–98. [PubMed] [Google Scholar]
- 80.Asrat T, Lewis DF, Garite TJ, Major CA, Nageotte MP, Towers CV, et al. Rate of recurrence of preterm premature rupture of membranes in consecutive pregnancies. Am J Obstet Gynecol. 1991;165:1111–15. doi: 10.1016/0002-9378(91)90481-6. [DOI] [PubMed] [Google Scholar]
- 81.Ekwo EE, Gosselink CA, Moawad A. Unfavorable outcome in penultimate pregnancy and premature rupture of membranes in successive pregnancy. Obstet Gynecol. 1992;80:166–72. [PubMed] [Google Scholar]
- 82.Mercer BM, Goldenberg RL, Meis PJ, Moawad AH, Shellhaas C, Das A, et al. The Preterm Prediction Study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. The National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;183:738–45. doi: 10.1067/mob.2000.106766. [DOI] [PubMed] [Google Scholar]
- 83.Romero R, Tromp G. High-dimensional biology in obstetrics and gynecology: functional genomics in microarray studies. Am J Obstet Gynecol. 2006;195:360–63. doi: 10.1016/j.ajog.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 84.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Ultrasonographic assessment of the uterine cervix and interleukin-8 concentrations in cervical secretions predict intrauterine infection in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 1998;12:86–92. doi: 10.1046/j.1469-0705.1998.12020086.x. [DOI] [PubMed] [Google Scholar]
- 85.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678–89. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 86.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000;15:778–84. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 87.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001;185:586–92. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 88.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–44. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 89.Evaldson G, Malmborg AS, Nord CE, Ostensson K. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest. 1983;15:230–41. doi: 10.1159/000299415. [DOI] [PubMed] [Google Scholar]
- 90.Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- 91.Svinarich DM, Wolf NA, Gomez R, Gonik B, Romero R. Detection of human defensin 5 in reproductive tissues. Am J Obstet Gynecol. 1997;176:470–75. doi: 10.1016/s0002-9378(97)70517-9. [DOI] [PubMed] [Google Scholar]
- 92.Romero R, Gomez R, Araneda H, et al. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol. 1993:A57. [Google Scholar]
- 93.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 94.Yoon BH, Romero R, Moon JB, Oh SY, Han SY, Kim JC, et al. The frequency and clinical significance of intra-amniotic inflammation in patients with a positive cervical fetal fibronectin. Am J Obstet Gynecol. 2001;185:1137–42. doi: 10.1067/mob.2001.118162. [DOI] [PubMed] [Google Scholar]
- 95.Leitich H, Kaider A. Fetal fibronectin--how useful is it in the prediction of preterm birth? BJOG. 2003;110(Suppl;%20):66–70. [PubMed] [Google Scholar]
- 96.Chang TC, Chew TS, Pang M, Tan AC, Yeo GS. Cervicovaginal foetal fibronectin in the prediction of preterm labour in a low-risk population. Ann Acad Med Singapore. 1997;26:776–80. [PubMed] [Google Scholar]
- 97.Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Meis PJ, Das A, et al. The preterm prediction study: patterns of cervicovaginal fetal fibronectin as predictors of spontaneous preterm delivery. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1997;177:8–12. doi: 10.1016/s0002-9378(97)70430-7. [DOI] [PubMed] [Google Scholar]
- 98.Malak TM, Sizmur F, Bell SC, Taylor DJ. Fetal fibronectin in cervicovaginal secretions as a predictor of preterm birth. Br J Obstet Gynaecol. 1996;103:648–53. doi: 10.1111/j.1471-0528.1996.tb09832.x. [DOI] [PubMed] [Google Scholar]
- 99.Parker J, Bell R, Brennecke S. Fetal fibronectin in the cervicovaginal fluid of women with threatened preterm labour as a predictor of delivery before 34 weeks’ gestation. Aust N Z J Obstet Gynaecol. 1995;35:257–61. doi: 10.1111/j.1479-828x.1995.tb01976.x. [DOI] [PubMed] [Google Scholar]
- 100.Burrus DR, Ernest JM, Veille JC. Fetal fibronectin, interleukin-6, and C-reactive protein are useful in establishing prognostic subcategories of idiopathic preterm labor. Am J Obstet Gynecol. 1995;173:1258–62. doi: 10.1016/0002-9378(95)91366-1. [DOI] [PubMed] [Google Scholar]
- 101.Chuileannain FN, Bell R, Brennecke S. Cervicovaginal fetal fibronectin testing in threatened preterm labour--translating research findings into clinical practice. Aust N Z J Obstet Gynaecol. 1998;38:399–402. doi: 10.1111/j.1479-828x.1998.tb03096.x. [DOI] [PubMed] [Google Scholar]
- 102.Goffeng AR, Holst E, Milsom I, Lindstedt G, Lundberg PA, Andersch B. Fetal fibronectin and microorganisms in vaginal fluid of women with complicated pregnancies. Acta Obstet Gynecol Scand. 1997;76:521–27. doi: 10.3109/00016349709024576. [DOI] [PubMed] [Google Scholar]
- 103.Lopez RL, Francis JA, Garite TJ, Dubyak JM. Fetal fibronectin detection as a predictor of preterm birth in actual clinical practice. Am J Obstet Gynecol. 2000;182:1103–06. doi: 10.1067/mob.2000.105411. [DOI] [PubMed] [Google Scholar]
- 104.Nageotte MP, Casal D, Senyei AE. Fetal fibronectin in patients at increased risk for premature birth. Am J Obstet Gynecol. 1994;170:20–25. doi: 10.1016/s0002-9378(94)70376-0. [DOI] [PubMed] [Google Scholar]
- 105.Oliveira T, de SE, Mariani-Neto C, Camano L. Fetal fibronectin as a predictor of preterm delivery in twin gestations. Int J Gynaecol Obstet. 1998;62:135–39. doi: 10.1016/s0020-7292(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 106.Morrison JC, Naef RW, III, Botti JJ, Katz M, Belluomini JM, McLaughlin BN. Prediction of spontaneous preterm birth by fetal fibronectin and uterine activity. Obstet Gynecol. 1996;87:649–55. doi: 10.1016/0029-7844(96)00041-5. [DOI] [PubMed] [Google Scholar]
- 107.Wennerholm UB, Holm B, Mattsby-Baltzer I, Nielsen T, Platz-Christensen J, Sundell G, et al. Fetal fibronectin, endotoxin, bacterial vaginosis and cervical length as predictors of preterm birth and neonatal morbidity in twin pregnancies. Br J Obstet Gynaecol. 1997;104:1398–404. doi: 10.1111/j.1471-0528.1997.tb11010.x. [DOI] [PubMed] [Google Scholar]
- 108.Tolino A, Ronsini S, Zullo F, Pellicano M, Regine V, Nappi C. Fetal fibronectin as a screening test for premature delivery in multiple pregnancies. Int J Gynaecol Obstet. 1996;52:3–7. doi: 10.1016/0020-7292(95)02529-4. [DOI] [PubMed] [Google Scholar]
- 109.Goldenberg RL, Iams JD, Miodovnik M, Van Dorsten JP, Thurnau G, Bottoms S, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047–53. doi: 10.1016/s0002-9378(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 110.Sciscione A, Hoffman MK, DeLuca S, O’Shea A, Benson J, Pollock M, et al. Fetal fibronectin as a predictor of vaginal birth in nulliparas undergoing preinduction cervical ripening. Obstet Gynecol. 2005;106:980–85. doi: 10.1097/01.AOG.0000185288.75896.98. [DOI] [PubMed] [Google Scholar]
- 111.Imai M, Tani A, Saito M, Saito K, Amano K, Nisijima M. Significance of fetal fibronectin and cytokine measurement in the cervicovaginal secretions of women at term in predicting term labor and post-term pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001;97:53–58. doi: 10.1016/s0301-2115(00)00483-8. [DOI] [PubMed] [Google Scholar]
- 112.Mijovic JE, Demianczuk N, Olson DM, Zakar T. Prostaglandin endoperoxide H synthase mRNA expression in the fetal membranes correlates with fetal fibronectin concentration in the cervico-vaginal fluids at term: evidence of enzyme induction before the onset of labour. BJOG. 2000;107:267–73. doi: 10.1111/j.1471-0528.2000.tb11699.x. [DOI] [PubMed] [Google Scholar]
- 113.Rozenberg P, Goffinet F, Hessabi M. Comparison of the Bishop score, ultrasonographically measured cervical length, and fetal fibronectin assay in predicting time until delivery and type of delivery at term. Am J Obstet Gynecol. 2000;182:108–13. doi: 10.1016/s0002-9378(00)70498-4. [DOI] [PubMed] [Google Scholar]
- 114.Kiss H, Ahner R, Hohlagschwandtner M, Leitich H, Husslein P. Fetal fibronectin as a predictor of term labor: a literature review. Acta Obstet Gynecol Scand. 2000;79:3–7. [PubMed] [Google Scholar]
- 115.Garite TJ, Casal D, Garcia-Alonso A, Kreaden U, Jimenez G, Ayala JA, et al. Fetal fibronectin: a new tool for the prediction of successful induction of labor. Am J Obstet Gynecol. 1996;175:1516–21. doi: 10.1016/s0002-9378(96)70100-x. [DOI] [PubMed] [Google Scholar]
- 116.Ahner R, Egarter C, Kiss H, Heinzl K, Zeillinger R, Schatten C, et al. Fetal fibronectin as a selection criterion for induction of term labor. Am J Obstet Gynecol. 1995;173:1513–17. doi: 10.1016/0002-9378(95)90641-x. [DOI] [PubMed] [Google Scholar]
- 117.Ahner R, Kiss H, Egarter C, Zeillinger R, Eppel W, Karas H, et al. Fetal fibronectin as a marker to predict the onset of term labor and delivery. Am J Obstet Gynecol. 1995;172:134–37. doi: 10.1016/0002-9378(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 118.Lockwood CJ, Senyei AE, Dische MR, Casal D, Shah KD, Thung SN, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669–74. doi: 10.1056/NEJM199109053251001. [DOI] [PubMed] [Google Scholar]
- 119.Brady K, Duff P, Yancey MK. Plasma fibronectin concentrations during normal term labor. Obstet Gynecol. 1990;75:619–21. [PubMed] [Google Scholar]
- 120.Grant A. Cervical cerclage to prolong pregnancy. In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective care in pregnancy and childbirth. Vol. 1. New York, NY: Oxford University Press; 1989. pp. 633–46. [Google Scholar]
- 121.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–67. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 122.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 123.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–17. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 124.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–07. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 125.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–67. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 126.Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol. 1990;162:991–93. doi: 10.1016/0002-9378(90)91302-s. [DOI] [PubMed] [Google Scholar]
- 127.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol. 1992;2:402–09. doi: 10.1046/j.1469-0705.1992.02060402.x. [DOI] [PubMed] [Google Scholar]
- 128.Guzman ER, Rosenberg JC, Houlihan C, Ivan J, Waldron R, Knuppel R. A new method using vaginal ultrasound and transfundal pressure to evaluate the asymptomatic incompetent cervix. Obstet Gynecol. 1994;83:248–52. [PubMed] [Google Scholar]
- 129.Guzman ER, Pisatowski DM, Vintzileos AM, Benito CW, Hanley ML, Ananth CV. A comparison of ultrasonographically detected cervical changes in response to transfundal pressure, coughing, and standing in predicting cervical incompetence. Am J Obstet Gynecol. 1997;177:660–65. doi: 10.1016/s0002-9378(97)70161-3. [DOI] [PubMed] [Google Scholar]
- 130.Guzman ER, Vintzileos AM, McLean DA, Martins ME, Benito CW, Hanley ML. The natural history of a positive response to transfundal pressure in women at risk for cervical incompetence. Am J Obstet Gynecol. 1997;176:634–38. doi: 10.1016/s0002-9378(97)70560-x. [DOI] [PubMed] [Google Scholar]
- 131.Guzman ER, Forster JK, Vintzileos AM, Ananth CV, Walters C, Gipson K. Pregnancy outcomes in women treated with elective versus ultrasound-indicated cervical cerclage. Ultrasound Obstet Gynecol. 1998;12:323–27. doi: 10.1046/j.1469-0705.1998.12050323.x. [DOI] [PubMed] [Google Scholar]
- 132.Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks’ gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol. 1998;92:31–37. doi: 10.1016/s0029-7844(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 133.Berghella V, Daly SF, Tolosa JE, DiVito MM, Chalmers R, Garg N, et al. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high-risk pregnancies: does cerclage prevent prematurity? Am J Obstet Gynecol. 1999;181:809–15. doi: 10.1016/s0002-9378(99)70306-6. [DOI] [PubMed] [Google Scholar]
- 134.Hassan SS, Romero R, Maymon E, Berry SM, Blackwell SC, Treadwell MC, et al. Does cervical cerclage prevent preterm delivery in patients with a short cervix? Am J Obstet Gynecol. 2001;184:1325–29. doi: 10.1067/mob.2001.115119. [DOI] [PubMed] [Google Scholar]
- 135.Macdonald R, Smith P, Vyas S. Cervical incompetence: the use of transvaginal sonography to provide an objective diagnosis. Ultrasound Obstet Gynecol. 2001;18:211–16. doi: 10.1046/j.1469-0705.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- 136.Rust OA, Atlas RO, Reed J, van GJ, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 137.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–03. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 138.Berghella V, Haas S, Chervoneva I, Hyslop T. Patients with prior second-trimester loss: prophylactic cerclage or serial transvaginal sonograms? Am J Obstet Gynecol. 2002;187:747–51. doi: 10.1067/mob.2002.124289. [DOI] [PubMed] [Google Scholar]
- 139.Williams M, Iams JD. Cervical length measurement and cervical cerclage to prevent preterm birth. Clin Obstet Gynecol. 2004;47:775–83. doi: 10.1097/01.grf.0000141895.85221.be. [DOI] [PubMed] [Google Scholar]
- 140.Althuisius SM, Dekker GA. A five century evolution of cervical incompetence as a clinical entity. Curr Pharm Des. 2005;11:687–97. doi: 10.2174/1381612053381909. [DOI] [PubMed] [Google Scholar]
- 141.Ramin KD, Ogburn PL, Jr, Mulholland TA, Breckle RJ, Ramsey PS. Ultrasonographic assessment of cervical length in triplet pregnancies. Am J Obstet Gynecol. 1999;180:1442–45. doi: 10.1016/s0002-9378(99)70033-5. [DOI] [PubMed] [Google Scholar]
- 142.Souka AP, Heath V, Flint S, Sevastopoulou I, Nicolaides KH. Cervical length at 23 weeks in twins in predicting spontaneous preterm delivery. Obstet Gynecol. 1999;94:450–54. doi: 10.1016/s0029-7844(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 143.Guzman ER, Walters C, O’reilly-Green C, Kinzler WL, Waldron R, Nigam J, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103–07. doi: 10.1067/mob.2000.108896. [DOI] [PubMed] [Google Scholar]
- 144.Guzman ER, Walters C, O’reilly-Green C, Meirowitz NB, Gipson K, Nigam J, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in triplet gestations. Am J Obstet Gynecol. 2000;183:1108–13. doi: 10.1067/mob.2000.108875. [DOI] [PubMed] [Google Scholar]
- 145.To MS, Skentou C, Cicero S, Liao AW, Nicolaides KH. Cervical length at 23 weeks in triplets: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2000;16:515–18. doi: 10.1046/j.1469-0705.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 146.Yang JH, Kuhlman K, Daly S, Berghella V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound Obstet Gynecol. 2000;15:288–91. doi: 10.1046/j.1469-0705.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 147.Maymon R, Herman A, Jauniaux E, Frenkel J, Ariely S, Sherman D. Transvaginal sonographic assessment of cervical length changes during triplet gestation. Hum Reprod. 2001;16:956–60. doi: 10.1093/humrep/16.5.956. [DOI] [PubMed] [Google Scholar]
- 148.Skentou C, Souka AP, To MS, Liao AW, Nicolaides KH. Prediction of preterm delivery in twins by cervical assessment at 23 weeks. Ultrasound Obstet Gynecol. 2001;17:7–10. doi: 10.1046/j.1469-0705.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 149.Vayssiere C, Favre R, Audibert F, Chauvet MP, Gaucherand P, Tardif D, et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596–604. doi: 10.1067/mob.2002.127380. [DOI] [PubMed] [Google Scholar]
- 150.PARIKH MN, MEHTA AC. Internal cervical os during the second half of pregnancy. J Obstet Gynaecol Br Emp. 1961;68:818–21. doi: 10.1111/j.1471-0528.1961.tb02817.x. [DOI] [PubMed] [Google Scholar]
- 151.Guzman ER, Mellon R, Vintzileos AM, Ananth CV, Walters C, Gipson K. Relationship between endocervical canal length between 15–24 weeks gestation and obstetric history. J Matern Fetal Med. 1998;7:269–72. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<269::AID-MFM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 152.Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J Clin Ultrasound. 1991;19:77–83. doi: 10.1002/jcu.1870190204. [DOI] [PubMed] [Google Scholar]
- 153.Romero R. Prenatal Medicine: the child is the father of the man. Prenatal and Neonatal Medicine. 1996;1:8–11. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 154.Moinian M, Andersch B. Does cervix conization increase the risk of complications in subsequent pregnancies? Acta Obstet Gynecol Scand. 1982;61:101–03. doi: 10.3109/00016348209156537. [DOI] [PubMed] [Google Scholar]
- 155.Kristensen J, Langhoff-Roos J, Wittrup M, Bock JE. Cervical conization and preterm delivery/low birth weight. A systematic review of the literature Acta. Obstet Gynecol Scand. 1993;72:640–44. doi: 10.3109/00016349309021157. [DOI] [PubMed] [Google Scholar]
- 156.Raio L, Ghezzi F, Di Naro E, Gomez R, Luscher KP. Duration of pregnancy after carbon dioxide laser conization of the cervix: influence of cone height. Obstet Gynecol. 1997;90:978–82. doi: 10.1016/s0029-7844(97)00489-4. [DOI] [PubMed] [Google Scholar]
- 157.Craig CJ. Congenital abnormalities of the uterus and foetal wastage. S Afr Med J. 1973;47:2000–05. [PubMed] [Google Scholar]
- 158.Mangan CE, Borow L, Burtnett-Rubin MM, Egan V, Giuntoli RL, Mikuta JJ. Pregnancy outcome in 98 women exposed to diethylstilbestrol in utero, their mothers, and unexposed siblings. Obstet Gynecol. 1982;59:315–19. [PubMed] [Google Scholar]
- 159.Ludmir J, Landon MB, Gabbe SG, Samuels P, Mennuti MT. Management of the diethylstilbestrol-exposed pregnant patient: a prospective study. Am J Obstet Gynecol. 1987;157:665–69. doi: 10.1016/s0002-9378(87)80025-x. [DOI] [PubMed] [Google Scholar]
- 160.Levine RU, Berkowitz KM. Conservative management and pregnancy outcome in diethylstilbestrol-exposed women with and without gross genital tract abnormalities. Am J Obstet Gynecol. 1993;169:1125–29. doi: 10.1016/0002-9378(93)90267-m. [DOI] [PubMed] [Google Scholar]
- 161.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–55. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 162.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–91. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 163.Bengtsson LP. Cervical insufficiency. Acta Obstet Gynecol Scand. 1968;47(Suppl-35) doi: 10.3109/00016346809156843. [DOI] [PubMed] [Google Scholar]
- 164.Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol. 1978;130:414–18. doi: 10.1016/0002-9378(78)90282-x. [DOI] [PubMed] [Google Scholar]
- 165.Sherman AI. Hormonal therapy for control of the incompetent os of pregnancy. Obstet Gynecol. 1966;28:198–205. doi: 10.1097/00003081-196608000-00010. [DOI] [PubMed] [Google Scholar]
- 166.Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192:554–57. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 167.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 168.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Blackmore-Prince C, Kieke B, Jr, Kugaraj KA, Ferre C, Elam-Evans LD, Krulewitch CJ, et al. Racial differences in the patterns of singleton preterm delivery in the 1988 National Maternal and Infant Health Survey. Matern Child Health J. 1999;3:189–97. doi: 10.1023/a:1022373205005. [DOI] [PubMed] [Google Scholar]
- 170.Simhan HN, Bodnar LM. Prepregnancy body mass index, vaginal inflammation, and the racial disparity in preterm birth. Am J Epidemiol. 2006;163:459–66. doi: 10.1093/aje/kwj053. [DOI] [PubMed] [Google Scholar]
- 171.Ananth CV, Misra DP, Demissie K, Smulian JC. Rates of preterm delivery among Black women and White women in the United States over two decades: an age-period-cohort analysis. Am J Epidemiol. 2001;154:657–65. doi: 10.1093/aje/154.7.657. [DOI] [PubMed] [Google Scholar]
- 172.State-specific changes in singleton preterm births among black and white women--United States, 1990 and 1997. MMWR Morb Mortal Wkly Rep. 2000;49:837–40. [PubMed] [Google Scholar]
- 173.Schieve LA, Handler A. Preterm delivery and perinatal death among black and white infants in a Chicago-area perinatal registry. Obstet Gynecol. 1996;88:356–63. doi: 10.1016/0029-7844(96)00203-7. [DOI] [PubMed] [Google Scholar]
- 174.Blackmore CA, Savitz DA, Edwards LJ, Harlow SD, Bowes WA., Jr Racial differences in the patterns of preterm delivery in central North Carolina, USA. Paediatr Perinat Epidemiol. 1995;9:281–95. doi: 10.1111/j.1365-3016.1995.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 175.Schulman ED. Preterm delivery among women in the South Carolina Medicaid High Risk Channeling Project. J Health Care Poor Underserved. 1995;6:352–67. doi: 10.1353/hpu.2010.0053. [DOI] [PubMed] [Google Scholar]
- 176.Savitz DA, Dole N, Herring AH, Kaczor D, Murphy J, Siega-Riz AM, et al. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19:97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 177.Merlino A, Laffineuse L, Collin M, Mercer B. Impact of weight loss between pregnancies on recurrent preterm birth. Am J Obstet Gynecol. 2006;195:818–21. doi: 10.1016/j.ajog.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 178.Yost NP, Owen J, Berghella V, Thom E, Swain M, Dildy GA, III, et al. Effect of coitus on recurrent preterm birth. Obstet Gynecol. 2006;107:793–97. doi: 10.1097/01.AOG.0000206757.92602.b5. [DOI] [PubMed] [Google Scholar]
- 179.Klebanoff MA, Nugent RP, Rhoads GG. Coitus during pregnancy: is it safe? Lancet. 1984;%20;2:914–17. doi: 10.1016/s0140-6736(84)90665-2. [DOI] [PubMed] [Google Scholar]
- 180.Berghella V, Klebanoff M, McPherson C, Carey JC, Hauth JC, Ernest JM, et al. Sexual intercourse association with asymptomatic bacterial vaginosis and Trichomonas vaginalis treatment in relationship to preterm birth. Am J Obstet Gynecol. 2002;187:1277–82. doi: 10.1067/mob.2002.127134. [DOI] [PubMed] [Google Scholar]
- 181.Kurki T, Ylikorkala O. Coitus during pregnancy is not related to bacterial vaginosis or preterm birth. Am J Obstet Gynecol. 1993;169:1130–34. doi: 10.1016/0002-9378(93)90268-n. [DOI] [PubMed] [Google Scholar]
- 182.Read JS, Klebanoff MA. Sexual intercourse during pregnancy and preterm delivery: effects of vaginal microorganisms. The Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1993;168:514–19. doi: 10.1016/0002-9378(93)90484-z. [DOI] [PubMed] [Google Scholar]
- 183.Brustman LE, Raptoulis M, Langer O, Anyaegbunam A, Merkatz IR. Changes in the pattern of uterine contractility in relationship to coitus during pregnancies at low and high risk for preterm labor. Obstet Gynecol. 1989;73:166–68. [PubMed] [Google Scholar]
- 184.Wagner NN, Butler JC, Sanders JP. Prematurity and orgasmic coitus during pregnancy: data on a small sample. Fertil Steril. 1976;27:911–15. doi: 10.1016/s0015-0282(16)42011-x. [DOI] [PubMed] [Google Scholar]
- 185.Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, et al. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178:562–67. doi: 10.1016/s0002-9378(98)70439-9. [DOI] [PubMed] [Google Scholar]
- 186.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195:1557–63. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 187.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995;173:1231–35. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 188.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 189.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 190.Romero R, Sepulveda W, Baumann P, Yoon BH, Brandt F, Gomez R, et al. The preterm labor syndrome: Biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J Obstet Gynecol. 1993:288. [Google Scholar]
- 191.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–16. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 192.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 193.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Idiopathic vaginal bleeding during pregnancy as the only clinical manifestation of intrauterine infection. J Matern Fetal Neonatal Med. 2005;18:31–37. doi: 10.1080/14767050500217863. [DOI] [PubMed] [Google Scholar]
- 194.Espinoza J, Goncalves LF, Romero R, Nien JK, Stites S, Kim YM, et al. The prevalence and clinical significance of amniotic fluid ‘sludge’ in patients with preterm labor and intact membranes. Ultrasound Obstet Gynecol. 2005;25:346–52. doi: 10.1002/uog.1871. [DOI] [PubMed] [Google Scholar]
- 195.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–28. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 196.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 197.Edwards RK, Clark P, Locksmith GJ, Duff P. Performance characteristics of putative tests for subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 2001;9:209–14. doi: 10.1155/S1064744901000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 199.Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. The diagnostic value of interleukin-8 and fetal fibronectin concentrations in cervical secretions in patients with preterm labor and intact membranes. J Perinat Med. 1997;25:461–68. doi: 10.1515/jpme.1997.25.6.461. [DOI] [PubMed] [Google Scholar]
- 200.Garry D, Figueroa R, guero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–41. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 201.Rizzo G, Capponi A, Rinaldo D, Tedeschi D, Arduini D, Romanini C. Interleukin-6 concentrations in cervical secretions identify microbial invasion of the amniotic cavity in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1996;175:812–17. doi: 10.1016/s0002-9378(96)80004-4. [DOI] [PubMed] [Google Scholar]
- 202.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand. 1996;75:624–27. doi: 10.3109/00016349609054686. [DOI] [PubMed] [Google Scholar]
- 203.Mazor M, Furman B, Wiznitzer A, Shoham-Vardi I, Cohen J, Ghezzi F. Maternal and perinatal outcome of patients with preterm labor and meconium-stained amniotic fluid. Obstet Gynecol. 1995;86:830–33. doi: 10.1016/0029-7844(95)00265-S. [DOI] [PubMed] [Google Scholar]
- 204.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, et al. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184–93. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 205.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–16. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 206.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–31. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 207.Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 208.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 209.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–66. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 210.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769–78. doi: 10.1097/00003081-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 211.Romero R, Baumann P, Gomez R, Salafia C, Rittenhouse L, Barberio D, et al. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol. 1993;168:1654–64. doi: 10.1016/0002-9378(93)90675-9. [DOI] [PubMed] [Google Scholar]
- 212.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–42. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 213.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–69. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 214.Lyall F. Mechanisms regulating cytotrophoblast invasion in normal pregnancy and pre-eclampsia. Aust N Z J Obstet Gynaecol. 2006;46:266–73. doi: 10.1111/j.1479-828X.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 215.Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol. 1967;93:581–92. doi: 10.1002/path.1700930219. [DOI] [PubMed] [Google Scholar]
- 216.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:876–81. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 217.Sheppard BL, Bonnar J. Ultrastructural abnormalities of placental villi in placentae from pregnancies complicated by intrauterine fetal growth retardation: their relationship to decidual spiral arterial lesions. Placenta. 1980;1:145–56. doi: 10.1016/s0143-4004(80)80023-3. [DOI] [PubMed] [Google Scholar]
- 218.Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–99. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- 219.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 220.Redman CW. Immunological aspects of pre-eclampsia. Baillieres Clin Obstet Gynaecol. 1992;6:601–15. doi: 10.1016/s0950-3552(05)80012-4. [DOI] [PubMed] [Google Scholar]
- 221.Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9 (Suppl 1):131–61. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 222.Gorodeski IG, Geier A, Lunenfeld B, Beery R, Bahary CM. Progesterone (P) receptor dynamics in estrogen primed normal human cervix following P injection. Fertil Steril. 1987;47:108–13. doi: 10.1016/s0015-0282(16)49944-9. [DOI] [PubMed] [Google Scholar]
- 223.Stjernholm Y, Sahlin L, Akerberg S, Elinder A, Eriksson HA, Malmstrom A, et al. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol. 1996;174:1065–71. doi: 10.1016/s0002-9378(96)70352-6. [DOI] [PubMed] [Google Scholar]
- 224.Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675–80. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- 225.Hill LM, Johnson CE, Lee RA. Prophylactic use of hydroxyprogesterone caproate in abdominal surgery during pregnancy. A retrospective evaluation. Obstet Gynecol. 1975;46:287–90. [PubMed] [Google Scholar]
- 226.Breart G, Lanfranchi M, Chavigny C, Rumeau-Rouquette C, Sureau C. A comparative study of the efficiency of hydroxyprogesterone caproate and of chlormadinone acetate in the prevention of premature labor. Int J Gynaecol Obstet. 1979;16:381–84. doi: 10.1002/j.1879-3479.1979.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 227.Johnson JW, Lee PA, Zachary AS, Calhoun S, Migeon CJ. High-risk prematurity--progestin treatment and steroid studies. Obstet Gynecol. 1979;54:412–18. [PubMed] [Google Scholar]
- 228.Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol. 1980;56:692–95. [PubMed] [Google Scholar]
- 229.Hauth JC, Gilstrap LC, III, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983;146:187–90. doi: 10.1016/0002-9378(83)91051-7. [DOI] [PubMed] [Google Scholar]
- 230.Yemini M, Borenstein R, Dreazen E, Apelman Z, Mogilner BM, Kessler I, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151:574–77. doi: 10.1016/0002-9378(85)90141-3. [DOI] [PubMed] [Google Scholar]
- 231.Goldstein P, Berrier J, Rosen S, Sacks HS, Chalmers TC. A meta-analysis of randomized control trials of progestational agents in pregnancy. Br J Obstet Gynaecol. 1989;96:265–74. doi: 10.1111/j.1471-0528.1989.tb02385.x. [DOI] [PubMed] [Google Scholar]
- 232.Daya S. Efficacy of progesterone support for pregnancy in women with recurrent miscarriage. A meta-analysis of controlled trials. Br J Obstet Gynaecol. 1989;96:275–80. doi: 10.1111/j.1471-0528.1989.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 233.Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol. 1990;97:149–54. doi: 10.1111/j.1471-0528.1990.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 234.Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol. 1990;97:149–54. doi: 10.1111/j.1471-0528.1990.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 235.Noblot G, Audra P, Dargent D, Faguer B, Mellier G. The use of micronized progesterone in the treatment of menace of preterm delivery. Eur J Obstet Gynecol Reprod Biol. 1991;40:203–09. doi: 10.1016/0028-2243(91)90118-5. [DOI] [PubMed] [Google Scholar]
- 236.Iams JD. Supplemental progesterone to prevent preterm birth. Am J Obstet Gynecol. 2003;188:303. doi: 10.1067/mob.2003.194. [DOI] [PubMed] [Google Scholar]
- 237.Brancazio LR, Murtha AP, Heine RP. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;349:1087–88. doi: 10.1056/NEJM200309113491115. [DOI] [PubMed] [Google Scholar]
- 238.Doggrell SA. Recurrent hope for the treatment of preterm delivery. Expert Opin Pharmacother. 2003;4:2363–66. doi: 10.1517/14656566.4.12.2363. [DOI] [PubMed] [Google Scholar]
- 239.Einstein FH, Bracero LA. Progesterone and preterm birth. Am J Obstet Gynecol. 2004;190:1798–99. doi: 10.1016/j.ajog.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 240.Keirse MJ. Progesterone and preterm: seventy years of “deja vu” or “still to be seen”? Birth. 2004;31:230–35. doi: 10.1111/j.0730-7659.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 241.Di Renzo GC, Mattei A, Gojnic M, Gerli S. Progesterone and pregnancy. Curr Opin Obstet Gynecol. 2005;17:598–600. doi: 10.1097/01.gco.0000191899.84567.4d. [DOI] [PubMed] [Google Scholar]
- 242.Ness A, Dias T, Damus K, Burd I, Berghella V. Impact of the recent randomized trials on the use of progesterone to prevent preterm birth: a 2005 follow-up survey. Am J Obstet Gynecol. 2006;195:1174–79. doi: 10.1016/j.ajog.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 243.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–24. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 244.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–85. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 245.Spong CY, Meis PJ, Thom EA, Sibai B, Dombrowski MP, Moawad AH, et al. Progesterone for prevention of recurrent preterm birth: impact of gestational age at previous delivery. Am J Obstet Gynecol. 2005;193:1127–31. doi: 10.1016/j.ajog.2005.05.077. [DOI] [PubMed] [Google Scholar]
- 246.Caritis S, Rouse DJ. A Randomized Controlled Trial of 17-Hydroxiprogesterone Caproate (17-OHPC) for the Prevention of Preterm Birth in Twins. Am J Obstet Gynecol. 2007;6:S2. doi: 10.1097/AOG.0b013e318193c677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth. Cochrane Database Syst Rev. 2006:CD004947. doi: 10.1002/14651858.CD004947.pub2. [DOI] [PubMed] [Google Scholar]
- 248.Dodd JM, Crowther CA, Cincotta R, Flenady V, Robinson JS. Progesterone supplementation for preventing preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2005;84:526–33. doi: 10.1111/j.0001-6349.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 249.Sanchez-Ramos L, Kaunitz AM, Delke I. Progestational agents to prevent preterm birth: a meta-analysis of randomized controlled trials. Obstet Gynecol. 2005;105:273–79. doi: 10.1097/01.AOG.0000150559.59531.b2. [DOI] [PubMed] [Google Scholar]
- 250.Odibo AO, Stamilio DM, Macones GA, Polsky D. 17alpha-hydroxyprogesterone caproate for the prevention of preterm delivery: A cost-effectiveness analysis. Obstet Gynecol. 2006;108:492–99. doi: 10.1097/01.AOG.0000232503.92206.d8. [DOI] [PubMed] [Google Scholar]
- 251.Advisory Committee for Reproductive Health Drugs Meeting - the safety and efficacy of New Drug Application (NDA) 21-945), proposed trade name Gestiva, 17 alpha-hydroxyprogesterone caproate injection, 250 mg/mL, Adeza Biomedical, for the proposed indication prevention of preterm delivery in women with a history of a prior preterm delivery. http://wwwfdagov/ohrms/dockets/ac/06/minutes/2006-4227M1pdf. 8-29-2006.
- 252.MS, Christian RLBPC. Embryo–fetal toxicity signals for 17a-hydroxyprogesterone caproate in high-risk pregnancies: A review of the non-clinical literature for embryo–fetal toxicity with progestins. J Matern Fetal Neonatal Med. 2007;20:89–112. doi: 10.1080/14767050601178758. [DOI] [PubMed] [Google Scholar]
- 253.McDonald H, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007:CD000262. doi: 10.1002/14651858.CD000262.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 255.Carey JC, Klebanoff MA. What have we learned about vaginal infections and preterm birth? Semin Perinatol. 2003;27:212–16. doi: 10.1016/s0146-0005(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 256.Riggs MA, Klebanoff MA. Treatment of vaginal infections to prevent preterm birth: a meta-analysis. Clin Obstet Gynecol. 2004;47:796–807. doi: 10.1097/01.grf.0000141450.61310.81. [DOI] [PubMed] [Google Scholar]
- 257.Odendaal HJ, Popov I, Schoeman J, Smith M, Grove D. Preterm labour--is bacterial vaginosis involved? S Afr Med J. 2002;92:231–34. [PubMed] [Google Scholar]
- 258.Kekki M, Kurki T, Pelkonen J, Kurkinen-Raty M, Cacciatore B, Paavonen J. Vaginal clindamycin in preventing preterm birth and peripartal infections in asymptomatic women with bacterial vaginosis: a randomized, controlled trial. Obstet Gynecol. 2001;97:643–48. doi: 10.1016/s0029-7844(01)01321-7. [DOI] [PubMed] [Google Scholar]
- 259.Lamont RF, Jones BM, Mandal D, Hay PE, Sheehan M. The efficacy of vaginal clindamycin for the treatment of abnormal genital tract flora in pregnancy. Infect Dis Obstet Gynecol. 2003;11:181–89. doi: 10.1080/10647440300025519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kekki M, Kurki T, Kotomaki T, Sintonen H, Paavonen J. Cost-effectiveness of screening and treatment for bacterial vaginosis in early pregnancy among women at low risk for preterm birth. Acta Obstet Gynecol Scand. 2004;83:27–36. doi: 10.1111/j.1600-0412.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 261.Larsson PG, Fahraeus L, Carlsson B, Jakobsson T, Forsum U. Late miscarriage and preterm birth after treatment with clindamycin: a randomised consent design study according to Zelen. BJOG. 2006;113:629–37. doi: 10.1111/j.1471-0528.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 262.Morency AM, Bujold E. The effect of second-trimester antibiotic therapy on the rate of preterm birth. J Obstet Gynaecol Can. 2007;29:35–44. doi: 10.1016/s1701-2163(16)32350-7. [DOI] [PubMed] [Google Scholar]
- 263.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstet Gynecol. 2006;194:617–23. doi: 10.1016/j.ajog.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 264.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194:630–37. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 265.Briggs RM, Thompson WB., Jr Treatment of the incompetent cervix. Obstet Gynecol. 1960;16:414–18. [Google Scholar]
- 266.Seppala M, Vara P. Cervical cerclage in the treatment of incompetent cervix. A retrospective analysis of the indications and results of 164 operations Acta. Obstet Gynecol Scand. 1970;49:343–46. doi: 10.3109/00016347009157264. [DOI] [PubMed] [Google Scholar]
- 267.Robboy MS. The management of cervical incompetence. UCLA experience with cerclage procedures. Obstet Gynecol. 1973;41:108–12. [PubMed] [Google Scholar]
- 268.Crombleholme WR, Minkoff HL, Delke I, Schwarz RH. Cervical cerclage: an aggressive approach to threatened or recurrent pregnancy wastage. Am J Obstet Gynecol. 1983;146:168–74. doi: 10.1016/0002-9378(83)91048-7. [DOI] [PubMed] [Google Scholar]
- 269.Lazar P, Gueguen S, Dreyfus J, Renaud R, Pontonnier G, Papiernik E. Multicentred controlled trial of cervical cerclage in women at moderate risk of preterm delivery. Br J Obstet Gynaecol. 1984;91:731–35. doi: 10.1111/j.1471-0528.1984.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 270.Rush RW, Isaacs S, McPherson K, Jones L, Chalmers I, Grant A. A randomized controlled trial of cervical cerclage in women at high risk of spontaneous preterm delivery. Br J Obstet Gynaecol. 1984;91:724–30. doi: 10.1111/j.1471-0528.1984.tb04840.x. [DOI] [PubMed] [Google Scholar]
- 271.Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. MRC/RCOG Working Party on Cervical Cerclage. Br J Obstet Gynaecol. 1993;100:516–23. doi: 10.1111/j.1471-0528.1993.tb15300.x. [DOI] [PubMed] [Google Scholar]
- 272.Ayhan A, Mercan R, Tuncer ZS, Tuncer R, Kisnisci HA. Postconceptional cervical cerclage. Int J Gynaecol Obstet. 1993;42:243–46. doi: 10.1016/0020-7292(93)90218-l. [DOI] [PubMed] [Google Scholar]
- 273.Golan A, Wolman I, Arieli S, Barnan R, Sagi J, David MP. Cervical cerclage for the incompetent cervical os. Improving the fetal salvage rate. J Reprod Med. 1995;40:367–70. [PubMed] [Google Scholar]
- 274.Olatunbosun OA, al Nuaim L, Turnell RW. Emergency cerclage compared with bed rest for advanced cervical dilatation in pregnancy. Int Surg. 1995;80:170–74. [PubMed] [Google Scholar]
- 275.Guzman ER, Houlihan C, Vintzileos A, Ivan J, Benito C, Kappy K. The significance of transvaginal ultrasonographic evaluation of the cervix in women treated with emergency cerclage. Am J Obstet Gynecol. 1996;175:471–76. doi: 10.1016/s0002-9378(96)70164-3. [DOI] [PubMed] [Google Scholar]
- 276.Kurup M, Goldkrand JW. Cervical incompetence: elective, emergent, or urgent cerclage. Am J Obstet Gynecol. 1999;181:240–46. doi: 10.1016/s0002-9378(99)70542-9. [DOI] [PubMed] [Google Scholar]
- 277.Althuisius SM, Dekker GA, van Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial (CIPRACT): study design and preliminary results. Am J Obstet Gynecol. 2000;183:823–29. doi: 10.1067/mob.2000.108874. [DOI] [PubMed] [Google Scholar]
- 278.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–12. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 279.Guzman ER, Ananth CV. Cervical length and spontaneous prematurity: laying the foundation for future interventional randomized trials for the short cervix. Ultrasound Obstet Gynecol. 2001;18:195–99. doi: 10.1046/j.0960-7692.2001.525.x. [DOI] [PubMed] [Google Scholar]
- 280.Novy MJ, Gupta A, Wothe DD, Gupta S, Kennedy KA, Gravett MG. Cervical cerclage in the second trimester of pregnancy: a historical cohort study. Am J Obstet Gynecol. 2001;184:1447–54. doi: 10.1067/mob.2001.114854. [DOI] [PubMed] [Google Scholar]
- 281.Althuisius SM, Dekker GA, van Geijn HP. Cervical incompetence: a reappraisal of an obstetric controversy. Obstet Gynecol Surv. 2002;57:377–87. doi: 10.1097/00006254-200206000-00023. [DOI] [PubMed] [Google Scholar]
- 282.To MS, Palaniappan V, Skentou C, Gibb D, Nicolaides KH. Elective cerclage vs. ultrasound-indicated cerclage in high-risk pregnancies. Ultrasound Obstet Gynecol. 2002;19:475–77. doi: 10.1046/j.1469-0705.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 283.Althuisius SM, Dekker GA, Hummel P, van Geijn HP. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2003;189:907–10. doi: 10.1067/s0002-9378(03)00718-x. [DOI] [PubMed] [Google Scholar]
- 284.Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clin Obstet Gynecol. 2003;46:947–62. doi: 10.1097/00003081-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 285.Odibo AO, Elkousy M, Ural SH, Macones GA. Prevention of preterm birth by cervical cerclage compared with expectant management: a systematic review. Obstet Gynecol Surv. 2003;58:130–36. doi: 10.1097/01.OGX.0000047740.21512.FC. [DOI] [PubMed] [Google Scholar]
- 286.To MS, Alfirevic Z, Heath VC, Cicero S, Cacho AM, Williamson PR, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–53. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 287.Belej-Rak T, Okun N, Windrim R, Ross S, Hannah ME. Effectiveness of cervical cerclage for a sonographically shortened cervix: a systematic review and meta-analysis. Am J Obstet Gynecol. 2003;189:1679–87. doi: 10.1016/s0002-9378(03)00871-8. [DOI] [PubMed] [Google Scholar]
- 288.Drakeley AJ, Roberts D, Alfirevic Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane Database Syst Rev. 2003:CD003253. doi: 10.1002/14651858.CD003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Drakeley AJ, Roberts D, Alfirevic Z. Cervical cerclage for prevention of preterm delivery: meta-analysis of randomized trials. Obstet Gynecol. 2003;102:621–27. doi: 10.1016/s0029-7844(03)00673-2. [DOI] [PubMed] [Google Scholar]
- 290.Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183:830–35. doi: 10.1067/mob.2000.109040. [DOI] [PubMed] [Google Scholar]
- 291.Higgins SP, Kornman LH, Bell RJ, Brennecke SP. Cervical surveillance as an alternative to elective cervical cerclage for pregnancy management of suspected cervical incompetence. Aust N Z J Obstet Gynaecol. 2004;44:228–32. doi: 10.1111/j.1479-828X.2004.00220.x. [DOI] [PubMed] [Google Scholar]
- 292.Sakai M, Shiozaki A, Tabata M, Sasaki Y, Yoneda S, Arai T, et al. Evaluation of effectiveness of prophylactic cerclage of a short cervix according to interleukin-8 in cervical mucus. Am J Obstet Gynecol. 2006;194:14–19. doi: 10.1016/j.ajog.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 293.Goldenberg RL, Klebanoff M, Carey JC, Macpherson C. Metronidazole treatment of women with a positive fetal fibronectin test result. Am J Obstet Gynecol. 2001;185:485–86. doi: 10.1067/mob.2001.115998. [DOI] [PubMed] [Google Scholar]
- 294.Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study. BJOG. 2006;113:65–74. doi: 10.1111/j.1471-0528.2005.00788.x. [DOI] [PubMed] [Google Scholar]